Immune Modulation by Inhibitors of the HO System
Abstract
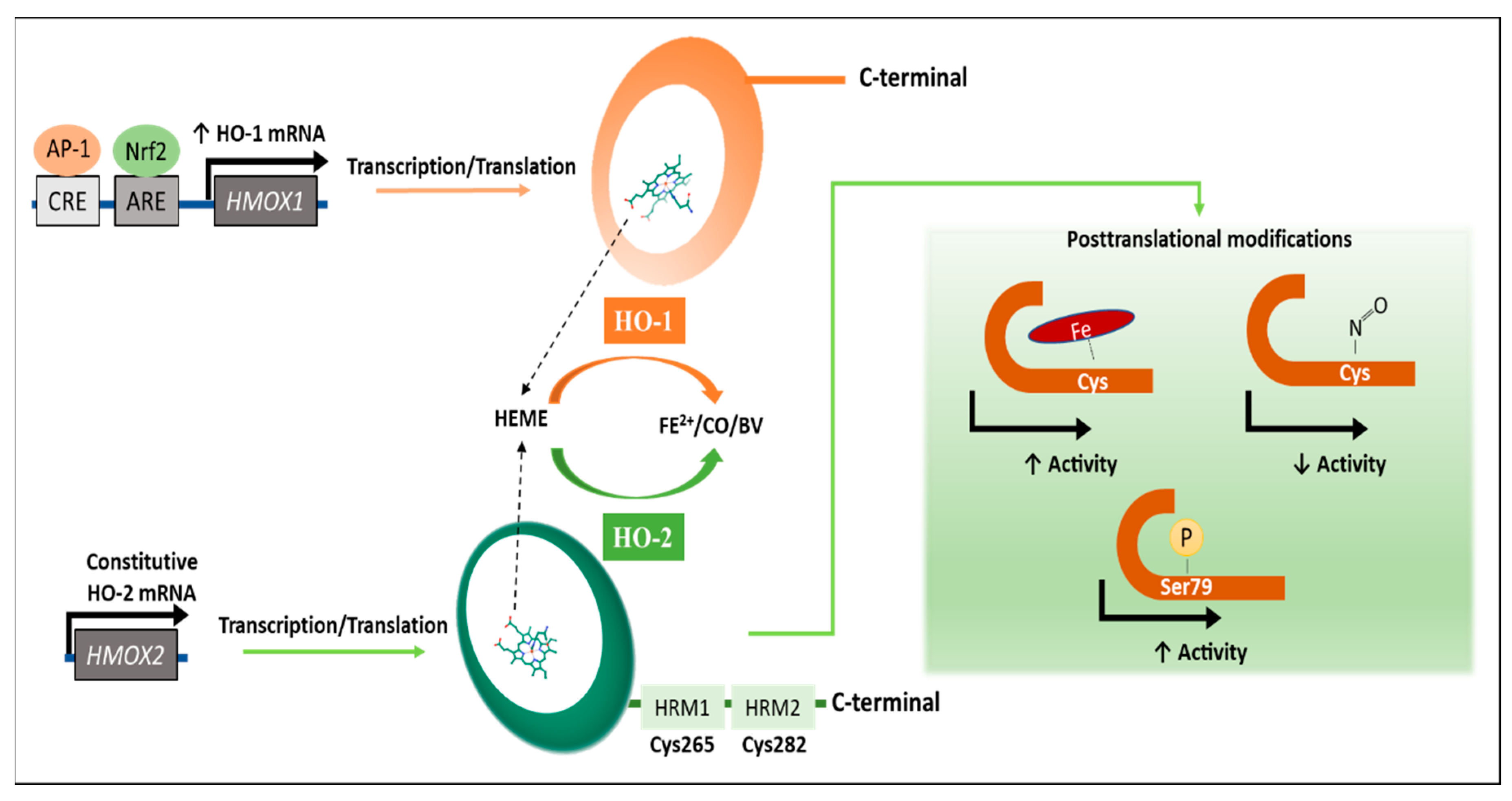
1. Introduction
2. Regulation of the HO Activity
3. Inhibitors of the HO System
3.1. First Generation of HO Inhibitors
3.2. Second Generation of HO Inhibitors
3.3. Synthetic Small Inhibitory Molecules
3.4. Inhibition by Genetic Engineering Approaches
4. Therapeutic Implications of HO Inhibitors
4.1. Cancer
4.2. Alzheimer’s Disease
4.3. Infections
4.3.1. Viral Infections
4.3.2. Bacterial Infections
4.3.3. Parasite Infections
4.3.4. Fungal Infections
5. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tenhunen, R.; Marver, H.S.; Schmid, R. Microsomal heme oxygenase. Characterization of the enzyme. J. Biol. Chem. 1969, 244, 6388–6394. [Google Scholar] [PubMed]
- Abraham, N.G.; Kappas, A. Pharmacological and Clinical Aspects of Heme Oxygenase. Pharmacol. Rev. 2008, 60, 79–127. [Google Scholar] [CrossRef] [PubMed]
- Kochert, B.A.; Fleischhacker, A.S.; Wales, T.E.; Becker, D.F.; Engen, J.R.; Ragsdale, S.W. Dynamic and structural differences between heme oxygenase-1 and -2 are due to differences in their C-terminal regions. J. Biol. Chem. 2019, 294, 8259–8272. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz, M.; Fernandez, P.; Guillen, M. Anti-Inflammatory Actions of the Heme Oxygenase-1 Pathway. Curr. Pharm. Des. 2005, 9, 2541–2551. [Google Scholar] [CrossRef] [PubMed]
- Ward, S. Heme oxygenase-1: A novel anti-inflammatory mediator. Trends Immunol. 2002, 23, 430. [Google Scholar] [CrossRef]
- Davis, S.J.; Bhoo, S.H.; Durski, A.M.; Walker, J.M.; Vierstra, R.D. The heme-oxygenase family required for phytochrome chromophore biosynthesis is necessary for proper photomorphogenesis in higher plants. Plant Physiol. 2001, 126, 656–669. [Google Scholar] [CrossRef]
- Kaizaki, A.; Tanaka, S.; Ishige, K.; Numazawa, S.; Yoshida, T. The neuroprotective effect of heme oxygenase (HO) on oxidative stress in HO-1 siRNA-transfected HT22 cells. Brain Res. 2006, 1108, 39–44. [Google Scholar] [CrossRef]
- Loboda, A.; Jozkowicz, A.; Dulak, J. HO-1/CO system in tumor growth, angiogenesis and metabolism—Targeting HO-1 as an anti-tumor therapy. Vascul. Pharmacol. 2015, 74, 11–22. [Google Scholar] [CrossRef]
- Salerno, L.; Pittalà, V.; Romeo, G.; Modica, M.N.; Siracusa, M.A.; Di Giacomo, C.; Acquaviva, R.; Barbagallo, I.; Tibullo, D.; Sorrenti, V. Evaluation of novel aryloxyalkyl derivatives of imidazole and 1,2,4-triazole as heme oxygenase-1 (HO-1) inhibitors and their antitumor properties. Bioorganic Med. Chem. 2013, 21, 5145–5153. [Google Scholar] [CrossRef]
- Scapagnini, G.; D’Agata, V.; Calabrese, V.; Pascale, A.; Colombrita, C.; Alkon, D.; Cavallaro, S. Gene expression profiles of heme oxygenase isoforms in the rat brain. Brain Res. 2002, 954, 51–59. [Google Scholar] [CrossRef]
- Funes, S.C.; Rios, M.; Fernández-Fierro, A.; Covián, C.; Bueno, S.M.; Riedel, C.A.; Mackern-Oberti, J.P.; Kalergis, A.M. Naturally Derived Heme-Oxygenase 1 Inducers and Their Therapeutic Application to Immune-Mediated Diseases. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Drummond, G.S.; Baum, J.; Greenberg, M.; Lewis, D.; Abraham, N.G. HO-1 overexpression and underexpression: Clinical implications. Arch. Biochem. Biophys. 2019, 673, 108073. [Google Scholar] [CrossRef] [PubMed]
- Was, H.; Sokolowska, M.; Sierpniowska, A.; Dominik, P.; Skrzypek, K.; Lackowska, B.; Pratnicki, A.; Grochot-Przeczek, A.; Taha, H.; Kotlinowski, J.; et al. Effects of heme oxygenase-1 on induction and development of chemically induced squamous cell carcinoma in mice. Free Radic. Biol. Med. 2011, 51, 1717–1726. [Google Scholar] [CrossRef] [PubMed]
- Balla, G.; Jacob, H.S.; Balla, J.; Rosenberg, M.; Nath, K.; Apple, F.; Eaton, J.W.; Vercellotti, G.M. Ferritin: A cytoprotective antioxidant strategem of endothelium. J. Biol. Chem. 1992, 267, 18148–18153. [Google Scholar] [PubMed]
- Ayer, A.; Zarjou, A.; Agarwa, A.; Stocker, R. Heme oxygenases in cardiovascular health and disease. Physiol. Rev. 2016, 96, 1449–1508. [Google Scholar] [CrossRef]
- Ryter, S.W.; Choi, A.M.K. Targeting heme oxygenase-1 and carbon monoxide for therapeutic modulation of inflammation. Transl. Res. 2016, 167, 7–34. [Google Scholar] [CrossRef]
- Cheng, H.T.; Yen, C.J.; Chang, C.C.; Huang, K.T.; Chen, K.H.; Zhang, R.Y.; Lee, P.Y.; Miaw, S.C.; Huang, J.W.; Chiang, C.K.; et al. Ferritin heavy chain mediates the protective effect of heme oxygenase-1 against oxidative stress. Biochim. Biophys. Acta Gen. Subj. 2015, 1850, 2506–2517. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef]
- Huang, J.; Guo, P.; Ma, D.; Lin, X.; Fang, Q.; Wang, J. Overexpression of heme oxygenase-1 induced by constitutively activated NF-B as a potential therapeutic target for activated B-cell-like diffuse large B-cell lymphoma. Int. J. Oncol. 2016, 49, 253–264. [Google Scholar] [CrossRef]
- Ricchetti, G.A.; Williams, L.M.; Foxwell, B.M.J. Heme oxygenase 1 expression induced by IL-10 requires STAT-3 and phosphoinositol-3 kinase and is inhibited by lipopolysaccharide. J. Leukoc. Biol. 2004, 76, 719–726. [Google Scholar] [CrossRef]
