High-Molecular-Weight Glutenin Subunits: Genetics, Structures, and Relation to End Use Qualities
Abstract
1. Introduction
2. Genetics of HMW-GS
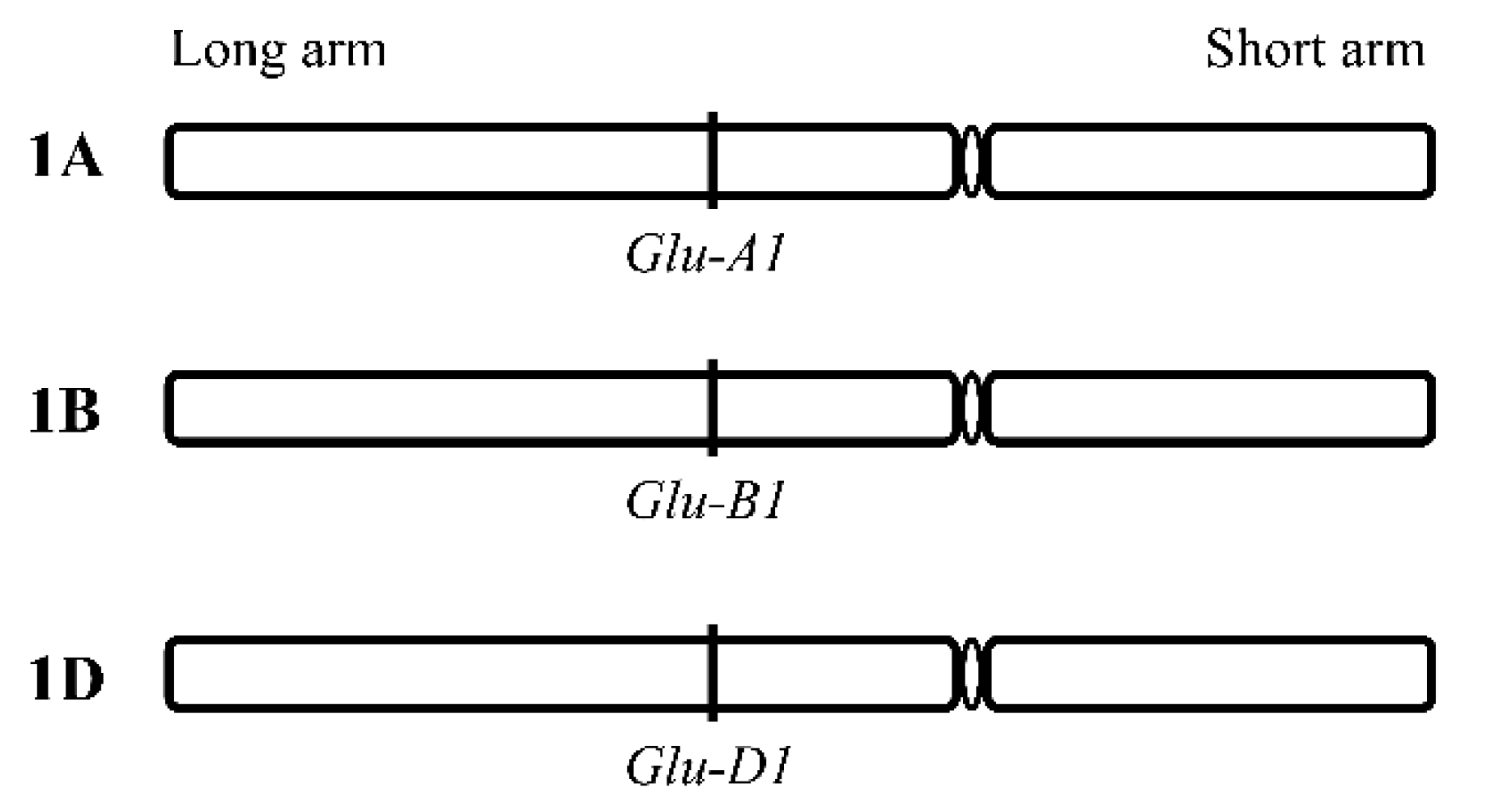
2.1. Allelic Variations of Glu-1 Loci
2.2. Gene Expression
2.2.1. Progress on the Ay Gene
2.2.2. Progress on the Bx7 Gene
3. Structures of HMW-GS
3.1. The Primary Structures
3.2. Cysteine Residues and Disulfide Bonds
3.3. Secondary Structures of HMW-GS
4. Relationship to End Use Qualities
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| HMW-GS | High-molecular-weight glutenin subunit |
| SDS-PAGE | Sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| LMW-GS | Low-molecular-weight glutenin subunit |
| PCR | Polymerase chain reaction |
| GUS | β-glucuronidase |
| Wis | Wheat insertion sequence |
| LTR | Long terminal repeat |
| 1Dx5-N | N-terminal domain of 1Dx5 |
| LC-MS/MS | liquid chromatograph-mass spectrometer/mass spectrometry |
| GSH | glutathione |
| PSSG | protein bound glutathione |
| FTIR | Fourier transform infrared spectroscopy |
| NMR | Nuclear magnetic resonance |
References
- Agricultural Market Information System. Available online: http://www.amis-outlook.org/#jfmulticontent_c363419-2 (accessed on 8 October 2020).
- Organisation for Economic Co-operation and Development and Food and Agriculture Organization of United Nations Agricultural Outlook 2019–2028. Available online: https://stats.oecd.org/viewhtml.aspx?datasetcode=HIGH_AGLINK_2019&lang=en# (accessed on 16 July 2019).
