FOXE1 Gene Dosage Affects Thyroid Cancer Histology and Differentiation In Vivo
Abstract
:1. Introduction
2. Results
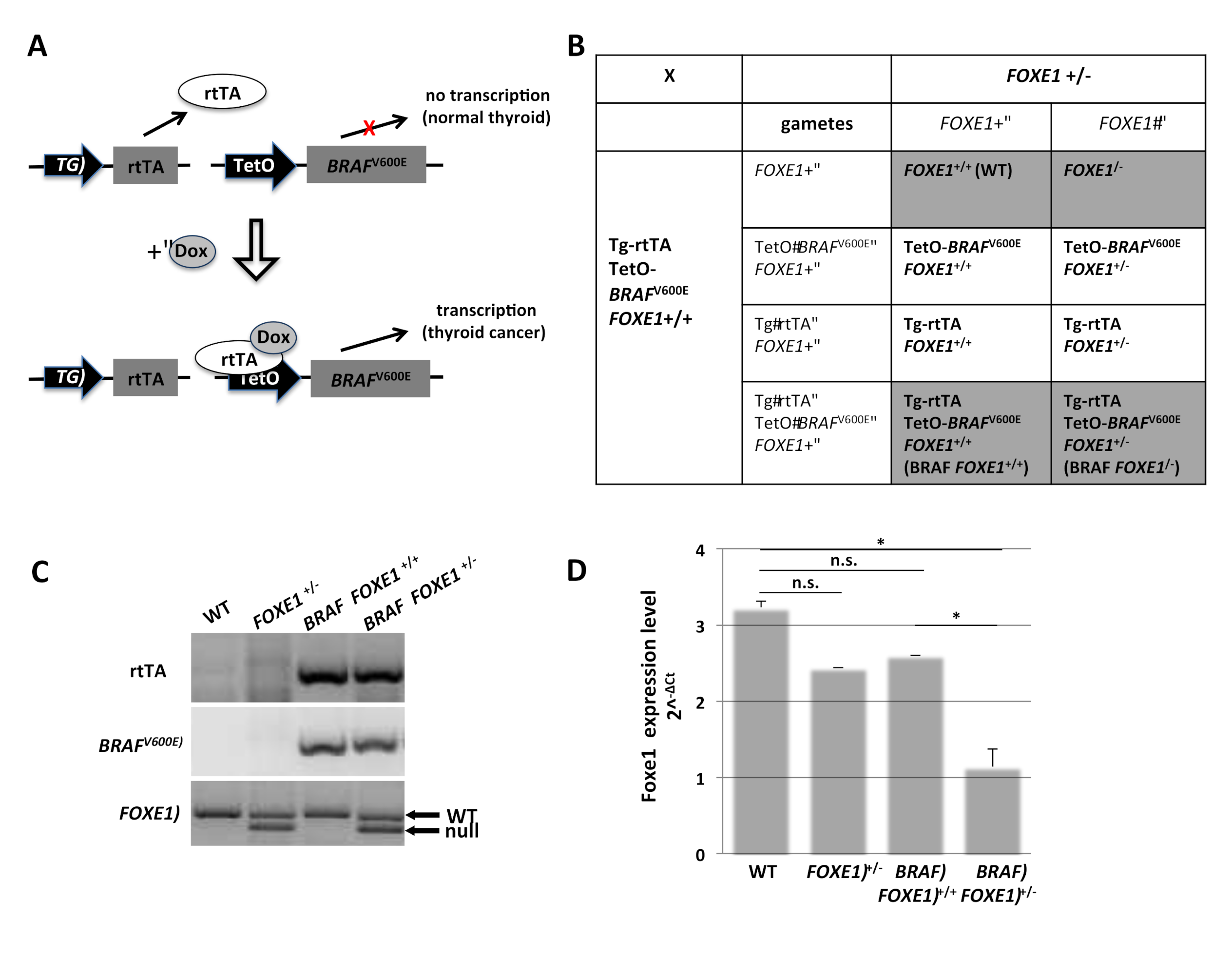
2.1. Generation of FOXE1 Heterozygous Knockout Mouse Model of Thyroid Cancer
2.2. Thyroid Cancer Histology in BRAF FOXE1 +/− Mice
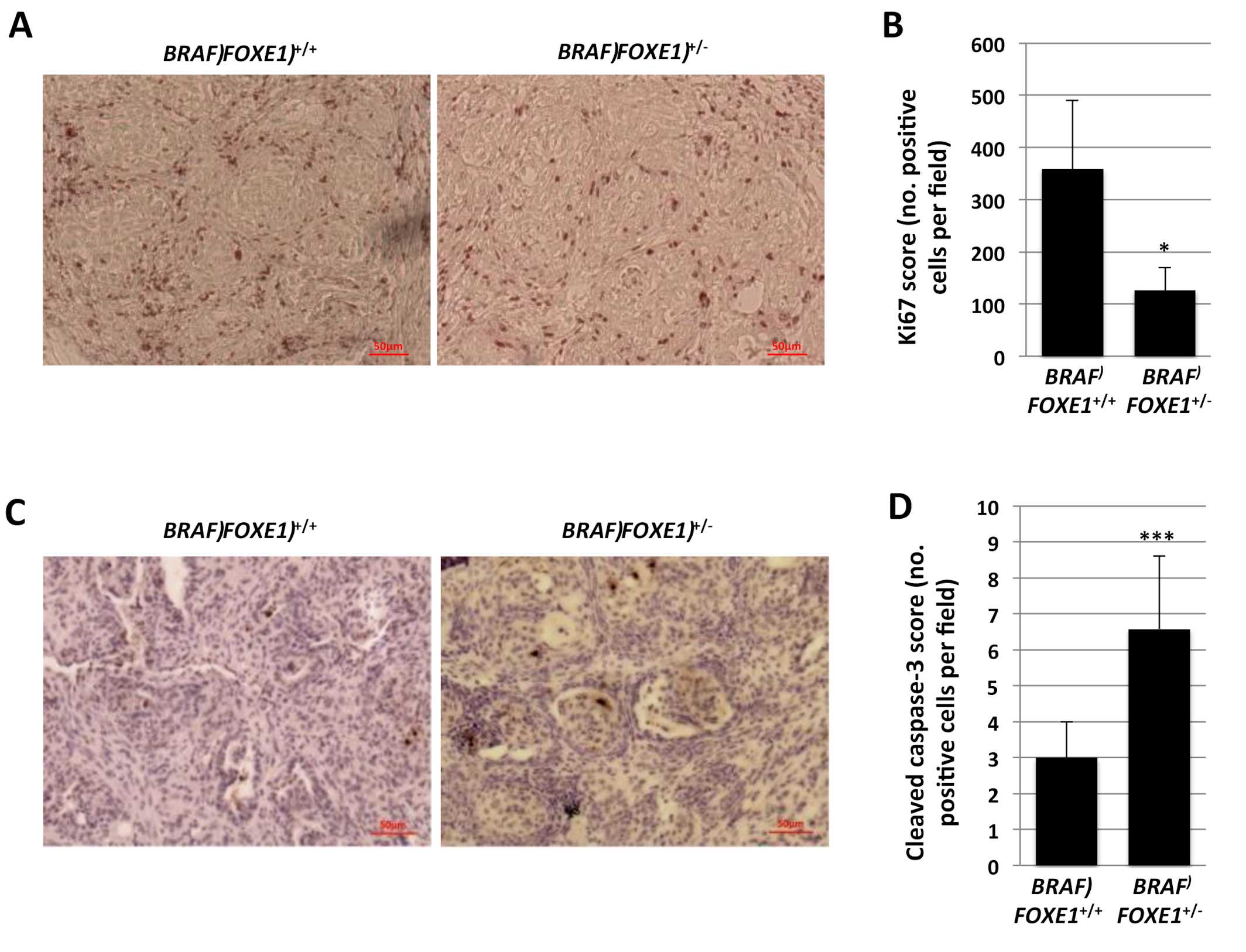
2.3. Thyroid Cancer Cell Growth Suppression and Apoptosis in BRAF FOXE1+/− Mice
2.4. Differentiated Thyroid Gene Expression in BRAF FOXE1+/− Cancers
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Genotyping
4.3. Quantitative Real-Time PCR
4.4. Staining and Immunohistochemistry
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cohen, S.M.; Mukerji, R.; Timmermann, B.N.; Samadi, A.K.; Cohen, M.S. A novel combination of withaferin A and sorafenib shows synergistic efficacy against both papillary and anaplastic thyroid cancers. Am. J. Surg. 2012, 204, 895–900; discussion 900–891. [Google Scholar] [CrossRef] [PubMed]
- Chrisoulidou, A.; Boudina, M.; Tzemailas, A.; Doumala, E.; Iliadou, P.K.; Patakiouta, F.; Pazaitou-Panayiotou, K. Histological subtype is the most important determinant of survival in metastatic papillary thyroid cancer. Thyroid Res. 2011, 4, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondo, T.; Ezzat, S.; Asa, S.L. Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nat. Reviews. Cancer 2006, 6, 292–306. [Google Scholar] [CrossRef] [PubMed]
- Rebaï, M.; Rebaï, A. Molecular genetics of thyroid cancer. Genet. Res. 2016, 98, e7. [Google Scholar] [CrossRef] [PubMed]
- Penna-Martinez, M.; Epp, F.; Kahles, H.; Ramos-Lopez, E.; Hinsch, N.; Hansmann, M.L.; Selkinski, I.; Grunwald, F.; Holzer, K.; Bechstein, W.O.; et al. FOXE1 association with differentiated thyroid cancer and its progression. Thyroid Off. J. Am. Thyroid Assoc. 2014, 24, 845–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; He, H.; Li, W.; Phay, J.; Shen, R.; Yu, L.; Hancioglu, B.; de la Chapelle, A. MYH9 binds to lncRNA gene PTCSC2 and regulates FOXE1 in the 9q22 thyroid cancer risk locus. Proc. Natl. Acad. Sci. USA 2017, 114, 474–479. [Google Scholar] [CrossRef] [Green Version]
