Extracellular Vesicles from Red Blood Cells and Their Evolving Roles in Health, Coagulopathy and Therapy
Abstract
:1. Introduction
2. EV Biogenesis
2.1. Exosome Biogenesis
2.2. MV Biogenesis
3. Molecular Composition of RBCEVs
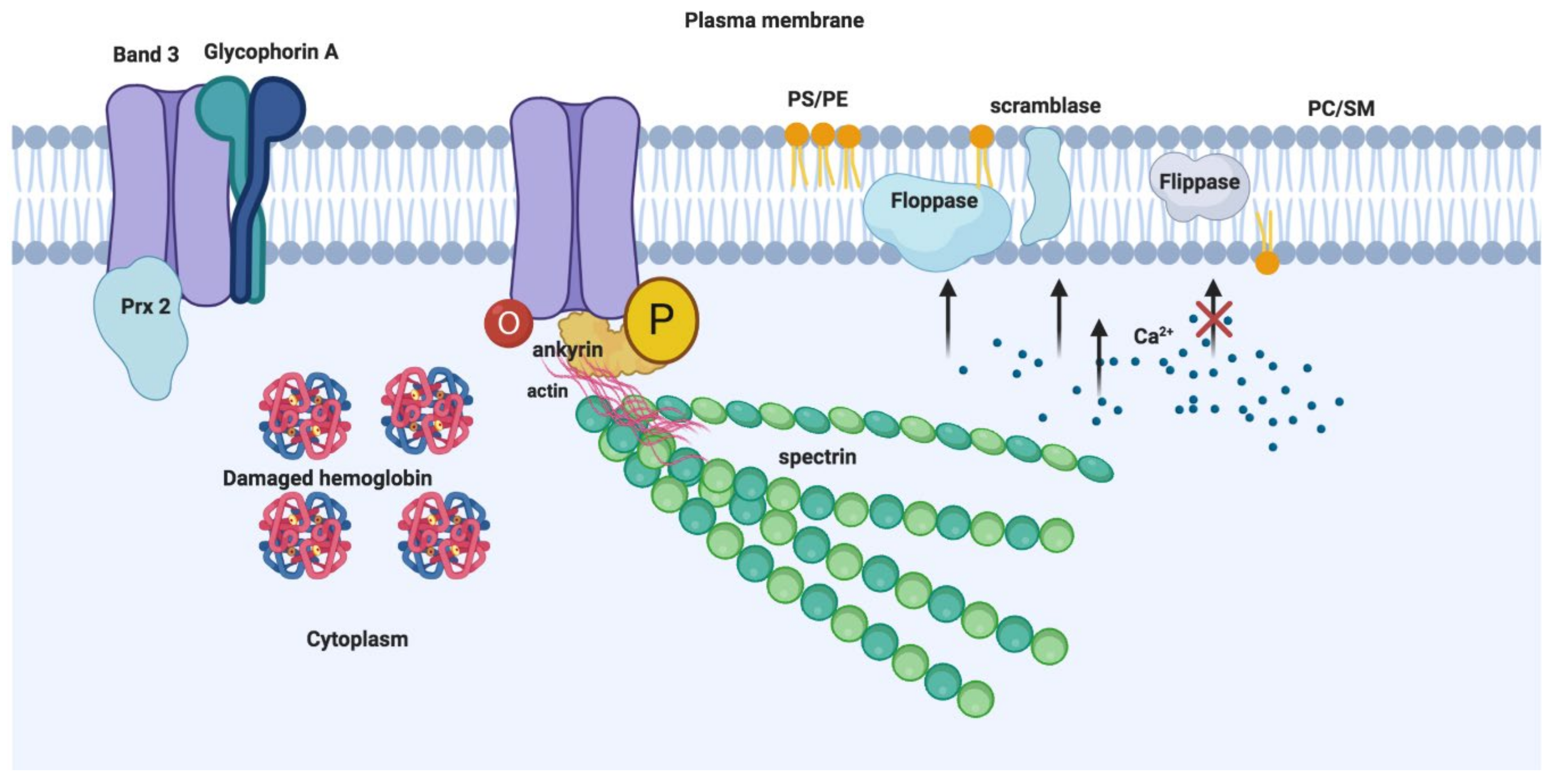
4. Biological Roles of RBCEVs
4.1. Nitric Oxide Homeostasis
4.2. Redox Balance
4.3. Immunomodulation
4.4. Critical Role for RBCEVs in Coagulopathy
4.4.1. Pro-Coagulant RBCEVs Generated under Blood Banking Conditions
4.4.2. Pro-Coagulant RBCEVs Generated in Health and Disease
5. Therapeutic Opportunities for RBCEVs
6. Conclusions
Supplementary Materials
Funding
Conflicts of Interest
Abbreviations
| AChE | Acetylcholinesterase |
| AFM | Atomic force microscopy |
| AML | Acute myeloid leukemia |
| ARRDC | Arrestin-domain-containing protein 1 |
| ARMMs | Arrestin-domain-containing protein 1 (ARRDC1) -mediated microvesicles |
| ATP | Adenosine triphosphate |
| BT | Beta thalassemia |
| CNS | Central nervous system |
| DLS | Dynamic light scattering |
| DPG | 2,3-diphosphoglycerate |
| EM | Electron microscopy |
| EM | Exosome mimetics |
| ESCRT | Endosomal sorting complex required for transport |
| ETP | Endogenous thrombin potential |
| EVs | Extracellular vesicles |
| FC | Flow-cytometry |
| GPI | Glycosylphosphatidylinositol |
| Hb | Hemoglobin |
| HbSC | Hemoglobin SC |
| HRP II | Plasmodium falciparum histidine rich protein II |
| Ig | Immunoglobulins |
| ILVs | Intra-luminal vesicles |
| ITP | Immune thrombocytopenia |
| LC-MS | Tandem mass spectrometry |
| LPA | lysophosphatidic acid |
| miRNA | MicroRNA |
| MNP | Magnetic nanoparticles |
| MVs | Microvesicles |
| MVB | Multivesicular bodies |
| NO | Nitric oxide |
| NP | Nanoparticles |
| NTA | Nano-tracker analysis |
| PBMCs | Peripheral blood mononuclear cells |
| PC | Phosphatidylcholine |
| PCA-ELISA | Perchloric acid- enzyme linked immunosorbent assay |
| PE | Phosphatidylethanolamine |
| PG | Phosphatidylglycerol |
| PI | Phosphoinositides |
| PM | Plasma Membrane |
| PMA | Phorbol-12-myristate-13-acetate |
| PNH | Paroxysmal nocturnal hemoglobinuria |
| pRBCs | Plasmodium falciparum red blood cells |
| PS | Phosphatidylserine |
| RBCs | Red blood cells |
| ROS | Reactive oxygen species |
| RV | RBC membrane-derived vesicles |
| SCD | Sickle cell disease |
| SM | Sphingomyelin |
| S1P | Sphingosine 1-phosphate |
| T2MD | Type 2 diabetes |
| TEM | Transmission electron microscope |
| TG | Thrombin generation |
| TI | Thalassemia intermedia |
| TF | Tissue factor |
| TLC | Thin-layer chromatography |
| TRPS | Tunable Resistive Pulse Sensing |
| UC | Ultracentrifugation |
References
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Horder, E. Blood dust or blood granules: A new constituent of the blood? Lancet 1899, 154, 1015. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, D.B.; Ly, T.B.T.; Bernhardt, I. Microvesicles Released from Human Red Blood Cells: Properties and Potential Applications; IntechOpen: Rijeka, Croatia, 2017; Volume 10. [Google Scholar]
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar] [PubMed]
- Yáñez-Mó, M.; Siljander, P.R.M.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [Green Version]
- Szatanek, R.; Baj-Krzyworzeka, M.; Zimoch, J.; Lekka, M.; Siedlar, M.; Baran, J. The Methods of Choice for Extracellular Vesicles (EVs) Characterization. Int. J. Mol. Sci. 2017, 18, 1153. [Google Scholar] [CrossRef] [PubMed]
- Robbins, P.D.; Morelli, A.E. Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 2014, 14, 195–208. [Google Scholar] [CrossRef] [Green Version]
- Harding, C.; Heuser, J.; Stahl, P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J. Cell Biol. 1983, 97, 329–339. [Google Scholar] [CrossRef]
- Pan, B.T.; Johnstone, R.M. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: Selective externalization of the receptor. Cell 1983, 33, 967–978. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [Green Version]
- Buschow, S.I.; Nolte-‘t Hoen, E.N.M.; van Niel, G.; Pols, M.S.; ten Broeke, T.; Lauwen, M.; Ossendorp, F.; Melief, C.J.M.; Raposo, G.; Wubbolts, R.; et al. MHC II in dendritic cells is targeted to lysosomes or T cell-induced exosomes via distinct multivesicular body pathways. Traffic 2009, 10, 1528–1542. [Google Scholar] [CrossRef] [PubMed]
- Theos, A.C.; Truschel, S.T.; Tenza, D.; Hurbain, I.; Harper, D.C.; Berson, J.F.; Thomas, P.C.; Raposo, G.; Marks, M.S. A lumenal domain-dependent pathway for sorting to intralumenal vesicles of multivesicular endosomes involved in organelle morphogenesis. Dev. Cell 2006, 10, 343–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villarroya-Beltri, C.; Baixauli, F.; Mittelbrunn, M.; Fernández-Delgado, I.; Torralba, D.; Moreno-Gonzalo, O.; Baldanta, S.; Enrich, C.; Guerra, S.; Sánchez-Madrid, F. ISGylation controls exosome secretion by promoting lysosomal degradation of MVB proteins. Nat. Commun. 2016, 7, 13588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edgar, J.R.; Manna, P.T.; Nishimura, S.; Banting, G.; Robinson, M.S. Tetherin is an exosomal tether. Elife 2016, 5, e17180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guix, F.X.; Sannerud, R.; Berditchevski, F.; Arranz, A.M.; Horré, K.; Snellinx, A.; Thathiah, A.; Saido, T.; Saito, T.; Rajesh, S.; et al. Tetraspanin 6: A pivotal protein of the multiple vesicular body determining exosome release and lysosomal degradation of amyloid precursor protein fragments. Mol. Neurodegener. 2017, 12, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamai, K.; Tanaka, N.; Nakano, T.; Kakazu, E.; Kondo, Y.; Inoue, J.; Shiina, M.; Fukushima, K.; Hoshino, T.; Sano, K.; et al. Exosome secretion of dendritic cells is regulated by Hrs, an ESCRT-0 protein. Biochem. Biophys. Res. Commun. 2010, 399, 384–390. [Google Scholar] [CrossRef]
- Blanc, L.; Liu, J.; Vidal, M.; Chasis, J.A.; An, X.; Mohandas, N. The water channel aquaporin-1 partitions into exosomes during reticulocyte maturation: Implication for the regulation of cell volume. Blood 2009, 114, 3928–3934. [Google Scholar] [CrossRef] [Green Version]
- Wolf, P. The nature and significance of platelet products in human plasma. Br. J. Haematol. 1967, 13, 269–288. [Google Scholar] [CrossRef]
- Hristov, M.; Erl, W.; Linder, S.; Weber, P.C. Apoptotic bodies from endothelial cells enhance the number and initiate the differentiation of human endothelial progenitor cells in vitro. Blood 2004, 104, 2761–2766. [Google Scholar] [CrossRef]
- Hurley, J.H.; Hanson, P.I. Membrane budding and scission by the ESCRT machinery: It’s all in the neck. Nat. Rev. Mol. Cell Biol. 2010, 11, 556–566. [Google Scholar] [CrossRef]
- Penalva, M. Faculty Opinions recommendation of Escrt-III: An endosome-associated heterooligomeric protein complex required for mvb sorting. Fac. Opin. Post Publ. Peer Rev. Biomed. Lit. 2002. [Google Scholar] [CrossRef]
- Babst, M.; Katzmann, D.J.; Snyder, W.B.; Wendland, B.; Emr, S.D. Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body. Dev. Cell 2002, 3, 283–289. [Google Scholar] [CrossRef] [Green Version]
- Bilodeau, P.S.; Winistorfer, S.C.; Kearney, W.R.; Robertson, A.D.; Piper, R.C. Vps27-Hse1 and ESCRT-I complexes cooperate to increase efficiency of sorting ubiquitinated proteins at the endosome. J. Cell Biol. 2003, 163, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Katzmann, D.J.; Babst, M.; Emr, S.D. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell 2001, 106, 145–155. [Google Scholar] [CrossRef] [Green Version]
