Newly Developed Recombinant Antithrombin Protects the Endothelial Glycocalyx in an Endotoxin-Induced Rat Model of Sepsis
Abstract
:1. Introduction
2. Results
2.1. Changes in Leukocyte Adhesion and Blood Flow
2.2. Changes in Perfused Boundary Region (PBR) and Red Blood Cell (RBC) Filling Percentage
2.3. Laboratory Data
2.4. Hyaluronan and Syndecan-1 Measurements
3. Discussion
Limitations
4. Materials and Methods
4.1. Lipopolysaccharide Administration and the Sepsis Model
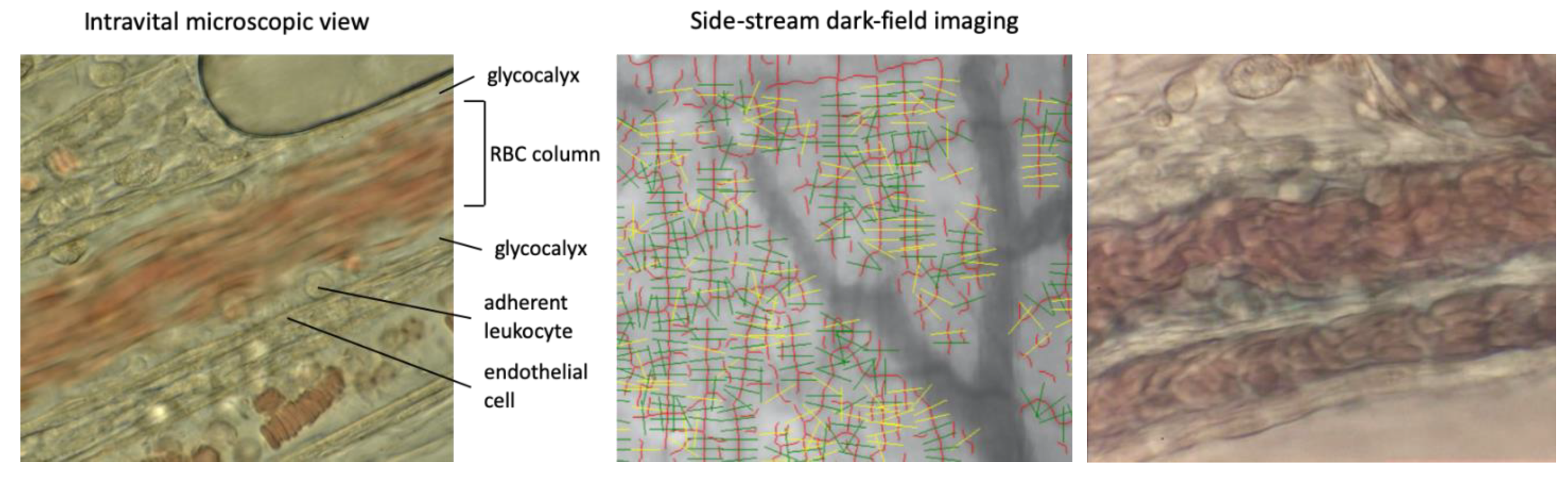
4.2. Evaluation of the Microcirculation Using Intravital Microscopy
4.3. Glycocalyx Evaluation Using Side-Stream Dark-Field Imaging
4.4. Blood Sampling and Measurement
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DIC | disseminated intravascular coagulation |
| AT-γ | recombinant antithrombin |
| RBC | red blood cell |
| PBR | perfused boundary region |
| APTT | activated partial thromboplastin time |
| ALT | alanine aminotransferase |
| BUN | blood urea nitrogen |
| ELISA | enzyme-linked immunosorbent assay |
| FUT8 | α-1,6-fucosyltransferase |
References
- Engelmann, B.; Massberg, S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. 2013, 13, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Esmon, C.T.; Xu, J.; Lupu, F. Innate immunity and coagulation. J. Thromb. Haemost. 2011, 9, 182–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simmons, J.; Pittet, J.F. The coagulopathy of acute sepsis. Curr. Opin. Anaesthesiol. 2015, 28, 227–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iba, T.; Levy, J.H. Inflammation and thrombosis: Roles of neutrophils, platelets and endothelial cells and their interactions in thrombus formation during sepsis. J. Thromb. Haemost. 2018, 16, 231–241. [Google Scholar] [CrossRef]
- Lee, D.H.; Dane, M.J.; van den Berg, B.M.; Boels, M.G.; van Teeffelen, J.W.; de Mutsert, R.; den Heijer, M.; Rosendaal, F.R.; van der Vlag, J.; van Zonneveld, A.J.; et al. Deeper penetration of erythrocytes into the endothelial glycocalyx is associated with impaired microvascular perfusion. PLoS ONE 2014, 9, e96477. [Google Scholar] [CrossRef] [Green Version]
- Iba, T.; Nakarai, E.; Takayama, T.; Nakajima, K.; Sasaoka, T.; Ohno, Y. Combination effect of antithrombin and recombinant human soluble thrombomodulin in a lipopolysaccharide induced rat sepsis model. Crit. Care 2009, 13, R203. [Google Scholar] [CrossRef] [Green Version]
- Sobczak, A.I.S.; Pitt, S.J.; Stewart, A.J. Glycosaminoglycan Neutralization in Coagulation Control. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1258–1270. [Google Scholar] [CrossRef] [Green Version]
- Becker, B.F.; Jacob, M.; Leipert, S.; Salmon, A.H.; Chappell, D. Degradation of the endothelial glycocalyx in clinical settings: Searching for the sheddases. Br. J. Clin. Pharmacol. 2015, 80, 389–402. [Google Scholar] [CrossRef]
- Myers, G.J.; Wegner, J. Endothelial Glycocalyx and Cardiopulmonary Bypass. J. Extra Corpor. Technol. 2017, 49, 174–181. [Google Scholar]
- Iba, T.; Di Nisio, M.; Thachil, J.; Wada, H.; Asakura, H.; Sato, K.; Kitamura, N.; Saitoh, D. Revision of the Japanese Association for Acute Medicine (JAAM) disseminated intravascular coagulation (DIC) diagnostic criteria using antithrombin activity. Crit. Care 2016, 20, 287. [Google Scholar] [CrossRef] [Green Version]