- Ryter, S.W.; Xi, S.; Hartsfield, C.L.; Choi, A.M.K. Mitogen activated protein kinase (MAPK) pathway regulates heme oxygenase-1 gene expression by hypoxia in vascular cells. Antioxid. Redox Signal. 2002, 4, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Køllgaard, T.; Kornblit, B.; Petersen, J.; Klausen, T.W.; Mortensen, B.K.; Brñndstrup, P.; Sengeløv, H.; Høgdall, E.; Müller, K.; Vindeløv, L.; et al. GTn repeat polymorphism in heme oxygenase-1 (HO-1) correlates with clinical outcome after myeloablative or nonmyeloablative allogeneic hematopoietic cell transplantation. PLoS ONE 2016, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Boehning, D.; Moon, C.; Sharma, S.; Hurt, K.J.; Hester, L.D.; Ronnett, G.V.; Shugar, D.; Snyder, S.H. Carbon monoxide neurotransmission activated by CK2 phosphorylation of heme oxygenase-2. Neuron 2003, 40, 129–137. [Google Scholar] [CrossRef]
- Yi, L.; Ragsdale, S.W. Evidence That the Heme Regulatory Motifs in Heme Oxygenase-2 Serve as a Thiol/Disulfide Redox Switch Regulating Heme Binding. J. Biol. Chem. 2007. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; McCoubrey, W.K.; Maines, M.D. Interaction of heme oxygenase-2 with nitric oxide donors. Is the oxygenase an intracellular “sink” for NO? Eur. J. Biochem. 1999, 264, 854–861. [Google Scholar] [CrossRef]
- Maines, M.D.; Panahian, N. The heme oxygenase system and cellular defense mechanisms. In Hypoxia; Springer: Boston, MA, USA, 2001; pp. 249–272. [Google Scholar] [CrossRef]
- Vukomanovic, D.; Rahman, M.; Maines, M.; Ozolinš, T.; Szarek, W.; Jia, Z.; Nakatsu, K. Cysteine-independent activation/inhibition of heme oxygenase-2. Med. Gas Res. 2016, 6, 10–13. [Google Scholar] [CrossRef]
- Bouton, C.; Demple, B. Nitric Oxide-inducible Expression of Heme Oxygenase-1 in Human Cells. J. Biol. Chem. 2000, 275, 32688–32693. [Google Scholar] [CrossRef]
- Maines, M.D. Heme oxygenase: Function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988, 2, 2557–2568. [Google Scholar] [CrossRef]
- Prawan, A.; Kundu, J.K.; Surh, Y.-J. Molecular Basis of Heme Oxygenase-1 Induction: Implications for Chemoprevention and Chemoprotection. Antioxid. Redox Signal. 2005, 7, 1688–1703. [Google Scholar] [CrossRef]
- Dennery, P.A. Pharmacological interventions for the treatment of neonatal jaundice. Semin. Neonatol. 2002, 7, 111–119. [Google Scholar] [CrossRef]
- Maisels, M.J. Managing the jaundiced newborn: A persistent challenge. CMAJ 2015, 187, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, T.; Sassa, S.; Kappas, A. Purification and properties of bovine spleen heme oxygenase. Amino acid composition and sites of action of inhibitors of heme oxidation. J. Biol. Chem. 1982, 257, 7778–7785. [Google Scholar] [PubMed]
- Drummond, G.S.; Kappas, A. Sn-protoporphyrin inhibition of fetal and neonatal brain heme oxygenase. Transplacental passage of the metalloporphyrin and prenatal suppression of hyperbilirubinemia in the newborn animal. J. Clin. Investig. 1986, 77, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Bhutani, V.K.; Poland, R.; Meloy, L.D.; Hegyi, T.; Fanaroff, A.A.; Maisels, M.J. Clinical trial of tin mesoporphyrin to prevent neonatal hyperbilirubinemia. J. Perinatol. 2016, 36, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Keino, H.; Nagae, H.; Mimura, S.; Watanabe, K.; Kashiwamata, S. Dangerous effects of tin-protoporphyrin plus photoirradiation on neonatal rats. Eur. J. Pediatr. 1990, 149, 278–279. [Google Scholar] [CrossRef]
- Vlahakis, J.Z.; Kinobe, R.T.; Bowers, R.J.; Brien, J.F.; Nakatsu, K.; Szarek, W.A. Synthesis and evaluation of azalanstat analogues as heme oxygenase inhibitors. Bioorganic Med. Chem. Lett. 2005, 15, 1457–1461. [Google Scholar] [CrossRef]
- Intagliata, S.; Salerno, L.; Ciaffaglione, V.; Leonardi, C.; Fallica, A.N.; Carota, G.; Amata, E.; Marrazzo, A.; Pittalà, V.; Romeo, G. Heme Oxygenase-2 (HO-2) as a therapeutic target: Activators and inhibitors. Eur. J. Med. Chem. 2019, 183, 111703. [Google Scholar] [CrossRef]
- Liu, Y.S.; Li, H.S.; Qi, D.F.; Zhang, J.; Jiang, X.C.; Shi, K.; Zhang, X.J.; Zhang, X.H. Zinc protoporphyrin IX enhances chemotherapeutic response of hepatoma cells to cisplatin. World J. Gastroenterol. 2014, 20, 8572–8582. [Google Scholar] [CrossRef]
- Mucha, O.; Podkalicka, P.; Mikulski, M.; Barwacz, S.; Andrysiak, K.; Biela, A.; Mieczkowski, M.; Kachamakova-Trojanowska, N.; Ryszawy, D.; Białas, A.; et al. Development and characterization of a new inhibitor of heme oxygenase activity for cancer treatment. Arch. Biochem. Biophys. 2019, 671, 130–142. [Google Scholar] [CrossRef]
- Fernández-Mendívil, C.; Arreola, M.A.; Hohsfield, L.A.; Green, K.N.; Lopez, M.G. Aging and progression of beta-amyloid pathology in alzheimer’s disease correlates with microglial heme-oxygenase-1 overexpression. Antioxidants 2020, 9, 644. [Google Scholar] [CrossRef]