- Katzmann, D.J.; Stefan, C.J.; Babst, M.; Emr, S.D. Vps27 recruits ESCRT machinery to endosomes during MVB sorting. J. Cell Biol. 2003, 162, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Baietti, M.F.; Zhang, Z.; Mortier, E.; Melchior, A.; Degeest, G.; Geeraerts, A.; Ivarsson, Y.; Depoortere, F.; Coomans, C.; Vermeiren, E.; et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 2012, 14, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Stuffers, S.; Sem Wegner, C.; Stenmark, H.; Brech, A. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic 2009, 10, 925–937. [Google Scholar] [CrossRef]
- van Niel, G.; Charrin, S.; Simoes, S.; Romao, M.; Rochin, L.; Saftig, P.; Marks, M.S.; Rubinstein, E.; Raposo, G. The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev. Cell 2011, 21, 708–721. [Google Scholar] [CrossRef] [Green Version]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brügger, B.; Simons, M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008, 319, 1244–1247. [Google Scholar] [CrossRef]
- Perez-Hernandez, D.; Gutiérrez-Vázquez, C.; Jorge, I.; López-Martín, S.; Ursa, A.; Sánchez-Madrid, F.; Vázquez, J.; Yáñez-Mó, M. The intracellular interactome of tetraspanin-enriched microdomains reveals their function as sorting machineries toward exosomes. J. Biol. Chem. 2013, 288, 11649–11661. [Google Scholar] [CrossRef] [Green Version]
- Kajimoto, T.; Okada, T.; Miya, S.; Zhang, L.; Nakamura, S.-I. Ongoing activation of sphingosine 1-phosphate receptors mediates maturation of exosomal multivesicular endosomes. Nat. Commun. 2013, 4, 2712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Niel, G.; Bergam, P.; Di Cicco, A.; Hurbain, I.; Lo Cicero, A.; Dingli, F.; Palmulli, R.; Fort, C.; Potier, M.C.; Schurgers, L.J.; et al. Apolipoprotein E Regulates Amyloid Formation within Endosomes of Pigment Cells. Cell Rep. 2015, 13, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, S.A.; Pérez-González, R.; Sharma, A.; Huang, F.-K.; Alldred, M.J.; Pawlik, M.; Kaur, G.; Ginsberg, S.D.; Neubert, T.A.; Levy, E. Enhanced exosome secretion in Down syndrome brain—A protective mechanism to alleviate neuronal endosomal abnormalities. Acta Neuropathol. Commun. 2017, 5, 65. [Google Scholar] [CrossRef] [PubMed]
- Odintsova, E.; van Niel, G.; Conjeaud, H.; Raposo, G.; Iwamoto, R.; Mekada, E.; Berditchevski, F. Metastasis suppressor tetraspanin CD82/KAI1 regulates ubiquitylation of epidermal growth factor receptor. J. Biol. Chem. 2013, 288, 26323–26334. [Google Scholar] [CrossRef] [Green Version]
- Vidal, M.; Mangeat, P.; Hoekstra, D. Aggregation reroutes molecules from a recycling to a vesicle-mediated secretion pathway during reticulocyte maturation. J. Cell Sci. 1997, 110 Pt 16, 1867–1877. [Google Scholar]
- Blanc, L.; De Gassart, A.; Géminard, C.; Bette-Bobillo, P.; Vidal, M. Exosome release by reticulocytes—An integral part of the red blood cell differentiation system. Blood Cells Mol. Dis. 2005, 35, 21–26. [Google Scholar] [CrossRef]
- Ney, P.A. Normal and disordered reticulocyte maturation. Curr. Opin. Hematol. 2011, 18, 152–157. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Varela, M.; de Menezes-Neto, A.; Perez-Zsolt, D.; Gámez-Valero, A.; Seguí-Barber, J.; Izquierdo-Useros, N.; Martinez-Picado, J.; Fernández-Becerra, C.; Del Portillo, H.A. Proteomics study of human cord blood reticulocyte-derived exosomes. Sci. Rep. 2018, 8, 14046. [Google Scholar] [CrossRef] [Green Version]
- Carayon, K.; Chaoui, K.; Ronzier, E.; Lazar, I.; Bertrand-Michel, J.; Roques, V.; Balor, S.; Terce, F.; Lopez, A.; Salomé, L.; et al. Proteolipidic composition of exosomes changes during reticulocyte maturation. J. Biol. Chem. 2011, 286, 34426–34439. [Google Scholar] [CrossRef] [Green Version]
- Bryk, A.H.; Wiśniewski, J.R. Quantitative Analysis of Human Red Blood Cell Proteome. J. Prot. Res. 2017, 16, 2752–2761. [Google Scholar] [CrossRef] [Green Version]
- Leal, J.K.F.; Adjobo-Hermans, M.J.W.; Bosman, G.J.C.G.M. Red Blood Cell Homeostasis: Mechanisms and Effects of Microvesicle Generation in Health and Disease. Front. Physiol. 2018, 9, 703. [Google Scholar] [CrossRef] [PubMed]
- Willekens, F.L.A.; Roerdinkholder-Stoelwinder, B.; Groenen-Döpp, Y.A.M.; Bos, H.J.; Bosman, G.J.C.G.M.; van den Bos, A.G.; Verkleij, A.J.; Werre, J.M. Hemoglobin loss from erythrocytes in vivo results from spleen-facilitated vesiculation. Blood 2003, 101, 747–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willekens, F.L.; Bosch, F.H.; Roerdinkholder-Stoelwinder, B.; Groenen-Döpp, Y.A.; Werre, J.M. Quantification of loss of haemoglobin components from the circulating red blood cell in vivo. Eur. J. Haematol. 1997, 58, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Willekens, F.L.A.; Werre, J.M.; Groenen-Döpp, Y.A.M.; Roerdinkholder-Stoelwinder, B.; de Pauw, B.; Bosman, G.J.C.G.M. Erythrocyte vesiculation: A self-protective mechanism? Br. J. Haematol. 2008, 141, 549–556. [Google Scholar] [CrossRef]
- Bosman, G.J.C.G.M.; Lasonder, E.; Groenen-Döpp, Y.A.M.; Willekens, F.L.A.; Werre, J.M. The proteome of erythrocyte-derived microparticles from plasma: New clues for erythrocyte aging and vesiculation. J. Prot. 2012, 76, 203–210. [Google Scholar] [CrossRef]
- Lux, S.E.T. Anatomy of the red cell membrane skeleton: Unanswered questions. Blood 2016, 127, 187–199. [Google Scholar] [CrossRef] [Green Version]
- Ursitti, J.A.; Wade, J.B. Ultrastructure and immunocytochemistry of the isolated human erythrocyte membrane skeleton. Cell Motil. Cytoskeleton 1993, 25, 30–42. [Google Scholar] [CrossRef]
- Liu, S.C.; Windisch, P.; Kim, S.; Palek, J. Oligomeric states of spectrin in normal erythrocyte membranes: Biochemical and electron microscopic studies. Cell 1984, 37, 587–594. [Google Scholar] [CrossRef]
- Salomao, M.; An, X.; Guo, X.; Gratzer, W.B.; Mohandas, N.; Baines, A.J. Mammalian alpha I-spectrin is a neofunctionalized polypeptide adapted to small highly deformable erythrocytes. Proc. Natl. Acad. Sci. USA 2006, 103, 643–648. [Google Scholar] [CrossRef] [Green Version]
- Picart, C.; Dalhaimer, P.; Discher, D.E. Actin protofilament orientation in deformation of the erythrocyte membrane skeleton. Biophys. J. 2000, 79, 2987–3000. [Google Scholar] [CrossRef] [Green Version]
- An, X.; Salomao, M.; Guo, X.; Gratzer, W.; Mohandas, N. Tropomyosin modulates erythrocyte membrane stability. Blood 2007, 109, 1284–1288. [Google Scholar] [CrossRef] [PubMed]
- Moyer, J.D.; Nowak, R.B.; Kim, N.E.; Larkin, S.K.; Peters, L.L.; Hartwig, J.; Kuypers, F.A.; Fowler, V.M. Tropomodulin 1-null mice have a mild spherocytic elliptocytosis with appearance of tropomodulin 3 in red blood cells and disruption of the membrane skeleton. Blood 2010, 116, 2590–2599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Matsuoka, Y.; Bennett, V. Adducin Preferentially Recruits Spectrin to the Fast Growing Ends of Actin Filaments in a Complex Requiring the MARCKS-related Domain and a Newly Defined Oligomerization Domain. J. Biol. Chem. 1998, 273, 19329–19338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.A.; Hanada, T.; Mohseni, M.; Jeong, J.-J.; Zeng, L.; Gaetani, M.; Li, D.; Reed, B.C.; Speicher, D.W.; Chishti, A.H. Dematin and adducin provide a novel link between the spectrin cytoskeleton and human erythrocyte membrane by directly interacting with glucose transporter-1. J. Biol. Chem. 2008, 283, 14600–14609. [Google Scholar] [CrossRef] [Green Version]
- Han, B.G.; Nunomura, W.; Takakuwa, Y.; Mohandas, N.; Jap, B.K. Protein 4.1R core domain structure and insights into regulation of cytoskeletal organization. Nat. Struct. Biol. 2000, 7, 871–875. [Google Scholar] [CrossRef]
- Manno, S.; Takakuwa, Y.; Mohandas, N. Modulation of erythrocyte membrane mechanical function by protein 4.1 phosphorylation. J. Biol. Chem. 2005, 280, 7581–7587. [Google Scholar] [CrossRef] [Green Version]