- Anjum, F.M.; Khan, M.R.; Din, A.; Saeed, M.; Pasha, I.; Arshad, M.U. Wheat Gluten: High Molecular Weight Glutenin Subunits–Structure, Genetics, and Relation to Dough Elasticity. J. Food Sci. 2007, 72, R56–R63. [Google Scholar] [CrossRef] [PubMed]
- Osborne, T.B. The Vegetable Proteins, 2nd ed.; Longmans Green and Co.: London, UK, 1924. [Google Scholar]
- MacRitchie, F. Evaluation of Contributions from Wheat Protein Fractions to Dough Mixing and Breadmaking. J. Cereal Sci. 1987, 6, 259–268. [Google Scholar] [CrossRef]
- MacRitchie, F. Physicochemical properties of wheat proteins in relation to functionality. Adv. Food Nutr. Res. 1992, 36, 1–87. [Google Scholar]
- Weegels, P.L.; Hamer, R.J.; Schofield, J.D. Functional Properties of Wheat Glutenin. J. Cereal Sci. 1996, 23, 1–18. [Google Scholar] [CrossRef]
- Wieser, H. Chemistry of gluten proteins. Food Microbiol. 2007, 24, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Lambourne, J.; Tosi, P.; Marsh, J.; Bhandari, D.; Green, R.; Frazier, R.; Shewry, P.R. Characterisation of an s-type low molecular weight glutenin subunit of wheat and its proline and glutamine-rich repetitive domain. J. Cereal Sci. 2010, 51, 96–104. [Google Scholar] [CrossRef]
- Belton, P.S. Mini review: On the Elasticity of Wheat Gluten. J. Cereal Sci. 1999, 29, 103–107. [Google Scholar] [CrossRef]
- D’Oovidio, R. The low-molecular-weight glutenin subunits of wheat gluten. J. Cereal Sci. 2004, 39, 321–339. [Google Scholar] [CrossRef]
- Zhang, Q.; Dong, Y.M.; An, X.L.; Wang, A.; Zhang, Y.Z.; Li, X.H.; Gao, L.Y.; Xia, X.C.; He, Z.H.; Yan, Y.M. Characterization of HMW glutenin subunits in common wheat and related species by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS). J. Cereal Sci. 2008, 47, 252–261. [Google Scholar] [CrossRef]
- Liu, L.; Wang, A.L.; Appels, R.; Ma, J.H.; Xia, X.C.; Lan, P.; He, Z.H.; Bekes, F.; Yan, Y.M.; Ma, W.J. A MALDI-TOF based analysis of high molecular weight glutenin subunits for wheat breeding. J. Cereal Sci. 2009, 50, 295–301. [Google Scholar] [CrossRef]
- Peng, Y.C.; Yu, K.; Zhang, Y.J.; Islam, S.; Sun, D.F.; Ma, W.J. Two novel y-type high molecular weight glutenin genes in Chinese wheat landraces of the Yangtze-River region. PLoS ONE 2015, 10, e0142348. [Google Scholar] [CrossRef] [PubMed]
- Bietz, J.A.; Shepherd, K.W.; Wall, J.S. Single-kernel analysis of glutenin: Use in wheat genetics and breeding. Cereal Chem. 1975, 52, 513–532. [Google Scholar]
- Payne, P.I.; Nightingale, M.A.; Krattiger, A.F.; Holt, L.M. The relationship between HMW glutenin subunit composition and the bread-making quality of British-grown wheat varieties. J. Sci. Food Agric. 1987, 40, 51–65. [Google Scholar] [CrossRef]
- Payne, P.I.; Holt, L.M.; Jackson, E.A.; Law, C. Wheat storage proteins: Their genetics and their potential for manipulation by plant breeding. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1984, 304, 359–371. [Google Scholar]
- Lawrence, G.J.; Shepherd, K.W. Chromosomal location of genes controlling seed proteins in species related to wheat. Theor. Appl. Genet. 1981, 59, 25–31. [Google Scholar] [CrossRef]
- Payne, P.I.; Corfield, K.G.; Holt, L.M.; Blackman, J.A. Correlations between the inheritance of certain high-molecular weight subunits of glutenin and bread-making quality in progenies of six crosses of bread wheat. J. Sci. Food Agric. 1981, 32, 51–60. [Google Scholar] [CrossRef]
- Payne, P.I.; Holt, L.M.; Lawrence, G.J.; Law, C.N. The genetics of gliadin and glutenin, the major storage proteins of the wheat endosperm. Plant Foods Hum. Nutr. 1982, 31, 229–241. [Google Scholar] [CrossRef]
- Shewry, P.R.; Lafiandra, D.; Tamás, L.; Békés, F. Genetic manipulation of gluten structure and function. In Gliadin and Glutenin the Unique Balance of Wheat Quality; Wrigley, C., Békés, F., Bushuk, W., Eds.; American Association of Cereal Chemists, Inc. (AACC): St. Paul, MN, USA, 2006; pp. 363–385. [Google Scholar]
- Thompson, R.D.; Bartels, D.; Harberd, N.P. Nucleotide sequence of a gene from chromosome 1D of wheat encoding a HMW-glutenin subunit. Nucleic Acids Res. 1985, 13, 6833–6846. [Google Scholar] [CrossRef][Green Version]
- Halford, N.G.; Forde, J.; Anderson, O.D.; Greene, F.C.; Shewry, P.R. The nucleotide and deduced amino acid sequences of an HMW glutenin subunit gene from chromosome 1B of bread wheat (Triticum aestivum L.) and comparison with those of genes from chromosomes 1A and 1D. Theor. Appl. Genet. 1987, 75, 117–126. [Google Scholar] [CrossRef]
- Payne, P.I.; Lawrence, G.J. Catalogue of alleles for the complex gene loci, Glu-A1, Glu-B1, and Glu-D1 which code for high-molecular-weight subunits of glutenin in hexaploid wheat. Cereal Res. Comm. 1983, 11, 29–35. [Google Scholar]