- Schipper, H.M.; Cissé, S.; Stopa, E.G. Expression of heme oxygenase-1 in the senescent and alzheimer-diseased brain. Ann. Neurol. 1995, 37, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Schipper, H.M.; Liberman, A.; Stopa, E.G. Neural heme oxygenase-1 expression in idiopathic Parkinson’s disease. Exp. Neurol. 1998, 150, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.L.; Amaral, E.P.; Namasivayam, S.; Mittereder, L.R.; Fisher, L.; Bonfim, C.C.; Sardinha-Silva, A.; Thompson, R.W.; Hieny, S.E.; Andrade, B.B.; et al. Heme oxygenase-1 inhibition promotes IFNγ- and NOS2-mediated control of Mycobacterium tuberculosis infection. Mucosal Immunol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Carasi, P.; Rodríguez, E.; da Costa, V.; Frigerio, S.; Brossard, N.; Noya, V.; Robello, C.; Anegón, I.; Freire, T. Heme-oxygenase-1 expression contributes to the immunoregulation induced by Fasciola hepatica and promotes infection. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef]
- Gobert, A.P.; Verriere, T.; Asim, M.; Barry, D.P.; Piazuelo, M.B.; de Sablet, T.; Delgado, A.G.; Bravo, L.E.; Correa, P.; Peek, R.M.; et al. Heme Oxygenase-1 Dysregulates Macrophage Polarization and the Immune Response to Helicobacter pylori. J. Immunol. 2014, 193, 3013–3022. [Google Scholar] [CrossRef]
- Navarathna, D.H.M.L.P.; Roberts, D.D. Candida albicans heme oxygenase and its product CO contribute to pathogenesis of candidemia and alter systemic chemokine and cytokine expression. Free Radic. Biol. Med. 2010, 49, 1561–1573. [Google Scholar] [CrossRef]
- Furci, L.M.; Lopes, P.; Eakanunkul, S.; Zhong, S.; MacKerell, A.D.; Wilks, A. Inhibition of the bacterial heme oxygenases from Pseudomonas aeruginosa and Neisseria meningitidis: Novel antimicrobial targets. J. Med. Chem. 2007, 50, 3804–3813. [Google Scholar] [CrossRef]
- Podkalicka, P.; Mucha, O.; Józkowicz, A.; Dulak, J.; Łoboda, A. Heme oxygenase inhibition in cancers: Possible tools and targets. Wspolczesna Onkol. 2017, 2, 23–32. [Google Scholar] [CrossRef]
- Jerry Kaneko, J. Porphyrins and the Porphyrias. Clin. Biochem. Domest. Anim. 2008, 241–258. [Google Scholar] [CrossRef]
- Yang, P.S.; Hsu, Y.C.; Lee, J.J.; Chen, M.J.; Huang, S.Y.; Cheng, S.P. Heme oxygenase-1 inhibitors induce cell cycle arrest and suppress tumor growth in thyroid cancer cells. Int. J. Mol. Sci. 2018, 19, 2502. [Google Scholar] [CrossRef]
- Vreman, H.J.; Ekstrand, B.C.; Stevenson, D.K. Selection of metalloporphyrin heme oxygenase inhibitors based on potency and photoreactivity. Pediatr. Res. 1993, 33, 195–200. [Google Scholar] [CrossRef]
- Wong, R.J.; Vreman, H.J.; Schulz, S.; Kalish, F.S.; Pierce, N.W.; Stevenson, D.K. In vitro inhibition of heme oxygenase isoenzymes by metalloporphyrins. J. Perinatol. 2011, 31, S35–S41. [Google Scholar] [CrossRef] [PubMed]
- Roman, G.; Riley, J.G.; Vlahakis, J.Z.; Kinobe, R.T.; Brien, J.F.; Nakatsu, K.; Szarek, W.A. Heme oxygenase inhibition by 2-oxy-substituted 1-(1H-imidazol-1-yl)-4-phenylbutanes: Effect of halogen substitution in the phenyl ring. Bioorganic Med. Chem. 2007, 15, 3225–3234. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.N.; Vlahakis, J.Z.; Roman, G.; Vukomanovic, D.; Szarek, W.A.; Nakatsu, K.; Jia, Z. Structural characterization of human heme oxygenase-1 in complex with azole-based inhibitors. J. Inorg. Biochem. 2010, 104, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Vlahakis, J.Z.; Kinobe, R.T.; Bowers, R.J.; Brien, J.F.; Nakatsu, K.; Szarek, W.A. Imidazole-dioxolane compounds as isozyme-selective heme oxygenase inhibitors. J. Med. Chem. 2006, 49, 4437–4441. [Google Scholar] [CrossRef] [PubMed]
- Kinobe, R.T.; Dercho, R.A.; Vlahakis, J.Z.; Brien, J.F.; Szarek, W.A.; Nakatsu, K. Inhibition of the enzymatic activity of heme oxygenases by azole-based antifungal drugs. J. Pharmacol. Exp. Ther. 2006, 319, 277–284. [Google Scholar] [CrossRef]
- Vlahakis, J.Z.; Vukomanovic, D.; Nakatsu, K.; Szarek, W.A. Selective inhibition of heme oxygenase-2 activity by analogs of 1-(4-chlorobenzyl)-2-(pyrrolidin-1-ylmethyl)-1H-benzimidazole (clemizole): Exploration of the effects of substituents at the N-1 position. Bioorganic Med. Chem. 2013, 21, 6788–6795. [Google Scholar] [CrossRef]
- Kong, X.; Vukomanovic, D.; Nakatsu, K.; Szarek, W.A. Structure-Activity Relationships of 1,2-Disubstituted Benzimidazoles: Selective Inhibition of Heme Oxygenase-2 Activity. ChemMedChem 2015, 10, 1435–1441. [Google Scholar] [CrossRef]
- Hom, K.; Heinzl, G.A.; Eakanunkul, S.; Lopes, P.E.M.; Xue, F.; MacKerell, A.D.; Wilks, A. Small molecule antivirulents targeting the iron-regulated heme oxygenase (HemO) of P. aeruginosa. J. Med. Chem. 2013, 56, 2097–2109. [Google Scholar] [CrossRef]
- O’Neill, M.J.; Wilks, A. The P. aeruginosa heme binding protein PhuS is a heme oxygenase titratable regulator of heme uptake. ACS Chem. Biol. 2013, 8, 1794–1802. [Google Scholar] [CrossRef]
- Marvig, R.L.; Damkiær, S.; Hossein Khademi, S.M.; Markussen, T.M.; Molin, S.; Jelsbak, L. Within-host evolution of Pseudomonas aeruginosa reveals adaptation toward iron acquisition from hemoglobin. MBio 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Giardina, B.J.; Shahzad, S.; Huang, W.; Wilks, A. Heme uptake and utilization by hypervirulent Acinetobacter baumannii LAC-4 is dependent on a canonical heme oxygenase (abHemO). Arch. Biochem. Biophys. 2019, 672, 108066. [Google Scholar] [CrossRef] [PubMed]