- Low, P.S.; Willardson, B.M.; Mohandas, N.; Rossi, M.; Shohet, S. Contribution of the band 3-ankyrin interaction to erythrocyte membrane mechanical stability. Blood 1991, 77, 1581–1586. [Google Scholar] [CrossRef]
- Westerman, M.; Pizzey, A.; Hirschman, J.; Cerino, M.; Weil-Weiner, Y.; Ramotar, P.; Eze, A.; Lawrie, A.; Purdy, G.; Mackie, I.; et al. Microvesicles in haemoglobinopathies offer insights into mechanisms of hypercoagulability, haemolysis and the effects of therapy. Br. J. Haematol. 2008, 142, 126–135. [Google Scholar] [CrossRef]
- Ferru, E.; Pantaleo, A.; Carta, F.; Mannu, F.; Khadjavi, A.; Gallo, V.; Ronzoni, L.; Graziadei, G.; Cappellini, M.D.; Turrini, F. Thalassemic erythrocytes release microparticles loaded with hemichromes by redox activation of p72Syk kinase. Haematologica 2014, 99, 570–578. [Google Scholar] [CrossRef]
- Willekens, F.L.A.; Werre, J.M.; Kruijt, J.K.; Roerdinkholder-Stoelwinder, B.; Groenen-Döpp, Y.A.M.; van den Bos, A.G.; Bosman, G.J.C.G.M.; van Berkel, T.J.C. Liver Kupffer cells rapidly remove red blood cell-derived vesicles from the circulation by scavenger receptors. Blood 2005, 105, 2141–2145. [Google Scholar] [CrossRef] [Green Version]
- Daleke, D.L. Regulation of phospholipid asymmetry in the erythrocyte membrane. Curr. Opin. Hematol. 2008, 15, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Said, A.S.; Rogers, S.C.; Doctor, A. Physiologic Impact of Circulating RBC Microparticles upon Blood-Vascular Interactions. Front. Physiol. 2017, 8, 1120. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, L.J.; Gibbons, E.; Bailey, R.W.; Fairbourn, J.; Nguyen, T.; Smith, S.K.; Best, K.B.; Nelson, J.; Judd, A.M.; Bell, J.D. The influence of membrane physical properties on microvesicle release in human erythrocytes. PMC Biophys. 2009, 2, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, D.B.; Ly, T.B.T.; Wesseling, M.C.; Hittinger, M.; Torge, A.; Devitt, A.; Perrie, Y.; Bernhardt, I. Characterization of Microvesicles Released from Human Red Blood Cells. Cell. Physiol. Biochem. 2016, 38, 1085–1099. [Google Scholar] [CrossRef] [PubMed]
- Wesseling, M.C.; Wagner-Britz, L.; Nguyen, D.B.; Asanidze, S.; Mutua, J.; Mohamed, N.; Hanf, B.; Ghashghaeinia, M.; Kaestner, L.; Bernhardt, I. Novel Insights in the Regulation of Phosphatidylserine Exposure in Human Red Blood Cells. Cell. Physiol. Biochem. 2016, 39, 1941–1954. [Google Scholar] [CrossRef]
- Matte, A.; Bertoldi, M.; Mohandas, N.; An, X.; Bugatti, A.; Brunati, A.M.; Rusnati, M.; Tibaldi, E.; Siciliano, A.; Turrini, F.; et al. Membrane association of peroxiredoxin-2 in red cells is mediated by the N-terminal cytoplasmic domain of band 3. Free Radic. Biol. Med. 2013, 55, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Gulbins, E.; Lerche, H.; Huber, S.M.; Kempe, D.S.; Foller, M. Eryptosis, a window to systemic disease. Cell. Physiol. Biochem. 2008, 22, 373–380. [Google Scholar] [CrossRef]
- Salzer, U.; Hinterdorfer, P.; Hunger, U.; Borken, C.; Prohaska, R. Ca++-dependent vesicle release from erythrocytes involves stomatin-specific lipid rafts, synexin (annexin VII), and sorcin. Blood 2002, 99, 2569–2577. [Google Scholar] [CrossRef]
- Wesseling, M.C.; Wagner-Britz, L.; Huppert, H.; Hanf, B.; Hertz, L.; Nguyen, D.B.; Bernhardt, I. Phosphatidylserine Exposure in Human Red Blood Cells Depending on Cell Age. Cell. Physiol. Biochem. 2016, 38, 1376–1390. [Google Scholar] [CrossRef]
- Sudnitsyna, J.; Skverchinskaya, E.; Dobrylko, I.; Nikitina, E.; Gambaryan, S.; Mindukshev, I. Microvesicle Formation Induced by Oxidative Stress in Human Erythrocytes. Antioxidants 2020, 9, 929. [Google Scholar] [CrossRef]
- Stowell, S.R.; Smith, N.H.; Zimring, J.C.; Fu, X.; Palmer, A.F.; Fontes, J.; Banerjee, U.; Yazer, M.H. Addition of ascorbic acid solution to stored murine red blood cells increases posttransfusion recovery and decreases microparticles and alloimmunization. Transfusion 2013, 53, 2248–2257. [Google Scholar] [CrossRef] [PubMed]
- Kuo, L.; Freed, E.O. ARRDC1 as a mediator of microvesicle budding. Proc. Natl. Acad. Sci. USA 2012, 109, 4025–4026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, B.; Fu, Y.; Liu, Y.; Agvanian, S.; Wirka, R.C.; Baum, R.; Zhou, K.; Shaw, R.M.; Hong, T. The ESCRT-III pathway facilitates cardiomyocyte release of cBIN1-containing microparticles. PLoS Biol. 2017, 15, e2002354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Antonyak, M.A.; Zhang, J.; Cerione, R.A. RhoA triggers a specific signaling pathway that generates transforming microvesicles in cancer cells. Oncogene 2012, 31, 4740–4749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, K.F.; Erickson, J.W.; Antonyak, M.A.; Cerione, R.A. Rho GTPases and their roles in cancer metabolism. Trends Mol. Med. 2013, 19, 74–82. [Google Scholar] [CrossRef] [Green Version]
- Del Conde, I.; Shrimpton, C.N.; Thiagarajan, P.; López, J.A. Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood 2005, 106, 1604–1611. [Google Scholar] [CrossRef]
- McConnell, R.E.; Higginbotham, J.N.; Shifrin, D.A., Jr.; Tabb, D.L.; Coffey, R.J.; Tyska, M.J. The enterocyte microvillus is a vesicle-generating organelle. J. Cell Biol. 2009, 185, 1285–1298. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.-K.; Lee, J.; Simpson, R.J.; Lötvall, J.; Gho, Y.S. EVpedia: A community web resource for prokaryotic and eukaryotic extracellular vesicles research. Semin. Cell Dev. Biol. 2015, 40, 4–7. [Google Scholar] [CrossRef]
- Kalra, H.; Simpson, R.J.; Ji, H.; Aikawa, E.; Altevogt, P.; Askenase, P.; Bond, V.C.; Borràs, F.E.; Breakefield, X.; Budnik, V.; et al. Vesiclepedia: A compendium for extracellular vesicles with continuous community annotation. PLoS Biol. 2012, 10, e1001450. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.-K.; Kang, B.; Kim, O.Y.; Choi, D.-S.; Lee, J.; Kim, S.R.; Go, G.; Yoon, Y.J.; Kim, J.H.; Jang, S.C.; et al. EVpedia: An integrated database of high-throughput data for systemic analyses of extracellular vesicles. J. Extracell. Vesicles 2013, 2. [Google Scholar] [CrossRef]
- Keerthikumar, S.; Chisanga, D.; Ariyaratne, D.; Al Saffar, H.; Anand, S.; Zhao, K.; Samuel, M.; Pathan, M.; Jois, M.; Chilamkurti, N.; et al. ExoCarta: A Web-Based Compendium of Exosomal Cargo. J. Mol. Biol. 2016, 428, 688–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prudent, M.; Delobel, J.; Hübner, A.; Benay, C.; Lion, N.; Tissot, J.-D. Proteomics of Stored Red Blood Cell Membrane and Storage-Induced Microvesicles Reveals the Association of Flotillin-2 With Band 3 Complexes. Front. Physiol. 2018, 9, 421. [Google Scholar] [CrossRef] [PubMed]
- Rubin, O.; Crettaz, D.; Canellini, G.; Tissot, J.D.; Lion, N. Microparticles in stored red blood cells: An approach using flow cytometry and proteomic tools. Vox Sang. 2008, 95, 288–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonelou, M.H.; Seghatchian, J. Update on extracellular vesicles inside red blood cell storage units: Adjust the sails closer to the new wind. Transfus. Apher. Sci. 2016, 55, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Tissot, J.-D.; Canellini, G.; Rubin, O.; Angelillo-Scherrer, A.; Delobel, J.; Prudent, M.; Lion, N. Blood microvesicles: From proteomics to physiology. Transl. Prot. 2013, 1, 38–52. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, A.J. MicroRNA in erythrocytes. Biochem. Soc. Trans. 2010, 38, 229–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aalto, A.P.; Pasquinelli, A.E. Small non-coding RNAs mount a silent revolution in gene expression. Curr. Opin. Cell Biol. 2012, 24, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Guerau-de-Arellano, M.; Alder, H.; Ozer, H.G.; Lovett-Racke, A.; Racke, M.K. miRNA profiling for biomarker discovery in multiple sclerosis: From microarray to deep sequencing. J. Neuroimmunol. 2012, 248, 32–39. [Google Scholar] [CrossRef] [Green Version]
