P2 Receptors in Cardiac Myocyte Pathophysiology and Mechanotransduction
Abstract
:1. Introduction
2. ATP as an Extracellular Chemical Messenger
3. P2 Receptors in Cardiac Muscle and Their Pharmacological Properties
4. Regulation of Cardiac Contractility by ATP and Roles of P2 Receptors
5. Regulation of Heart Rate by P2 Receptors
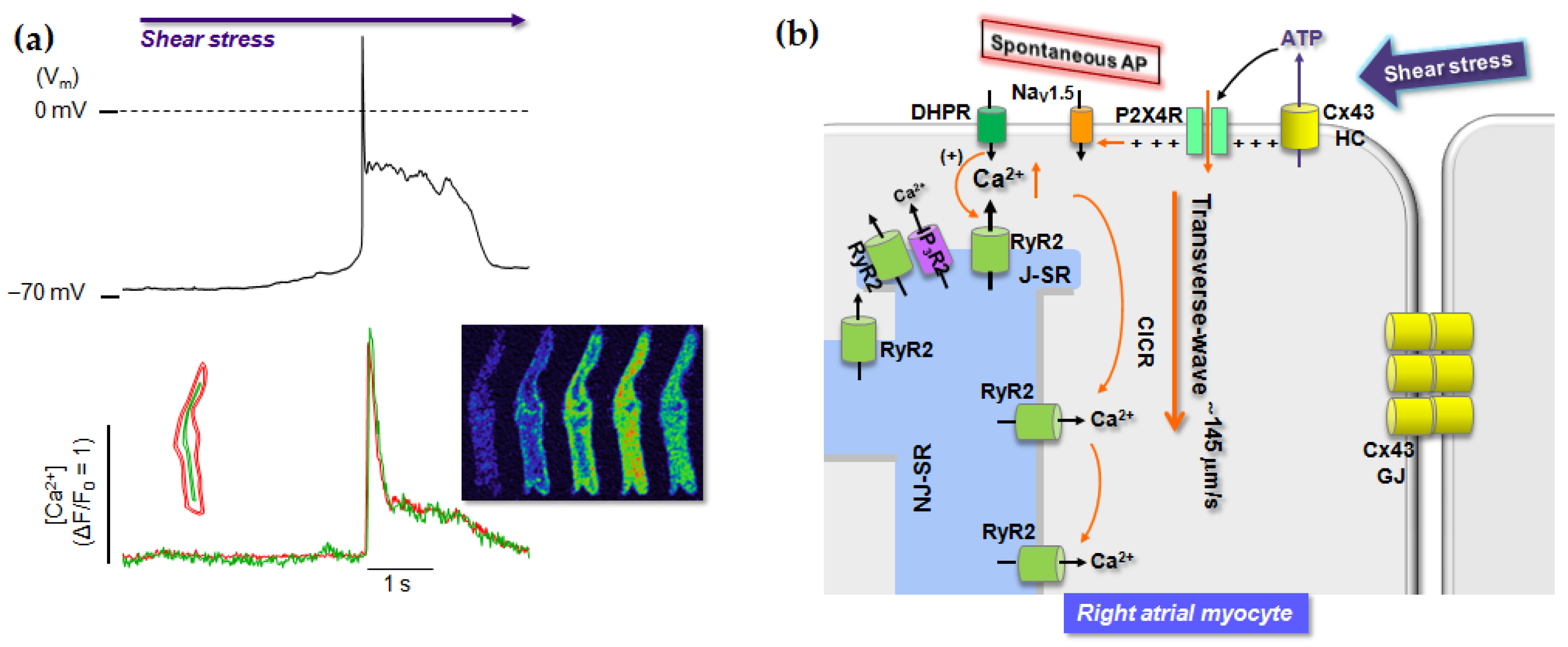
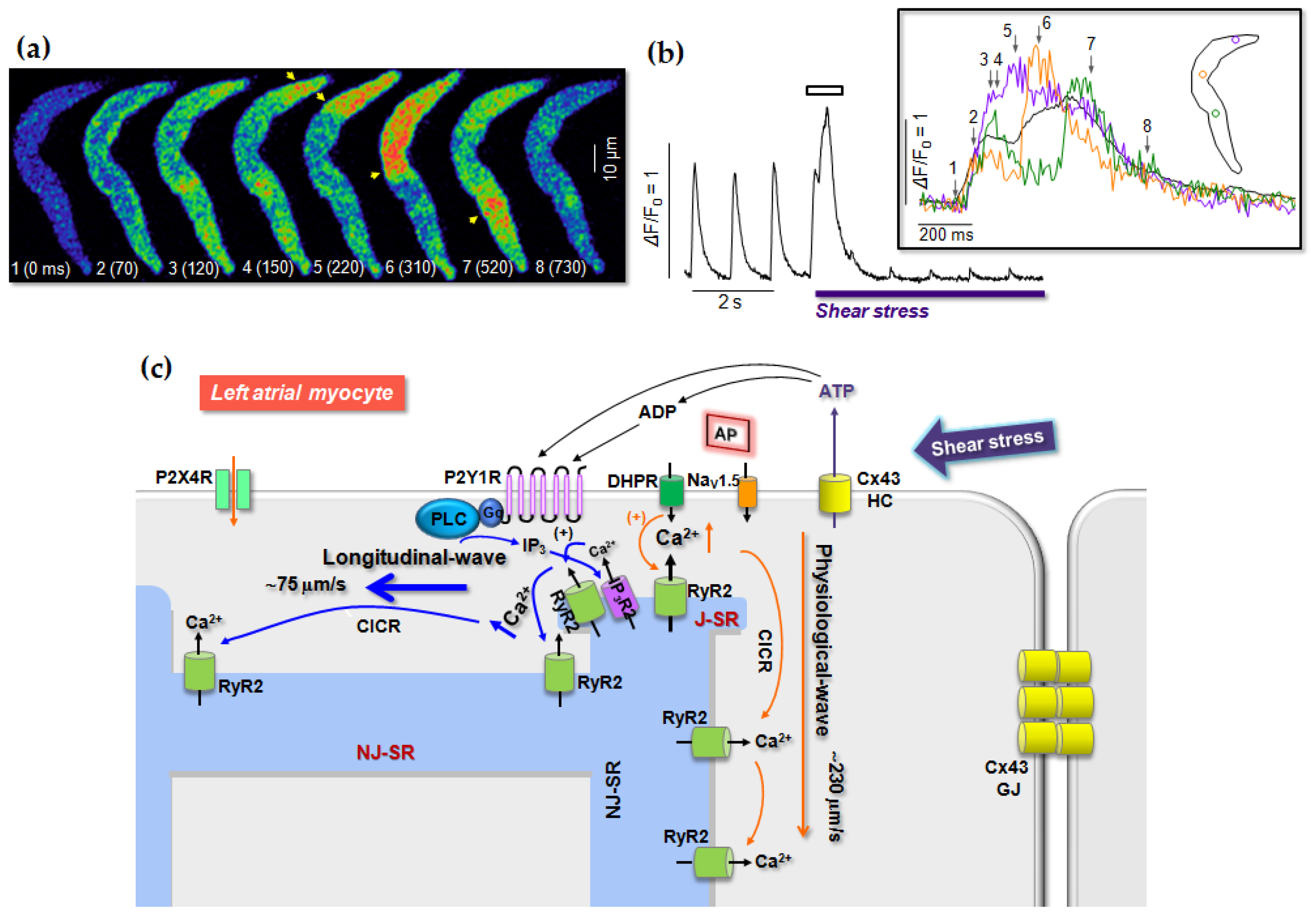
6. Role of P2 Receptors in Cardiac Stress Responses
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2-Cl-ATP | 2-chloro-ATP |
| 2-MeS-ADP | 2-methylthio-ADP |
| 2-MeS-ATP | 2-methylthio-ATP |
| 5-Br-UDP | 5-bromo-UDP |
| ADP | Adenosine diphosphate |
| AMP | Adenosine monophosphate |
| ANP | Atrial natriuretic peptide |
| AP | Action potential |
| Ap4A | Diadenosine tetraphosphate |
| ARC67085 | 2-propylthio-β,γ-dichloromethylene-D-ATP |
| ATP | Adenosine triphosphate |
| ATPγS | Adenosine-(O-3-thiotriphosphate) |
| BzATP | Benzoyl–benzoyl–ATP |
| cAMP | Cyclic AMP |
| cGMP | Cyclic guanosine monophosphate |
| CICR | Ca2+-induced Ca2+ release |
| Cx43 | Connexin 43 |
| DPCPX | 1,3-dipropyl-8-cyclopentylxanthine |
| eNOS | Endothelial nitric oxide synthase |
| ERK | Extracellular signal-regulated kinase |
| INS37217 | P1-(uridine 5′)-P4-(2′-deoxycytidine-5′)-tetraphosphate |
| IP3R | Inositol 1,4,5-trisphosphate receptor |
| KAch | Acetylcholine activated K+ channels |
| LA | Left atrial |
| LV | Left ventricle |
| MI | Myocardial infarction |
| MLC-2 | Myosin light chain-2 |
| MRS 2179 | 2’-deoxy-N6-methyladenosine-3’,5’-bisphosphate |
| (N)-mc-2-MeSADP | (N)-methanocarba-2-methylthio-ADP |
| NO | Nitric oxide |
| PCR | Polymerase chain reaction |
| PLC | Phospholipase C |
| PPADS | Pyridoxal phosphate-6-azo(bensene-2,4-disulfonic acid) tetrasodium |
| RA | Right atrial |
| ROI | Region-of-interest |
| RyR2 | Ryanodine receptor type 2 |
| SAN | Sinoatrial node |
| SR | Sarcoplasmic reticulum |
| UDP | Uridine diphosphate |
| UTP | Uridine triphosphate |
| UTPγS | Uridine-(O-3-thiotriphosphate) |
References
- Burnstock, G. Historical review: ATP as a neurotransmitter. Trends Pharmacol. Sci. 2006, 27, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Vassort, G. Adenosine 5′-triphosphate: A P2-Purinergic agonist in the myocardium. Physiol. Rev. 2001, 81, 767–806. [Google Scholar] [CrossRef] [PubMed]
- Fischer, Y.; Becker, C.; Löken, C. Purinergic inhibition of glucose transport in cardiomyocytes. J. Biol. Chem. 1999, 274, 755–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, A.L.; Kudlow, B.A.; Marrs, K.L.; Gruenert, D.C.; Guggino, W.B.; Schwiebert, E.M. Bioluminescence detection of ATP release mechanisms in epithelia. Am. J. Physiol. Cell Physiol. 1998, 275, C1391–C1406. [Google Scholar] [CrossRef] [PubMed]
- Beigi, R.; Kobatake, E.; Aizawa, M.; Dubyak, G.R. Detection of local ATP release from activated platelets using cell surface-attached firefly luciferase. Am. J. Physiol. Cell Physiol. 1999, 276, C267–C278. [Google Scholar] [CrossRef]
- Schneider, S.W.; Egan, M.E.; Jena, B.P.; Guggino, W.B.; Oberleithner, H.; Geibel, J.P. Continuous detection of extracellular ATP on living cells by using atomic force microscopy. Proc. Natl. Acad. Sci. USA 1999, 96, 12180–12185. [Google Scholar] [CrossRef] [Green Version]
- Nishida, M.; Sato, Y.; Uemura, A.; Narita, Y.; Tozaki-Saitoh, H.; Nakaya, M.; Ide, T.; Suzuki, K.; Inoue, K.; Nagao, T.; et al. P2Y6 receptor-Gα12/13 signalling in cardiomyocytes triggers pressure overload-induced cardiac fibrosis. EMBO J. 2008, 27, 3104–3115. [Google Scholar] [CrossRef]
- Oishi, S.; Sasano, T.; Tateishi, Y.; Tamura, N.; Isobe, M.; Furukawa, T. Stretch of atrial myocytes stimulates recruitment of macrophages via ATP released through gap-junction channels. J. Pharmacol. Sci. 2012, 120, 296–304. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.C.; Son, M.J.; Woo, S.H. Ca2+ Signaling Triggered by Shear-Autocrine P2X Receptor Pathway in Rat Atrial Myocytes. Cell. Physiol. Biochem. 2018, 50, 2296–2313. [Google Scholar] [CrossRef]
- Allen, T.G. The ‘sniffer-patch’ technique for detection of neurotransmitter release. Trends Neurosci. 1997, 20, 192–197. [Google Scholar] [CrossRef]
- Le, Q.A.; Kim, J.C.; Kim, K.H.; Van Vu, A.T.; Woo, S.H. Distinct shear-induced Ca2+ signaling in the left and right atrial myocytes: Role of P2 receptor context. J. Mol. Cell. Cardiol. 2020, 143, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Bell, P.D.; Lapointe, J.Y.; Sabirov, R.; Hayashi, S.; Peti-Peterdi, J.; Manabe, K.; Kovacs, G.; Okada, Y. Macula densa cell signaling involves ATP release through a maxi anion channel. Proc. Natl. Acad. Sci. USA 2003, 100, 4322–4327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, S.; Hazama, A.; Dutta, A.K.; Sabirov, R.Z.; Okada, Y. Detecting ATP release by a biosensor method. Sci. Signal. 2004, 2004, pl14. [Google Scholar] [CrossRef] [PubMed]
- Stout, C.E.; Costantin, J.L.; Naus, C.C.; Charles, A.C. Intercellular calcium signaling in astrocytes via ATP signaling in astrocytes. Anal. Chem. 2000, 72, 10482–10488. [Google Scholar]
- Abraham, E.H.; Prat, A.G.; Gerweck, L.; Seneveratne, T.; Arceci, R.J.; Kramer, R.; Guidotti, G.; Cantiello, H.F. The multidrug resistance (mdr1) gene product functions as an ATP channel. Proc. Natl. Acad. Sci. USA 1993, 90, 312–316. [Google Scholar] [CrossRef] [Green Version]
- Abraham, E.H.; Okunieff, P.; Scala, S.; Vos, P.; Oosterveld, M.J.; Chen, A.Y.; Shrivastav, B. Cystic fibrosis transmembrane conductance regulator and adenosine triphosphate. Science 1997, 275, 1324–1326. [Google Scholar] [CrossRef] [Green Version]
- Al-Awqati, Q. Regulation of ion channels by ABC transporters that secrete ATP. Science 1995, 269, 805–806. [Google Scholar] [CrossRef]