- Shewry, P.R.; Gilbert, S.M.; Savage, A.W.; Tatham, A.S.; Wan, Y.F.; Belton, P.S.; Wellner, N.; D’Ovidio, R.; Bekes, F.; Halford, N.G. Sequence and properties of HMW subunit 1Bx20 from pasta wheat (Triticum durum) which is associated with poor end use properties. Theor. Appl. Genet. 2003, 106, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Wang, D.; Shewry, P.R.; Halford, N.G. Isolation and characterization of five novel high molecular weight subunit of glutenin genes from Triticum timopheevi and Aegilops cylindrica. Theor. Appl. Genet. 2002, 104, 828–839. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yan, Z.; Wan, Y.; Liu, K.; Zheng, Y.; Wang, D. Analysis of HMW glutenin subunits and their coding sequences in two diploid Aegilops species. Theor. Appl. Genet. 2003, 106, 1368–1378. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Q.; Zhang, X.Y. An approach for isolating high-molecular-weight glutenin subunit genes using monoclonal antibodies. Genome 2006, 49, 181–189. [Google Scholar] [CrossRef]
- McIntosh, R.A.; Yamazaki, Y.; Devos, K.M.; Dubcovsky, J.; Rogers, W.J.; Appels, R. Catalogue of gene symbols for wheat. In Proceedings of the 10th International Wheat Genetics Symposium, SIMI, Rome, Italy, 1–6 September 2003. [Google Scholar]
- Bekes, F.; Cavanagh, C.R.; Martinov, S.; Bushuk, S.; Wrigley, C.W.F. PART II. Composition table for the HMW subunits of glutenin. In The Gluten Composition of Wheat Varieties and Genotypes; AACC International: St. Paul, MN, USA, 2008. [Google Scholar]
- Li, X.; Liu, D.C.; Sun, J.Z.; Yang, W.L.; Guo, X.L.; Wang, D.W.; Zhang, A.M. Characterization of novel high-molecular-weight glutenin subunits and their coding sequences in Aegilops markgrafii. J. Cereal Sci. 2015, 65, 9–18. [Google Scholar] [CrossRef]
- Du, X.; Zhang, X. Molecular cloning and functional characterization of two novel high molecular weight glutenin subunit genes in Aegilops markgrafii. J. Genet. 2017, 96, 563–570. [Google Scholar] [CrossRef]
- Hou, W.Q.; Feng, W.; Yu, G.H.; Du, X.Y.; Ren, M.J. Cloning and functional analysis of a novel x-type high-molecular-weight glutenin subunit with altered cysteine residues from Aegilops umbellulata. Crop Pasture Sci. 2017, 68, 409–414. [Google Scholar] [CrossRef]
- Hu, J.X.; Wang, J.; Deng, X.; Yan, Y.M. Cloning and characterization of special HMW glutenin subunit genes from Aegilops longissima L. and their potential for wheat quality improvement. 3 Biotech 2019, 9, 267. [Google Scholar] [CrossRef]
- Wang, H.J.; Zhang, H.J.; Li, B.; Yu, Z.H.; Li, G.R.; Zhang, J.; Yang, Z.J. Molecular cytogenetic characterization of new wheat—Dasypyrum breviaristatum introgression lines for improving grain quality of wheat. Front. Plant Sci. 2018, 9, 365. [Google Scholar] [CrossRef]
- Du, X.Y.; Jia, Z.; Yu, Y.; Wang, S.; Che, B.J.; Ni, F.; Bao, Y.G. A wheat-Aegilops umbellulata addition line improves wheat agronomic traits and processing quality. Breeding Sci. 2019, 69, 503–507. [Google Scholar] [CrossRef]
- Yang, Y.H.; Li, S.M.; Zhang, K.P.; Dong, Z.Y.; Li, Y.W.; An, X.L.; Chen, J.; Chen, Q.F.; Jiao, Z.; Liu, X.; et al. Efficient isolation of ion beam-induced mutants for homoeologous loci in common wheat and comparison of the contributions of Glu-1 loci to gluten functionality. Theor. Appl. Genet. 2014, 127, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, G.J.; MacRitchie, F.; Wrigley, C.W. Dough and baking quality of wheat lines deficient in glutenin subunits controlled by the Glu-A1, Glu-B1 and Glu-D1 loci. J. Cereal Sci. 1988, 7, 109–112. [Google Scholar] [CrossRef]
- Zhang, L.J.; Chen, Q.F.; Su, M.J.; Yan, B.; Zhang, X.Q.; Jiao, Z. High-molecular-weight glutenin subunit-deficient mutants induced by ion beam and the effects of Glu-1 loci deletion on wheat quality properties. J. Food Sci. Agric. 2015, 96, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.H.; Xue, J.S.; Duan, L.N.; Gu, Y.S.; Mu, J.Y.; Han, S.C.; Chen, L.; Li, Y.X.; Ma, W.J.; Yan, Y.M.; et al. Effects of high-molecular-weight glutenin subunit combination in common wheat on the quality of crumb structure. J. Sci. Food Agric. 2019, 99, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Brönneke, V.; Zimmermann, G.; Killermann, B. Effect of high molecular weight glutenins and D-zone gliadins on bread-making quality in German wheat varieties. Cereal Res. Commun. 2000, 28, 187–194. [Google Scholar] [CrossRef]
- Liu, H.Y.; Wang, K.; Xiao, L.L.; Wang, S.L.; Du, L.P.; Cao, X.Y.; Zhang, X.X.; Zhou, Y.; Yan, Y.M.; Ye, X.G. Comprehensive identification and bread-making quality evaluation of common wheat somatic variation line AS208 on glutenin composition. PLoS ONE 2016, 11, e0146933. [Google Scholar] [CrossRef][Green Version]
- Wang, Z.J.; Li, Y.W.; Yang, Y.H.; Liu, X.; Qin, H.J.; Dong, Z.Y.; Zheng, S.H.; Zhang, K.P.; Wang, D.W. New insight into the function of wheat glutenin proteins as investigated with two series of genetic mutants. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Gao, X.; Liu, T.H.; Ding, M.Y.; Wang, J.; Li, C.L.; Wang, Z.H.; Li, X.J. Effects of HMW-GS Ax1 or Dx2 absence on the glutenin polymerization and gluten micro structure of wheat (Triticum aestivum L.). Food Chem. 2018, 240, 626–633. [Google Scholar] [CrossRef]