- Karius, T.; Schnekenburger, M.; Dicato, M.; Diederich, M. MicroRNAs in cancer management and their modulation by dietary agents. Biochem. Pharmacol. 2012, 83, 1591–1601. [Google Scholar] [CrossRef]
- Saki, N.; Abroun, S.; Soleimani, M.; Hajizamani, S.; Shahjahani, M.; Kast, R.E.; Mortazavi, Y. Involvement of MicroRNA in T-Cell Differentiation and Malignancy. Int. J. Hematol. Oncol. Stem Cell Res. 2015, 9, 33–49. [Google Scholar]
- Sun, L.; Yu, Y.; Niu, B.; Wang, D. Red Blood Cells as Potential Repositories of MicroRNAs in the Circulatory System. Front. Genet. 2020, 11, 442. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhu, J.; Fan, L.; Lin, Q.; Fu, D.; Wei, B.; Wei, S. MicroRNA Profiling of Exosomes Derived from Red Blood Cell Units: Implications in Transfusion-Related Immunomodulation. Biomed Res. Int. 2019, 2019, 2045915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasheed, Z.; Rasheed, N.; Abdulmonem, W.A.; Khan, M.I. Author Correction: MicroRNA-125b-5p regulates IL-1β induced inflammatory genes via targeting TRAF6-mediated MAPKs and NF-κB signaling in human osteoarthritic chondrocytes. Sci. Rep. 2019, 9, 14729. [Google Scholar] [CrossRef] [PubMed]
- Diao, W.; Lu, L.; Li, S.; Chen, J.; Zen, K.; Li, L. MicroRNA-125b-5p modulates the inflammatory state of macrophages via targeting B7-H4. Biochem. Biophys. Res. Commun. 2017, 491, 912–918. [Google Scholar] [CrossRef]
- Yang, D.; Yuan, Q.; Balakrishnan, A.; Bantel, H.; Klusmann, J.-H.; Manns, M.P.; Ott, M.; Cantz, T.; Sharma, A.D. MicroRNA-125b-5p mimic inhibits acute liver failure. Nat. Commun. 2016, 7, 11916. [Google Scholar] [CrossRef] [Green Version]
- Morelli, E.; Leone, E.; Cantafio, M.E.G.; Di Martino, M.T.; Amodio, N.; Biamonte, L.; Gullà, A.; Foresta, U.; Pitari, M.R.; Botta, C.; et al. Selective targeting of IRF4 by synthetic microRNA-125b-5p mimics induces anti-multiple myeloma activity in vitro and in vivo. Leukemia 2015, 29, 2173–2183. [Google Scholar] [CrossRef] [Green Version]
- Doss, J.F.; Corcoran, D.L.; Jima, D.D.; Telen, M.J.; Dave, S.S.; Chi, J.-T. A comprehensive joint analysis of the long and short RNA transcriptomes of human erythrocytes. BMC Genomics 2015, 16, 952. [Google Scholar] [CrossRef] [Green Version]
- Dore, L.C.; Amigo, J.D.; Dos Santos, C.O.; Zhang, Z.; Gai, X.; Tobias, J.W.; Yu, D.; Klein, A.M.; Dorman, C.; Wu, W.; et al. A GATA-1-regulated microRNA locus essential for erythropoiesis. Proc. Natl. Acad. Sci. USA 2008, 105, 3333–3338. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Fan, F.; Li, R.; Niu, B.; Zhu, L.; Yu, S.; Wang, S.; Li, C.; Wang, D. Different Erythrocyte MicroRNA Profiles in Low- and High-Altitude Individuals. Front. Physiol. 2018, 9, 1099. [Google Scholar] [CrossRef]
- Hua, Z.; Lv, Q.; Ye, W.; Wong, C.-K.A.; Cai, G.; Gu, D.; Ji, Y.; Zhao, C.; Wang, J.; Yang, B.B.; et al. MiRNA-directed regulation of VEGF and other angiogenic factors under hypoxia. PLoS ONE 2006, 1, e116. [Google Scholar] [CrossRef] [Green Version]
- Kulshreshtha, R.; Ferracin, M.; Wojcik, S.E.; Garzon, R.; Alder, H.; Agosto-Perez, F.J.; Davuluri, R.; Liu, C.-G.; Croce, C.M.; Negrini, M.; et al. A microRNA signature of hypoxia. Mol. Cell. Biol. 2007, 27, 1859–1867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piriyapongsa, J.; Jordan, I.K.; Conley, A.B.; Ronan, T.; Smalheiser, N.R. Transcription factor binding sites are highly enriched within microRNA precursor sequences. Biol. Direct 2011, 6, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hebert, C.; Norris, K.; Scheper, M.A.; Nikitakis, N.; Sauk, J.J. High mobility group A2 is a target for miRNA-98 in head and neck squamous cell carcinoma. Mol. Cancer 2007, 6, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juzenas, S.; Venkatesh, G.; Hübenthal, M.; Hoeppner, M.P.; Du, Z.G.; Paulsen, M.; Rosenstiel, P.; Senger, P.; Hofmann-Apitius, M.; Keller, A.; et al. A comprehensive, cell specific microRNA catalogue of human peripheral blood. Nucleic Acids Res. 2017, 45, 9290–9301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eich, R.F.; Li, T.; Lemon, D.D.; Doherty, D.H.; Curry, S.R.; Aitken, J.F.; Mathews, A.J.; Johnson, K.A.; Smith, R.D.; Phillips, G.N., Jr.; et al. Mechanism of NO-induced oxidation of myoglobin and hemoglobin. Biochemistry 1996, 35, 6976–6983. [Google Scholar] [CrossRef] [PubMed]
- Doherty, D.H.; Doyle, M.P.; Curry, S.R.; Vali, R.J.; Fattor, T.J.; Olson, J.S.; Lemon, D.D. Rate of reaction with nitric oxide determines the hypertensive effect of cell-free hemoglobin. Nat. Biotechnol. 1998, 16, 672–676. [Google Scholar] [CrossRef]
- Donadee, C.; Raat, N.J.H.; Kanias, T.; Tejero, J.; Lee, J.S.; Kelley, E.E.; Zhao, X.; Liu, C.; Reynolds, H.; Azarov, I.; et al. Nitric Oxide Scavenging by Red Blood Cell Microparticles and Cell-Free Hemoglobin as a Mechanism for the Red Cell Storage Lesion. Circulation 2011, 124, 465–476. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, W.; Christ, G.J.; Gladwin, M.T.; Kim-Shapiro, D.B. Nitric oxide scavenging by red cell microparticles. Free Radic. Biol. Med. 2013, 65, 1164–1173. [Google Scholar] [CrossRef] [Green Version]
- Herring, J.M.; McMichael, M.A.; Smith, S.A. Microparticles in health and disease. J. Vet. Intern. Med. 2013, 27, 1020–1033. [Google Scholar] [CrossRef]
- Poisson, J.; Tanguy, M.; Davy, H.; Camara, F.; El Mdawar, M.B.; Kheloufi, M.; Dagher, T.; Devue, C.; Lasselin, J.; Plessier, A.; et al. Erythrocyte-derived microvesicles induce arterial spasms in JAK2V617F myeloproliferative neoplasm. J. Clin. Investig. 2020, 130, 2630–2643. [Google Scholar] [CrossRef] [Green Version]
- Harisa, G.I.; Badran, M.M.; Alanazi, F.K. Erythrocyte nanovesicles: Biogenesis, biological roles and therapeutic approach: Erythrocyte nanovesicles. Saudi Pharm. J. 2017, 25, 8–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhn, V.; Diederich, L.; Keller, T.C.S.t.; Kramer, C.M.; Lückstädt, W.; Panknin, C.; Suvorava, T.; Isakson, B.E.; Kelm, M.; Cortese-Krott, M.M. Red Blood Cell Function and Dysfunction: Redox Regulation, Nitric Oxide Metabolism, Anemia. Antioxid. Redox Signal. 2017, 26, 718–742. [Google Scholar] [CrossRef] [PubMed]
- Jank, H.; Salzer, U. Vesicles Generated during Storage of Red Blood Cells Enhance the Generation of Radical Oxygen Speciesin Activated Neutrophils. Sci. World J. 2011, 11, 173–185. [Google Scholar] [CrossRef] [Green Version]
- Danesh, A.; Inglis, H.C.; Jackman, R.P.; Wu, S.; Deng, X.; Muench, M.O.; Heitman, J.W.; Norris, P.J. Exosomes from red blood cell units bind to monocytes and induce proinflammatory cytokines, boosting T-cell responses in vitro. Blood 2014, 123, 687–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hezel, M.E.V.; Nieuwland, R.; Bruggen, R.V.; Juffermans, N.P. The Ability of Extracellular Vesicles to Induce a Pro-Inflammatory Host Response. Int. J. Mol. Sci. 2017, 18, 1285. [Google Scholar] [CrossRef] [Green Version]
- Almizraq, R.J.; Seghatchian, J.; Acker, J.P. Extracellular vesicles in transfusion-related immunomodulation and the role of blood component manufacturing. Transf. Apher. Sci. 2016, 55, 281–291. [Google Scholar] [CrossRef]
- Wannez, A.; Devalet, B.; Chatelain, B.; Chatelain, C.; Dogné, J.-M.; Mullier, F. Extracellular Vesicles in Red Blood Cell Concentrates: An Overview. Transf. Med. Rev. 2019, 33, 125–130. [Google Scholar] [CrossRef]
- Sadallah, S.; Eken, C.; Schifferli, J.A. Ectosomes as modulators of inflammation and immunity. Clin. Exp. Immunol. 2011, 163, 26–32. [Google Scholar] [CrossRef]