- Pasyk, E.A.; Foskett, J.K. Cystic fibrosis transmembrane conductance regulator-associated ATP and adenosine 3′-phosphate 5′-phosphosulfate channels in endoplasmic reticulum and plasma membranes. J. Biol. Chem. 1997, 272, 7746–7751. [Google Scholar] [CrossRef] [Green Version]
- Schwiebert, E.M.; Egan, M.E.; Hwang, T.H.; Fulmer, S.B.; Allen, S.S.; Cutting, G.R.; Guggino, W.B. CFTR regulates outwardly rectifying chloride channels through an autocrine mechanism involving ATP. Cell 1995, 81, 1063–1073. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Roman, R.; Lidofsky, S.D.; Fitz, J.G. Autocrine signaling through ATP release represents a novel mechanism for cell volume regulation. Proc. Natl. Acad. Sci. USA 1996, 93, 12020–12025. [Google Scholar] [CrossRef] [Green Version]
- Sugita, M.; Yue, Y.; Foskett, J.K. CFTR Cl- channel and CFTR-associated ATP channel: Distinct pores regulated by common gates. EMBO J. 1998, 17, 898–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabirov, R.Z.; Okada, Y. Wide nanoscopic pore of maxi-anion channel suits its function as an ATP-conductive pathway. Biophys. J. 2004, 87, 1672–1685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barzu, T.; Huerta, F.; Pourrias, B. The chronotropic effect of adenosine and ATP in dogs. The antagonism by theophylline. J. Pharmacol. 1985, 16, 197–211. [Google Scholar] [PubMed]
- Gordon, J.L. Extracellular ATP: Effects, sources and fate. Biochem. J. 1986, 233, 309–319. [Google Scholar] [CrossRef]
- Yamamoto, K.; Sokabe, T.; Ohura, N.; Nakatsuka, H.; Kamiya, A.; Ando, J. Endogenously released ATP mediates shear stress-induced Ca2+ influx into pulmonary artery endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H793–H803. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, K.; Furuya, K.; Nakamura, M.; Kobatake, E.; Sokabe, M.; Ando, J. Visualization of flow-induced ATP release and triggering of Ca2+ waves at caveolae in vascular endothelial cells. J. Cell Sci. 2011, 124, 3477–3483. [Google Scholar] [CrossRef] [Green Version]
- Burnstock, G. Purinergic nerves. Pharmacol. Rev. 1972, 24, 509–581. [Google Scholar]
- Kyösola, K.; Partanen, S.; Korkala, O.; Merikallio, E.; Penttilä, O.; Siltanen, P. Fluorescence histochemical and electron-microscopical observations on the innervation of the atrial myocardium of the adult human heart. Virchows Arch. 1976, 371, 101–119. [Google Scholar] [CrossRef]
- Burnstock, G. Noradrenaline and ATP as co-transmitters in sympathetic nerves. Neurochem. Int. 1990, 17, 357–368. [Google Scholar] [CrossRef]
- Holton, P. The liberation of adenosine triphosphate on antidromic stimulation of sensory nerves. J. Physiol. 1959, 145, 494–504. [Google Scholar] [CrossRef]
- Richardson, P.J.; Brown, S.J. ATP release from affinity-purified rat cholinergic nerve terminals. J. Neurochem. 1987, 48, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Berne, R.M. Cardiac nucleotides in hypoxia: Possible role in regulation of coronary blood flow. Am. J. Physiol. 1963, 204, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Forrester, T.; Williams, C.A. Release of adenosine triphosphate from isolated adult heart cells in response to hypoxia. J. Physiol. 1977, 268, 371–390. [Google Scholar] [CrossRef] [PubMed]
- Paddle, B.M.; Burnstock, G. Release of ATP from perfused heart during coronary vasodilatation. J. Vasc. Res. 1974, 11, 110–119. [Google Scholar] [CrossRef]
- Williams, C.A.; Forrester, T. Possible source of adenosine triphosphate released from rat myocytes in response to hypoxia and acidosis. Cardiovasc. Res. 1983, 17, 301–312. [Google Scholar] [CrossRef]
- Bodin, P.; Bailey, D.; Burnstock, G. Increased flow-induced ATP release from isolated vascular endothelial cells but not smooth muscle cells. Br. J. Pharmacol. 1991, 103, 1203–1205. [Google Scholar] [CrossRef] [Green Version]
- Ralevic, V.; Burnstock, G. Receptors for purrines and pyrimidines. Pharmacol. Rev. 1998, 50, 413–492. [Google Scholar]
- Yang, S.; Cheek, D.J.; Westfall, D.P.; Buxton, I.L. Purinergic axis in cardiac blood vessels. Agonist-mediated release of ATP from cardiac endothelial cells. Circ. Res. 1994, 74, 401–417. [Google Scholar] [CrossRef] [Green Version]
- Katsuragi, T.; Tokunaga, T.; Usune, S.; Sato, C.; Furukawa, T. Neurotransmitter-mediated ATP release from smooth muscles. In Role of Adenosine and Adenine Nucleotides in the Biological System; Imai, S., Nakazawa, M., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1991; pp. 407–414. [Google Scholar]
- Pearson, J.D.; Gordon, J.L. Vascular endothelial and smooth muscle cells in culture selectively release adenine nucleotides. Nature 1979, 281, 384–386. [Google Scholar] [CrossRef]
- Day, H.J.; Holmsen, H. Concepts of the blood platelet release reaction. Ser. Hematol. 1971, 4, 3–27. [Google Scholar]
- Holmsen, H. Platelet metabolism and activation. Semin. Hematol. 1985, 22, 219–240. [Google Scholar] [PubMed]
- Mills, D.C.; Robb, I.A.; Roberts, G.C. The release of nucleotides, 5-hydroxytryptamine and enzymes from human blood platelets during aggregation. J. Physiol. 1968, 195, 715–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borst, M.M.; Schrader, J. Adenine nucleotide release from isolated perfused guinea pig hearts and extracellular formation of adenosine. Circ. Res. 1991, 68, 797–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jorgensen, S. Breakdown of adenine and hypoxanthine nucleotides and nucleosides in human plasma. Acta Pharmacol. Toxicol. 1956, 12, 294–302. [Google Scholar] [CrossRef]
- Welford, L.A.; Cusack, N.J.; Hourani, S.M. The structure-activity relationships of ectonucleotidases and of excitatory P2-purinoceptors: Evidence that dephosphorylation of ATP analogues reduces pharmacological potency. Eur. J. Pharmacol. 1987, 141, 123–130. [Google Scholar] [CrossRef]