- Song, L.J.; Li, L.Q.; Zhao, L.Y.; Liu, Z.Z.; Xie, T.T.; Li, X.J. Absence of Dx2 at Glu-D1 locus weakens gluten quality potentially regulated by expression of nitrogen metabolism enzymes and glutenin-related genes in wheat. Int. J. Mol. Sci. 2020, 21, 1383. [Google Scholar] [CrossRef]
- Chen, H.Q.; Li, S.J.; Liu, Y.W.; Liu, J.X.; Ma, X.L.; Du, L.P.; Wang, K.; Ye, X.G. Effects of 1Dy12 subunit silencing on seed storage protein accumulation and flour-processing quality in a common wheat somatic variation line. Food Chem. 2021, 335, 127663. [Google Scholar] [CrossRef]
- Duan, L.N.; Han, S.C.; Wang, K.; Jiang, P.H.; Gu, Y.S.; Chen, L.; Mu, J.Y.; Ye, X.G.; Li, Y.X.; Yan, Y.M.; et al. Analyzing the action of evolutionarily conserved modules on HMW-GS 1Ax1 promoter activity. Plant Mol. Biol. 2019, 102, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, X.; Zhao, Y.; Chen, F.G.; Xia, G.M. Structure, variation and expression analysis of glutenin gene promoters from Triticum aestivum cultivar Chinese Spring shows the distal region of promoter 1Bx7 is key regulatory sequence. Gene 2013, 527, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Ravel, C.; Fiquet, S.; Boudet, J.; Dardevet, M.; Vincent, J.; Merlino, M.; Michard, R.; Martre, P. Conserved cis-regulatory modules in promoters of genes encoding wheat high-molecular-weight glutenin subunits. Front. Plant Sci. 2014, 5, 621. [Google Scholar] [CrossRef] [PubMed]
- Makai, S.; Éva, C.; Tamás, L.; Juhász, A. Multiple elements controlling the expression of wheat high molecular weight glutenin paralogs. Funct. Integr. Genomics 2015, 15, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Albani, D.; Hammond-Kosack, M.C.; Smith, C.; Conlan, S.; Colot, V.; Holdsworth, M.; Bevan, M.W. The Wheat Transcriptional Activator SPA: A Seed-Specific bZIP Protein That Recognizes the GCN4-like Motif in the Bifactorial Endosperm Box of Prolamin Genes. Plant Cell 1997, 9, 171–184. [Google Scholar]
- Ravel, C.; Martre, P.; Romeuf, I.; Dardevet, M.; El-Malki, R.; Bordes, J.; Duchateau, N.; Brunel, D.; Balfourier, F.; Charmet, G. Nucleotide polymorphism in the wheat transcriptional activator Spa influences its pattern of expression and has pleiotropic effects on grain protein composition, dough viscoelasticity, and grain hardness. Plant Physiol. 2009, 151, 2133–2144. [Google Scholar] [CrossRef]
- Kumar, A.; Jaiswal, J.P.; Sharma, N.; Gupta, S.; Kumar, A. Understanding the molecular basis of differential grain protein accumulation in wheat (Triticum aestivum L.) through expression profiling of transcription factors related to seed nutrients storage. 3 Biotech 2018, 8, 112. [Google Scholar] [CrossRef]
- Guo, D.D.; Hou, Q.L.; Zhang, R.Q.; Lou, H.Y.; Li, Y.H.; Zhang, Y.F.; You, M.S.; Xie, C.J.; Liang, R.Q.; Li, B.Y. Over-Expressing TaSPA-B Reduces Prolamin and Starch Accumulation in Wheat (Triticum aestivum L.) Grains. Int. J. Mol. Sci. 2020, 21, 3257. [Google Scholar] [CrossRef]
- Boudet, J.; Merlino, M.; Plessis, A.; Gaudin, J.C.; Dardevet, M.; Perrochon, S.; Alvarez, D.; Risacher, T.; Martre, P.; Ravel, C. The bZIP transcription factor SPA Heterodimerizing Protein represses glutenin synthesis in Triticum aestivum. Plant J. 2019, 97, 858–871. [Google Scholar] [CrossRef]
- Johansson, E.; Henriksson, P.; Svensson, G.; Heneen, W. Detection, chromosomal location and evaluation of the functional value of a novel high Mr glutenin subunit found in Swedish wheats. J. Cereal Sci. 1993, 17, 237–245. [Google Scholar] [CrossRef]
- Margiotta, B.; Urbano, M.; Colaprico, G.; Johansson, E.; Buonocore, F.; D’Ovidio, R.; Lafiandra, D. Detection of y-type subunit at the Glu-A1 locus in some Swedish bread wheat lines. J. Cereal Sci. 1996, 23, 203–212. [Google Scholar] [CrossRef]
- Anjum, F.M.; Lookhart, G.L.; Walker, C.E. Electrophoretic identification of hard white spring wheats grown at different locations in Pakistan in different years. J. Sci. Food Agric. 2000, 80, 1155–1161. [Google Scholar] [CrossRef]
- Ciaffi, M.; Lafiandra, D.; Porceddu, E.; Benedettelli, S. Storage-protein variation in wild emmer wheat (Triticum turgidum ssp. dicoccoides) from Jordan and Turkey. I. Electrophoretic characterization of genotypes. Theor. Appl. Genet. 1993, 86, 474–480. [Google Scholar]
- Jiang, Q.T.; Ma, J.; Zhao, S.; Zhao, Q.Z.; Lan, X.J.; Dai, S.F.; Lu, Z.X.; Zheng, Y.L.; Wei, Y.M. Characterization of HMW-GSs and their gene inaction in tetraploid wheat. Genetica 2012, 140, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.B.; Song, S.Y.; Zhao, L.R.; Shen, L.S.; Song, Y.H.; Wang, X.; Yu, K.; Liu, Z.Y.; Li, Y.W.; Yang, W.L. Mechanisms, origin and heredity of Glu-1Ay silencing in wheat evolution and domestication. Theor. Appl. Genet. 2018, 131, 1561–1575. [Google Scholar] [CrossRef] [PubMed]
- Harberd, N.P.; Flavell, R.B.; Thompson, R.D. Identification of a transposon-like insertion in a Glu-1 allele of wheat. Mol. Gen. Genet. 1987, 209, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.M.; Yan, Y.M.; Jiang, Y.; Xiao, Y.H.; Hu, Y.K.; Cai, M.H.; Li, Y.X.; Hsam, S.L.K.; Zeller, F.J. Molecular cloning and comparative analysis of a y-type inactive HMW glutenin subunit gene from cultivated emmer wheat (Triticum dicoccum L.). Hereditas 2004, 141, 46–54. [Google Scholar] [CrossRef]