- Straat, M.; van Hezel, M.E.; Böing, A.; Boer, A.T.-D.; Weber, N.; Nieuwland, R.; van Bruggen, R.; Juffermans, N.P. Monocyte-mediated activation of endothelial cells occurs only after binding to extracellular vesicles from red blood cell products, a process mediated by β-integrin. Transfusion 2016, 56, 3012–3020. [Google Scholar] [CrossRef]
- Fischer, D.; Büssow, J.; Meybohm, P.; Weber, C.F.; Zacharowski, K.; Urbschat, A.; Müller, M.M.; Jennewein, C. Microparticles from stored red blood cells enhance procoagulant and proinflammatory activity. Transfusion 2017, 57, 2701–2711. [Google Scholar] [CrossRef]
- Sadallah, S.; Eken, C.; Schifferli, J.A. Erythrocyte-derived ectosomes have immunosuppressive properties. J. Leukoc. Biol. 2008, 84, 1316–1325. [Google Scholar] [CrossRef] [PubMed]
- Nazimek, K.; Bustos-Morán, E.; Blas-Rus, N.; Nowak, B.; Ptak, W.; Askenase, P.W.; Sánchez-Madrid, F.; Bryniarski, K. Syngeneic red blood cell–induced extracellular vesicles suppress delayed-type hypersensitivity to self-antigens in mice. Clin. Exp. Allergy 2019, 49, 1487–1499. [Google Scholar] [CrossRef] [PubMed]
- Bradlow, B.A. Liberation of material with platelet-like coagulant properties from intact red cells and particularly from reticulocytes. Br. J. Haematol. 1961, 7, 476–495. [Google Scholar] [CrossRef] [PubMed]
- Horne, M.K., 3rd; Cullinane, A.M.; Merryman, P.K.; Hoddeson, E.K. The effect of red blood cells on thrombin generation. Br. J. Haematol. 2006, 133, 403–408. [Google Scholar] [CrossRef]
- Diamant, M.; Tushuizen, M.E.; Sturk, A.; Nieuwland, R. Cellular microparticles: New players in the field of vascular disease? Eur. J. Clin. Investig. 2004, 34, 392–401. [Google Scholar] [CrossRef]
- Van Der Meijden, P.E.J.; Van Schilfgaarde, M.; Van Oerle, R.; Renné, T.; ten Cate, H.; Spronk, H.M.H. Platelet- and erythrocyte-derived microparticles trigger thrombin generation via factor XIIa. J. Thromb. Haemost. 2012, 10, 1355–1362. [Google Scholar] [CrossRef]
- Koshiar, R.L.; Somajo, S.; Norstrom, E.; Dahlback, B. Erythrocyte-derived microparticles supporting activated protein C-mediated regulation of blood coagulation. PLoS ONE 2014, 9, e104200. [Google Scholar] [CrossRef] [Green Version]
- Levin, G.; Sukhareva, E.; Lavrentieva, A. Impact of microparticles derived from erythrocytes on fibrinolysis. J. Thromb Thrombolysis 2016, 41, 452–458. [Google Scholar] [CrossRef]
- Hashemi Tayer, A.; Amirizadeh, N.; Ahmadinejad, M.; Nikougoftar, M.; Deyhim, M.R.; Zolfaghari, S. Procoagulant Activity of Red Blood Cell-Derived Microvesicles during Red Cell Storage. Transfus. Med. Hemother. 2019, 46, 224–230. [Google Scholar] [CrossRef]
- Tzounakas, V.L.; Georgatzakou, H.T.; Kriebardis, A.G.; Voulgaridou, A.I.; Stamoulis, K.E.; Foudoulaki-Paparizos, L.E.; Antonelou, M.H.; Papassideri, I.S. Donor variation effect on red blood cell storage lesion: A multivariable, yet consistent, story. Transfusion 2016, 56, 1274–1286. [Google Scholar] [CrossRef]
- Sweeney, J.; Kouttab, N.; Kurtis, J. Stored red blood cell supernatant facilitates thrombin generation. Transfusion 2009, 49, 1569–1579. [Google Scholar] [CrossRef] [PubMed]
- Rubin, O.; Delobel, J.; Prudent, M.; Lion, N.; Kohl, K.; Tucker, E.I.; Tissot, J.-D.; Angelillo-Scherrer, A. Red blood cell-derived microparticles isolated from blood units initiate and propagate thrombin generation. Transfusion 2013, 53, 1744–1754. [Google Scholar] [CrossRef] [PubMed]
- Camus, S.M.; De Moraes, J.A.; Bonnin, P.; Abbyad, P.; Le Jeune, S.; Lionnet, F.; Loufrani, L.; Grimaud, L.; Lambry, J.-C.; Charue, D.; et al. Circulating cell membrane microparticles transfer heme to endothelial cells and trigger vasoocclusions in sickle cell disease. Blood 2015, 125, 3805–3814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freedman, J.E.; Loscalzo, J. Nitric oxide and its relationship to thrombotic disorders: Nitric oxide and thrombotic disorders. J. Thromb. Haemost. 2003, 1, 1183–1188. [Google Scholar] [CrossRef]
- Loscalzo, J. Nitric oxide insufficiency, platelet activation, and arterial thrombosis. Circ. Res. 2001, 88, 756–762. [Google Scholar] [CrossRef] [Green Version]
- Devalet, B.; Mullier, F.; Chatelain, B.; Dogné, J.-M.; Chatelain, C. The central role of extracellular vesicles in the mechanisms of thrombosis in paroxysmal nocturnal haemoglobinuria: A review. J. Extracell Vesicles 2014, 3(1). [Google Scholar] [CrossRef]
- Yoshida, T.; Prudent, M.; D’Alessandro, A. Red blood cell storage lesion: Causes and potential clinical consequences. Blood Transfus. 2019, 17, 27–52. [Google Scholar] [CrossRef]
- Macey, M.G.; Enniks, N.; Bevan, S. Flow cytometric analysis of microparticle phenotype and their role in thrombin generation. Cytometry B Clin. Cytom. 2011, 80, 57–63. [Google Scholar] [CrossRef]
- Berckmans, R.J.; Nieuwland, R.; Böing, A.N.; Romijn, F.P.; Hack, C.E.; Sturk, A. Cell-derived microparticles circulate in healthy humans and support low grade thrombin generation. Thromb. Haemost. 2001, 85, 639–646. [Google Scholar]
- Zubairova, L.D.; Nabiullina, R.M.; Nagaswami, C.; Zuev, Y.F.; Mustafin, I.G.; Litvinov, R.I.; Weisel, J.W. Circulating Microparticles Alter Formation, Structure, and Properties of Fibrin Clots. Sci. Rep. 2015, 5, 17611. [Google Scholar] [CrossRef] [Green Version]
- Aharon, A.; Rebibo-Sabbah, A.; Tzoran, I.; Levin, C. Extracellular vesicles in hematological disorders. Rambam Maimonides Med. J. 2014, 5, e0032. [Google Scholar] [CrossRef] [PubMed]
- Ataga, K.I. Hypercoagulability and thrombotic complications in hemolytic anemias. Haematologica 2009, 94, 1481–1484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allan, D.; Limbrick, A.R.; Thomas, P.; Westerman, M.P. Release of spectrin-free spicules on reoxygenation of sickled erythrocytes. Nature 1982, 295, 612–613. [Google Scholar] [CrossRef] [PubMed]
- Shet, A.S.; Aras, O.; Gupta, K.; Hass, M.J.; Rausch, D.J.; Saba, N.; Koopmeiners, L.; Key, N.S.; Hebbel, R.P. Sickle blood contains tissue factor-positive microparticles derived from endothelial cells and monocytes. Blood 2003, 102, 2678–2683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Beers, E.J.; Schaap, M.C.L.; Berckmans, R.J.; Nieuwland, R.; Sturk, A.; van Doormaal, F.F.; Meijers, J.C.M.; Biemond, B.J.; group, C.S. Circulating erythrocyte-derived microparticles are associated with coagulation activation in sickle cell disease. Haematologica 2009, 94, 1513–1519. [Google Scholar] [CrossRef] [Green Version]
- Gerotziafas, G.T.; Van Dreden, P.; Chaari, M.; Galea, V.; Khaterchi, A.; Lionnet, F.; Stankovic-Stojanovic, K.; Blanc-Brude, O.; Woodhams, B.; Maier-Redelsperger, M.; et al. The acceleration of the propagation phase of thrombin generation in patients with steady-state sickle cell disease is associated with circulating erythrocyte-derived microparticles. Thromb. Haemost. 2012, 107, 1044–1052. [Google Scholar] [CrossRef]
- Mahfoudhi, E.; Lecluse, Y.; Driss, F.; Abbes, S.; Flaujac, C.; Garçon, L. Red cells exchanges in sickle cells disease lead to a selective reduction of erythrocytes-derived blood microparticles. Br. J. Haematol. 2012, 156, 545–547. [Google Scholar] [CrossRef]
- Tantawy, A.A.G.; Adly, A.A.M.; Ismail, E.A.R.; Habeeb, N.M.; Farouk, A. Circulating platelet and erythrocyte microparticles in young children and adolescents with sickle cell disease: Relation to cardiovascular complications. Platelets 2013, 24, 605–614. [Google Scholar] [CrossRef]
- Nebor, D.; Bowers, A.; Connes, P.; Hardy-Dessources, M.-D.; Knight-Madden, J.; Cumming, V.; Reid, M.; Romana, M. Plasma concentration of platelet-derived microparticles is related to painful vaso-occlusive phenotype severity in sickle cell anemia. PLoS ONE 2014, 9, e87243. [Google Scholar] [CrossRef]