- Tagami, T.; Matsui, H.; Horiguchi, H.; Fushimi, K.; Yasunaga, H. Antithrombin and mortality in severe pneumonia patients with sepsis-associated disseminated intravascular coagulation: An observational nationwide study. J. Thromb. Haemost. 2014, 12, 1470–1479. [Google Scholar] [CrossRef] [PubMed]
- Wada, H.; Asakura, H.; Okamoto, K.; Iba, T.; Uchiyama, T.; Kawasugi, K.; Koga, S.; Mayumi, T.; Koike, K.; Gando, S.; et al. Expert consensus for the treatment of disseminated intravascular coagulation in Japan. Thromb. Res. 2010, 125, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Iba, T.; Saitoh, D.; Wada, H.; Asakura, H. Efficacy and bleeding risk of antithrombin supplementation in septic disseminated intravascular coagulation: A secondary survey. Crit. Care 2014, 18, 497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirose, M.; Tsukada, M.; Hirayama, F.; Kubo, Y.; Kajii, M.; Mochizuki, S.; Hamato, N.; Ohi, H. Recombinant human antithrombin expressed in Chinese hamster ovary cells shows in vivo efficacy on rat DIC model smilarly to plasma-derived antithrombin regardless of different N-glycosylation. Thromb. Res. 2007, 119, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Yamane-Ohnuki, N.; Kinoshita, S.; Inoue-Urakubo, M.; Kusunoki, M.; Iida, S.; Nakano, R.; Wakitani, M.; Niwa, R.; Sakurada, M.; Uchida, K.; et al. Establishment of FUT8 knockout Chinese hamster ovary cells: An ideal host cell line for producing completely defucosylated antibodies with enhanced antibody-dependentcellular cytotoxicity. Biotechnol. Bioeng. 2004, 87, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Endo, S.; Shimazaki, R. An open-label, randomized, phase 3 study of the efficacy and safety of antithrombin gamma in patients with sepsis-induced disseminated intravascular coagulation syndrome. J. Intensiv. Care 2018, 6, 75. [Google Scholar] [CrossRef] [Green Version]
- Iba, T.; Hirota, T.; Sato, K.; Nagaoka, I. Protective effect of a newly developed fucose-deficient recombinant antithrombin against histone-induced endothelial damage. Int. J. Hematol. 2018, 107, 528–534. [Google Scholar] [CrossRef]
- Woodcock, T.E.; Woodcock, T.M. Revised Starling equation and the glycocalyx model of transvascular fluid exchange: An improved paradigm for prescribing intravenous fluid therapy. Br. J. Anaesth. 2012, 108, 384–394. [Google Scholar] [CrossRef] [Green Version]
- Becker, B.F.; Chappell, D.; Bruegger, D.; Annecke, T.; Jacob, M. Therapeutic strategies targeting the endothelial glycocalyx: Acute deficits, but great potential. Cardiovasc. Res. 2010, 87, 300–310. [Google Scholar] [CrossRef] [Green Version]
- Chappell, D.; Dörfler, N.; Jacob, M.; Rehm, M.; Welsch, U.; Conzen, P.; Becker, B.F. Glycocalyx protection reduces leukocyte adhesion after ischemia/reperfusion. Shock 2010, 34, 133–139. [Google Scholar] [CrossRef]
- Grundmann, S.; Fink, K.; Rabadzhieva, L.; Bourgeois, N.; Schwab, T.; Moser, M.; Bode, C.; Busch, H.J. Perturbation of the endothelial glycocalyx in post cardiac arrest syndrome. Resuscitation 2012, 83, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Johansson, P.I.; Stensballe, J.; Rasmussen, L.S.; Ostrowski, S.R. A high admission syndecan-1 level, a marker of endothelial glycocalyx degradation, is associated with inflammation, protein C depletion, fibrinolysis, and increased mortality in trauma patients. Ann. Surg. 