- Jiang, Q.T.; Wei, Y.M.; Wang, F.; Wang, J.R.; Yan, Z.H.; Zheng, Y.L. Characterization and comparative analysis of HMW glutenin 1Ay alleles with differential expressions. BMC Plant Biol. 2009, 9, 16. [Google Scholar] [CrossRef]
- Gu, Y.Q.; Coleman-Derr, D.; Kong, X.Y.; Anderson, O.D. Rapid genome evolution revealed by comparative sequence analysis of orthologous regions from four triticeae genomes. Plant Physiol. 2004, 135, 459–470. [Google Scholar] [CrossRef]
- Wang, Z.Z.; Huang, L.; Wu, B.H.; Hu, J.L.; Jiang, Z.L.; Qi, P.F.; Zheng, Y.L.; Liu, D.C. Characterization of an Integrated Active Glu-1Ay Allele in Common Wheat from Wild Emmer and Its Potential Role in Flour Improvement. Int. J. Mol. Sci. 2018, 19, 923. [Google Scholar] [CrossRef]
- Roy, N.; Islam, S.; Ma, J.; Lu, M.; Torok, K.; Tomoskozi, S.; Bekes, F.; Lafiandra, D.; Appels, R.; Ma, W.J. Expressed Ay HMW glutenin subunit in Australian wheat cultivars indicates a positive effect on wheat quality. J. Cereal Sci. 2017, 79, 494–500. [Google Scholar] [CrossRef]
- Roy, N.; Islam, S.; Yu, Z.T.; Lu, M.Q.; Lafiandra, D.; Zhao, Y.; Anwar, M.; Mayer, J.E.; Ma, W.J. Introgression of an expressed HMW 1Ay glutenin subunit allele into bread wheat cv. Lincoln increases grain protein content and breadmaking quality without yield penalty. Theor. Appl. Genet. 2019, 133, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Marchylo, B.A.; Lukow, O.M.; Kruger, J.E. Quantitative variation in high molecular weight glutenin subunit 7 in some Canadian wheats. J. Cereal Sci. 1992, 15, 29–37. [Google Scholar] [CrossRef]
- Lukow, O.M.; Forsyth, S.A.; Payne, P.I. Over-production of HMW [High Molecular Weight] glutenin subunits coded on chromosome 1B in common wheat—Triticum aestivum [in Israel and Canada]. J. Genet. Breeding Sci. 1992, 46, 187–192. [Google Scholar]
- D’Ovidio, R.; Masci, S.; Porceddu1, E.; Kasarda, D.D. Duplication of the Bx7 high-molecular-weight glutenin subunit gene in bread wheat (Triticum aestivum L.) cultivar‘Red River 68’. Plant Breeding 1997, 116, 525–531. [Google Scholar] [CrossRef]
- Juhàsz, A.; Larroque, O.R.; Tamas, L.; Hsam, S.L.K.; Zeller, F.J.; Békés, F.; Bedo, Z. Bánkúti 1201-an old Hungarian wheat variety with special storage protein composition. Theor. Appl. Genet. 2003, 107, 697–704. [Google Scholar] [CrossRef]
- Radovanovic, N.; Cloutier, S.; Brown, D.; Humphreys, D.G.; Lukow, O.M. Genetic Variance for Gluten Strength Contributed by High Molecular Weight Glutenin Proteins. Cereal Chem. 2002, 79, 843–849. [Google Scholar] [CrossRef]
- Gao, X.; Appelbee, M.J.; Mekuria, G.T.; Chalmers, K.J.; Mather, D.E. A second ‘overexpression’ allele at the Glu-B1 high-molecular-weight glutenin locus of wheat: Sequence characterisation and functional effects. Theor. Appl. Genet. 2012, 124, 333–343. [Google Scholar] [CrossRef]
- Butow, B.J.; Gras, P.W.; Haraszi, R.; Bekes, F. Effects of different salts on mixing and extension parameters on a diverse group of wheat cultivars using 2-g mixograph and extensigraph methods. Cereal Chem. 2002, 79, 826–833. [Google Scholar] [CrossRef]
- Butow, B.J.; Ma, W.; Gale, K.R.; Cornish, G.B.; Rampling, L.; Larroque, O.; Morell, M.K.; Bekes, F. Molecular discrimination of Bx7 alleles demonstrates that a highly expressed high molecular weight glutenin allele has a major impact on wheat flour dough strength. Theor. Appl. Genet. 2003, 107, 1524–1532. [Google Scholar] [CrossRef]
- Ragupathy, R.; Naeem, H.A.; Reimer, E.; Lukow, O.M.; Sapirstein, H.D.; Cloutier, S. Evolutionary origin of the segmental duplication encompassing the wheat GLU-B1 locus encoding the overexpressed Bx7 (Bx7OE) high molecular weight glutenin subunit. Theor. Appl. Genet. 2007, 116, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Cloutier, S.; Banks, T.; Nilmalgoda, S. Molecular understanding of wheat evolution at the Glu-B1 locus. In Proceedings of the International Conference on Plant Genomics and Biotechnology: Challenges and Opportunities, Raipur, India, 26–28 October 2005; p. 40. [Google Scholar]
- Shewry, P.R.; Halford, N.G.; Tatham, A.S. High molecular weight subunits of wheat glutenin. J. Cereal Sci. 1992, 15, 105–120. [Google Scholar] [CrossRef]
- Tatham, A.S.; Drake, A.F.; Shewry, P.R. Conformational studies of synthetic peptides corresponding to the repetitive regions of the high molecular weight (HMW) glutenin subunits of wheat. J. Cereal Sci. 1990, 11, 189–200. [Google Scholar] [CrossRef]
- Shewry, P.R.; Halford, N.G.; Tatham, A.S. The high molecular weight subunits of wheat, barley and rye: Genetics, molecular biology, chemistry and role in wheat gluten structure and functionality. In Oxford Surveys of Plant and Molecular Cell Biology; Miflin, B.J., Ed.; Oxford University Press: London, UK, 1989; pp. 163–219. [Google Scholar]
