Curcumin Metabolite Tetrahydrocurcumin in the Treatment of Eye Diseases
Abstract
1. Introduction
2. Curcumin
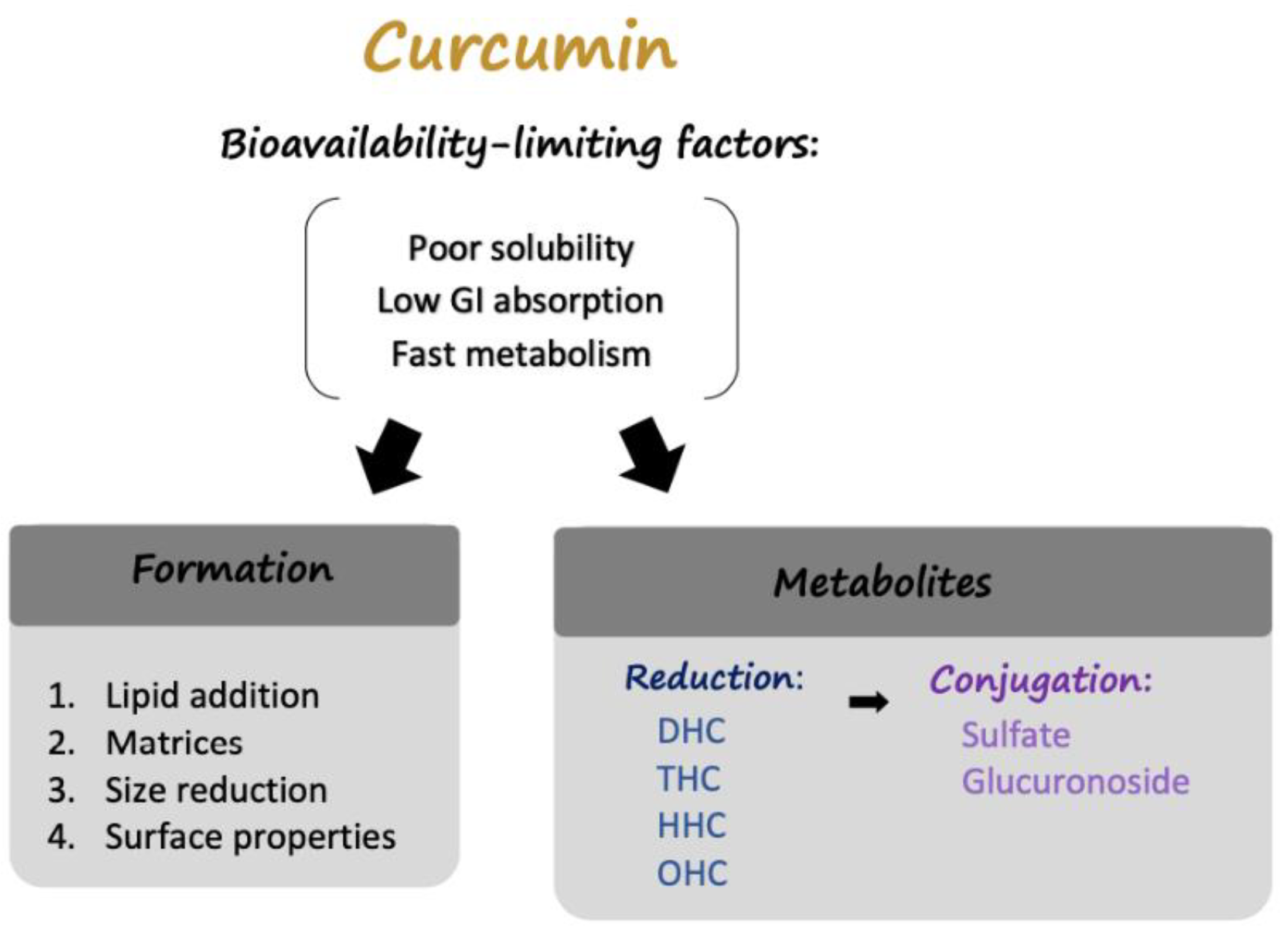
2.1. Curcumin: Limitations
2.2. Modulation of Curcumin: Delivery Formulations
2.2.1. Lipid Addition
2.2.2. Absorption and Dispersion on Matrices
2.2.3. Particle Size Reduction
2.2.4. Surface Property Modulation
2.3. Modulation of Curcumin: Curcumin Metabolites
3. Therapeutic Potential of Curcumin in Ophthalmology
3.1. Main Ophthalmic Conditions in Modern Society
3.1.1. Age-Related Cataract
3.1.2. Glaucoma
3.1.3. AMD
3.1.4. DR
3.2. Therapeutic Potential of the THC Pathway
3.3. Effect of THC on Antioxidative Stress
3.3.1. THC has an Anti-Inflammatory Effect
3.3.2. The Anti-VEGF Effect of THC
3.3.3. The Neuroprotective Effect of THC
3.3.4. The Inhibitory Effect of THC on Platelet Aggregation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviation
| AMD | age-related macular degeneration |
| ATF | activating transcription factor |
| Chop | C/EBP homologous protein |
| COX | cyclooxygenase |
| Cur-BSA-NPs-Gel | curcumin, bovine serum albumin, nanoparticles, gel |
| CUR-CNLC-GEL | curcumin, cationic nanostructured lipid carriers, nanogel |
| DR | diabetes retinopathy |
| eNOS | endothelial nitric oxide synthase |
| ER stress | endoplasmic reticulum stress |
| ERK | extracellular signal-regulated kinases |
| GCLC | glutamate-cysteine ligase catalytic subunit |
| GCLM | glutamate-cysteine ligase modifier subunit |
| GSH | glutathione |
| HIF-1α | hypoxia-inducible factor—1 alpha |
| HO-1 | heme oxygenase-1 |
| IL-1β, IL-6 | Interleukin—1 beta, interleukin—6 |
| IRE1α | Inositol-requiring transmembrane kinase/endoribonuclease 1α |
| Keap1 | kelch-like ECH-associated protein 1 |
| LC3 | microtubule-associated protein 1A/1B-light chain 3 |
| LPO | lipid peroxidation |
| MAPK | mitogen-activated protein kinase |
| MePEG-PCL | methoxypoly (ethylene glycol)-poly(caprolactone) |
| MMP | matrix metalloproteinases |
| MSVI | moderate to severe visual impairment |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NQO1 | NAD(P)H quinone dehydrogenase 1 |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| NSAIDs | nonsteroidal anti-inflammatory drugs |
| OHC | octahydrocurcumin |
| P62 | ubiquitin-binding protein p62 |
| PEG | polyethylene glycol |
| PERK | protein kinase RNA-like endoplasmic reticulum kinase |
| PGE2 | prostaglandin E2 |
| PLGA | poly (lactic-co-glycolic acid) |
| ROS | reactive oxygen species |
| RPE | retinal pigment epithelium |
| SIRT1 | sirtuin-1 |
| Smad3 | mothers against decapentaplegic homolog 3 |
| SOD | superoxide dismutase |
| TGF-β1 | transforming growth factor-beta 1 |
| THC | tetrahydrocurcumim |
| TNF-α | tumor necrosis factor alpha |
| UPR | unfolded protein response |
| VEGF | vascular endothelial growth factor |
References
- Thapa, R.; Bajimaya, S.; Paudyal, G.; Khanal, S.; Tan, S.; Thapa, S.S.; van Rens, G. Population awareness of diabetic eye disease and age related macular degeneration in Nepal: The Bhaktapur Retina Study. BMC Ophthalmol. 2015, 15, 188. [Google Scholar] [CrossRef] [PubMed]
- Stevens, G.A.; White, R.A.; Flaxman, S.R.; Price, H.; Jonas, J.B.; Keeffe, J.; Leasher, J.; Naidoo, K.; Pesudovs, K.; Resnikoff, S.; et al. Global prevalence of vision impairment and blindness: Magnitude and temporal trends, 1990–2010. Ophthalmology 2013, 120, 2377–2384. [Google Scholar] [CrossRef] [PubMed]