2011, 254, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Holzmann, M.S.; Winkler, M.S.; Strunden, M.S.; Izbicki, J.R.; Schoen, G.; Greiwe, G.; Pinnschmidt, H.O.; Poppe, A.; Saugel, B.; Daum, G.; et al. Syndecan-1 as a biomarker for sepsis survival after major abdominal surgery. Biomark. Med. 2018, 12, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Cerny, V.; Astapenko, D.; Brettner, F.; Benes, J.; Hyspler, R.; Lehmann, C.; Zadak, Z. Targeting the endothelial glycocalyx in acute critical illness as a challenge for clinical and laboratory medicine. Crit. Rev. Clin. Lab. Sci. 2017, 54, 343–357. [Google Scholar] [CrossRef]
- Murphy, L.S.; Wickersham, N.; McNeil, J.B.; Shaver, C.M.; May, A.K.; Bastarache, J.A.; Ware, L.B. Endothelial glycocalyx degradation is more severe in patients with non-pulmonary sepsis compared to pulmonary sepsis and associates with risk of ARDS and other organ dysfunction. Ann. Intensiv. Care 2017, 7, 102. [Google Scholar] [CrossRef]
- Ikeda, M.; Matsumoto, H.; Ogura, H.; Hirose, T.; Shimizu, K.; Yamamoto, K.; Maruyama, I.; Shimazu, T. Circulating syndecan-1 predicts the development of disseminated intravascular coagulation in patients with sepsis. J. Crit. Care 2018, 43, 48–53. [Google Scholar] [CrossRef]
- Chappell, D.; Hofmann-Kiefer, K.; Jacob, M.; Rehm, M.; Briegel, J.; Welsch, U.; Conzen, P.; Becker, B.F. TNF-alpha induced shedding of the endothelial glycocalyx is prevented by hydrocortisone and antithrombin. Basic Res. Cardiol. 2009, 104, 78–89. [Google Scholar] [CrossRef]
- Song, J.W.; Zullo, J.A.; Liveris, D.; Dragovich, M.; Zhang, X.F.; Goligorsky, M.S. Therapeutic Restoration of Endothelial Glycocalyx in Sepsis. J. Pharmacol. Exp. Ther. 2017, 361, 115–121. [Google Scholar] [CrossRef] [Green Version]
- Alphonsus, C.S.; Rodseth, R.N. The endothelial glycocalyx: A review of the vascular barrier. Anaesthesia 2014, 69, 777–784. [Google Scholar] [CrossRef]
- Schött, U.; Solomon, C.; Fries, D.; Bentzer, P. The endothelial glycocalyx and its disruption, protection and regeneration: A narrative review. Scand. J. Trauma Resusc. Emerg. Med. 2016, 24, 48. [Google Scholar] [CrossRef] [Green Version]
- Chappell, D.; Jacob, M.; Hofmann-Kiefer, K.; Rehm, M.; Welsch, U.; Conzen, P.; Becker, B.F. Antithrombin reduces shedding of the endothelial glycocalyx following ischaemia/reperfusion. Cardiovasc. Res. 2009, 83, 388–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iba, T.; Sasaki, T.; Ohshima, T.; Sato, K.; Nagaoka, I.; Thachil, J. The comparison of the protective effects of a and β-antithrombin against vascular endothelial cell damage induced by histone in vitro. Thromb. Haemost. Open 2017, 1, e3–e10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aldecoa, C.; Llau, J.V.; Nuvials, X.; Artigas, A. Role of albumin in the preservation of endothelial glycocalyx integrity and the microcirculation: A review. Ann. Intensiv. Care 2020, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Vlahu, C.A.; Lemkes, B.A.; Struijk, D.G.; Koopman, M.G.; Krediet, R.T.; Vink, H. Damage of the endothelial glycocalyx in dialysis patients. J. Am. Soc. Nephrol. 2012, 23, 1900–1908. [Google Scholar] [CrossRef] [Green Version]
- Ushiyama, A.; Kataoka, H.; Iijima, T. Glycocalyx and its involvement in clinical pathophysiologies. J. Intensiv. Care 2016, 4, 59. [Google Scholar] [CrossRef] [Green Version]