- Anderson, O.D.; Greene, F.C. The characterization and comparative analysis of high-molecular-weight glutenin genes from genomes A and B of a hexaploid bread wheat. Theor. Appl. Genet. 1989, 77, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Shewry, P.R.; Halford, N.G.; Tatham, A.S.; Popineau, Y.; Lafiandra, D.; Belton, P.S. The high molecular weight subunits of wheat glutenin and their role in determining wheat processing properties. Adv. Food Nutr. Res. 2003, 45, 219–302. [Google Scholar] [PubMed]
- D’Ovidio, R.; Masci, S.; Porceddu, E. Development of a set of oligonucleotide primers specific for genes at the Glu-1 complex loci of wheat. Theor. Appl. Genet. 1995, 91, 189–194. [Google Scholar] [CrossRef]
- Halford, N.G.; Field, J.M.; Blair, H.; Urwin, P.; Moore, K.; Robert, L.; Thompson, R.; Flavell, R.B.; Tatham, A.S.; Shewry, P.R. Analysis of HMW glutenin subunits encoded by chromosome 1A of bread wheat (Triticum aestivum L.) indicates quantitative effects on grain quality. Theor. Appl. Genet. 1992, 83, 373–378. [Google Scholar] [CrossRef]
- Gao, J.H.; Yu, P.X.; Liang, H.R.; Fu, J.H.; Luo, Z.Y.; Yang, D. The wPDI Redox Cycle Coupled Conformational Change of the Repetitive Domain of the HMW-GS 1Dx5-A Computational Study. Molecules 2020, 25, 4393. [Google Scholar] [CrossRef]
- Anderson, O.D.; Greene, F.C.; Yip, R.E.; Halford, N.G.; Shewry, P.R.; Malpica-Romero, J. Nucleotide sequences of the two high-molecular-weight glutenin genes from the D-genome of a hexaploid bread wheat, Triticum aestivum L. cv Cheyenne. Nucleic Acids Res. 1989, 17, 461–462. [Google Scholar] [CrossRef]
- Kasarda, D.D. Glutenin structure in relation to wheat quality. In Wheat is Unique; Pomeranz, Y., Ed.; AACC: St. Paul, MN, USA, 1989; Volume 277, pp. 277–302. [Google Scholar]
- Buonocore, F.; Caporale, C.; Lafiandra, D. Purification and Characterisation of High Mr Glutenin Subunit 20 and its Linked y-type Subunit from Durum Wheat. J. Cereal Sci. 1996, 23, 195–201. [Google Scholar] [CrossRef]
- Li, W.; Wan, Y.; Liu, Z.; Liu, K.; Liu, X.; Li, B.; Li, Z.; Zhang, X.; Dong, Y.; Wang, D. Molecular characterization of HMW glutenin subunit allele 1Bx14: Further insights into the evolution of Glu-B1-1 alleles in wheat and related species. Theor. Appl. Genet. 2004, 109, 1093–1104. [Google Scholar] [CrossRef] [PubMed]
- Tatham, A.S.; Field, J.M.; Keen, J.N.; Jackson, P.J.; Shewry, P.R. Purification and characterization of HMW glutenin subunits encoded by chromosome 1B of durum wheat (Triticum durum). J. Cereal Sci. 1991, 14, 111–116. [Google Scholar] [CrossRef]
- Grosch, W.; Wieser, H. Redox Reactions in Wheat Dough as Affected by Ascorbic Acid. J. Cereal Sci. 1999, 29, 1–16. [Google Scholar] [CrossRef]
- Wrigley, C.W. Giant proteins with flour power. Nature 1996, 72, 738–739. [Google Scholar] [CrossRef]
- Wang, L.; Yu, J.J.; Wang, C.C. Protein disulfide isomerase is regulated in multiple ways: Consequences for conformation, activities, and pathophysiological functions. BioEssays 2020. [Google Scholar] [CrossRef]
- Wieser, H. The use of redox agents in breadmaking. In Breadmaking, 2nd ed.; Cauvain, S.P., Ed.; Woodhead Publishing: Sawston, UK, 2012; pp. 447–469. [Google Scholar]
- Shewry, P.R.; Popineau, Y.; Lafiandra, D.; Belton, P. Wheat glutenin subunits and dough elasticity: Findings of the EUROWHEAT project. Trends Food Sci. Technol. 2001, 11, 433–441. [Google Scholar] [CrossRef]
- Humphris, A.D.L.; McMaster, T.J.; Miles, M.J.; Gilbert, S.M.; Shewry, P.R.; Tatham, A.S. Atomic Force Microscopy (AFM) Study of Interactions of HMW Subunits of Wheat Glutenin. Cereal Chem. 2000, 77, 107–110. [Google Scholar] [CrossRef]
- Zhao, C.F.; Luo, Z.Y.; Li, M.Z.; Gao, J.H.; Liang, Z.X.; Sun, S.Y.; Wang, X.; Yang, D. Wheat protein disulfide isomerase improves bread properties via different mechanisms. Food Chem. 2020, 315, 126242. [Google Scholar] [CrossRef]
- Lindsay, M.P.; Tamas, L.; Appels, R.; Skerritt, J.H. Direct Evidence that the Number and Location of Cysteine Residues affect Glutenin Polymer Structure. J. Cereal Sci. 2000, 31, 321–333. [Google Scholar] [CrossRef]
- Ferrer, E.G.; Gómez, A.V.; Añón, M.C.; Puppo, M.C. Structural changes in gluten protein structure after addition of emulsifier. A Raman spectroscopy study. Spectrochimi. Acta A 2011, 79, 278–281. [Google Scholar] [CrossRef]
- Li, X.J.; Liu, T.H.; Song, L.J.; Zhang, H.; Li, L.Q.; Gao, X. Influence of high-molecular-weight glutenin subunit composition at Glu-A1 and Glu-D1 loci on secondary and micro structures of gluten in wheat (Triticum aestivum L.). Food Chem. 2016, 213, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Keck, B.; Köhler, P.; Wieser, H. Disulphide bonds in wheat gluten: Cystine peptides derived from gluten proteins following peptic and thermolytic digestion. Z. Lebensm. Unters. F. 1995, 200, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Lutz, E.; Wieser, H.; Koehler, P. Identification of disulfide bonds in wheat gluten proteins by means of mass spectrometry/electron transfer dissociation. J. Agric. Food Chem. 2012, 60, 3708–3716. [Google Scholar] [CrossRef] [PubMed]