- Huynh, T.P.; Mann, S.N.; Mandal, N.A. Botanical compounds: Effects on major eye diseases. Evid. Based Complement. Alternat. Med. 2013, 2013, 549174. [Google Scholar] [CrossRef] [PubMed]
- Hatcher, H.; Planalp, R.; Cho, J.; Torti, F.M.; Torti, S.V. Curcumin: From ancient medicine to current clinical trials. Cell. Mol. Life Sci. 2008, 65, 1631–1652. [Google Scholar] [CrossRef]
- Nowak, M.S.; Smigielski, J. The prevalence and causes of visual impairment and blindness among older adults in the city of Lodz, Poland: Erratum. Medicine 2015, 94, 1. [Google Scholar] [CrossRef]
- Bourne, R.R.A.; Flaxman, S.R.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H.; Leasher, J.; Limburg, H.; et al. Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e888–e897. [Google Scholar] [CrossRef]
- Barchitta, M.; Maugeri, A.; Favara, G.; Magnano San Lio, R.; Evola, G.; Agodi, A.; Basile, G. Nutrition and Wound Healing: An Overview Focusing on the Beneficial Effects of Curcumin. Int. J. Mol. Sci. 2019, 20, 1119. [Google Scholar] [CrossRef]
- Du, Z.Y.; Wei, X.; Huang, M.T.; Zheng, X.; Liu, Y.; Conney, A.H.; Zhang, K. Anti-proliferative, anti-inflammatory and antioxidant effects of curcumin analogue A(2). Arch. Pharm. Res. 2013, 36, 1204–1210. [Google Scholar] [CrossRef]
- Hussain, Z.; Thu, H.E.; Amjad, M.W.; Hussain, F.; Ahmed, T.A.; Khan, S. Exploring recent developments to improve antioxidant, anti-inflammatory and antimicrobial efficacy of curcumin: A review of new trends and future perspectives. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 1316–1326. [Google Scholar] [CrossRef]
- Rahimi, H.R.; Nedaeinia, R.; Sepehri Shamloo, A.; Nikdoust, S.; Kazemi Oskuee, R. Novel delivery system for natural products: Nano-curcumin formulations. Avicenna J. Phytomed. 2016, 6, 383–398. [Google Scholar]
- Gupta, S.C.; Patchva, S.; Koh, W.; Aggarwal, B.B. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin. Exp. Pharmacol. Physiol. 2012, 39, 283–299. [Google Scholar] [CrossRef] [PubMed]
- Stohs, S.J.; Chen, O.; Ray, S.D.; Ji, J.; Bucci, L.R.; Preuss, H.G. Highly Bioavailable Forms of Curcumin and Promising Avenues for Curcumin-Based Research and Application: A Review. Molecules 2020, 25, 1397. [Google Scholar] [CrossRef] [PubMed]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Antony, B.; Merina, B.; Iyer, V.S.; Judy, N.; Lennertz, K.; Joyal, S.A.A. Pilot Cross-Over Study to Evaluate Human Oral Bioavailability of BCM-95CG (Biocurcumax), A Novel Bioenhanced Preparation of Curcumin. Indian J. Pharm. Sci. 2008, 70, 445–449. [Google Scholar] [CrossRef]
- Allegri, P.; Mastromarino, A.; Neri, P. Management of chronic anterior uveitis relapses: Efficacy of oral phospholipidic curcumin treatment. Long-term follow-up. Clin. Ophthalmol. 2010, 4, 1201–1206. [Google Scholar]
- Khalil, N.M.; do Nascimento, T.C.; Casa, D.M.; Dalmolin, L.F.; de Mattos, A.C.; Hoss, I.; Romano, M.A.; Mainardes, R.M. Pharmacokinetics of curcumin-loaded PLGA and PLGA-PEG blend nanoparticles after oral administration in rats. Colloids Surf. B Biointerfaces 2013, 101, 353–360. [Google Scholar] [CrossRef]
- Gangwar, R.K.; Tomar, G.B.; Dhumale, V.A.; Zinjarde, S.; Sharma, R.B.; Datar, S. Curcumin conjugated silica nanoparticles for improving bioavailability and its anticancer applications. J. Agric. Food Chem. 2013, 61, 9632–9637. [Google Scholar] [CrossRef]