- Kasar, M.; Boğa, C.; Yeral, M.; Asma, S.; Kozanoglu, I.; Ozdogu, H. Clinical significance of circulating blood and endothelial cell microparticles in sickle-cell disease. J. Thromb. Thrombolysis 2014, 38, 167–175. [Google Scholar] [CrossRef]
- Piccin, A.; Murphy, C.; Eakins, E.; Kunde, J.; Corvetta, D.; Di Pierro, A.; Negri, G.; Guido, M.; Sainati, L.; Mc Mahon, C.; et al. Circulating microparticles, protein C, free protein S and endothelial vascular markers in children with sickle cell anaemia. J. Extracell Vesicles 2015, 4, 28414. [Google Scholar] [CrossRef] [PubMed]
- Hierso, R.; Lemonne, N.; Villaescusa, R.; Lalanne-Mistrih, M.-L.; Charlot, K.; Etienne-Julan, M.; Tressières, B.; Lamarre, Y.; Tarer, V.; Garnier, Y.; et al. Exacerbation of oxidative stress during sickle vaso-occlusive crisis is associated with decreased anti-band 3 autoantibodies rate and increased red blood cell-derived microparticle level: A prospective study. Br. J. Haematol. 2017, 176, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Nébor, D.; Romana, M.; Santiago, R.; Vachiery, N.; Picot, J.; Broquere, C.; Chaar, V.; Doumdo, L.; Odièvre, M.-H.; Benkerrou, M.; et al. Fetal hemoglobin and hydroxycarbamide moduate both plasma concentration and cellular origin of circulating microparticles in sickle cell anemia children. Haematologica 2013, 98, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Garnier, Y.; Ferdinand, S.; Etienne-Julan, M.; Elana, G.; Petras, M.; Doumdo, L.; Tressières, B.; Lalanne-Mistrih, M.-L.; Hardy-Dessources, M.-D.; Connes, P.; et al. Differences of microparticle patterns between sickle cell anemia and hemoglobin SC patients. PLoS ONE 2017, 12, e0177397. [Google Scholar] [CrossRef]
- Romana, M.; Connes, P.; Key, N.S. Microparticles in sickle cell disease. Clin. Hemorheol. Microcirc. 2018, 68, 319–329. [Google Scholar] [CrossRef]
- Taher, A.T.; Otrock, Z.K.; Uthman, I.; Cappellini, M.D. Thalassemia and hypercoagulability. Blood Rev. 2008, 22, 283–292. [Google Scholar] [CrossRef]
- Musallam, K.M.; Taher, A.T. Thrombosis in thalassemia: Why are we so concerned? Hemoglobin 2011, 35, 503–510. [Google Scholar] [CrossRef]
- Pattanapanyasat, K.; Gonwong, S.; Chaichompoo, P.; Noulsri, E.; Lerdwana, S.; Sukapirom, K.; Siritanaratkul, N.; Fucharoen, S. Activated platelet-derived microparticles in thalassaemia. Br. J. Haematol. 2007, 136, 462–471. [Google Scholar] [CrossRef]
- Pattanapanyasat, K.; Noulsri, E.; Fucharoen, S.; Lerdwana, S.; Lamchiagdhase, P.; Siritanaratkul, N.; Webster, H.K. Flow cytometric quantitation of red blood cell vesicles in thalassemia. Cytometry B Clin. Cytom. 2004, 57, 23–31. [Google Scholar] [CrossRef]
- Habib, A.; Kunzelmann, C.; Shamseddeen, W.; Zobairi, F.; Freyssinet, J.-M.; Taher, A. Elevated levels of circulating procoagulant microparticles in patients with beta-thalassemia intermedia. Haematologica 2008, 93, 941–942. [Google Scholar] [CrossRef] [Green Version]
- Chaichompoo, P.; Kumya, P.; Khowawisetsut, L.; Chiangjong, W.; Chaiyarit, S.; Pongsakul, N.; Sirithanaratanakul, N.; Fucharoen, S.; Thongboonkerd, V.; Pattanapanyasat, K. Characterizations and proteome analysis of platelet-free plasma-derived microparticles in β-thalassemia/hemoglobin E patients. J. Proteomics 2012, 76, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Agouti, I.; Cointe, S.; Robert, S.; Judicone, C.; Loundou, A.; Driss, F.; Brisson, A.; Steschenko, D.; Rose, C.; Pondarré, C.; et al. Platelet and not erythrocyte microparticles are procoagulant in transfused thalassaemia major patients. Br. J. Haematol. 2015, 171, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Tantawy, A.A.G.; Adly, A.A.M.; Ismail, E.A.R.; Habeeb, N.M. Flow cytometric assessment of circulating platelet and erythrocytes microparticles in young thalassemia major patients: Relation to pulmonary hypertension and aortic wall stiffness. Eur. J. Haematol. 2013, 90, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Kheansaard, W.; Phongpao, K.; Paiboonsukwong, K.; Pattanapanyasat, K.; Chaichompoo, P.; Svasti, S. Microparticles from β-thalassaemia/HbE patients induce endothelial cell dysfunction. Sci. Rep. 2018, 8, 13033. [Google Scholar] [CrossRef] [Green Version]
- Tripodi, A.; Cappellini, M.D.; Chantarangkul, V.; Padovan, L.; Fasulo, M.R.; Marcon, A.; Mannucci, P.M. Hypercoagulability in splenectomized thalassemic patients detected by whole-blood thromboelastometry, but not by thrombin generation in platelet-poor plasma. Haematologica 2009, 94, 1520–1527. [Google Scholar] [CrossRef] [Green Version]
- Peacock-Young, B.; Macrae, F.L.; Newton, D.J.; Hill, A.; Ariëns, R.A.S. The prothrombotic state in paroxysmal nocturnal hemoglobinuria: A multifaceted source. Haematologica 2018, 103, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Hill, A.; Kelly, R.J.; Hillmen, P. Thrombosis in paroxysmal nocturnal hemoglobinuria. Blood 2013, 121, 4985–4996. [Google Scholar] [CrossRef] [Green Version]
- Kozuma, Y.; Sawahata, Y.; Takei, Y.; Chiba, S.; Ninomiya, H. Procoagulant properties of microparticles released from red blood cells in paroxysmal nocturnal haemoglobinuria. Br. J. Haematol. 2011, 152, 631–639. [Google Scholar] [CrossRef]
- Hugel, B.; Socié, G.; Vu, T.; Toti, F.; Gluckman, E.; Freyssinet, J.M.; Scrobohaci, M.L. Elevated levels of circulating procoagulant microparticles in patients with paroxysmal nocturnal hemoglobinuria and aplastic anemia. Blood 1999, 93, 3451–3456. [Google Scholar] [CrossRef]
- Simak, J.; Holada, K.; Risitano, A.M.; Zivny, J.H.; Young, N.S.; Vostal, J.G. Elevated circulating endothelial membrane microparticles in paroxysmal nocturnal haemoglobinuria. Br. J. Haematol. 2004, 125, 804–813. [Google Scholar] [CrossRef]
- Ovchynnikova, E.; Aglialoro, F.; von Lindern, M.; van den Akker, E. The Shape Shifting Story of Reticulocyte Maturation. Front. Physiol. 2018, 9, 829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mankelow, T.J.; Griffiths, R.E.; Trompeter, S.; Flatt, J.F.; Cogan, N.M.; Massey, E.J.; Anstee, D.J. Autophagic vesicles on mature human reticulocytes explain phosphatidylserine-positive red cells in sickle cell disease. Blood 2015, 126, 1831–1834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalluri, R. The biology and function of exosomes in cancer. J. Clin. Investig. 2016, 126, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Fitts, C.A.; Ji, N.; Li, Y.; Tan, C. Exploiting Exosomes in Cancer Liquid Biopsies and Drug Delivery. Adv. Healthc. Mater. 2019, 8, e1801268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, Y.-W.; Zheng, L.; Wang, Q. Characteristics and Roles of Exosomes in Cardiovascular Disease. DNA Cell Biol. 2017, 36, 202–211. [Google Scholar] [CrossRef]
- Jansen, F.; Li, Q. Exosomes as Diagnostic Biomarkers in Cardiovascular Diseases. Exosomes Cardiovasc. Dis. 2017, 998, 61–70. [Google Scholar] [CrossRef]
- Kanninen, K.M.; Bister, N.; Koistinaho, J.; Malm, T. Exosomes as new diagnostic tools in CNS diseases. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2016, 1862, 403–410. [Google Scholar] [CrossRef]
- Alipoor, S.D.; Mortaz, E.; Garssen, J.; Movassaghi, M.; Mirsaeidi, M.; Adcock, I.M. Exosomes and Exosomal miRNA in Respiratory Diseases. Med. Inflamm. 2016, 2016, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Masyuk, A.I.; Masyuk, T.V.; LaRusso, N.F. Exosomes in the pathogenesis, diagnostics and therapeutics of liver diseases. J. Hepatol. 2013, 59, 621–625. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Zhou, X.; Zhang, H.; Yao, Q.; Liu, Y.; Dong, Z. Extracellular vesicles in diagnosis and therapy of kidney diseases. Am. J. Physiol. Renal Physiol. 2016, 311, F844–F851. [Google Scholar] [CrossRef] [Green Version]