- Köhler, P.; Belitz, H.-D.; Wieser, H. Disulphide bonds in wheat gluten: Further cystine peptides from high molecular weight (HMW) and low molecular weight (LMW) subunits of glutenin and from γ-gliadins. Z. Lebensm. Unters. F. 1993, 196, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Köhler, P.; Bettina, K.G.; Wieser, H.; Kasarda, D.D. Molecular Modeling of the N-terminal Regions of High Molecular Weight Glutenin Subunits 7 and 5 in Relation to Intramolecular Disulfide Bond Formation. Cereal Chem. 1997, 74, 154–158. [Google Scholar] [CrossRef]
- Cazalis, R.; Aussenac, T.; Rhazi, L.; Marin, A.; Gibrat, J.F. Homology modeling and molecular dynamics simulations of the N-terminal domain of wheat high molecular weight glutenin subunit 10. Protein Sci. 2003, 12, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Liu, G.; Huang, Y.B.; Zeng, Q.H.; Hu, S.Q. Dissecting the disulfide linkage of the N-terminal domain of HMW 1Dx5 and its contributions to dough functionality. J. Agric. Food Chem. 2017, 65, 6264. [Google Scholar] [CrossRef]
- Wang, J.J.; Liu, G.; Huang, Y.B.; Zeng, Q.H.; Song, G.S.; Hou, Y.; Li, L.; Hu, S.Q. Role of N-terminal domain of HMW 1Dx5 in the functional and structural properties of wheat dough. Food Chem. 2016, 213, 682–690. [Google Scholar] [CrossRef]
- Popineau, Y.; Deshayes, G.; Lefebvre, J.; Fido, R.; Tatham, A.S.; Shewry, P.R. Prolamin aggregation, gluten viscoelasticity, and mixing properties of transgenic wheat lines expressing 1Ax and 1Dx high molecular weight glutenin subunit transgenes. J. Agric. Food Chem. 2001, 49, 395–401. [Google Scholar] [CrossRef]
- Margiotta, B.; Pfluger, L.; Roth, M.R.; MacRitchie, F.; Lafiandra, D. Isogenic bread wheat lines differing in number and type of high Mr glutenin subunits. In Proceedings of the 7th International Workshop Gluten 2000, Bristol, UK, 2–6 April 2000; pp. 29–33. [Google Scholar]
- Zhang, D.L.; He, T.T.; Liang, H.H.; Huang, L.Y.; Su, Y.Z.; Li, Y.G.; Li, S.P. Flour quality and related molecular characterization of high molecular weight glutenin subunit genes from wild emmer wheat accession TD-256. J. Agric. Food Chem. 2016, 64, 5128–5136. [Google Scholar] [CrossRef]
- Joye, I.J.; Lagrain, B.; Delcour, J.A. Endogenous redox agents and enzymes that affect protein network formation during breadmaking—A review. J. Cereal Sci. 2009, 50, 1–10. [Google Scholar] [CrossRef]
- Li, W.L.; Tsiami, A.A.; Schofield, J.D. Redox Reactions during Dough Mixing and Dough Resting: Effect of Reduced and Oxidised Glutathione and L-Ascorbic Acid on Rheological Properties of Gluten. In Wheat Gluten; Shewry, P.R., Tatham, A.S., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2000; pp. 239–243. [Google Scholar]
- Gilbert, S.M.; Wellner, N.; Belton, P.S.; Greenfield, J.A.; Siligardi, G.; Shewry, P.R.; Tatham, A.S. Expression and characterisation of a highly repetitive peptide derived from a wheat seed storage protein. BBA-Protein Struct. Mol. Enzym. 2000, 1479, 135–146. [Google Scholar] [CrossRef]
- Tatham, A.S.; Shewry, P.R.; Miflin, B.J. Wheat gluten elasticity: A similar molecular basis to elastin? FEBS Lett. 1984, 177, 205–208. [Google Scholar] [CrossRef]
- Tatham, A.S.; Miflin, B.J.; Shewry, P.R. The β-turn conformation in wheat gluten proteins: Relationship to gluten elasticity. Cereal Chem. 1985, 62, 405–412. [Google Scholar]
- Van Dijk, A.A.; Van Wijk, L.L.; Van Swieten, E.; Robillard, G.T.; Vliet, A.V.; Tesser, G.I.; Haris, P. Structure characterization of the central repetitive domain of high molecular weight gluten proteins. I. Model studies using cyclic and linear peptides. Protein Sci. 1997, 6, 637–648. [Google Scholar] [CrossRef][Green Version]
- Miles, M.J.; Carr, H.J.; McMaster, T.C.; I’Anson, K.J.; Belton, P.S.; Morris, V.J.; Field, J.M.; Shewry, P.R.; Tatham, A.S. Scanning tunneling microscopy of a wheat seed storage protein reveals details of an unusual supersecondary structure. PNAS 1991, 88, 68–71. [Google Scholar] [CrossRef]
- Field, J.M.; Tatham, A.S.; Shewry, P.R. The structure of a high-Mr subunit of durum-wheat (Triticum durum) gluten. Biochem. J. 1987, 247, 215–221. [Google Scholar] [CrossRef]
- Matsushima, N.; Danno, G.I.; Sasaki, N.; Izumi, Y. Small-angle X-ray scattering study by synchrotron orbital radiation reveals that high molecular weight subunit of glutenin is a very anisotropic molecule. Biochem. Biophys. Res. Commun. 1992, 186, 1057–1064. [Google Scholar] [CrossRef]
- Belton, P.S.; Colquhoun, I.J.; Grant, A.; Wellner, N.; Field, J.M.; Shewry, P.R.; Tatham, A.S. FTIR and NMR studies on the hydration of a high-Mr subunit of glutenin. Int. J. Biol. Macromol. 1995, 17, 74–80. [Google Scholar] [CrossRef]
- Georget, D.M.R.; Belton, P.S. Effects of temperature and water content on the secondary structure of wheat gluten studied by FTIR spectroscopy. Biomacromolecules 2006, 7, 469–475. [Google Scholar] [CrossRef]