- Kuzmin, A.I.; Lakomkin, V.L.; Kapelko, V.I.; Vassort, G. Interstitial ATP level and degradation in control and postmyocardial infarcted rats. Am. J. Physiol. 1998, 275, C766–C771. [Google Scholar] [CrossRef]
- Darius, H.; Stahl, G.L.; Lefer, A.M. Pharmacologic modulation of ATP release from isolated rat hearts in response to vasoconstrictor stimuli using a continuous flow technique. J. Pharmacol. Exp. Ther. 1987, 240, 542–547. [Google Scholar]
- Katsuragi, T.; Tokunaga, T.; Ohba, M.; Sato, C.; Furukawa, T. Implication of ATP released from atrial, but not papillary, muscle segments of guinea pig by isoproterenol and forskolin. Life Sci. 1993, 53, 961–967. [Google Scholar] [CrossRef]
- Vial, C.; Owen, P.; Opie, L.H.; Posel, D. Significance of release of adenosine triphosphate and adenosine induced by hypoxia or adrenaline in perfused rat heart. J. Mol. Cell. Cardiol. 1987, 19, 187–197. [Google Scholar] [CrossRef]
- Uozumi, H.; Kudoh, S.; Zou, Y.; Harada, K.; Yamazaki, T.; Komuro, I. Autocrine release of ATP mediates mechanical stress-induced cardiomyocyte hypertrophy. Circulation 1998, 98, I-624. [Google Scholar]
- Vials, A.J.; Burnstock, G. ATP release from the isolated perfused guinea pig heart in response to increased flow. J. Vasc. Res. 1996, 33, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Doyle, T.B.; Forrester, T. Appearance of adenosine triphosphate in the perfusate from working frog heart. Pflüg. Arch. 1985, 405, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Kunapuli, S.P.; Daniel, J.L. P2 receptor subtypes in the cardiovascular system. Biochem. J. 1998, 336, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G.; Kennedy, C. P2X Receptors in Health and Disease. In Advances in Pharmacology; Kenneth, A.J., Joel, J., Eds.; Academic Press: Cambridge, MA, USA, 2011; Volume 61, pp. 333–372. [Google Scholar]
- North, R.A. Molecular physiology of P2X receptors. Physiol. Rev. 2002, 82, 1013–1067. [Google Scholar] [CrossRef] [PubMed]
- Khakh, B.S.; North, R.A. Neuromodulation by extracellular ATP and P2X receptors in the CNS. Neuron 2012, 76, 51–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnard, E.A. Receptor classes and the transmitter-gated ion channels. Trends Biochem. Sci. 1992, 17, 368–374. [Google Scholar] [CrossRef]
- Brake, A.J.; Julius, D. Signaling by extracellular nucleotides. Annu. Rev. Cell Dev. Biol. 1996, 12, 519–541. [Google Scholar] [CrossRef]
- Buell, G.; Collo, G.; Rassendren, F. P2X receptors: An emerging channel family. Eur. J. Neurosci. 1996, 8, 2221–2228. [Google Scholar] [CrossRef]
- Burnstock, G.; Meghji, P. Distribution of P1- and P2-purinoceptors in the guinea-pig and frog heart. Br. J. Pharmacol. 1981, 73, 879–885. [Google Scholar] [CrossRef] [Green Version]
- Fredholm, B.B.; Abbracchio, M.P.; Burnstock, G.; Daly, J.W.; Harden, T.K.; Jacobson, K.A.; Leff, P.; Williams, M. Nomenclature and classification of purinoceptors. Pharmacol. Rev. 1994, 46, 143–156. [Google Scholar]
- Kügelgen, I. Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol. Ther. 2006, 110, 415–432. [Google Scholar] [CrossRef] [PubMed]
- Brake, A.J.; Wagenbach, M.J.; Julius, D. New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature 1994, 371, 519–523. [Google Scholar] [CrossRef]
- Hattori, M.; Gouaux, E. Molecular mechanism of ATP binding and ion channel activation in P2X receptors. Nature 2012, 485, 207–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waldo, G.L.; Harden, T.K. Agonist binding and Gq-stimulating activities of the purified human P2Y1 receptor. Mol. Pharmacol. 2004, 65, 426–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodor, E.T.; Waldo, G.L.; Hooks, S.B.; Corbitt, J.; Boyer, J.L.; Harden, T.K. Purification and functional reconstitution of the human P2Y12 receptor. Mol. Pharmacol. 2003, 64, 1210–1216. [Google Scholar] [CrossRef]
- Michel, A.D.; Humphrey, P.P. Distribution and characterisation of [3H]alpha,beta-methylene ATP binding sites in the rat. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1993, 348, 608–617. [Google Scholar] [CrossRef]
- Froldi, G.; Varani, K.; Chinellato, A.; Ragazzi, E.; Caparrotta, L.; Borea, P.A. P2X-purinoceptors in the heart: Actions of ATP and UTP. Life Sci. 1997, 60, 1419–1430. [Google Scholar] [CrossRef]
- Dhulipala, P.D.; Wang, Y.X.; Kotlikoff, M.I. The human P2X4 receptor gene is alternatively spliced. Gene 1998, 207, 259–266. [Google Scholar] [CrossRef]
- Vulchanova, L.; Arvidsson, U.; Riedl, M.; Wang, J.; Buell, G.; Surprenant, A.; North, R.A.; Elde, R. Differential distribution of two ATP-gated channels (P2X receptors) determined by immunocytochemistry. Proc. Natl. Acad. Sci. USA 1996, 93, 8063–8067. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; Mei, Q.B.; Yao, X.J.; Smith, E.; Barry, W.H.; Liang, B.T. A novel contractile phenotype with cardiac transgenic expression of the human P2X4 receptor. FASEB J. 2001, 15, 2739–2741. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; Senkler, C.; Yang, A.; Soto, F.; Liang, B.T. P2X4 receptor is a glycosylated cardiac receptor mediating a positive inotropic respense to ATP. J. Biol. Chem. 2002, 277, 15752–15757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bo, X.; Kim, M.; Nori, S.L.; Schoepfer, R.; Burnstock, G.; North, R.A. Tissue distribution of P2X4 receptors studied with an ectodomain antibody. Cell Tissue Res. 2003, 313, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Musa, H.; Tellez, J.O.; Chandler, N.J.; Greener, I.D.; Maczewski, M.; Mackiewicz, U.; Beresewicz, A.; Molenaar, P.; Boyett, M.R.; Dobrzynski, H. P2 purinergic receptor mRNA in rat and human sinoatrial node and other heart regions. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2009, 379, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Sartiani, L.; Bochet, P.; Cerbai, E.; Mugelli, A.; Fischmeister, R. Functional expression of the hyperpolarization-activated, non-selective cation current If in immortalized HL-1 cardiomyocytes. J. Physiol. 2002, 545, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Claycomb, W.C.; Lanson, N.A., Jr.; Stallworth, B.S.; Egeland, D.B.; Delcarpio, J.B.; Bahinski, A.; Izzo, N.J., Jr. HL-1 cells: A cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc. Natl. Acad. Sci. USA 1998, 95, 2979–2984. [Google Scholar] [CrossRef] [Green Version]