- Anand, D.; Ray, S.; Srivastava, L.M.; Bhargava, S. Evolution of serum hyaluronan and syndecan levels in prognosis of sepsis patients. Clin. Biochem. 2016, 49, 768–776. [Google Scholar] [CrossRef]
- Smart, L.; Macdonald, S.P.J.; Burrows, S.; Bosio, E.; Arendts, G.; Fatovich, D.M. Endothelial glycocalyx biomarkers increase in patients with infection during Emergency Department treatment. J. Crit. Care 2017, 42, 304–309. [Google Scholar] [CrossRef]
- Curry, F.E.; Adamson, R.H. Endothelial glycocalyx: Permeability barrier and mechanosensor. Ann. Biomed. Eng. 2012, 40, 828–839. [Google Scholar] [CrossRef] [Green Version]
- van der Poll, T.; Levi, M. Crosstalk between inflammation and coagulation: The lessons of sepsis. Curr. Vasc. Pharmacol. 2012, 10, 632–638. [Google Scholar] [CrossRef]
- Iba, T.; Saitoh, D. Efficacy of antithrombin in preclinical and clinical applications for sepsis-associated disseminated intravascular coagulation. J. Intensiv. Care 2014, 2, 66. [Google Scholar] [CrossRef] [Green Version]
- Martens, R.J.; Vink, H.; van Oostenbrugge, R.J.; Staals, J. Sublingual microvascular glycocalyx dimensions in lacunar stroke patients. Cerebrovasc Dis. 2013, 35, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Conway, E.M. Thrombin: Coagulation’s master regulator of innate immunity. J. Thromb. Haemost. 2019, 17, 1785–1789. [Google Scholar] [CrossRef] [PubMed]



| Parameters | Platelet count (×103/mm3) | APTT (second) | Fibrinogen (mg/dL) | AT activity (%) | ALT (IU/L) | BUN (mg/dL) | Albumin (mg/dL) | Lactate (mmol/L) |
|---|---|---|---|---|---|---|---|---|
| Normal group | 110–120 | 16.1–16.7 | 210–220 | 100–120 | 15–32 | 12.7–14.5 | 4.7–5.1 | 0.8–0.9 |
| Control group | 17 ± 2 | 33 ± 3 | 82 ± 10 | 41 ± 10 | 144 ± 21 | 52 ± 3 | 2.3 ± 0.1 | 4.6 ± 0.3 |
| Low-dose group | 27 ± 3 * | 31 ± 3 | 109 ± 11 | 82 ± 8 * | 100 ± 13 | 35 ± 3 * | 2.8 ± 0.1 | 3.6 ± 0.3 |
| High-dose group | 27 ± 3 * | 24 ± 3 * | 122 ± 12 * | 101 ± 11 * | 93 ± 16 | 37 ± 4 * | 3.1 ± 0.2 * | 3.4 ± 0.3 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iba, T.; Levy, J.H.; Aihara, K.; Kadota, K.; Tanaka, H.; Sato, K.; Nagaoka, I. Newly Developed Recombinant Antithrombin Protects the Endothelial Glycocalyx in an Endotoxin-Induced Rat Model of Sepsis. Int. J. Mol. Sci. 2021, 22, 176. https://doi.org/10.3390/ijms22010176
Iba T, Levy JH, Aihara K, Kadota K, Tanaka H, Sato K, Nagaoka I. Newly Developed Recombinant Antithrombin Protects the Endothelial Glycocalyx in an Endotoxin-Induced Rat Model of Sepsis. International Journal of Molecular Sciences. 2021; 22(1):176. https://doi.org/10.3390/ijms22010176
Chicago/Turabian StyleIba, Toshiaki, Jerrold H. Levy, Koichiro Aihara, Katsuhiko Kadota, Hiroshi Tanaka, Koichi Sato, and Isao Nagaoka. 2021. "Newly Developed Recombinant Antithrombin Protects the Endothelial Glycocalyx in an Endotoxin-Induced Rat Model of Sepsis" International Journal of Molecular Sciences 22, no. 1: 176. https://doi.org/10.3390/ijms22010176
APA StyleIba, T., Levy, J. H., Aihara, K., Kadota, K., Tanaka, H., Sato, K., & Nagaoka, I. (2021). Newly Developed Recombinant Antithrombin Protects the Endothelial Glycocalyx in an Endotoxin-Induced Rat Model of Sepsis. International Journal of Molecular Sciences, 22(1), 176. https://doi.org/10.3390/ijms22010176