- Rachmawati, H.; Al Shaal, L.; Muller, R.H.; Keck, C.M. Development of curcumin nanocrystal: Physical aspects. J. Pharm. Sci. 2013, 102, 204–214. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Fast-dissolving antioxidant curcumin/cyclodextrin inclusion complex electrospun nanofibrous webs. Food Chem. 2020, 317, 126397. [Google Scholar] [CrossRef]
- Liu, R.; Sun, L.; Fang, S.; Wang, S.; Chen, J.; Xiao, X.; Liu, C. Thermosensitive in situ nanogel as ophthalmic delivery system of curcumin: Development, characterization, in vitro permeation and in vivo pharmacokinetic studies. Pharm. Dev. Technol. 2016, 21, 576–582. [Google Scholar] [CrossRef]
- Lou, J.; Sun, L.; Fang, S.; Wang, S.; Chen, J.; Xiao, X.; Liu, C. Optimization and evaluation of a thermoresponsive ophthalmic in situ gel containing curcumin-loaded albumin nanoparticles. Int. J. Nanomedicine 2014, 9, 2517–2525. [Google Scholar] [PubMed]
- Pradhan, N.; Guha, R.; Chowdhury, S.; Nandi, S.; Konar, A.; Hazra, S. Curcumin nanoparticles inhibit corneal neovascularization. J. Mol. Med. 2015, 93, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Huang, T.M.; Lin, J.K. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab. Dispos. 1999, 27, 486–494. [Google Scholar] [PubMed]
- Holder, G.M.; Plummer, J.L.; Ryan, A.J. The metabolism and excretion of curcumin (1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) in the rat. Xenobiotica 1978, 8, 761–768. [Google Scholar] [CrossRef]
- Zhang, Z.B.; Luo, D.D.; Xie, J.H.; Xian, Y.F.; Lai, Z.Q.; Liu, Y.H.; Liu, W.H.; Chen, J.N.; Lai, X.P.; Lin, Z.X.; et al. Curcumin’s Metabolites, Tetrahydrocurcumin and Octahydrocurcumin, Possess Superior Anti-inflammatory Effects in vivo Through Suppression of TAK1-NF-kappaB Pathway. Front. Pharmacol. 2018, 9, 1181. [Google Scholar] [CrossRef]
- Yeung, A.W.K.; Horbanczuk, M.; Tzvetkov, N.T.; Mocan, A.; Carradori, S.; Maggi, F.; Marchewka, J.; Sut, S.; Dall’Acqua, S.; Gan, R.Y.; et al. Curcumin: Total-Scale Analysis of the Scientific Literature. Molecules 2019, 24, 1393. [Google Scholar] [CrossRef]
- Ackland, P.; Resnikoff, S.; Bourne, R. World blindness and visual impairment: Despite many successes, the problem is growing. Community Eye Health 2017, 30, 71–73. [Google Scholar]
- Flaxman, S.R.; Bourne, R.R.A.; Resnikoff, S.; Ackland, P.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H.; et al. Global causes of blindness and distance vision impairment 1990-2020: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e1221–e1234. [Google Scholar] [CrossRef]
- Nangia, V.; Jonas, J.B.; George, R.; Lingam, V.; Ellwein, L.; Cicinelli, M.V.; Das, A.; Flaxman, S.R.; Keeffe, J.E.; Kempen, J.H.; et al. Prevalence and causes of blindness and vision impairment: Magnitude, temporal trends and projections in South and Central Asia. Br. J. Ophthalmol. 2019, 103, 871–877. [Google Scholar] [CrossRef]
- Berendschot, T.T.; Broekmans, W.M.; Klopping-Ketelaars, I.A.; Kardinaal, A.F.; Van Poppel, G.; Van Norren, D. Lens aging in relation to nutritional determinants and possible risk factors for age-related cataract. Arch. Ophthalmol. 2002, 120, 1732–1737. [Google Scholar] [CrossRef]