- Pfleger, C.; Ebeling, G.; Bläsche, R.; Patton, M.; Patel, H.H.; Kasper, M.; Barth, K. Detection of caveolin-3/caveolin-1/P2X7R complexes in mice atrial cardiomyocytes in vivo and in vitro. Histochem. Cell Biol. 2012, 138, 231–241. [Google Scholar] [CrossRef] [Green Version]
- Adinolfi, E.; Amoroso, F.; Giuliani, A.L. P2X7 Receptor Function in Bone-Related Cancer. J. Osteoporos. 2012, 2012, 637863. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.B.; Pappano, A.J.; Liang, B.T. Extracellular ATP-stimulated current in wild-type and P2X4 receptor transgenic mouse ventricular myocytes: Implications for a cardiac physiologic role of P2X4 receptors. FASEB J. 2006, 20, 277–284. [Google Scholar] [CrossRef]
- Simon, J.; Webb, T.E.; King, B.F.; Burnstock, G.; Barnard, E.A. Characterisation of a recombinant P2Y purinoceptor. Eur. J. Pharmacol. 1995, 291, 281–289. [Google Scholar] [CrossRef]
- Lustig, K.D.; Shiau, A.K.; Brake, A.J.; Julius, D. Expression cloning of an ATP receptor from mouse neuroblastoma cells. Proc. Natl. Acad. Sci. USA 1993, 90, 5113–5117. [Google Scholar] [CrossRef] [Green Version]
- Webb, T.E.; Boluyt, M.O.; Barnard, E.A. Molecular biology of P2Y purinoceptors: Expression in rat heart. J. Auton. Pharmacol. 1996, 16, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.K.; Huang, W.; Jiang, L.; Barden, J.A.; Allen, D.G. ATP modulates intracellular Ca2+ and firing rate through a P2Y1 purinoceptor in cane toad pacemaker cells. J. Physiol. 2003, 552, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Tokuyama, Y.; Hara, M.; Jones, E.M.; Fan, Z.; Bell, G.I. Cloning of rat and mouse P2Y purinoceptors. Biochem. Biophys. Res. Commun. 1995, 211, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Vöhringer, C.; Schäfer, R.; Reiser, G. A chimeric rat brain P2Y1 receptor tagged with green-fluorescent protein: High-affinity ligand recognition of adenosine diphosphates and triphosphates and selectivity identical to that of the wild-type receptor. Biochem. Pharmacol. 2000, 59, 791–800. [Google Scholar] [CrossRef]
- Henderson, D.J.; Elliot, D.G.; Smith, G.M.; Webb, T.E.; Dainty, I.A. Cloning and characterisation of a bovine P2Y receptor. Biochem. Biophys. Res. Commun. 1995, 212, 648–656. [Google Scholar] [CrossRef]
- Ayyanathan, K.; Webb, T.E.; Sandhu, A.K.; Athwal, R.S.; Barnard, E.A.; Kunapuli, S.P. Cloning and chromosomal localization of the human P2Y1 purinoceptor. Biochem. Biophys. Res. Commun. 1996, 218, 783–788. [Google Scholar] [CrossRef]
- Janssens, R.; Communi, D.; Pirotton, S.; Samson, M.; Parmentier, M.; Boeynaems, J.M. Cloning and tissue distribution of the human P2Y1 receptor. Biochem. Biophys. Res. Commun. 1996, 221, 588–593. [Google Scholar] [CrossRef]
- Leon, C.; Vial, C.; Cazenave, J.P.; Gachet, C. Cloning and sequencing of a human cDNA encoding endothelial P2Y1 purinoceptor. Gene 1996, 171, 295–297. [Google Scholar] [CrossRef]
- Leon, C.; Hechler, B.; Vial, C.; Leray, C.; Cazenave, J.P.; Gachet, C. The P2Y1 receptor is an ADP receptor antagonized by ATP and expressed in platelets and megakaryoblastic cells. FEBS Lett. 1997, 403, 26–30. [Google Scholar] [CrossRef] [Green Version]
- Palmer, R.K.; Boyer, J.L.; Schachter, J.B.; Nicholas, R.A.; Harden, T.K. Agonist action of adenosine triphosphates at the human P2Y1 receptor. Mol. Pharmacol. 1998, 54, 1118–1123. [Google Scholar] [CrossRef] [Green Version]
- Chhatriwala, M.; Ravi, R.G.; Patel, R.I.; Boyer, J.L.; Jacobson, K.A.; Harden, T.K. Induction of novel agonist selectivity for the ADPactivated P2Y1 receptor versus the ADP-activated P2Y12 and P2Y13 receptors by conformational constraint of an ADP analog. J. Pharmacol. Exp. Ther. 2004, 311, 1038–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rice, W.R.; Burton, F.M.; Fiedeldey, D.T. Cloning and expression of the alveolar type II cell P2u-purinergic receptor. Am. J. Respir. Cell Mol. Biol. 1995, 12, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.P.; Krull, N.; Xu, S.; Levy, A.; Lightman, S.L. Molecular cloning and functional characterization of a rat pituitary G protein-coupled adenosine triphosphate (ATP) receptor. Endocrinology 1996, 137, 1833–1840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wildman, S.S.; Unwin, R.J.; King, B.F. Extended pharmacological profiles of rat P2Y2 and rat P2Y4 receptors and their sensitivity to extracellular H+ and Zn2+ ions. Br. J. Pharmacol. 2003, 140, 1177–1186. [Google Scholar] [CrossRef] [Green Version]
- Zambon, A.C.; Hughes, R.J.; Meszaros, J.G.; Wu, J.J.; Torres, B.; Brunton, L.L.; Insel, P.A. P2Y(2) receptor of MDCK cells: Cloning, expression, and cell-specific signaling. Am. J. Physiol. Ren. Physiol. 2000, 279, F1045–F1052. [Google Scholar] [CrossRef]
- Shen, J.; Seye, C.I.; Wang, M.; Weisman, G.A.; Wilden, P.A.; Sturek, M. Cloning, up-regulation, and mitogenic role of porcine P2Y2 receptor in coronary artery smooth muscle cells. Mol. Pharmacol. 2004, 66, 1265–1274. [Google Scholar] [CrossRef] [Green Version]
- Parr, C.E.; Sullivan, D.M.; Paradiso, A.M.; Lazarowski, E.R.; Burch, L.H.; Olsen, J.C.; Erb, L.; Weisman, G.A.; Boucher, R.C.; Turner, J.T. Cloning and expression of a human P2U nucleotide receptor, a target for cystic fibrosis pharmacotherapy. Proc. Natl. Acad. Sci. USA 1994, 91, 3275–3279. [Google Scholar] [CrossRef] [Green Version]