- Soldano, A.; Klinke, S.; Otero, L.H.; Rivera, M.; Catalano-Dupuy, D.L.; Ceccarelli, E.A. Structural and mutational analyses of the Leptospira interrogans virulence-related heme oxygenase provide insights into its catalytic mechanism. PLoS ONE 2017, 12, e0182535. [Google Scholar] [CrossRef] [PubMed]
- Brewitz, H.H.; Hagelueken, G.; Imhof, D. Structural and functional diversity of transient heme binding to bacterial proteins. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 683–697. [Google Scholar] [CrossRef] [PubMed]
- Heinzl, G.A.; Huang, W.; Yu, W.; Giardina, B.J.; Zhou, Y.; MacKerell, A.D.; Wilks, A.; Xue, F. Iminoguanidines as allosteric inhibitors of the iron-regulated heme oxygenase (HemO) of Pseudomonas aeruginosa. J. Med. Chem. 2016, 59, 6929–6942. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.E.; Simionatto, C.S.; Drummond, G.S.; Kappas, A. Tissue distribution and disposition of tin-protoporphyrin, a potent competitive inhibitor of heme oxygenase. J. Pharmacol. Exp. Ther. 1984, 228, 327–333. [Google Scholar]
- Vzorov, A.N.; Dixon, D.W.; Trommel, J.S.; Marzilli, L.G.; Compans, R.W. Inactivation of human immunodeficiency virus type 1 by porphyrins. Antimicrob. Agents Chemother. 2002, 46, 3917–3925. [Google Scholar] [CrossRef]
- Lin, L.; Hu, J. Inhibition of Hepadnavirus Reverse Transcriptase-ε RNA Interaction by Porphyrin Compounds. J. Virol. 2008, 82, 2305–2312. [Google Scholar] [CrossRef]
- Cheng, Y.; Tsou, L.K.; Cai, J.; Aya, T.; Dutschman, G.E.; Gullen, E.A.; Grill, S.P.; Chen, A.P.C.; Lindenbach, B.D.; Hamilton, A.D.; et al. A novel class of meso-tetrakis-porphyrin derivatives exhibits potent activities against hepatitis C virus genotype 1b replicons in vitro. Antimicrob. Agents Chemother. 2010, 54, 197–206. [Google Scholar] [CrossRef]
- Benati, F.J.; Lauretti, F.; Faccin, L.C.; Nodari, B.; Ferri, D.V.; Mantovani, M.S.; Linhares, R.E.C.; Nozawa, C. Effects of chlorophyllin on replication of poliovirus and bovine herpesvirus in vitro. Lett. Appl. Microbiol. 2009, 49, 791–795. [Google Scholar] [CrossRef]
- Kinobe, R.T.; Vlahakis, J.Z.; Vreman, H.J.; Stevenson, D.K.; Brien, J.F.; Szarek, W.A.; Nakatsu, K. Selectivity of imidazole-dioxolane compounds for in vitro inhibition of microsomal haem oxygenase isoforms. Br. J. Pharmacol. 2006, 147, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Vlahakis, J.Z.; Hum, M.; Rahman, M.N.; Jia, Z.; Nakatsu, K.; Szarek, W.A. Synthesis and evaluation of imidazole-dioxolane compounds as selective heme oxygenase inhibitors: Effect of substituents at the 4-position of the dioxolane ring. Bioorganic Med. Chem. 2009, 17, 2461–2475. [Google Scholar] [CrossRef] [PubMed]
- Amata, E.; Marrazzo, A.; Dichiara, M.; Modica, M.N.; Salerno, L.; Prezzavento, O.; Nastasi, G.; Rescifina, A.; Romeo, G.; Pittalà, V. Heme Oxygenase Database (HemeOxDB) and QSAR Analysis of Isoform 1 Inhibitors. ChemMedChem 2017, 12, 1873–1881. [Google Scholar] [CrossRef] [PubMed]
- Floresta, G.; Amata, E.; Gentile, D.; Romeo, G.; Marrazzo, A.; Pittalà, V.; Salerno, L.; Rescifina, A. Fourfold filtered statistical/computational approach for the identification of imidazole compounds as HO-1 inhibitors from natural products. Mar. Drugs 2019, 17, 113. [Google Scholar] [CrossRef]
- Barrangou, R.; Birmingham, A.; Wiemann, S.; Beijersbergen, R.L.; Hornung, V.; Smith, A.V.B. Advances in CRISPR-Cas9 genome engineering: Lessons learned from RNA interference. Nucleic Acids Res. 2015, 43, 3407–3419. [Google Scholar] [CrossRef]
- Cheng, T.L.; Chang, W.T. Construction of simple and efficient DNA vector-based short hairpin RNA expression systems for specific gene silencing in mammalian cells. Methods Mol. Biol. 2007, 408, 223–241. [Google Scholar] [CrossRef]
- O’Keefe, E.P. siRNAs and shRNAs: Tools for Protein Knockdown by Gene Silencing. Mater. Methods 2013, 3. [Google Scholar] [CrossRef]
- Horvath, P.; Barrangou, R. CRISPR/Cas, the immune system of Bacteria and Archaea. Science 2010, 327, 167–170. [Google Scholar] [CrossRef]
- Hsu, P.D.; Lander, E.S.; Zhang, F. Development and Applications of CRISPR-Cas9 for Genome Engineering. Cell 2014, 157, 1262–1278. [Google Scholar] [CrossRef]
- Mucha, O.; Podkalicka, P.; Czarnek, M.; Biela, A.; Mieczkowski, M.; Kachamakova-Trojanowska, N.; Stepniewski, J.; Jozkowicz, A.; Dulak, J.; Loboda, A. Pharmacological versus genetic inhibition of heme oxygenase-1—The comparison of metalloporphyrins, shRNA and CRISPR/Cas9 system. Acta Biochim. Pol. 2018, 65, 277–286. [Google Scholar] [CrossRef]
- Sass, G.; Leukel, P.; Schmitz, V.; Raskopf, E.; Ocker, M.; Neureiter, D.; Meissnitzer, M.; Tasika, E.; Tannapfel, A.; Tiegs, G. Inhibition of heme oxygenase 1 expression by small interfering RNA decreases orthotopic tumor growth in livers of mice. Int. J. Cancer 2008, 123, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Naito, Y.; Takagi, T.; Higashimura, Y. Heme oxygenase-1 and anti-inflammatory M2 macrophages. Arch. Biochem. Biophys. 2014, 564, 83–88. [Google Scholar] [CrossRef]
