Abstract
Synovial mesenchymal stem cell (SMSC) is the promising cell source of cartilage regeneration but has several issues to overcome such as limited cell proliferation and heterogeneity of cartilage regeneration ability. Previous reports demonstrated that basic fibroblast growth factor (bFGF) can promote proliferation and cartilage differentiation potential of MSCs in vitro, although no reports show its beneficial effect in vivo. The purpose of this study is to investigate the promoting effect of bFGF on cartilage regeneration using human SMSC in vivo. SMSCs were cultured with or without bFGF in a growth medium, and 2 × 105 cells were aggregated to form a synovial pellet. Synovial pellets were implanted into osteochondral defects induced in the femoral trochlea of severe combined immunodeficient mice, and histological evaluation was performed after eight weeks. The presence of implanted SMSCs was confirmed by the observation of human vimentin immunostaining-positive cells. Interestingly, broad lacunae structures and cartilage substrate stained by Safranin-O were observed only in the bFGF (+) group. The bFGF (+) group had significantly higher O’Driscoll scores in the cartilage repair than the bFGF (−) group. The addition of bFGF to SMSC growth culture may be a useful treatment option to promote cartilage regeneration in vivo.
1. Introduction
Articular cartilage has a poor self-healing ability and the conventional surgical treatments and cell therapies for cartilage injuries, such as bone marrow stimulation, autologous osteochondral transplantation, and autologous chondrocyte implantation (ACI), have not yielded full recovery of normal cartilage [1]. Furthermore, ACI requires two-step surgery and the sacrifice of normal cartilage tissue to supply new cartilaginous tissue to the defect. Therefore, cartilage regeneration using mesenchymal stem cells (MSCs) has been widely researched [2,3]. Synovial MSCs (SMSCs) are an attractive cell source for cartilage regeneration because they are easily collected as surplus tissue during joint surgery and show favorable chondrogenesis and proliferation potential both in vitro [4,5] and in vivo [6,7]. During the development of commercialization of an allogenic SMSC bank for cartilage repair, we have reported that SMSCs obtained even from young patients showed a large variation in their proliferation and cartilage differentiation potentials [8]. In fact, our human clinical trial revealed that autologous SMSC implantation for the repair of a cartilage defect failed to recover full articular cartilage [9]. Therefore, various pretreatment methods to enhance the cartilage differentiation potential of MSCs have been reported [10,11,12]; we showed that a low oxygen tension prevented cellular senescence and promoted the proliferative and chondrogenic differentiation capacity of human SMSCs in vitro [13]. However, none of these reports succeed to demonstrate the positive effects in vivo. The basic fibroblast growth factor (bFGF) has been widely reported to enhance the potential of proliferation and cartilaginous differentiation of MSCs in vitro [14,15,16]. Studies of embryonic, neural, and bone marrow stem cells reported that bFGF regulates undifferentiated state and multipotent differentiation of stem cells through the fibroblast growth factor receptor 3 (FGFR3)-mediated signaling [14,17,18]. FGFR3 is known as one of the markers of mesenchymal pre-cartilaginous stem cells and was detected at the margins of the cartilage nodules of synovial chondromatosis and SMSCs [19,20,21]. However, no positive effects have been documented in vivo. We hypothesized that SMSCs pretreated with bFGF may demonstrate enhanced cartilaginous differentiation in vivo. The purpose of this study was to investigate whether bFGF can promote cartilage repair using human SMSCs in vivo.