- Lazarowski, E.R.; Watt, W.C.; Stutts, M.J.; Boucher, R.C.; Harden, T.K. Pharmacological selectivity of the cloned human P2U-purinoceptor: Potent activation by diadenosine tetraphosphate. Br. J. Pharmacol. 1995, 116, 1619–1627. [Google Scholar] [CrossRef] [Green Version]
- Nicholas, R.A.; Watt, W.C.; Lazarowski, E.R.; Li, Q.; Harden, K. Uridine nucleotide selectivity of three phospholipase C-activating P2 receptors: Identification of a UDP-selective, a UTP-selective, and an ATP- and UTP-specific receptor. Mol. Pharmacol. 1996, 50, 224–229. [Google Scholar]
- Yerxa, B.R.; Sabater, J.R.; Davis, C.W.; Stutts, M.J.; Lang-Furr, M.; Picher, M.; Jones, A.C.; Cowlen, M.; Dougherty, R.; Boyer, J.; et al. Pharmacology of INS37217 [P1-(uridine 5V)-P4-(2′-deoxycytidine 5′) tetraphosphate, tetrasodium salt], a next-generation P2Y2 receptor agonist for the treatment of cystic fibrosis. J. Pharmacol. Exp. Ther. 2002, 302, 871–880. [Google Scholar] [CrossRef]
- Bogdanov, Y.D.; Wildman, S.S.; Clements, M.P.; King, B.F.; Burnstock, G. Molecular cloning and characterization of rat P2Y4 nucleotide receptor. Br. J. Pharmacol. 1998, 124, 428–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webb, T.E.; Henderson, D.J.; Roberts, J.A.; Barnard, E.A. Molecular cloning and characterization of the rat P2Y4 receptor. J. Neurochem. 1998, 71, 1348–1357. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, C.; Qi, A.D.; Herold, C.L.; Harden, T.K.; Nicholas, R.A. ATP, an agonist at the rat P2Y4 receptor, is an antagonist at the human P2Y4 receptor. Mol. Pharmacol. 2000, 57, 926–931. [Google Scholar] [PubMed]
- Suarez-Huerta, N.; Pouillon, V.; Boeynaems, J.; Robaye, B. Molecular cloning and characterization of the mouse P2Y4 nucleotide receptor. Eur. J. Pharmacol. 2000, 416, 197–202. [Google Scholar] [CrossRef]
- Communi, D.; Pirotton, S.; Parmentier, M.; Boeynaems, J.M. Cloning and functional expression of a human uridine nucleotide receptor. J. Biol. Chem. 1995, 270, 30849–30852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Communi, D.; Motte, S.; Boeynaems, J.M.; Pirotton, S. Pharmacological characterization of the human P2Y4 receptor. Eur. J. Pharmacol. 1996, 317, 383–389. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.; Erb, L.; Weisman, G.A.; Marchese, A.; Heng, H.H.; Garrad, R.C.; George, S.R.; Turner, J.T.; O’Dowd, B.F. Cloning, expression, and chromosomal localization of the human uridine nucleotide receptor gene. J. Biol. Chem. 1995, 270, 30845–30848. [Google Scholar] [CrossRef] [Green Version]
- Herold, C.L.; Qi, A.D.; Harden, T.K.; Nicholas, R.A. Agonist versus antagonist action of ATP at the P2Y4 receptor is determined by the second extracellular loop. J. Biol. Chem. 2004, 279, 11456–11464. [Google Scholar] [CrossRef] [Green Version]
- Chang, K.; Hanaoka, K.; Kumada, M.; Takuwa, Y. Molecular cloning and functional analysis of a novel P2 nucleotide receptor. J. Biol. Chem. 1995, 270, 26152–26158. [Google Scholar] [CrossRef] [Green Version]
- Lazarowski, E.R.; Rochelle, L.G.; O’Neal, W.K.; Ribeiro, C.M.; Grubb, B.R.; Zhang, V.; Harden, T.K.; Boucher, R.C. Cloning and functional characterization of two murine uridine nucleotide receptors reveal a potential target for correcting ion transport deficiency in cystic fibrosis gallbladder. J. Pharmacol. Exp. Ther. 2001, 297, 43–49. [Google Scholar]
- Communi, D.; Parmentier, M.; Boeynaems, J.M. Cloning, functional expression and tissue distribution of the human P2Y6 receptor. Biochem. Biophys. Res. Commun. 1996, 222, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Southey, M.C.; Hammet, F.; Hutchins, A.M.; Paidhungat, M.; Somers, G.R.; Venter, D.J. Molecular cloning and sequencing of a novel human P2 nucleotide receptor. Biochim. Biophys. Acta 1996, 1309, 77–80. [Google Scholar] [CrossRef]
- Maier, R.; Glatz, A.; Mosbacher, J.; Bilbe, G. Cloning of P2Y6 cDNAs and identification of a pseudogene: Comparison of P2Y receptor subtype expression in bone and brain tissues. Biochem. Biophys. Res. Commun. 1997, 240, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Qi, A.D.; Zambon, A.C.; Insel, P.A.; Nicholas, R.A. An arginine/glutamine difference at the juxtaposition of transmembrane domain 6 and the third extracellular loop contributes to the markedly different nucleotide selectivities of human and canine P2Y11 receptors. Mol. Pharmacol. 2001, 60, 1375–1382. [Google Scholar] [CrossRef]
- Zambon, A.C.; Brunton, L.L.; Barrett, K.E.; Hughes, R.J.; Torres, B.; Insel, P.A. Cloning, expression, signaling mechanisms, and membrane targeting of P2Y11 receptors in Madin Darby canine kidney cells. Mol. Pharmacol. 2001, 60, 26–35. [Google Scholar] [CrossRef]
- Communi, D.; Govaerts, C.; Parmentier, M.; Boeynaems, J.M. Cloning of a human purinergic P2Y receptor coupled to phospholipase C and adenylyl cyclase. J. Biol. Chem. 1997, 272, 31969–31973. [Google Scholar] [CrossRef] [Green Version]
- Communi, D.; Robaye, B.; Boeynaems, J.M. Pharmacological characterization of the human P2Y11 receptor. Br. J. Pharmacol. 1999, 128, 1199–1206. [Google Scholar] [CrossRef]
- White, P.J.; Webb, T.E.; Boarder, M.R. Characterization of a Ca2+ response to both UTP and ATP at human P2Y11 receptors: Evidence for agonist-specific signaling. Mol. Pharmacol. 2003, 63, 1356–1363. [Google Scholar] [CrossRef]
- Hollopeter, G.; Jantzen, H.M.; Vincent, D.; Li, G.; England, L.; Ramakrishnan, V.; Yang, R.B.; Nurden, P.; Nurden, A.; Julius, D.; et al. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature 2001, 409, 202–207. [Google Scholar] [CrossRef] [Green Version]
- Simon, J.; Filippov, A.K.; Goransson, S.; Wong, Y.H.; Frelin, C.; Michel, A.D.; Brown, D.A.; Barnard, E.A. Characterization and channel coupling of the P2Y(12) nucleotide receptor of brain capillary endothelial cells. J. Biol. Chem. 2002, 277, 31390–31400. [Google Scholar] [CrossRef] [Green Version]