- Vijayan, V.; Wagener, F.A.D.T.G.; Immenschuh, S. The macrophage heme-heme oxygenase-1 system and its role in inflammation. Biochem. Pharmacol. 2018, 153, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Tzima, S.; Victoratos, P.; Kranidioti, K.; Alexiou, M.; Kollias, G. Myeloid heme oxygenase-1 regulates innate immunity and autoimmunity by modulating IFN-β production. J. Exp. Med. 2009, 206, 1167–1179. [Google Scholar] [CrossRef] [PubMed]
- Otterbein, L.E.; Bach, F.H.; Alam, J.; Soares, M.; Tao Lu, H.; Wysk, M.; Davis, R.J.; Flavell, R.A.; Choi, A.M.K. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat. Med. 2000, 6, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.-H.; Chen, H.-A.; Gau, R.-J.; Yen, J.-H.; Suen, J.-L. Heme Oxygenase-1-Expressing Dendritic Cells Promote Foxp3+ Regulatory T Cell Differentiation and Induce Less Severe Airway Inflammation in Murine Models. PLoS ONE 2016, 11, e0168919. [Google Scholar] [CrossRef] [PubMed]
- Kapturczak, M.H.; Wasserfall, C.; Brusko, T.; Campbell-Thompson, M.; Ellis, T.M.; Atkinson, M.A.; Agarwal, A. Heme oxygenase-1 modulates early inflammatory responses: Evidence from the heme oxygenase-1-deficient mouse. Am. J. Pathol. 2004, 165, 1045–1053. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, D.; Wang, J.; Li, Y.; Ma, D.; Chai, X.; Kang, Q. Identification of Heme Oxygenase-1 as a Novel Predictor of Hematopoietic Stem Cell Transplantation Outcomes in Acute Leukemia. Cell. Physiol. Biochem. 2016, 39, 1495–1502. [Google Scholar] [CrossRef]
- Immenschuh, S.; Shan, Y.; Kroll, H.; Santoso, S.; Wössmann, W.; Bein, G.; Bonkovsky, H.L. Marked hyperbilirubinemia associated with the heme oxygenase-1 gene promoter microsatellite polymorphism in a boy with autoimmune hemolytic anemia. Pediatrics 2007, 119. [Google Scholar] [CrossRef]
- Arnold, J.N.; Magiera, L.; Kraman, M.; Fearon, D.T. Tumoral immune suppression by macrophages expressing fibroblast activation protein-α and heme oxygenase-1. Cancer Immunol. Res. 2014, 2, 121–126. [Google Scholar] [CrossRef]
- Alaluf, E.; Vokaer, B.; Detavernier, A.; Azouz, A.; Splittgerber, M.; Carrette, A.; Boon, L.; Libert, F.; Soares, M.; Le Moine, A.; et al. Heme oxygenase-1 orchestrates the immunosuppressive program of tumor-associated macrophages. JCI Insight 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yang, H.; Yahui, P.; Tang, L.; Jin, J.; He, R.; Li, Y.; Zhang, S.; Li, L.; Zhou, Y.; et al. Overexpression of heme Oxygenase 1 causes cognitive decline and affects pathways for tauopathy in Mice. J. Alzheimer’s Dis. 2015, 43, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Stolt, C.; Schmidt, I.H.E.; Sayfart, Y.; Steinmetz, I.; Bast, A. Heme Oxygenase-1 and Carbon Monoxide Promote Burkholderia pseudomallei Infection. J. Immunol. 2016, 197, 834–846. [Google Scholar] [CrossRef] [PubMed]
- Goodman, A.I.; Choudhury, M.; Da Silva, J.L.; Schwartzman, M.L.; Abraham, N.G. Overexpression of the heme oxygenase gene in renal cell carcinoma. Proc. Soc. Exp. Biol. Med. 1997, 214, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Mayerhofer, M.; Florian, S.; Krauth, M.T.; Aichberger, K.J.; Bilban, M.; Marculescu, R.; Printz, D.; Fritsch, G.; Wagner, O.; Selzer, E.; et al. Identification of Heme Oxygenase-1 As a Novel BCR/ABL-Dependent Survival Factor in Chronic Myeloid Leukemia. Cancer Res. 2004, 64, 3148–3154. [Google Scholar] [CrossRef] [PubMed]
- Brouard, S.; Otterbein, L.E.; Anrather, J.; Tobiasch, E.; Bach, F.H.; Choi, A.M.K.; Soares, M.P. Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J. Exp. Med. 2000, 192, 1015–1025. [Google Scholar] [CrossRef]
- Berberat, P.O.; Dambrauskas, Z.; Gulbinas, A.; Giese, T.; Giese, N.; Künzli, B.; Autschbach, F.; Meuer, S.; Büchler, M.W.; Friess, H. Inhibition of heme oxygenase-1 increases responsiveness of pancreatic cancer cells to anticancer treatment. Clin. Cancer Res. 2005, 11, 3790–3798. [Google Scholar] [CrossRef]
- Hill, M.; Pereira, V.; Chauveau, C.; Zagani, R.; Remy, S.; Tesson, L.; Mazal, D.; Ubillos, L.; Brion, R.; Ashgar, K.; et al. Heme oxygenase-1 inhibits rat and human breast cancer cell proliferation: Mutual cross inhibition with indoleamine 2,3-dioxygenase. FASEB J. 2005, 19, 1957–1968. [Google Scholar] [CrossRef]
- Sunamura, M.; Duda, D.G.; Ghattas, M.H.; Lozonschi, L.; Motoi, F.; Yamauchi, J.I.; Matsuno, S.; Shibahara, S.; Abraham, N.G. Heme oxygenase-1 accelerates tumor angiogenesis of human pancreatic cancer. Angiogenesis 2003, 6, 15–24. [Google Scholar] [CrossRef]
- Nowis, D.; Legat, M.; Grzela, T.; Niderla, J.; Wilczek, E.; Wilczynski, G.M.; Głodkowska, E.; Mrówka, P.; Issat, T.; Dulak, J.; et al. Heme oxygenase-1 protects tumor cells against photodynamic therapy-mediated cytotoxicity. Oncogene 2006, 25, 3365–3374. [Google Scholar] [CrossRef]
- Andersen, M.H.; Sørensen, R.B.; Brimnes, M.K.; Svane, I.M.; Becker, J.C.; Straten, P.T. Identification of heme oxygenase-1-specific regulatory CD8+ T cells in cancer patients. J. Clin. Investig. 2009, 119, 2245–2256. [Google Scholar] [CrossRef] [PubMed]