- Katakowski, M.; Buller, B.; Zheng, X.; Lu, Y.; Rogers, T.; Osobamiro, O.; Shu, W.; Jiang, F.; Chopp, M. Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett. 2013, 335, 201–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohno, S.-I.; Takanashi, M.; Sudo, K.; Ueda, S.; Ishikawa, A.; Matsuyama, N.; Fujita, K.; Mizutani, T.; Ohgi, T.; Ochiya, T.; et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol. Ther. 2013, 21, 185–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamerkar, S.; LeBleu, V.S.; Sugimoto, H.; Yang, S.; Ruivo, C.F.; Melo, S.A.; Lee, J.J.; Kalluri, R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 2017, 546, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Mendt, M.; Kamerkar, S.; Sugimoto, H.; McAndrews, K.M.; Wu, C.-C.; Gagea, M.; Yang, S.; Rodriges Blanko, E.V.; Peng, Q.; Ma, X.; et al. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight 2018, 3, e99263. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Kundrat, L.; Pishesha, N.; Bilate, A.; Theile, C.; Maruyama, T.; Dougan, S.K.; Ploegh, H.L.; Lodish, H.F. Engineered red blood cells as carriers for systemic delivery of a wide array of functional probes. Proc. Natl. Acad. Sci. USA 2014, 111, 10131–10136. [Google Scholar] [CrossRef] [Green Version]
- Usman, W.M.; Pham, T.C.; Kwok, Y.Y.; Vu, L.T.; Ma, V.; Peng, B.; Chan, Y.S.; Wei, L.; Chin, S.M.; Azad, A.; et al. Efficient RNA drug delivery using red blood cell extracellular vesicles. Nat. Commun. 2018, 9, 2359. [Google Scholar] [CrossRef]
- Malhotra, S.; Dumoga, S.; Sirohi, P.; Singh, N. Red Blood Cells-Derived Vesicles for Delivery of Lipophilic Drug Camptothecin. ACS Appl. Mater. Interfaces 2019, 11, 22141–22151. [Google Scholar] [CrossRef]
- Chang, M.; Hsiao, J.-K.; Yao, M.; Chien, L.-Y.; Hsu, S.-C.; Ko, B.-S.; Chen, S.-T.; Liu, H.-M.; Chen, Y.-C.; Yang, C.-S.; et al. Homologous RBC-derived vesicles as ultrasmall carriers of iron oxide for magnetic resonance imaging of stem cells. Nanotechnology 2010, 21, 235103. [Google Scholar] [CrossRef]
- Gangadaran, P.; Hong, C.M.; Oh, J.M.; Rajendran, R.L.; Kalimuthu, S.; Son, S.H.; Gopal, A.; Zhu, L.; Baek, S.H.; Jeong, S.Y.; et al. In vivo Non-invasive Imaging of Radio-Labeled Exosome-Mimetics Derived from Red Blood Cells in Mice. Front. Pharmacol. 2018, 9, 817. [Google Scholar] [CrossRef] [Green Version]
- Iwamoto, T. Clinical Application of Drug Delivery Systems in Cancer Chemotherapy: Review of the Efficacy and Side Effects of Approved Drugs. Biol. Pharm. Bull. 2013, 36, 715–718. [Google Scholar] [CrossRef] [Green Version]
- Veronese, F.M.; Pasut, G. PEGylation, successful approach to drug delivery. Drug Discov. Today 2005, 10, 1451–1458. [Google Scholar] [CrossRef]
- Harris, J.M.; Chess, R.B. Effect of pegylation on pharmaceuticals. Nat. Rev. Drug Discov. 2003, 2, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Ihler, G.M.; Glew, R.H.; Schnure, F.W. Enzyme Loading of Erythrocytes. Proc. Natl. Acad. Sci. USA 1973, 70, 2663–2666. [Google Scholar] [CrossRef] [PubMed]
- Pierigè, F.; Serafini, S.; Rossi, L.; Magnani, M. Cell-based drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, M.; Tajerzadeh, H. Carrier Erythrocytes: An Overview. Drug Deliv. 2003, 10, 9–20. [Google Scholar] [CrossRef]
- Dehaini, D.; Wei, X.; Fang, R.H.; Masson, S.; Angsantikul, P.; Luk, B.T.; Zhang, Y.; Ying, M.; Jiang, Y.; Kroll, A.V.; et al. Erythrocyte–Platelet Hybrid Membrane Coating for Enhanced Nanoparticle Functionalization. Adv. Mater. 2017, 29, 1606209. [Google Scholar] [CrossRef] [Green Version]
- Yoo, J.-W.; Irvine, D.J.; Discher, D.E.; Mitragotri, S. Bio-inspired, bioengineered and biomimetic drug delivery carriers. Nat. Rev. Drug Discov. 2011, 10, 521–535. [Google Scholar] [CrossRef]
- Doshi, N.; Zahr, A.S.; Bhaskar, S.; Lahann, J.; Mitragotri, S. Red blood cell-mimicking synthetic biomaterial particles. Proc. Natl. Acad. Sci. USA 2009, 106, 21495–21499. [Google Scholar] [CrossRef] [Green Version]
- Tsai, R.K.; Rodriguez, P.L.; Discher, D.E. Self inhibition of phagocytosis: The affinity of ‘marker of self’ CD47 for SIRPα dictates potency of inhibition but only at low expression levels. Blood Cells Mol. Dis. 2010, 45, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Barclay, A.N.; Van den Berg, T.K. The Interaction Between Signal Regulatory Protein Alpha (SIRPα) and CD47: Structure, Function, and Therapeutic Target. Annu. Rev. Immunol. 2014, 32, 25–50. [Google Scholar] [CrossRef]
- Schönermark, S.; Rauterberg, E.W.; Shin, M.L.; Löke, S.; Roelcke, D.; Hänsch, G.M. Homologous species restriction in lysis of human erythrocytes: A membrane-derived protein with C8-binding capacity functions as an inhibitor. J. Immunol. 1986, 136, 1772–1776. [Google Scholar] [PubMed]
- Zalman, L.S.; Wood, L.M.; Müller-Eberhard, H.J. Isolation of a human erythrocyte membrane protein capable of inhibiting expression of homologous complement transmembrane channels. Proc. Natl. Acad. Sci. USA 1986, 83, 6975–6979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.D.; Miwa, T.; Kimura, Y.; Schwendener, R.A.; van Lookeren Campagne, M.; Song, W.-C. Deficiency of decay-accelerating factor and complement receptor 1–related gene/protein y on murine platelets leads to complement-dependent clearance by the macrophage phagocytic receptor CRIg. Blood 2008, 112, 1109–1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, R.H.; Hu, C.-M.J.; Zhang, L. Nanoparticles disguised as red blood cells to evade the immune system. Expert Opin. Biol. Therapy 2012, 12, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-M.J.; Zhang, L.; Aryal, S.; Cheung, C.; Fang, R.H.; Zhang, L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl. Acad. Sci. USA 2011, 108, 10980–10985. [Google Scholar] [CrossRef] [Green Version]
- Tajerzadeh, H.; Hamidi, M. Evaluation of hypotonic preswelling method for encapsulation of enalaprilat in intact human erythrocytes. Drug Dev. Ind. Pharm. 2000, 26, 1247–1257. [Google Scholar] [CrossRef]
- Lynch, A.L.; Chen, R.; Slater, N.K.H. pH-responsive polymers for trehalose loading and desiccation protection of human red blood cells. Biomaterials 2011, 32, 4443–4449. [Google Scholar] [CrossRef]
- Ren, X.; Zheng, R.; Fang, X.; Wang, X.; Zhang, X.; Yang, W.; Sha, X. Red blood cell membrane camouflaged magnetic nanoclusters for imaging-guided photothermal therapy. Biomaterials 2016, 92, 13–24. [Google Scholar] [CrossRef]
- Luk, B.T.; Hu, C.-M.J.; Fang, R.H.; Dehaini, D.; Carpenter, C.; Gao, W.; Zhang, L. Interfacial interactions between natural RBC membranes and synthetic polymeric nanoparticles. Nanoscale 2014, 6, 2730–2737. [Google Scholar] [CrossRef] [Green Version]
- Xia, Q.; Zhang, Y.; Li, Z.; Hou, X.; Feng, N. Red blood cell membrane-camouflaged nanoparticles: A novel drug delivery system for antitumor application. Acta Pharm. Sin. B 2019, 9, 675–689. [Google Scholar] [CrossRef]
- Miyazaki, J.-I.; Aihara, H. Gene Transfer into Muscle by Electroporation In Vivo. Gene Therapy Protocols 1998, 69, 49–62. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, W.; Fang, R.H.; Dong, A.; Zhang, L. Synthesis of Nanogels via Cell Membrane-Templated Polymerization. Small 2015, 11, 4309–4313. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Sun, H.; Meng, Q.; Yin, Q.; Tang, S.; Zhang, P.; Chen, Y.; Zhang, Z.; Yu, H.; Li, Y. Long Circulation Red-Blood-Cell-Mimetic Nanoparticles with Peptide-Enhanced Tumor Penetration for Simultaneously Inhibiting Growth and Lung Metastasis of Breast Cancer. Adv. Funct. Mater. 2016, 26, 1243–1252. [Google Scholar] [CrossRef]