- Foster, C.J.; Prosser, D.M.; Agans, J.M.; Zhai, Y.; Smith, M.D.; Lachowicz, J.E.; Zhang, F.L.; Gustafson, E.; Monsma, F.J., Jr.; Wiekowski, M.T.; et al. Molecular identification and characterization of the platelet ADP receptor targeted by thienopyridine antithrombotic drugs. J. Clin. Investig. 2001, 107, 1591–1598. [Google Scholar] [CrossRef] [PubMed]
- Von Kügelgen, I.; Kulick, M.; Bönisch, H.; Göthert, M.; Brüss, M. Cloning of the rat and mouse P2Y12-receptor from neuronal cells or tissues. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2001, 364, R30. [Google Scholar]
- Pausch, M.H.; Lai, M.; Tseng, E.; Paulsen, J.; Bates, B.; Kwak, S. Functional expression of human and mouse P2Y12 receptors in Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 2004, 324, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Ennion, S.J.; Powell, A.D.; Seward, E.P. Identification of the P2Y12 receptor in nucleotide inhibition of exocytosis from bovine chromaffin cells. Mol. Pharmacol. 2004, 66, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Takasaki, J.; Kamohara, M.; Saito, T.; Matsumoto, M.; Matsumoto, S.; Ohishi, T.; Soga, T.; Matsushime, H.; Furuichi, K. Molecular cloning of the platelet P2T(AC) ADP receptor: Pharmacological comparison with another ADP receptor, the P2Y(1) receptor. Mol. Pharmacol. 2001, 60, 432–439. [Google Scholar] [PubMed]
- Zhang, F.L.; Luo, L.; Gustafson, E.; Lachowicz, J.; Smith, M.; Qiao, X.; Liu, Y.H.; Chen, G.; Pramanik, B.; Laz, T.M.; et al. ADP is the cognate ligand for the orphan G protein-coupled receptor SP1999. J. Biol. Chem. 2001, 276, 8608–8615. [Google Scholar] [CrossRef] [Green Version]
- Fumagalli, M.; Trincavelli, L.; Lecca, D.; Martini, C.; Ciana, P.; Abbracchio, M.P. Cloning, pharmacological characterisation and distribution of the rat G-protein-coupled P2Y(13) receptor. Biochem. Pharmacol. 2004, 68, 113–124. [Google Scholar] [CrossRef]
- Zhang, F.L.; Luo, L.; Gustafson, E.; Palmer, K.; Qiao, X.; Fan, X.; Yang, S.; Laz, T.M.; Bayne, M.; Monsma, F., Jr. P2Y13: Identification and characterization of a novel Galphaicoupled ADP receptor from human and mouse. J. Pharmacol. Exp. Ther. 2002, 301, 705–713. [Google Scholar] [CrossRef]
- Communi, D.; Gonzalez, N.S.; Detheux, M.; Brezillon, S.; Lannoy, V.; Parmentier, M.; Boeynaems, J.M. Identification of a novel human ADP receptor coupled to Gi. J. Biol. Chem. 2001, 276, 41479–41485. [Google Scholar] [CrossRef] [Green Version]
- Marteau, F.; Le Poul, E.; Communi, D.; Communi, D.; Labouret, C.; Savi, P.; Boeynaems, J.; Gonzalez, N.S. Pharmacological characterization of the human P2Y13 receptor. Mol. Pharmacol. 2003, 64, 104–112. [Google Scholar] [CrossRef] [Green Version]
- Charlton, M.E.; Williams, A.S.; Fogliano, M.; Sweetnam, P.M.; Duman, R.S. The isolation and characterization of a novel G protein-coupled receptor regulated by immunologic challenge. Brain Res. 1997, 764, 141–148. [Google Scholar] [CrossRef]
- Freeman, K.; Tsui, P.; Moore, D.; Emson, P.C.; Vawter, L.; Naheed, S.; Lane, P.; Bawagan, H.; Herrity, N.; Murphy, K.; et al. Cloning, pharmacology, and tissue distribution of G-protein coupled receptor GPR105 (KIAA0001) rodent orthologs. Genomics 2001, 78, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.K.; Macdonald, L.E.; Sarau, H.M.; Ames, R.S.; Freeman, K.; Foley, J.J.; Zhu, Y.; McLaughlin, M.M.; Murdock, P.; McMillan, L.; et al. A G protein-coupled receptor for UDP-glucose. J. Biol. Chem. 2000, 275, 10767–10771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Froldi, G.; Pandolfo, L.; Chinellato, A.; Ragazzi, E.; Caparrotta, L.; Fassina, G. Dual effect of ATP and UTP on rat atria: Which types of receptors are involved? Naunyn-Schmiedeberg’s Arch. Pharmacol. 1994, 349, 381–386. [Google Scholar] [CrossRef]
- Gergs, U.; Boknik, P.; Schmitz, W.; Simm, A.; Silber, R.E.; Neumann, J. A positive inotropic effect of ATP in the human cardiac atrium. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H1716–H1723. [Google Scholar] [CrossRef] [Green Version]
- Gergs, U.; Simm, A.; Bushnaq, H.; Silber, R.E.; Neumann, J. A positive inotropic effect of UTP in the human cardiac atrium. Eur. J. Pharmacol. 2014, 724, 24–30. [Google Scholar] [CrossRef]
- Mantelli, L.; Amerini, S.; Filippi, S.; Ledda, F. Blockade of adenosine receptors unmasks a stimulatory effect of ATP on cardiac contractility. Br. J. Pharmacol. 1993, 109, 1268–1271. [Google Scholar] [CrossRef]
- Scamps, F.; Legssyer, A.; Mayoux, E.; Vassort, G. The mechanism of positive inotropy induced by adenosine triphosphate in rat heart. Circ. Res. 1990, 67, 1007–1016. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.M.; Liu, J.; Li, C.X. Intermedin protects against myocardial ischemia-reperfusion injury in hyperlipidemia rats. Genet. Mol. Res. 2014, 13, 8309–8319. [Google Scholar] [CrossRef]
- Bragança, B.; Nogueira-Marques, S.; Ferreirinha, F.; Fontes-Sousa, A.P.; Correia-de-Sá, P. The Ionotropic P2X4 Receptor has Unique Properties in the Heart by Mediating the Negative Chronotropic Effect of ATP While Increasing the Ventricular Inotropy. Front. Pharmacol. 2019, 10, 1103. [Google Scholar] [CrossRef]
- Vessey, D.A.; Li, L.; Kelley, M. Pannexin-I/P2X 7 purinergic receptor channels mediate the release of cardioprotectants induced by ischemic pre- and postconditioning. J. Cardiovasc. Pharmacol. Ther. 2010, 15, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Vessey, D.A.; Li, L.; Kelley, M. P2X7 receptor agonists pre- and postcondition the heart against ischemia-reperfusion injury by opening pannexin-1/P2X7 channels. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H881–H887. [Google Scholar] [CrossRef] [PubMed]
- Pham, T. UTP but not ATP causes hypertrophic growth in neonatal rat cardiomyocytes. J. Mol. Cell. Cardiol. 2003, 35, 287–292. [Google Scholar] [CrossRef]