- Funes, S.C.; Rios, M.; Escobar-Vera, J.; Kalergis, A.M. Implications of macrophage polarization in autoimmunity. Immunology 2018, 154, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Kongpetch, S.; Kukongviriyapan, V.; Prawan, A.; Senggunprai, L.; Kukongviriyapan, U.; Buranrat, B. Crucial role of heme oxygenase-1 on the sensitivity of cholangiocarcinoma cells to chemotherapeutic agents. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Jozkowicz, A.; Was, H.; Dulak, J. Heme Oxygenase-1 in Tumors: Is It a False Friend? Antioxid. Redox Signal. 2007, 9, 2099–2118. [Google Scholar] [CrossRef]
- Ciesla, M.; Marona, P.; Kozakowska, M.; Jez, M.; Seczynska, M.; Loboda, A.; Bukowska-Strakova, K.; Szade, A.; Walawender, M.; Kusior, M.; et al. Heme oxygenase-1 controls an HDAC4-MIR-206 pathway of oxidative stress in rhabdomyosarcoma. Cancer Res. 2016, 76, 5707–5718. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, W.; Ma, D.; Xiong, J.; Kuang, X.; Zhang, S.; Fang, Q.; Wang, J. Heme oxygenase-1 inhibition mediates Gas6 to enhance bortezomib-sensitivity in multiple myeloma via ERK/STAT3 axis. Aging 2020, 12, 6611–6629. [Google Scholar] [CrossRef]
- Fest, S.; Soldati, R.; Christiansen, N.M.; Zenclussen, M.L.; Kilz, J.; Berger, E.; Starke, S.; Lode, H.N.; Engel, C.; Zenclussen, A.C.; et al. Targeting of heme oxygenase-1 as a novel immune regulator of neuroblastoma. Int. J. Cancer 2016, 138, 2030–2042. [Google Scholar] [CrossRef]
- Salerno, L.; Pittalà, V.; Romeo, G.; Modica, M.N.; Marrazzo, A.; Siracusa, M.A.; Sorrenti, V.; Di Giacomo, C.; Vanella, L.; Parayath, N.N.; et al. Novel imidazole derivatives as heme oxygenase-1 (HO-1) and heme oxygenase-2 (HO-2) inhibitors and their cytotoxic activity in human-derived cancer cell lines. Eur. J. Med. Chem. 2015, 96, 162–172. [Google Scholar] [CrossRef]
- Wang, J.; Liu, P.; Xin, S.; Wang, Z.; Li, J. Nrf2 suppresses the function of dendritic cells to facilitate the immune escape of glioma cells. Exp. Cell Res. 2017, 360, 66–73. [Google Scholar] [CrossRef]
- Funes, S.C.; Ríos, M.; Gómez-Santander, F.; Fernández-Fierro, A.; Altamirano-Lagos, M.J.; Rivera-Perez, D.; Pulgar-Sepúlveda, R.; Jara, E.L.; Rebolledo-Zelada, D.; Villarroel, A.; et al. Tolerogenic dendritic cell transfer ameliorates systemic lupus erythematosus in mice. J Immunol. 2019, 158, 322–339. [Google Scholar] [CrossRef]
- DeTure, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System during Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Holtzman, D.M. Interplay between innate immunity and Alzheimer disease: APOE and TREM2 in the spotlight. Nat. Rev. Immunol. 2018, 18, 759–772. [Google Scholar] [CrossRef] [PubMed]
- Stahnke, T.; Stadelmann, C.; Netzler, A.; Brück, W.; Richter-Landsberg, C. Differential upregulation of heme oxygenase-1 (HSP32) in glial cells after oxidative stress and in demyelinating disorders. J. Mol. Neurosci. 2007, 32, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Ling, K.; Men, F.; Wang, W.C.; Zhou, Y.Q.; Zhang, H.W.; Ye, D.W. Carbon Monoxide and Its Controlled Release: Therapeutic Application, Detection, and Development of Carbon Monoxide Releasing Molecules (CORMs). J. Med. Chem. 2018, 61, 2611–2635. [Google Scholar] [CrossRef]
- Gupta, A.; Lacoste, B.; Pistel, P.J.; Ingram, D.K.; Hamel, E.; Alaoui-Jamali, M.A.; Szarek, W.A.; Vlahakis, J.Z.; Jie, S.; Song, W.; et al. Neurotherapeutic effects of novel HO-1 inhibitors in vitro and in a transgenic mouse model of Alzheimer’s disease. J. Neurochem. 2014, 131, 778–790. [Google Scholar] [CrossRef]
- Di Domenico, F.; Sultana, R.; Tiu, G.F.; Scheff, N.N.; Perluigi, M.; Cini, C.; Butterfield, D.A. Protein levels of heat shock proteins 27, 32, 60, 70, 90 and thioredoxin-1 in amnestic mild cognitive impairment: An investigation on the role of cellular stress response in the progression of Alzheimer disease. Brain Res. 2010, 1333, 72–81. [Google Scholar] [CrossRef]
- Song, W.; Su, H.; Song, S.; Paudel, H.K.; Schipper, H.M. Over-expression of heme oxygenase-1 promotes oxidative mitochondrial damage in rat astroglia. J. Cell. Physiol. 2006, 206, 655–663. [Google Scholar] [CrossRef]
- Fernández-Mendívil, C.; Luengo, E.; Trigo-Alonso, P.; García-Magro, N.; Negredo, P.; López, M.G. Protective role of microglial HO-1 blockade in aging: Implication of iron metabolism. Redox Biol. 2020, 38, 101789. [Google Scholar] [CrossRef]
- Cressatti, M.; Song, W.; Turk, A.Z.; Garabed, L.R.; Benchaya, J.A.; Galindez, C.; Liberman, A.; Schipper, H.M. Glial HMOX1 expression promotes central and peripheral α-synuclein dysregulation and pathogenicity in parkinsonian mice. Glia 2019, 67, 1730–1744. [Google Scholar] [CrossRef]
- Yet, S.-F.; Tian, R.; Layne, M.D.; Wang, Z.Y.; Maemura, K.; Solovyeva, M.; Ith, B.; Melo, L.G.; Zhang, L.; Ingwall, J.S.; et al. Cardiac-Specific Expression of Heme Oxygenase-1 Protects Against Ischemia and Reperfusion Injury in Transgenic Mice. Circ. Res. 2001, 89, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Araujo, J.A.; Zhang, M.; Yin, F. Heme oxygenase-1, oxidation, inflammation, and atherosclerosis. Front. Pharmacol. 2012, 3, 119. [Google Scholar] [CrossRef] [PubMed]