- Ellwein, L.B.; Kupfer, C. Strategic issues in preventing cataract blindness in developing countries. Bull. World Health Organ. 1995, 73, 681–690. [Google Scholar] [PubMed]
- Isaacs, R.; Ram, J.; Apple, D. Cataract blindness in the developing world: Is there a solution? J. Agromedicine 2004, 9, 207–220. [Google Scholar] [CrossRef]
- Grzybowski, A.; Och, M.; Kanclerz, P.; Leffler, C.; Moraes, C.G. Primary Open Angle Glaucoma and Vascular Risk Factors: A Review of Population Based Studies from 1990 to 2019. J. Clin. Med. 2020, 9, 761. [Google Scholar] [CrossRef] [PubMed]
- Le, A.; Mukesh, B.N.; McCarty, C.A.; Taylor, H.R. Risk factors associated with the incidence of open-angle glaucoma: The visual impairment project. Invest. Ophthalmol. Vis. Sci. 2003, 44, 3783–3789. [Google Scholar] [CrossRef]
- European Glaucoma Prevention Study Group. Predictive factors for open-angle glaucoma among patients with ocular hypertension in the European Glaucoma Prevention Study. Ophthalmology 2007, 114, 3–9. [Google Scholar] [CrossRef] [PubMed]
- McMonnies, C.W. Glaucoma history and risk factors. J. Optom. 2017, 10, 71–78. [Google Scholar] [CrossRef]
- Armstrong, R.A.; Mousavi, M. Overview of Risk Factors for Age-Related Macular Degeneration (AMD). J. Stem. Cells 2015, 10, 171–191. [Google Scholar]
- Yau, J.W.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef]
- Wat, N.; Wong, R.L.; Wong, I.Y. Associations between diabetic retinopathy and systemic risk factors. Hong Kong Med. J. 2016, 22, 589–599. [Google Scholar] [CrossRef]
- Zheng, Y.; He, M.; Congdon, N. The worldwide epidemic of diabetic retinopathy. Indian J. Ophthalmol. 2012, 60, 428–431. [Google Scholar]
- Babizhayev, M.A.; Deyev, A.I.; Linberg, L.F. Lipid peroxidation as a possible cause of cataract. Mech. Ageing Dev. 1988, 44, 69–89. [Google Scholar] [CrossRef]
- Carver, J.A.; Guerreiro, N.; Nicholls, K.A.; Truscott, R.J. On the interaction of alpha-crystallin with unfolded proteins. Biochim. Biophys. Acta. 1995, 1252, 251–260. [Google Scholar] [CrossRef]
- Reddy, G.B.; Kumar, P.A.; Kumar, M.S. Chaperone-like activity and hydrophobicity of alpha-crystallin. IUBMB Life 2006, 58, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Thiagarajan, R.; Manikandan, R. Antioxidants and cataract. Free Radic. Res. 2013, 47, 337–345. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Aspects Med. 2009, 30, 1–12. [Google Scholar] [CrossRef]
- Anathanaryanan, P.H. A comparative study of lens protein glycation in various forms of cataract. Indian J. Clin. Biochem. 2004, 19, 110–112. [Google Scholar]
- Dou, G.; Sreekumar, P.G.; Spee, C.; He, S.; Ryan, S.J.; Kannan, R.; Hinton, D.R. Deficiency of alphaB crystallin augments ER stress-induced apoptosis by enhancing mitochondrial dysfunction. Free Radic. Biol. Med. 2012, 53, 1111–1122. [Google Scholar] [CrossRef]
- Berthoud, V.M.; Minogue, P.J.; Lambert, P.A.; Snabb, J.I.; Beyer, E.C. The Cataract-linked Mutant Connexin50D47A Causes Endoplasmic Reticulum Stress in Mouse Lenses. J. Biol. Chem. 2016, 291, 17569–17578. [Google Scholar] [CrossRef]
- Gabelt, B.T.; Kaufman, L.P. Changes in aqueous humor dynamics with age and glaucoma. Prog. Retin. Eye Res. 2005, 24, 612–637. [Google Scholar] [CrossRef]
- Nickells, R.W. Retinal ganglion cell death in glaucoma: The how, the why, and the maybe. J. Glaucoma 1996, 5, 345–356. [Google Scholar] [CrossRef]