- Braun, O.O.; Jagroop, A.; Wang, L.; Mikhailidis, D.P.; Burnstock, G.; Erlinge, D. Increased platelet purinergic sensitivity in peripheral arterial disease-a pilot study. Platelets 2005, 16, 261–267. [Google Scholar] [CrossRef]
- Yamamoto, K.; Sokabe, T.; Matsumoto, T.; Yoshimura, K.; Shibata, M.; Ohura, N.; Fukuda, T.; Sato, T.; Sekine, K.; Kato, S.; et al. Impaired flow-dependent control of vascular tone and remodeling in P2X4-deficient mice. Nat. Med. 2006, 12, 133–137. [Google Scholar] [CrossRef]
- Woo, S.H.; Cleemann, L.; Morad, M. Ca2+ current-gated focal and local Ca2+ release in rat atrial myocytes: Evidence from rapid 2-D confocal imaging. J. Physiol. 2002, 543, 439–453. [Google Scholar] [CrossRef]
- Fabiato, A.; Fabiato, F. Effects of pH on the myofilaments and the sarcoplasmic reticulum of skinned cells from cardiace and skeletal muscles. J. Physiol. 1978, 276, 233–255. [Google Scholar] [CrossRef]
- Pucéat, M.; Clément-Chomienne, O.; Terzic, A.; Vassort, G. Alpha 1-adrenoceptor and purinoceptor agonists modulate Na-H antiport in single cardiac cells. Am. J. Physiol. 1993, 264, H310–H319. [Google Scholar]
- Saito, D.; Ueeda, M.; Abe, Y.; Tani, H.; Nakatsu, T.; Yoshida, H.; Haraoka, S.; Nagashima, H. Treatment of paroxysmal supraventricular tachycardia with intravenous injection of adenosine triphosphate. Br. Heart J. 1986, 55, 291–294. [Google Scholar] [CrossRef] [Green Version]
- Pfaffinger, P.J.; Martin, J.M.; Hunter, D.D.; Nathanson, N.M.; Hille, B. GTP-binding proteins couple cardiac muscarinic receptors to a K channel. Nature 1985, 317, 536–538. [Google Scholar] [CrossRef]
- Belardinelli, L.; Giles, W.R.; West, A. Ionic mechanisms of adenosine actions in pacemarker cells from rabbit heart. J. Physiol. 1988, 405, 615–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Kügelgen, I.; Wetter, A. Molecular pharmacology of P2Y-receptors. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2000, 362, 310–323. [Google Scholar] [CrossRef] [PubMed]
- Blomström-Lundqvist, C.; Scheinman, M.M.; Aliot, E.M.; Alpert, J.S.; Calkins, H.; Camm, A.J.; Campbell, W.B.; Haines, D.E.; Kuck, K.H.; Lerman, B.B.; et al. ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias--executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients with Supraventricular Arrhythmias). Circulation 2003, 108, 1871–1909. [Google Scholar]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [PubMed]
- Soto, F.; Garcia-Guznam, M.; Gomez-Hernandez, J.M.; Hollmann, M.; Karschin, C.; Stuhmer, W. P2X4: An ATP-activated ionotropic receptor cloned from rat brain. Proc. Natl. Acad. Sci. USA 1996, 93, 3684–3688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, C.A.; Chessell, I.P.; Simon, J.; Barnard, E.A.; Miller, K.J.; Michel, A.D.; Humphrey, P.P.A. Functional characterization of the P2X4 receptor orthologues. Br. J. Pharmacol. 2000, 129, 388–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, M.V.; Downey, J.M. Adenosine: Trigger and mediator of cardioprotection. Basic Res. Cardiol. 2007, 103, 203–215. [Google Scholar] [CrossRef]
- Peart, J.N.; Headrick, J.P. Adenosinergic cardioprotection: Multiple receptors, multiple pathways. Pharmacol. Ther. 2007, 114, 208–221. [Google Scholar] [CrossRef]
- Shen, J.B.; Shutt, R.; Pappano, A.; Liang, B.T. Characterization and mechanism of P2X receptor-mediated increase in cardiac myocyte contractility. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H3056–H3062. [Google Scholar] [CrossRef]
- Vessey, D.A.; Li, L.; Honbo, N.; Karliner, J.S. Sphingosine 1-phosphate is an important endogenous cardioprotectant released by ischemic pre- and postconditioning. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1429–H1435. [Google Scholar] [CrossRef]
- Scemes, E.; Suadicani, S.O.; Dahl, G.; Spray, D.C. Connexin and pannexin mediated cell-cell communication. Neuron Glia Biol. 2007, 3, 199–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahlenberg, J.M.; Lundberg, K.C.; Kertesy, S.B.; Qu, Y.; Dubyak, G.R. Potentiation of caspase-1 activation by the P2X7 receptor is dependent on TLR signals and requires NF-kappaB-driven protein synthesis. J. Immunol. 2005, 175, 7611–7622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merkle, S.; Frantz, S.; Schon, M.P.; Bauersachs, J.; Buitrago, M.; Frost, A.J.; Schmitteckert, E.M.; Lohse, M.J.; Engelhardt, S. A role for caspase-1 in heart failure. Circ. Res. 2007, 100, 645–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, V.; Sharma, A.; Saran, V.; Bernatchez, P.N.; Allard, M.F.; McNeill, J.H. β-receptor antagonist treatment prevents activation of cell death signaling in the diabetic heart independent of its metabolic actions. Eur. J. Pharmacol. 2011, 657, 117–125. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, T.; Bossuyt, J.; Li, X.; McKinsey, T.A.; Dedman, J.R.; Olson, E.N.; Chen, J.; Brown, J.H.; Bers, D.M. Local InsP3-dependent perinuclear Ca2+ signaling in cardiac myocyte excitation-transcription coupling. J. Clin. Investig. 2006, 116, 675–682. [Google Scholar] [CrossRef]
- Subedi, K.P.; Son, M.J.; Chidipi, B.; Kim, S.W.; Wang, J.; Kim, K.H.; Woo, S.H.; Kim, J.C. Signaling Pathway for endothelin-1- and phenylephrine-induced cAMP response element binding protein activation in rat ventricular myocytes: Role of inositol 1,4,5-trisphosphate receptors and CaMKII. Cell. Physiol. Biochem. 2017, 41, 399–412. [Google Scholar] [CrossRef]
- Sugden, P.; Clerk, A. Cellular mechanisms of cardiac hypertrophy. J. Mol. Med. 1980, 76, 725–742. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Boluyt, M.; Long, X.; O’Neill, L.; Lakatta, E.; Crow, M. Extracellular ATP inhibits adrenergic agonist-induced hypertrophy of neonatal cardiac myocytes. Circ. Res. 1996, 78, 525–535. [Google Scholar] [CrossRef]
- Shimoda, K.; Nishimura, A.; Sunggip, C.; Ito, T.; Nishiyama, K.; Kato, Y.; Tanaka, T.; Tozaki-Saitoh, H.; Tsuda, M.; Nishida, M. Modulation of P2Y6R expression exacerbates pressure overload-induced cardiac remodeling in mice. Sci. Rep. 2020, 10, 13926. [Google Scholar] [CrossRef]