- Wiesel, P.; Patel, A.P.; DiFonzo, N.; Marria, P.B.; Sim, C.U.; Pellacani, A.; Maemura, K.; LeBlanc, B.W.; Marino, K.; Doerschuk, C.M.; et al. Endotoxin-Induced Mortality Is Related to Increased Oxidative Stress and End-Organ Dysfunction, Not Refractory Hypotension, in Heme Oxygenase-1–Deficient Mice. Circulation 2000, 102, 3015–3022. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.W.; Hall, S.R.; Perrella, M.A. Role of haem oxygenase-1 in microbial host defence. Cell. Microbiol. 2009, 11, 199–207. [Google Scholar] [CrossRef]
- Espinoza, J.A.; González, P.A.; Kalergis, A.M. Modulation of Antiviral Immunity by Heme Oxygenase-1. Am. J. Pathol. 2017, 187, 487–493. [Google Scholar] [CrossRef]
- Tseng, C.K.; Lin, C.K.; Wu, Y.H.; Chen, Y.H.; Chen, W.C.; Young, K.C.; Lee, J.C. Human heme oxygenase 1 is a potential host cell factor against dengue virus replication. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Cummins, N.W.; Weaver, E.A.; May, S.M.; Croatt, A.J.; Foreman, O.; Kennedy, R.B.; Poland, G.A.; Barry, M.A.; Nath, K.A.; Badley, A.D. Heme oxygenase-1 regulates the immune response to influenza virus infection and vaccination in aged mice. FASEB J. 2012, 26, 2911–2918. [Google Scholar] [CrossRef]
- Espinoza, J.A.; León, M.A.; Céspedes, P.F.; Gómez, R.S.; Canedo-Marroquín, G.; Riquelme, S.A.; Salazar-Echegarai, F.J.; Blancou, P.; Simon, T.; Anegon, I.; et al. Heme Oxygenase-1 Modulates Human Respiratory Syncytial Virus Replication and Lung Pathogenesis during Infection. J. Immunol. 2017, 199, 212–223. [Google Scholar] [CrossRef]
- Ibáñez, F.J.; Farías, M.A.; Retamal-Díaz, A.; Espinoza, J.A.; Kalergis, A.M.; González, P.A. Pharmacological induction of heme oxygenase-1 impairs nuclear accumulation of herpes simplex virus capsids upon infection. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef]
- Riquelme, S.A.; Carreño, L.J.; Espinoza, J.A.; Mackern-Oberti, J.P.; Alvarez-Lobos, M.M.; Riedel, C.A.; Bueno, S.M.; Kalergis, A.M. Modulation of antigen processing by haem-oxygenase 1. Implications on inflammation and tolerance. J. Immunol. 2016, 149, 1–12. [Google Scholar] [CrossRef]
- Blancou, P.; Tardif, V.; Simon, T.; Rémy, S.; Carreño, L.; Kalergis, A.; Anegon, I. Immunoregulatory properties of heme oxygenase-1. Methods Mol. Biol. 2011, 677, 247–268. [Google Scholar] [CrossRef] [PubMed]
- Hayek, I.; Schatz, V.; Bogdan, C.; Jantsch, J.; Lührmann, A. Mechanisms controlling bacterial infection in myeloid cells under hypoxic conditions. Cell. Mol. Life Sci. 2020, 1, 3. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Deshane, J.S.; Crossman, D.K.; Bolisetty, S.; Yan, B.S.; Kramnik, I.; Agarwal, A.; Steyn, A.J.C. Heme oxygenase-1-derived carbon monoxide induces the Mycobacterium tuberculosis dormancy regulon. J. Biol. Chem. 2008, 283, 18032–18039. [Google Scholar] [CrossRef] [PubMed]
- Andrade, B.B.; Pavan Kumar, N.; Mayer-Barber, K.D.; Barber, D.L.; Sridhar, R.; Rekha, V.V.B.; Jawahar, M.S.; Nutman, T.B.; Sher, A.; Babu, S. Plasma Heme Oxygenase-1 Levels Distinguish Latent or Successfully Treated Human Tuberculosis from Active Disease. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Chinta, K.C.; Rahman, M.A.; Saini, V.; Glasgow, J.N.; Reddy, V.P.; Lever, J.M.; Nhamoyebonde, S.; Leslie, A.; Wells, R.M.; Traylor, A.; et al. Microanatomic Distribution of Myeloid Heme Oxygenase-1 Protects against Free Radical-Mediated Immunopathology in Human Tuberculosis. Cell Rep. 2018, 25, 1938–1952. [Google Scholar] [CrossRef]
- Costa, D.L.; Namasivayam, S.; Amaral, E.P.; Arora, K.; Chao, A.; Mittereder, L.R.; Maiga, M.; Boshoff, H.I.; Barry, C.E.; Goulding, C.W.; et al. Pharmacological inhibition of host heme oxygenase-1 suppresses mycobacterium tuberculosis infection in vivo by a mechanism dependent on T lymphocytes. MBio 2016, 7. [Google Scholar] [CrossRef]
- Abdalla, M.Y.; Ahmad, I.M.; Switzer, B.; Britigan, B.E. Induction of heme oxygenase-1 contributes to survival of Mycobacterium abscessus in human macrophages-like THP-1 cells. Redox Biol. 2015, 4, 328–339. [Google Scholar] [CrossRef][Green Version]
- Mitterstiller, A.M.; Haschka, D.; Dichtl, S.; Nairz, M.; Demetz, E.; Talasz, H.; Soares, M.P.; Einwallner, E.; Esterbauer, H.; Fang, F.C.; et al. Heme oxygenase 1 controls early innate immune response of macrophages to Salmonella Typhimurium infection. Cell. Microbiol. 2016, 18, 1374–1389. [Google Scholar] [CrossRef]
- Czaikoski, P.G.; Nascimento, D.C.; Sônego, F.; De Freitas, A.; Turato, W.M.; De Carvalho, M.A.; Santos, R.S.; De Oliveira, G.P.; Dos Santos Samary, C.; Tefe-Silva, C.; et al. Heme oxygenase inhibition enhances neutrophil migration into the bronchoalveolar spaces and improves the outcome of murine pneumonia-induced sepsis. Shock 2013, 39, 389–396. [Google Scholar] [CrossRef]
- Short, E.E.; Caminade, C.; Thomas, B.N. Climate Change Contribution to the Emergence or Re-Emergence of Parasitic Diseases. Infect. Dis. Res. Treat. 2017, 10, 117863361773229. [Google Scholar] [CrossRef]