- Martinive, P.; De Wever, J.; Bouzin, C.; Baudelet, C.; Sonveaux, P.; Grégoire, V.; Gallez, B.; Feron, O. Reversal of temporal and spatial heterogeneities in tumor perfusion identifies the tumor vascular tone as a tunable variable to improve drug delivery. Mol. Cancer Ther. 2006, 5, 1620–1627. [Google Scholar] [CrossRef] [Green Version]
- Sonveaux, P.; Dessy, C.; Martinive, P.; Havaux, X.; Jordan, B.F.; Gallez, B.; Grégoire, V.; Balligand, J.-L.; Feron, O. Endothelin-1 is a critical mediator of myogenic tone in tumor arterioles: Implications for cancer treatment. Cancer Res. 2004, 64, 3209–3214. [Google Scholar] [CrossRef] [Green Version]
- Hamid, Z.A.A.; Abdul Hamid, Z.A.; Tham, C.Y.; Ahmad, Z. Preparation and optimization of surface-engineered poly(lactic acid) microspheres as a drug delivery device. J. Mater. Sci. 2018, 53, 4745–4758. [Google Scholar] [CrossRef]
- Shive, M.S.; Anderson, J.M. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv. Drug Deliv. Rev. 1997, 28, 5–24. [Google Scholar] [CrossRef]
- McCall, R.L.; Sirianni, R.W. PLGA Nanoparticles Formed by Single- or Double-emulsion with Vitamin E-TPGS. J. Visualized Exp. 2013, 82, 51015. [Google Scholar] [CrossRef] [Green Version]
- Fang, R.H.; Hu, C.-M.J.; Luk, B.T.; Gao, W.; Copp, J.A.; Tai, Y.; O’Connor, D.E.; Zhang, L. Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett. 2014, 14, 2181–2188. [Google Scholar] [CrossRef]
- Lin, H.J.; Wang, J.H.; Wang, C.Y.; Wu, Y.C. The preparation and characteristic of poly(lactide co-glycolide) microspheres as novel antigen delivery systems. Int. J. Nanotechnol. 2013, 10, 870. [Google Scholar] [CrossRef]
- Xing, L.; Shi, Q.; Zheng, K.; Shen, M.; Ma, J.; Li, F.; Liu, Y.; Lin, L.; Tu, W.; Duan, Y.; et al. Ultrasound-Mediated Microbubble Destruction (UMMD) Facilitates the Delivery of CA19-9 Targeted and Paclitaxel Loaded mPEG-PLGA-PLL Nanoparticles in Pancreatic Cancer. Theranostics 2016, 6, 1573–1587. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.; Xing, R.; Xu, Z.; Hou, Y.; Gao, S.; Sun, S. ChemInform Abstract: Synthesis, Functionalization, and Biomedical Applications of Multifunctional Magnetic Nanoparticles. Chem. Inform. 2010, 41. [Google Scholar] [CrossRef]
- Sharifi, S.; Seyednejad, H.; Laurent, S.; Atyabi, F.; Saei, A.A.; Mahmoudi, M. Superparamagnetic iron oxide nanoparticles forin vivomolecular and cellular imaging. Contrast Med. Mol. Imaging 2015, 10, 329–355. [Google Scholar] [CrossRef] [PubMed]
- Yoshida , T.; Sasayama , T.; Enpuku , K. (Invited) Biological Applications of Magnetic Nanoparticles for Magnetic Immunoassay and Magnetic Particle Imaging. ECS Meet. Abstr. 2016. [Google Scholar] [CrossRef]
- Chu, M.; Shao, Y.; Peng, J.; Dai, X.; Li, H.; Wu, Q.; Shi, D. Near-infrared laser light mediated cancer therapy by photothermal effect of Fe3O4 magnetic nanoparticles. Biomaterials 2013, 34, 4078–4088. [Google Scholar] [CrossRef]
- Chen, H.; Burnett, J.; Zhang, F.; Zhang, J.; Paholak, H.; Sun, D. Highly crystallized iron oxide nanoparticles as effective and biodegradable mediators for photothermal cancer therapy. J. Mater. Chem. B Mater. Biol. Med. 2014, 2, 757–765. [Google Scholar] [CrossRef]
- Shen, S.; Wang, S.; Zheng, R.; Zhu, X.; Jiang, X.; Fu, D.; Yang, W. Magnetic nanoparticle clusters for photothermal therapy with near-infrared irradiation. Biomaterials 2015, 39, 67–74. [Google Scholar] [CrossRef]
- Su, J.; Sun, H.; Meng, Q.; Zhang, P.; Yin, Q.; Li, Y. Enhanced Blood Suspensibility and Laser-Activated Tumor-specific Drug Release of Theranostic Mesoporous Silica Nanoparticles by Functionalizing with Erythrocyte Membranes. Theranostics 2017, 7, 523–537. [Google Scholar] [CrossRef]
- Zhou, Y.; Quan, G.; Wu, Q.; Zhang, X.; Niu, B.; Wu, B.; Huang, Y.; Pan, X.; Wu, C. Mesoporous silica nanoparticles for drug and gene delivery. Acta Pharm. Sin. B 2018, 8, 165–177. [Google Scholar] [CrossRef]
- Cheng, L.; Yang, K.; Li, Y.; Chen, J.; Wang, C.; Shao, M.; Lee, S.-T.; Liu, Z. Facile preparation of multifunctional upconversion nanoprobes for multimodal imaging and dual-targeted photothermal therapy. Angew. Chem. Int. Ed. Engl. 2011, 50, 7385–7390. [Google Scholar] [CrossRef]
- Rieffel, J.; Chen, F.; Kim, J.; Chen, G.; Shao, W.; Shao, S.; Chitgupi, U.; Hernandez, R.; Graves, S.A.; Nickles, R.J.; et al. Hexamodal imaging with porphyrin-phospholipid-coated upconversion nanoparticles. Adv. Mater. 2015, 27, 1785–1790. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, Z.; Li, Z.; Ju, E.; Gao, N.; Zhou, L.; Ren, J.; Qu, X. Upconversion nanoprobes for efficiently in vitro imaging reactive oxygen species and in vivo diagnosing rheumatoid arthritis. Biomaterials 2015, 39, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Braun, G.B.; Shi, Y.; Zhang, Y.; Sun, X.; Reich, N.O.; Zhao, D.; Stucky, G. Fabrication of Ag@SiO(2)@Y(2)O(3):Er nanostructures for bioimaging: Tuning of the upconversion fluorescence with silver nanoparticles. J. Am. Chem. Soc. 2010, 132, 2850–2851. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, J.; Ao, Y.; Li, S.; Miao, Y.; Yu, Z.; Zhu, L.; Lan, X.; Zhu, Y.; Zhang, Y.; et al. Synergizing Upconversion Nanophotosensitizers with Hyperbaric Oxygen to Remodel the Extracellular Matrix for Enhanced Photodynamic Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 22985–22996. [Google Scholar] [CrossRef]
- Giljohann, D.A.; Seferos, D.S.; Daniel, W.L.; Massich, M.D.; Patel, P.C.; Mirkin, C.A. Gold Nanoparticles for Biology and Medicine. Angew. Chem. Int. Ed. 2010, 49, 3280–3294. [Google Scholar] [CrossRef] [Green Version]
- Piao, J.-G.; Wang, L.; Gao, F.; You, Y.-Z.; Xiong, Y.; Yang, L. Erythrocyte membrane is an alternative coating to polyethylene glycol for prolonging the circulation lifetime of gold nanocages for photothermal therapy. ACS Nano 2014, 8, 10414–10425. [Google Scholar] [CrossRef]
- Toth, M.; Fridman, R. Assessment of Gelatinases (MMP-2 and MMP-9) by Gelatin Zymography. Metastasis Res. Protocols 2012, 163–174. [Google Scholar] [CrossRef] [Green Version]
- Li, L.-L.; Xu, J.-H.; Qi, G.-B.; Zhao, X.; Yu, F.; Wang, H. Core–Shell Supramolecular Gelatin Nanoparticles for Adaptive and “On-Demand” Antibiotic Delivery. ACS Nano 2014, 8, 4975–4983. [Google Scholar] [CrossRef]
- Castro, F.; Martins, C.; Silveira, M.J.; Moura, R.P.; Pereira, C.L.; Sarmento, B. Advances on erythrocyte-mimicking nanovehicles to overcome barriers in biological microenvironments. Adv. Drug Deliv. Rev. 2020. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thangaraju, K.; Neerukonda, S.N.; Katneni, U.; Buehler, P.W. Extracellular Vesicles from Red Blood Cells and Their Evolving Roles in Health, Coagulopathy and Therapy. Int. J. Mol. Sci. 2021, 22, 153. https://doi.org/10.3390/ijms22010153
Thangaraju K, Neerukonda SN, Katneni U, Buehler PW. Extracellular Vesicles from Red Blood Cells and Their Evolving Roles in Health, Coagulopathy and Therapy. International Journal of Molecular Sciences. 2021; 22(1):153. https://doi.org/10.3390/ijms22010153
Chicago/Turabian StyleThangaraju, Kiruphagaran, Sabari Nath Neerukonda, Upendra Katneni, and Paul W. Buehler. 2021. "Extracellular Vesicles from Red Blood Cells and Their Evolving Roles in Health, Coagulopathy and Therapy" International Journal of Molecular Sciences 22, no. 1: 153. https://doi.org/10.3390/ijms22010153
APA StyleThangaraju, K., Neerukonda, S. N., Katneni, U., & Buehler, P. W. (2021). Extracellular Vesicles from Red Blood Cells and Their Evolving Roles in Health, Coagulopathy and Therapy. International Journal of Molecular Sciences, 22(1), 153. https://doi.org/10.3390/ijms22010153