- Nazir, S.A.; Lab, M.J. Mechanoelectric feedback in the atrium of the isolated guinea-pig heart. Cardiovasc. Res. 1996, 32, 112–119. [Google Scholar] [CrossRef]
- Nattel, S. New ideas about atrial fibrillation 50 years on. Nature 2002, 415, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.C.; Woo, S.H. Shear stress induces a longitudinal Ca2+ wave via autocrine activation of P2Y1 purinergic signalling in rat atrial myocytes. J. Physiol. 2015, 593, 5091–5109. [Google Scholar] [CrossRef] [Green Version]
- Ayettey, A.S.; Navaratnam, V. The T-tubule system in the specialized and general myocardium of the rat. J. Anat. 1978, 127, 125–140. [Google Scholar] [PubMed]
- Carl, S.L.; Felix, K.; Caswell, A.H.; Brandt, N.R.; Ball, W.J.; Vaghy, P.L.; Meissner, G.; Ferguson, D.G. Immunoloalization of sarcolemmal dihydropyridine receptor and sarcoplasmic reticular triadin and ryanodine receptor in rabbit ventricle and atrium. J. Cell Biol. 1995, 129, 673–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berlin, J.R. Spatiotemporal changes of Ca2+ during electrically evoked contractions in atrial and ventricular cells. Am. J. Physiol. 1995, 269, H1665–H1670. [Google Scholar] [CrossRef]
- Hüser, J.; Lipsius, S.L.; Blatter, L.A. Calcium gradients during excitation-contraction coupling in cat atrial myocytes. J. Physiol. 1996, 494, 641–651. [Google Scholar] [CrossRef]
- Kockskämper, J.; Sheehan, K.A.; Bare, D.J.; Lipsius, S.L.; Mignery, G.A.; Blatter, L.A. Activation and propagation of Ca2+ release during excitation-contraction coupling in atrial myocytes. Biophys. J. 2001, 81, 2590–2605. [Google Scholar] [CrossRef] [Green Version]
| Type | Species | Principal Agonists | Tissue Distribution | Selected References |
|---|---|---|---|---|
| P2Y1 | Rat | 2-MeS-ADP = 2-MeS-ATP > ADP | Heart, platelet, skeletal muscle, neuron, intestine | [85,86] |
| Mouse | 2-MeS-ATP > 2Cl-ATP > ATP | [85] | ||
| Bovine | 2-MeS-ATP > ADP > ATP | [87] | ||
| Human | (N)-mc-2-MeS-ADP > 2-MeS-ADP > ADP = ADPβS ATP | [66,88,89,90,91,92,93] | ||
| P2Y2 | Rat | UTP = ATP > CTP > GTP | Heart, lung, skeletal muscle, spleen, kidney | [94,95,96] |
| Mouse | UTP = ATP> Ap4A | [82] | ||
| Canine | UTP ≥ ATP > ADP > 2-MeS-ATP | [97] | ||
| Porcine | UTP > ITP > ATP > UDP | [98] | ||
| Human | UTP = ATP > INS37217 > Ap4A > ATPγS | [99,100,101,102] | ||
| P2Y4 | Rat | UTP = ATP = ITP = Ap4A | Placenta, lung, vascular smooth muscle, brain, liver | [96,103,104,105] |
| Mouse | UTP = ATP | [106] | ||
| Human | UTP > UTPγS | [101,107,108,109,110] | ||
| P2Y6 | Rat | UDP > UTP > ADP > 2-MeS-ATP | Heart, lung, spleen, placenta, thymus, intestine, brain | [101,111] |
| Mouse | UDP > UTP > ADP > 2-MeS-ATP | [112] | ||
| Human | UDP = 5-Br-UDP >> UTP > 2-MeS-ADP | [113,114,115] | ||
| P2Y11 | Canine | ADPβS = 2-MeS-ADP ≥ 2-MeS-ATP > ATP | Spleen, intestine, immune cells | [116,117] |
| Human | ARC67085 ≥ ATPγS = BzATP > ATP, (UTP) > 2-MeSAT | [116,117,118,119,120] | ||
| P2Y12 | Rat | 2-MeSADP >ADP > ATP | Neuron, platelet | [121,122] |
| Mouse | 2-MeSADP >ADP > ADPβS | [123,124,125] | ||
| Bovine | 2-MeS-ADP ≫ ADP, ATP | [126] | ||
| Human | 2-MeS-ADP >ADP >> (N)-mc-2-MeS-ADP | [121,127,128] | ||
| P2Y13 | Rat | ADP > 2-MeS-ADP >> HATP | Spleen, leucocytes, bone marrow, liver, brain | [129] |
| Mouse | ADP = 2-MeS-ADP = ADPβS > ATP | [130] | ||
| Human | 2-MeS-ADP > (=) ADP > ADPβS | [130,131,132] | ||
| P2Y14 | Rat | UDP-glucose | Placenta, adipose tissue, intestine, brain, spleen | [133] |
| Mouse | UDP-glucose | [134] | ||
| Human | UDP-glucose > UDP-galactose | [135] |
| Function | Cardiac Regions | Agonist | Effect | R | Species | References |
|---|---|---|---|---|---|---|
| Contraction | LA | ATP, ADP, UTP, Adenosine 2-MeS-ATP | Biphasic(Neg-pos) Neg Neg | A1 | Rat | [136] |
| A | ATP, ATPγS 2-MeS-ATP | Biphasic (neg-pos) Pos | P2X4(?) | Human | [137] | |
| A | UTP | Pos | P2Y | Human, rat, mouse | [136,137,138] | |
| A | ATP | Biphasic (pos-neg) | P2-A1 | Rat, guinea-pig | [136,139] | |
| A | 2-MeS-ATP | Neg | P2X | Rat | [136] | |
| A | 2-MeS-ATP | Pos | Mouse, chicken | [136] | ||
| V V | ATP 2-MeS-ATP, ivermectin | Pos Pos | P2X4 | Rat Mouse | [140] [72,141] | |
| Heart rate | A SAN RA | Adenosine ATP, 2-MeS-ADP ATP | Neg Biphasic (pos-neg) Neg | P2Y1-SR Ca2+ (↓) P2X4 | Frog Toad Rat | [61] [84] [72,142] |
| Pathology | V V V V A(HL-1) Fiboblast V V | ATP UTP ATP | Anti-HF IR-injury (↓) Inflammation (?) Hypertrophy Hypertrophy Fibrosis Fibrosis Antihypertensive | P2X4 P2X7 P2X7 P2Y P2Y(?) P2Y2 P2Y6 P2X4 | Mouse Rat Mouse Rat Mouse Human Mouse Mouse | [141] [143,144] [78] [145] [9] [146] [7] [147] |
| Mechano- transduction | RA | ATP | Shear stress, depolarization | P2X4 | Rat | [9,11] |
| LA | ATP | Shear stress, Ca2+ dysregulation | P2Y1 | Rat | [11,148] | |
| Endo | ATP | Shear stress | P2X4 | Mouse | [147] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woo, S.-H.; Trinh, T.N. P2 Receptors in Cardiac Myocyte Pathophysiology and Mechanotransduction. Int. J. Mol. Sci. 2021, 22, 251. https://doi.org/10.3390/ijms22010251
Woo S-H, Trinh TN. P2 Receptors in Cardiac Myocyte Pathophysiology and Mechanotransduction. International Journal of Molecular Sciences. 2021; 22(1):251. https://doi.org/10.3390/ijms22010251
Chicago/Turabian StyleWoo, Sun-Hee, and Tran Nguyet Trinh. 2021. "P2 Receptors in Cardiac Myocyte Pathophysiology and Mechanotransduction" International Journal of Molecular Sciences 22, no. 1: 251. https://doi.org/10.3390/ijms22010251
APA StyleWoo, S. -H., & Trinh, T. N. (2021). P2 Receptors in Cardiac Myocyte Pathophysiology and Mechanotransduction. International Journal of Molecular Sciences, 22(1), 251. https://doi.org/10.3390/ijms22010251