- Gudmundsson, J.; Sulem, P.; Gudbjartsson, D.F.; Jonasson, J.G.; Sigurdsson, A.; Bergthorsson, J.T.; He, H.; Blondal, T.; Geller, F.; Jakobsdottir, M.; et al. Common variants on 9q22.33 and 14q13.3 predispose to thyroid cancer in European populations. Nat. Genet. 2009, 41, 460–464. [Google Scholar] [CrossRef]
- He, H.; Li, W.; Liyanarachchi, S.; Srinivas, M.; Wang, Y.; Akagi, K.; Wang, Y.; Wu, D.; Wang, Q.; Jin, V.; et al. Multiple functional variants in long-range enhancer elements contribute to the risk of SNP rs965513 in thyroid cancer. Proc. Natl. Acad. Sci. USA 2015, 112, 6128–6133. [Google Scholar] [CrossRef] [Green Version]
- Tomaz, R.A.; Sousa, I.; Silva, J.G.; Santos, C.; Teixeira, M.R.; Leite, V.; Cavaco, B.M. FOXE1 polymorphisms are associated with familial and sporadic nonmedullary thyroid cancer susceptibility. Clin. Endocrinol. 2012, 77, 926–933. [Google Scholar] [CrossRef]
- Somuncu, E.; Karatas, A.; Ferahman, S.; Saygili, N.; Yilmaz, E.; Ozturk, O.; Kapan, M. The investigation of foxe1 variations in papillary thyroid carcinoma. Int. J. Clin. Exp. Pathol. 2015, 8, 13458–13464. [Google Scholar]
- Takahashi, M.; Saenko, V.A.; Rogounovitch, T.I.; Kawaguchi, T.; Drozd, V.M.; Takigawa-Imamura, H.; Akulevich, N.M.; Ratanajaraya, C.; Mitsutake, N.; Takamura, N.; et al. The FOXE1 locus is a major genetic determinant for radiation-related thyroid carcinoma in Chernobyl. Hum. Mol. Genet. 2010, 19, 2516–2523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landa, I.; Ruiz-Llorente, S.; Montero-Conde, C.; Inglada-Perez, L.; Schiavi, F.; Leskela, S.; Pita, G.; Milne, R.; Maravall, J.; Ramos, I.; et al. The variant rs1867277 in FOXE1 gene confers thyroid cancer susceptibility through the recruitment of USF1/USF2 transcription factors. Plos Genet. 2009, 5, e1000637. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Ding, Z.; Yang, Z.; Deng, X.; Kang, J.; Wu, B.; Zheng, Q. Expression and clinical significance of FOXE1 in papillary thyroid carcinoma. Mol. Med. Rep. 2013, 8, 123–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morillo-Bernal, J.; Fernández, L.P.; Santisteban, P. FOXE1 regulates migration and invasion in thyroid cancer cells and targets ZEB1. Endocr. Relat. Cancer 2020, 27, 137–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakravarty, D.; Santos, E.; Ryder, M.; Knauf, J.A.; Liao, X.H.; West, B.L.; Bollag, G.; Kolesnick, R.; Thin, T.H.; Rosen, N.; et al. Small-molecule MAPK inhibitors restore radioiodine incorporation in mouse thyroid cancers with conditional BRAF activation. J. Clin. Investig. 2011, 121, 4700–4711. [Google Scholar] [CrossRef] [Green Version]
- De Felice, M.; Ovitt, C.; Biffali, E.; Rodriguez-Mallon, A.; Arra, C.; Anastassiadis, K.; Macchia, P.E.; Mattei, M.G.; Mariano, A.; Scholer, H.; et al. A mouse model for hereditary thyroid dysgenesis and cleft palate. Nat. Genet. 1998, 19, 395–398. [Google Scholar] [CrossRef]
- Fernández, L.P.; López, M.A.; Santisteban, P. Thyroid transcription factors in development, differentiation and disease. Nat. Rev. Endocrinol. 2015, 11, 29–42. [Google Scholar] [CrossRef] [Green Version]
- Bass, A.J.; Watanabe, H.; Mermel, C.H.; Yu, S.; Perner, S.; Verhaak, R.G.; Kim, S.Y.; Wardwell, L.; Tamayo, P.; Gat-Viks, I.; et al. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat. Genet. 2009, 41, 1238–1242. [Google Scholar] [CrossRef]
- Kwei, K.A.; Kim, Y.H.; Girard, L.; Kao, J.; Pacyna-Gengelbach, M.; Salari, K.; Lee, J.; Choi, Y.L.; Sato, M.; Wang, P.; et al. Genomic profiling identifies TITF1 as a lineage-specific oncogene amplified in lung cancer. Oncogene 2008, 27, 3635–3640. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Silva, T.C.; Gull, N.; Yang, Q.; Plummer, J.T.; Chen, S.; Daigo, K.; Hamakubo, T.; Gery, S.; Ding, L.W.; et al. Lineage-Specific Epigenomic and Genomic Activation of Oncogene HNF4A Promotes Gastrointestinal Adenocarcinomas. Cancer Res. 2020, 80, 2722–2736. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, N.; Ito, E.; Azuma, S.; Honma, R.; Yanagisawa, Y.; Nishikawa, A.; Kawamura, M.; Imai, J.; Tatsuta, K.; Inoue, J.; et al. FoxA1 as a lineage-specific oncogene in luminal type breast cancer. Biochem. Biophys. Res. Commun. 2008, 365, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Credendino, S.C.; Bellone, M.L.; Lewin, N.; Amendola, E.; Sanges, R.; Basu, S.; Sepe, R.; Decaussin-Petrucci, M.; Tinto, N.; Fusco, A.; et al. A ceRNA Circuitry Involving the Long Noncoding RNA Klhl14-AS, Pax8, and Bcl2 Drives Thyroid Carcinogenesis. Cancer Res. 2019, 79, 5746–5757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Credendino, S.C.; Lewin, N.; de Oliveira, M.; Basu, S.; D’Andrea, B.; Amendola, E.; Di Guida, L.; Nardone, A.; Sanges, R.; De Felice, M.; et al. Tissue- and Cell Type-Specific Expression of the Long Noncoding RNA Klhl14-AS in Mouse. Int. J. Genom. 2017, 2017, 9769171. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Credendino, S.C.; Moccia, C.; Amendola, E.; D’Avino, G.; Di Guida, L.; Clery, E.; Greco, A.; Bellevicine, C.; Brunetti, A.; De Felice, M.; et al. FOXE1 Gene Dosage Affects Thyroid Cancer Histology and Differentiation In Vivo. Int. J. Mol. Sci. 2021, 22, 25. https://doi.org/10.3390/ijms22010025
Credendino SC, Moccia C, Amendola E, D’Avino G, Di Guida L, Clery E, Greco A, Bellevicine C, Brunetti A, De Felice M, et al. FOXE1 Gene Dosage Affects Thyroid Cancer Histology and Differentiation In Vivo. International Journal of Molecular Sciences. 2021; 22(1):25. https://doi.org/10.3390/ijms22010025
Chicago/Turabian StyleCredendino, Sara C., Carmen Moccia, Elena Amendola, Giuliana D’Avino, Luigi Di Guida, Eduardo Clery, Adelaide Greco, Claudio Bellevicine, Arturo Brunetti, Mario De Felice, and et al. 2021. "FOXE1 Gene Dosage Affects Thyroid Cancer Histology and Differentiation In Vivo" International Journal of Molecular Sciences 22, no. 1: 25. https://doi.org/10.3390/ijms22010025