- Belton, P.S. A hypothesis concerning the elasticity of high molecular weight subunits. In Wheat Kernel Proteins: Molecular Functional Aspects; Universita della Tuscia: Viterbo, Italy, 1994; pp. 159–165. [Google Scholar]
- Mita, T.; Matsumoto, H. Flow properties of aqueous gluten and gluten methyl ester dispersions. Cereal Chem. 1981, 58, 57–61. [Google Scholar]
- Bushuk, W. Interactions in wheat doughs. In The Keys to Cereal Quality; American Assn. of Cereal Chemists: St. Paul, MN, USA, 1998; pp. 1–16. [Google Scholar]
- Alberti, E.; Gilbert, S.M.; Tatham, A.S.; Shewry, P.R.; Gil, A.M. Study of high molecular weight wheat glutenin subunit 1Dx5 by 13C and 1H solid-state NMR spectroscopy. I. Role of covalent crosslinking. Biopolymers 2002, 67, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Feeney, K.A.; Wellner, N.; Gilbert, S.M.; Halford, N.G.; Tatham, A.S.; Shewry, P.R.; Belton, P.S. Molecular structures and interactions of repetitive peptides based on wheat glutenin subunits depend on chain length. Biopolymers 2003, 72, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Wellner, N.; Mills, E.N.C.; Brownsey, G.; Wilson, R.H.; Brown, N.; Freeman, J.; Halford, N.G.; Shewry, P.R.; Belton, P.S. Changes in Protein Secondary Structure during Gluten Deformation Studied by Dynamic Fourier Transform Infrared Spectroscopy. Biomacromolecules 2005, 6, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Liu, T.H.; Yu, J.; Li, L.Q.; Feng, Y.; Li, X.J. Influence of high-molecular-weight glutenin subunit composition at Glu-B1 locus on secondary and micro structures of gluten in wheat (Triticum aestivum L.). Food Chem. 2016, 197, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Li, S.P.; Liu, Y.C.; Tong, J.Y.; Yu, L.W.; Ding, M.Y.; Zhang, Z.M.; Rehman, A.-u.; Majzoobi, M.; Wang, Z.H.; Gao, X. The overexpression of high-molecular-weight glutenin subunit Bx7 improves the dough rheological properties by altering secondary and micro-structures of wheat gluten. Food Res. Int. 2020, 130, 108914. [Google Scholar] [CrossRef]
- Jin, M.; Xie, Z.Z.; Ge, P.; Li, J.; Jiang, S.S.; Subburaj, S.; Li, X.H.; Zeller, F.J.; Hsam, S.L.K.; Yan, Y.M. Identification and molecular characterisation of HMW glutenin subunit 1By16* in wild emmer. J. Appl. Genet. 2012, 53, 249–258. [Google Scholar] [CrossRef]
- Flavell, R.B.; Goldsbrough, A.P.; Robert, L.S.; Schnick, D.; Thompson, R.D. Genetic variation in wheat HMW glutenin subunits and the molecular basis of bread-making quality. Bio/Technology 1989, 7, 1281–1285. [Google Scholar] [CrossRef]
- Wang, K.; An, X.L.; Pan, L.P.; Dong, K.; Gao, L.Y.; Wang, S.L.; Xie, Z.Z.; Zhang, Z.; Appels, R.; Ma, W.; et al. Molecular characterization of HMW-GS 1Dx3t and 1Dx4t genes from Aegilops tauschii and their potential value for wheat quality improvement. Hereditas 2012, 149, 41–49. [Google Scholar] [CrossRef]
- Wang, S.; Yu, Z.T.; Cao, M.; Shen, X.X.; Li, N.; Li, X.H.; Ma, W.J.; Weißgerber, H.; Zeller, F.; Hsam, S.; et al. Molecular mechanisms of HMW glutenin subunits from 1Sl genome of Aegilops longissima positively affecting wheat breadmaking quality. PLoS ONE 2013, 8, e58947. [Google Scholar] [CrossRef]
- Gianibelli, M.C.; Larroque, O.R.; MacRitchie, F.; Wrigley, C.W. Biochemical, Genetic, and Molecular Characterization of Wheat Glutenin and Its Component Subunits. Cereal Chem. 2001, 78, 635–646. [Google Scholar] [CrossRef]
- Li, X.Y.; Li, Y.; Karim, H.; Li, Y.; Zhong, X.J.; Tang, H.P.; Qi, P.F.; Ma, J.; Wang, J.R.; Chen, G.Y.; et al. The production of wheat-Aegilops sharonensis 1Ssh chromosome substitution lines harboring alien novel high-molecular-weight glutenin subunits. Genome 2019, 63, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Gao, X.; Li, L.; Du, D.; Cheng, X.; Zhao, Y.; Liu, Y.; Li, X. Effects of HMW-GS at Glu-B1 locus on the polymerization of glutenin during grain development and on the secondary and micro-structures of gluten in wheat (Triticum aestivum L.). J. Cereal Sci. 2016, 72, 101–107. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Fu, J.; Shen, Q.; Yang, D. High-Molecular-Weight Glutenin Subunits: Genetics, Structures, and Relation to End Use Qualities. Int. J. Mol. Sci. 2021, 22, 184. https://doi.org/10.3390/ijms22010184
Li Y, Fu J, Shen Q, Yang D. High-Molecular-Weight Glutenin Subunits: Genetics, Structures, and Relation to End Use Qualities. International Journal of Molecular Sciences. 2021; 22(1):184. https://doi.org/10.3390/ijms22010184
Chicago/Turabian StyleLi, Yi, Jiahui Fu, Qun Shen, and Dong Yang. 2021. "High-Molecular-Weight Glutenin Subunits: Genetics, Structures, and Relation to End Use Qualities" International Journal of Molecular Sciences 22, no. 1: 184. https://doi.org/10.3390/ijms22010184
APA StyleLi, Y., Fu, J., Shen, Q., & Yang, D. (2021). High-Molecular-Weight Glutenin Subunits: Genetics, Structures, and Relation to End Use Qualities. International Journal of Molecular Sciences, 22(1), 184. https://doi.org/10.3390/ijms22010184