- Krizaj, D.; Ryskamp, D.A.; Tian, N.; Tezel, G.; Mitchell, C.H.; Slepak, V.Z.; Shestopalov, V.I. From mechanosensitivity to inflammatory responses: New players in the pathology of glaucoma. Curr. Eye Res. 2014, 39, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Yarmohammadi, A.; Zangwill, L.M.; Diniz-Filho, A.; Suh, M.H.; Yousefi, S.; Saunders, L.J.; Belghith, A.; Manalastas, P.I.; Medeiros, F.A.; Weinreb, R.N. Relationship between Optical Coherence Tomography Angiography Vessel Density and Severity of Visual Field Loss in Glaucoma. Ophthalmology 2016, 123, 2498–2508. [Google Scholar] [CrossRef] [PubMed]
- Stefan, C.; Timaru, C.M.; Iliescu, D.A.; Schmitzer, S.; De Algerino, S.; Batras, M.; Hosseini-Ramhormozi, J. Glaucoma after chemical burns and radiation. Rom. J. Ophthalmol. 2016, 60, 209–215. [Google Scholar] [PubMed]
- Quigley, H.A. Clinical trials for glaucoma neuroprotection are not impossible. Curr. Opin. Ophthalmol. 2012, 23, 144–154. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef]
- Doozandeh, A.; Yazdani, S. Neuroprotection in Glaucoma. J. Ophthalmic. Vis. Res. 2016, 11, 209–220. [Google Scholar] [CrossRef]
- Amoaku, W.M.; Chakravarthy, U.; Gale, R.; Gavin, M.; Ghanchi, F.; Gibson, J.; Harding, S.; Johnston, R.L.; Kelly, S.P.; Lotery, A.; et al. Defining response to anti-VEGF therapies in neovascular AMD. Eye 2015, 29, 1397–1398. [Google Scholar] [CrossRef]
- Miller, J.W.; Adamis, A.P.; Aiello, L.P. Vascular endothelial growth factor in ocular neovascularization and proliferative diabetic retinopathy. Diabetes Metab. Rev. 1997, 13, 37–50. [Google Scholar] [CrossRef]
- Stone, J.; Itin, A.; Alon, T.; Pe’er, J.; Gnessin, H.; Chan-Ling, T.; Keshet, E. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J. Neurosci. 1995, 15 Pt. 1, 4738–4747. [Google Scholar] [CrossRef]
- Wang, J.; Xu, X.; Elliott, M.H.; Zhu, M.; Le, Y.Z. Muller cell-derived VEGF is essential for diabetes-induced retinal inflammation and vascular leakage. Diabetes 2010, 59, 2297–2305. [Google Scholar] [CrossRef]
- Forsythe, J.A.; Jiang, B.H.; Iyer, N.V.; Agani, F.; Leung, S.W.; Koos, R.D.; Semenza, G.L. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol. 1996, 16, 4604–4613. [Google Scholar] [CrossRef] [PubMed]
- Penn, J.S.; Madan, A.; Caldwell, R.B.; Bartoli, M.; Caldwell, R.W.; Hartnett, M.E. Vascular endothelial growth factor in eye disease. Prog. Retin. Eye Res. 2008, 27, 331–371. [Google Scholar] [CrossRef] [PubMed]
- Geraldes, P.; King, G.L. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ. Res. 2010, 106, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Tang, J.; Li, G.; Berti-Mattera, L.; Lee, C.A.; Bartkowski, D.; Gale, D.; Monahan, J.; Niesman, M.R.; Alton, G.; et al. Effects of p38 MAPK inhibition on early stages of diabetic retinopathy and sensory nerve function. Invest. Ophthalmol. Vis. Sci. 2010, 51, 2158–2164. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Santos, J.M.; Mishra, M. Epigenetic modifications and diabetic retinopathy. Biomed. Res. Int. 2013, 2013, 635284. [Google Scholar] [CrossRef]
- Zhong, Q.; Kowluru, R.A. Epigenetic modification of Sod2 in the development of diabetic retinopathy and in the metabolic memory: Role of histone methylation. Invest. Ophthalmol. Vis. Sci. 2013, 54, 244–250. [Google Scholar] [CrossRef]