2. Results
2.1. Proliferative Capacity and Cellular Morphology of SMSCs Cultured in the Presence or Absence of bFGF
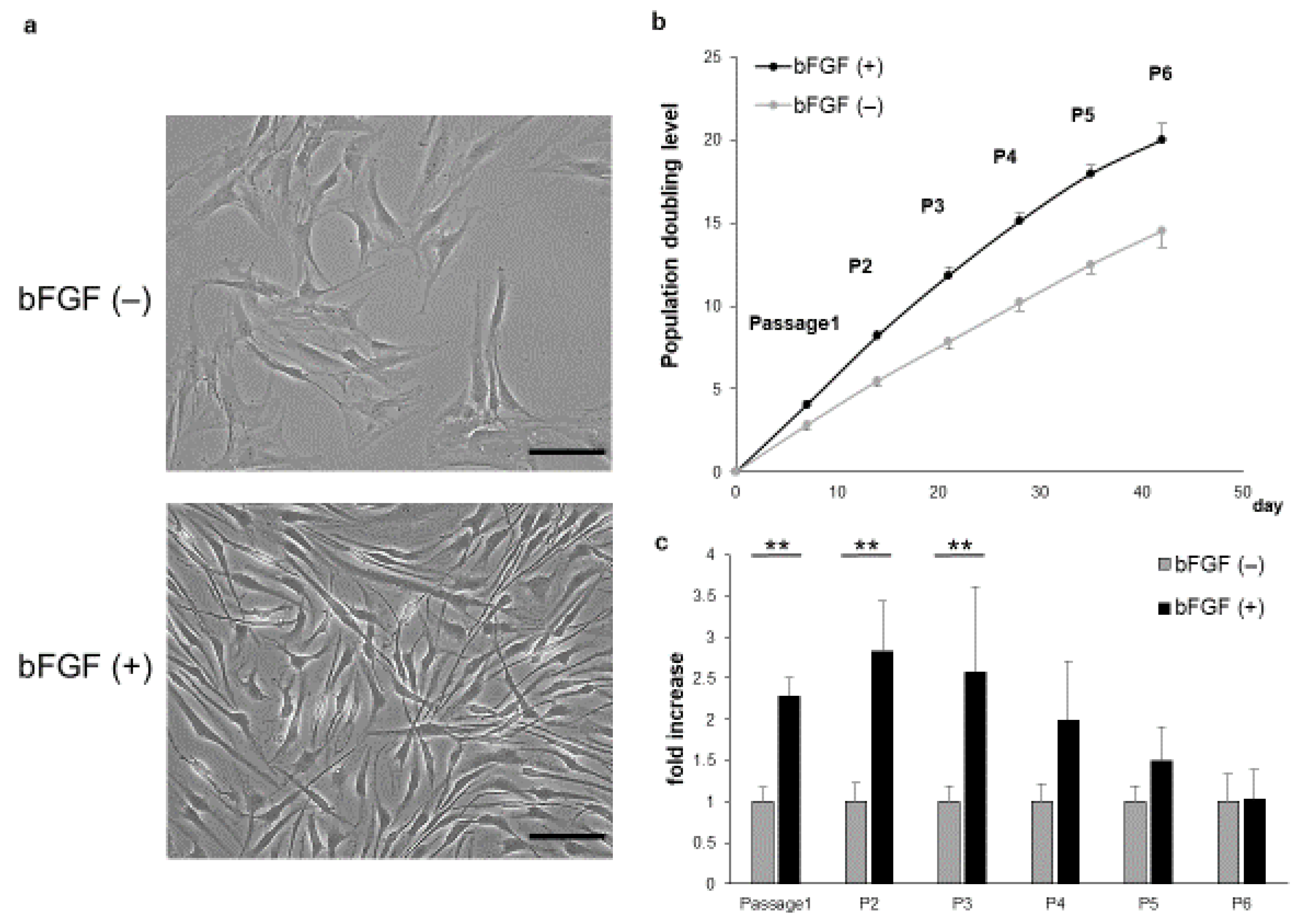
SMSCs of the bFGF (+) group showed a shrunken cytoplasm and enhanced proliferation compared with those of the bFGF (−) group (Figure 1a). In addition, the bFGF (+) group maintained higher growth rates than the bFGF (−) group (Figure 1b). The relative proliferation rate of SMSCs from the bFGF (+) group compared with that from the bFGF (−) group was significantly higher from passages 1 to 3 [P1: bFGF (−) vs. bFGF (+), 1.0 ± 0.18 vs. 2.3 ± 0.24-fold; p = 0.0075; P2: bFGF (−) vs. bFGF (+), 1.0 ± 0.23 vs. 2.8 ± 0.62-fold; p = 0.0075; P3: bFGF (−) vs. bFGF (+), 1.0 ± 0.19 vs. 2.6 ± 1.0-fold; p = 0.0075]. However, the difference between the two groups became non-significant after passage 4 (Figure 1c).
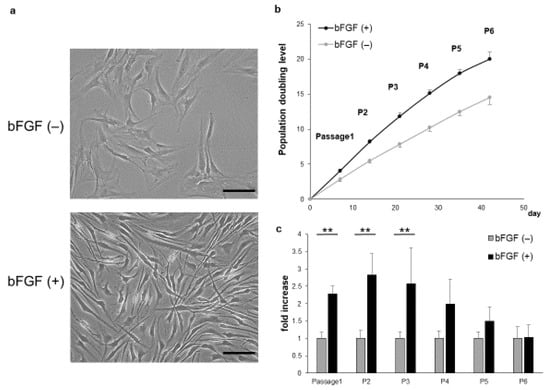
Figure 1.
Effect of bFGF on cell proliferation and morphology. (a) The morphology of SMSCs was observed under a light microscope at passage 3 (bar = 100 µm). (b) Population doubling level of SMSCs (patients #1 to #5). (c) Cell counts of SMSCs in the bFGF (+) group at each passage relative to those of the bFGF (−) group (patients #1 to #5). ** p < 0.01. bFGF, basic fibroblast growth factor; SMSCs, synovial mesenchymal stem cells.