- Kutzer, M.A.M.; Armitage, S.A.O. Maximising fitness in the face of parasites: A review of host tolerance. Zoology 2016, 119, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Campbell, N.K.; Williams, D.G.; Fitzgerald, H.K.; Barry, P.J.; Cunningham, C.C.; Nolan, D.P.; Dunne, A. Trypanosoma brucei Secreted Aromatic Ketoacids Activate the Nrf2/HO-1 Pathway and Suppress Pro-inflammatory Responses in Primary Murine Glia and Macrophages. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.C.M.C.; Travassos, L.H.; Paiva, C.N.; Bozza, M.T. Heme oxygenase-1 in protozoan infections: A tale of resistance and disease tolerance. PLoS Pathog. 2020, 16, e1008599. [Google Scholar] [CrossRef] [PubMed]
- Seixas, E.; Gozzelino, R.; Chora, Â.; Ferreira, A.; Silva, G.; Larsen, R.; Rebelo, S.; Penido, C.; Smith, N.R.; Coutinho, A.; et al. Heme oxygenase-1 affords protection against noncerebral forms of severe malaria. Proc. Natl. Acad. Sci. USA 2009, 106, 15837–15842. [Google Scholar] [CrossRef]
- Singh, N.; Ahmad, Z.; Baid, N.; Kumar, A. Host heme oxygenase-1: Friend or foe in tackling pathogens? IUBMB Life 2018, 70, 869–880. [Google Scholar] [CrossRef]
- Walther, M.; de Caul, A.; Aka, P.; Njie, M.; Amambua-Ngwa, A.; Walther, B.; Predazzi, I.M.; Cunnington, A.; Deininger, S.; Takem, E.N.; et al. HMOX1 gene promoter alleles and high HO-1 levels are associated with severe malaria in Gambian children. PLoS Pathog. 2012, 8. [Google Scholar] [CrossRef]
- Luz, N.F.; Andrade, B.B.; Feijó, D.F.; Araújo-Santos, T.; Carvalho, G.Q.; Andrade, D.; Abánades, D.R.; Melo, E.V.; Silva, A.M.; Brodskyn, C.I.; et al. Heme Oxygenase-1 Promotes the Persistence of Leishmania chagasi Infection. J. Immunol. 2012, 188, 4460–4467. [Google Scholar] [CrossRef]
- Wisplinghoff, H.; Seifert, H.; Tallent, S.M.; Bischoff, T.; Wenzel, R.P.; Edmond, M.B. Nosocomial bloodstream infections in pediatric patients in United States hospitals: Epidemiology, clinical features and susceptibilities. Pediatr. Infect. Dis. J. 2003, 22, 686–691. [Google Scholar] [CrossRef]
- Pendrak, M.L.; Yan, S.S.; Roberts, D.D. Sensing the host environment: Recognition of hemoglobin by the pathogenic yeast Candida albicans. Arch. Biochem. Biophys. 2004, 426, 148–156. [Google Scholar] [CrossRef]
- Mackern-Oberti, J.P.; Obreque, J.; Méndez, G.P.; Llanos, C.; Kalergis, A.M. Carbon monoxide inhibits T cell activation in target organs during systemic lupus erythematosus. Clin. Exp. Immunol. 2015, 182, 1–13. [Google Scholar] [CrossRef]
- Basuroy, S.; Bhattacharya, S.; Tcheranova, D.; Qu, Y.; Regan, R.F.; Leffler, C.W.; Parfenova, H. HO-2 provides endogenous protection against oxidative stress and apoptosis caused by TNF-α in cerebral vascular endothelial cells. Am. J. Physiol. Cell Physiol. 2006, 291, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Syapin, P.J. Regulation of haeme oxygenase-1 for treatment of neuroinflammation and brain disorders. Br. J. Pharmacol. 2008, 155, 623–640. [Google Scholar] [CrossRef] [PubMed]
- Morgan, D.; Gordon, M.N.; Tan, J.; Wilcock, D.; Rojiani, A.M. Dynamic Complexity of the Microglial Activation Response in Transgenic Models of Amyloid Deposition: Implications for Alzheimer Therapeutics. J. Neuropathol. Exp. Neurol. 2005, 64, 743–753. [Google Scholar] [CrossRef] [PubMed]
- López-Herce, J.; Borrego, R.; Bustinza, A.; Carrillo, A. Elevated carboxyhemoglobin associated with sodium nitroprusside treatment. Intensive Care Med. 2005, 31, 1235–1238. [Google Scholar] [CrossRef]
- The Nobel Prize in Chemistry 2020. Available online: https://www.nobelprize.org/prizes/chemistry/2020/summary/ (accessed on 9 October 2020).


| HO System Inhibitors | Name | Inhibitors Characteristics | References |
|---|---|---|---|
| First generation inhibitors | Metallo-protoporphyrins: Sn-, Zn-, Mn- |
| [23,33,38,51,52,53] |
| Metallo- mesoporphyrins: Cr- and Mn- |
| ||
| Second generation inhibitors | Azalanstat-derived imidazole-dioxolane compounds |
| [38,54,55] |
| Imidazole-derived antifungal agents: ketoconazole, terconazole, and sulconazole |
| [51,56,57] | |
| Clemizole and derive compounds |
| [58,59] | |
| Small molecules inhibiting microbial HO | Small antimicrobial molecules against heme oxygenase (HemO) expressed by microbes. |
| [48,60,61,62,63,64,65,66] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Fierro, A.; Funes, S.C.; Rios, M.; Covián, C.; González, J.; Kalergis, A.M. Immune Modulation by Inhibitors of the HO System. Int. J. Mol. Sci. 2021, 22, 294. https://doi.org/10.3390/ijms22010294
Fernández-Fierro A, Funes SC, Rios M, Covián C, González J, Kalergis AM. Immune Modulation by Inhibitors of the HO System. International Journal of Molecular Sciences. 2021; 22(1):294. https://doi.org/10.3390/ijms22010294
Chicago/Turabian StyleFernández-Fierro, Ayleen, Samanta C. Funes, Mariana Rios, Camila Covián, Jorge González, and Alexis M. Kalergis. 2021. "Immune Modulation by Inhibitors of the HO System" International Journal of Molecular Sciences 22, no. 1: 294. https://doi.org/10.3390/ijms22010294
APA StyleFernández-Fierro, A., Funes, S. C., Rios, M., Covián, C., González, J., & Kalergis, A. M. (2021). Immune Modulation by Inhibitors of the HO System. International Journal of Molecular Sciences, 22(1), 294. https://doi.org/10.3390/ijms22010294