- Maugeri, A.; Mazzone, M.G.; Giuliano, F.; Vinciguerra, M.; Basile, G.; Barchitta, M.; Agodi, A. Curcumin Modulates DNA Methyltransferase Functions in a Cellular Model of Diabetic Retinopathy. Oxid. Med. Cell. Longev. 2018, 2018, 5407482. [Google Scholar] [CrossRef]
- Dehdashtian, E.; Mehrzadi, S.; Yousefi, B.; Hosseinzadeh, A.; Reiter, R.J.; Safa, M.; Ghaznavi, H.; Naseripour, M. Diabetic retinopathy pathogenesis and the ameliorating effects of melatonin; involvement of autophagy, inflammation and oxidative stress. Life Sci. 2018, 193, 20–33. [Google Scholar] [CrossRef]
- Chapman, K.; Scorgie, F.E.; Ariyarajah, A.; Stephens, E.; Enjeti, A.K.; Lincz, L.F. The effects of tetrahydrocurcumin compared to curcuminoids on human platelet aggregation and blood coagulation in vitro. Thromb. Res. 2019, 179, 28–30. [Google Scholar] [CrossRef]
- Simo, R.; Hernandez, C. Intravitreous anti-VEGF for diabetic retinopathy: Hopes and fears for a new therapeutic strategy. Diabetologia 2008, 51, 1574–1580. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Deb, L.; Prasad, S. Curcumin differs from tetrahydrocurcumin for molecular targets, signaling pathways and cellular responses. Molecules 2014, 20, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.C.; Wilkinson Berka, J.L.; Deliyanti, D.; Hunter, D.; Fung, A.; Liew, G.; White, A. The role of reactive oxygen species in the pathogenesis and treatment of retinal diseases. Exp. Eye Res. 2020, 201, 108255. [Google Scholar] [CrossRef] [PubMed]
- Suryanarayana, P.; Krishnaswamy, K.; Reddy, G.B. Effect of curcumin on galactose-induced cataractogenesis in rats. Mol. Vis. 2003, 9, 223–230. [Google Scholar] [PubMed]
- Imamura, Y.; Noda, S.; Hashizume, K.; Shinoda, K.; Yamaguchi, M.; Uchiyama, S.; Shimizu, T.; Mizushima, Y.; Shirasawa, T.; Tsubota, K. Drusen, choroidal neovascularization, and retinal pigment epithelium dysfunction in SOD1-deficient mice: A model of age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2006, 103, 11282–11287. [Google Scholar] [CrossRef]
- Mao, H.; Seo, S.J.; Biswal, M.R.; Li, H.; Conners, M.; Nandyala, A.; Jones, K.; Le, Y.Z.; Lewin, A.S. Mitochondrial oxidative stress in the retinal pigment epithelium leads to localized retinal degeneration. Invest. Ophthalmol. Vis. Sci. 2014, 55, 4613–4627. [Google Scholar] [CrossRef]
- Li, K.; Zhai, M.; Jiang, L.; Song, F.; Zhang, B.; Li, J.; Li, H.; Li, B.; Xia, L.; Xu, L.; et al. Tetrahydrocurcumin Ameliorates Diabetic Cardiomyopathy by Attenuating High Glucose-Induced Oxidative Stress and Fibrosis via Activating the SIRT1 pathway. Oxid. Med. Cell. Longev. 2019, 2019, 6746907. [Google Scholar] [CrossRef]
- Cao, K.; Dong, Y.T.; Xiang, J.; Xu, Y.; Hong, W.; Song, H.; Guan, Z.Z. Reduced expression of SIRT1 and SOD-1 and the correlation between these levels in various regions of the brains of patients with Alzheimer’s disease. J. Clin. Pathol. 2018, 71, 1090–1099. [Google Scholar] [CrossRef]
- Zhou, M.; Luo, J.; Zhang, H. Role of Sirtuin 1 in the pathogenesis of ocular disease (Review). Int. J. Mol. Med. 2018, 42, 13–20. [Google Scholar] [CrossRef]
- Zuo, L.; Khan, R.S.; Lee, V.; Dine, K.; Wu, W.; Shindler, K.S. SIRT1 promotes RGC survival and delays loss of function following optic nerve crush. Invest. Ophthalmol. Vis. Sci. 2013, 54, 5097–5102. [Google Scholar] [CrossRef]
- Bertram, K.M.; Baglole, C.J.; Phipps, R.P.; Libby, R.T. Molecular regulation of cigarette smoke induced-oxidative stress in human retinal pigment epithelial cells: Implications for age-related macular degeneration. Am. J. Physiol. Cell. Physiol. 2009, 297, C1200–C1210. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [PubMed]
- Cano, M.; Thimmalappula, R.; Fujihara, M.; Nagai, N.; Sporn, M.; Wang, A.L.; Neufeld, A.H.; Biswal, S.; Handa, J.T. Cigarette smoking, oxidative stress, the antioxidant response through Nrf2 signaling, and Age-related Macular Degeneration. Vision Res. 2010, 50, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Chen, Y.; Wang, J.; Sternberg, P.; Freeman, M.L.; Grossniklaus, H.E.; Cai, J. Age-related retinopathy in NRF2-deficient mice. PLoS ONE 2011, 6, e19456. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, M.M.; Cano, M.; Handa, J.T. Nrf2 signaling is impaired in the aging RPE given an oxidative insult. Exp. Eye Res. 2014, 119, 111–114. [Google Scholar] [CrossRef]
- Luo, D.D.; Chen, J.F.; Liu, J.J.; Xie, J.H.; Zhang, Z.B.; Gu, J.Y.; Zhuo, J.Y.; Huang, S.; Su, Z.R.; Sun, Z.H. Tetrahydrocurcumin and octahydrocurcumin, the primary and final hydrogenated metabolites of curcumin, possess superior hepatic-protective effect against acetaminophen-induced liver injury: Role of CYP2E1 and Keap1-Nrf2 pathway. Food Chem. Toxicol. 2019, 123, 349–362. [Google Scholar] [CrossRef]
- Sennlaub, F.; Valamanesh, F.; Vazquez-Tello, A.; El-Asrar, A.M.; Checchin, D.; Brault, S.; Gobeil, F.; Beauchamp, M.H.; Mwaikambo, B.; Courtois, Y.; et al. Cyclooxygenase-2 in human and experimental ischemic proliferative retinopathy. Circulation 2003, 108, 198–204. [Google Scholar] [CrossRef]
- Lin, C.C.; Lee, I.T.; Yang, Y.L.; Lee, C.W.; Kou, Y.R.; Yang, C.M. Induction of COX-2/PGE(2)/IL-6 is crucial for cigarette smoke extract-induced airway inflammation: Role of TLR4-dependent NADPH oxidase activation. Free Radic. Biol. Med. 2010, 48, 240–254. [Google Scholar] [CrossRef]
- Rao, P.; Knaus, E.E. Evolution of nonsteroidal anti-inflammatory drugs (NSAIDs): Cyclooxygenase (COX) inhibition and beyond. J. Pharm. Pharm. Sci. 2008, 11, 81s–110s. [Google Scholar] [CrossRef]
- Jiang, B.H.; Agani, F.; Passaniti, A.; Semenza, G.L. V-SRC induces expression of hypoxia-inducible factor 1 (HIF-1) and transcription of genes encoding vascular endothelial growth factor and enolase 1: Involvement of HIF-1 in tumor progression. Cancer Res. 1997, 57, 5328–5335. [Google Scholar]
- Lin, C.; McGough, R.; Aswad, B.; Block, J.A.; Terek, R. Hypoxia induces HIF-1alpha and VEGF expression in chondrosarcoma cells and chondrocytes. J. Orthop. Res. 2004, 22, 1175–1181. [Google Scholar] [CrossRef]
- Bates, D.O. Vascular endothelial growth factors and vascular permeability. Cardiovasc. Res. 2010, 87, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Mansoor, S.; Sharma, A.; Sapkal, A.; Sheth, J.; Falatoonzadeh, P.; Kuppermann, B.; Kenney, M. Diabetic retinopathy and VEGF. Open Ophthalmol. J. 2013, 7, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Claesson-Welsh, L. Vascular permeability—the essentials. Ups. J. Med. Sci. 2015, 120, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Yoysungnoen, B.; Bhattarakosol, P.; Patumraj, S.; Changtam, C. Effects of tetrahydrocurcumin on hypoxia-inducible factor-1alpha and vascular endothelial growth factor expression in cervical cancer cell-induced angiogenesis in nude mice. Biomed. Res. Int. 2015, 2015, 391748. [Google Scholar] [CrossRef]
- Gao, Y.; Li, J.; Wu, L.; Zhou, C.; Wang, Q.; Li, X.; Zhou, M.; Wang, H. Tetrahydrocurcumin provides neuroprotection in rats after traumatic brain injury: Autophagy and the PI3K/AKT pathways as a potential mechanism. J. Surg. Res. 2016, 206, 67–76. [Google Scholar] [CrossRef]
- Tyagi, N.; Qipshidze, N.; Munjal, C.; Vacek, J.C.; Metreveli, N.; Givvimani, S.; Tyagi, S.C. Tetrahydrocurcumin ameliorates homocysteinylated cytochrome-c mediated autophagy in hyperhomocysteinemia mice after cerebral ischemia. J. Mol. Neurosci. 2012, 47, 128–138. [Google Scholar] [CrossRef]
- Munemasa, Y.; Kitaoka, Y. Autophagy in axonal degeneration in glaucomatous optic neuropathy. Prog. Retin. Eye Res. 2015, 47, 1–18. [Google Scholar] [CrossRef]
- Park, H.Y.; Kim, J.H.; Park, C.K. Activation of autophagy induces retinal ganglion cell death in a chronic hypertensive glaucoma model. Cell. Death Dis. 2012, 3, e290. [Google Scholar] [CrossRef]
- Borsey, D.Q.; Prowse, C.V.; Gray, R.S.; Dawes, J.; James, K.; Elton, R.A.; Clarke, B.F. Platelet and coagulation factors in proliferative diabetic retinopathy. J. Clin. Pathol. 1984, 37, 659–664. [Google Scholar] [CrossRef]
- Srivastava, K.C.; Bordia, A.; Verma, S.K. Curcumin, a major component of food spice turmeric (Curcuma longa) inhibits aggregation and alters eicosanoid metabolism in human blood platelets. Prostaglandins Leukot. Essent. Fatty Acids 1995, 52, 223–227. [Google Scholar] [CrossRef]



| Site | Pathogenesis | Treatment | ||
|---|---|---|---|---|
| Age-related cataract | Lens | Malfunctioning proteins on lens | (Present) removal of the cloudy lens (Future) antioxidant application | [44] |
| Glaucoma | Optic nerves | Ganglion cell degeneration | (Present) release of IOP (Future) neuroprotection | [54] |
| AMD | Retina (macula) | Macular degeneration and vascularization | (Future) anti-VEGF agents and reversal of angiogenesis | [57] |
| DR | Retina | Vascular abnormalities | (Traditional) laser or surgery (Future) anti-VEGF agents | [70] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kao, Y.-W.; Hsu, S.-K.; Chen, J.Y.-F.; Lin, I.-L.; Chen, K.-J.; Lee, P.-Y.; Ng, H.-S.; Chiu, C.-C.; Cheng, K.-C. Curcumin Metabolite Tetrahydrocurcumin in the Treatment of Eye Diseases. Int. J. Mol. Sci. 2021, 22, 212. https://doi.org/10.3390/ijms22010212
Kao Y-W, Hsu S-K, Chen JY-F, Lin I-L, Chen K-J, Lee P-Y, Ng H-S, Chiu C-C, Cheng K-C. Curcumin Metabolite Tetrahydrocurcumin in the Treatment of Eye Diseases. International Journal of Molecular Sciences. 2021; 22(1):212. https://doi.org/10.3390/ijms22010212
Chicago/Turabian StyleKao, Yu-Wen, Sheng-Kai Hsu, Jeff Yi-Fu Chen, I-Ling Lin, Kuo-Jen Chen, Po-Yen Lee, Hui-Suan Ng, Chien-Chih Chiu, and Kai-Chun Cheng. 2021. "Curcumin Metabolite Tetrahydrocurcumin in the Treatment of Eye Diseases" International Journal of Molecular Sciences 22, no. 1: 212. https://doi.org/10.3390/ijms22010212
APA StyleKao, Y.-W., Hsu, S.-K., Chen, J. Y.-F., Lin, I.-L., Chen, K.-J., Lee, P.-Y., Ng, H.-S., Chiu, C.-C., & Cheng, K.-C. (2021). Curcumin Metabolite Tetrahydrocurcumin in the Treatment of Eye Diseases. International Journal of Molecular Sciences, 22(1), 212. https://doi.org/10.3390/ijms22010212