2.2. Expression of FGFR3 Protein
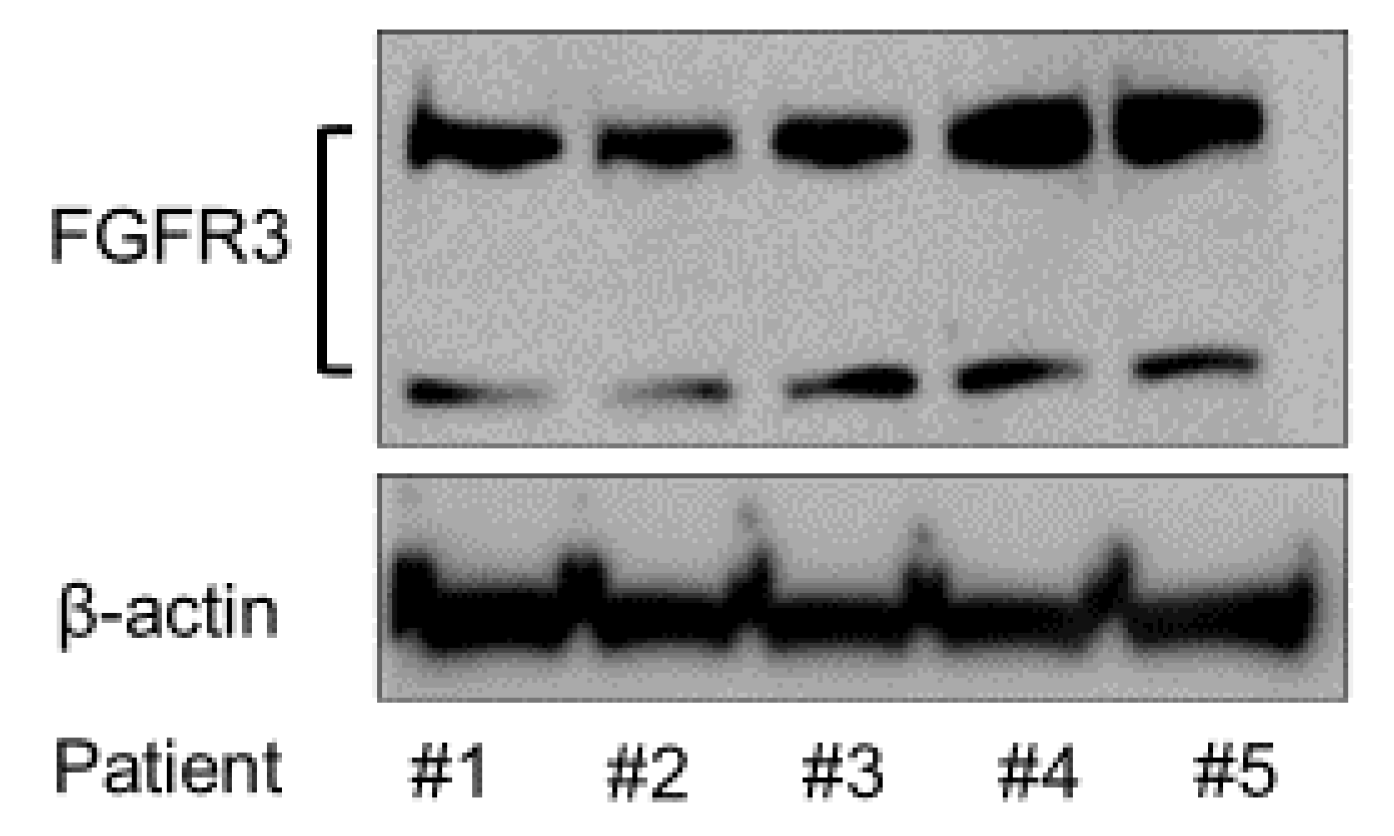
To analyze the expression of FGFR3 in the SMSCs from different patients, Western blotting was conducted using anti-FGFR3 antibody. The FGFR3 protein expression was confirmed in all of the patients’ SMSCs by Western blotting (Figure 2).
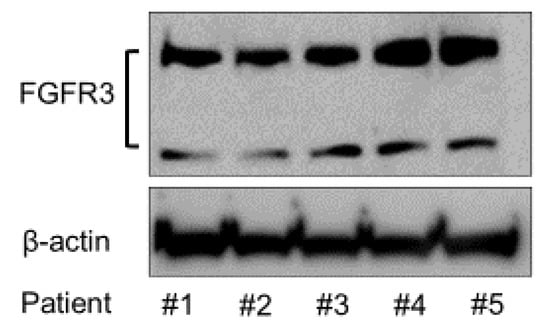
Figure 2.
Expression of FGFR3 protein in the SMSCs of all the patients.
2.3. Expression of Cell Surface Markers
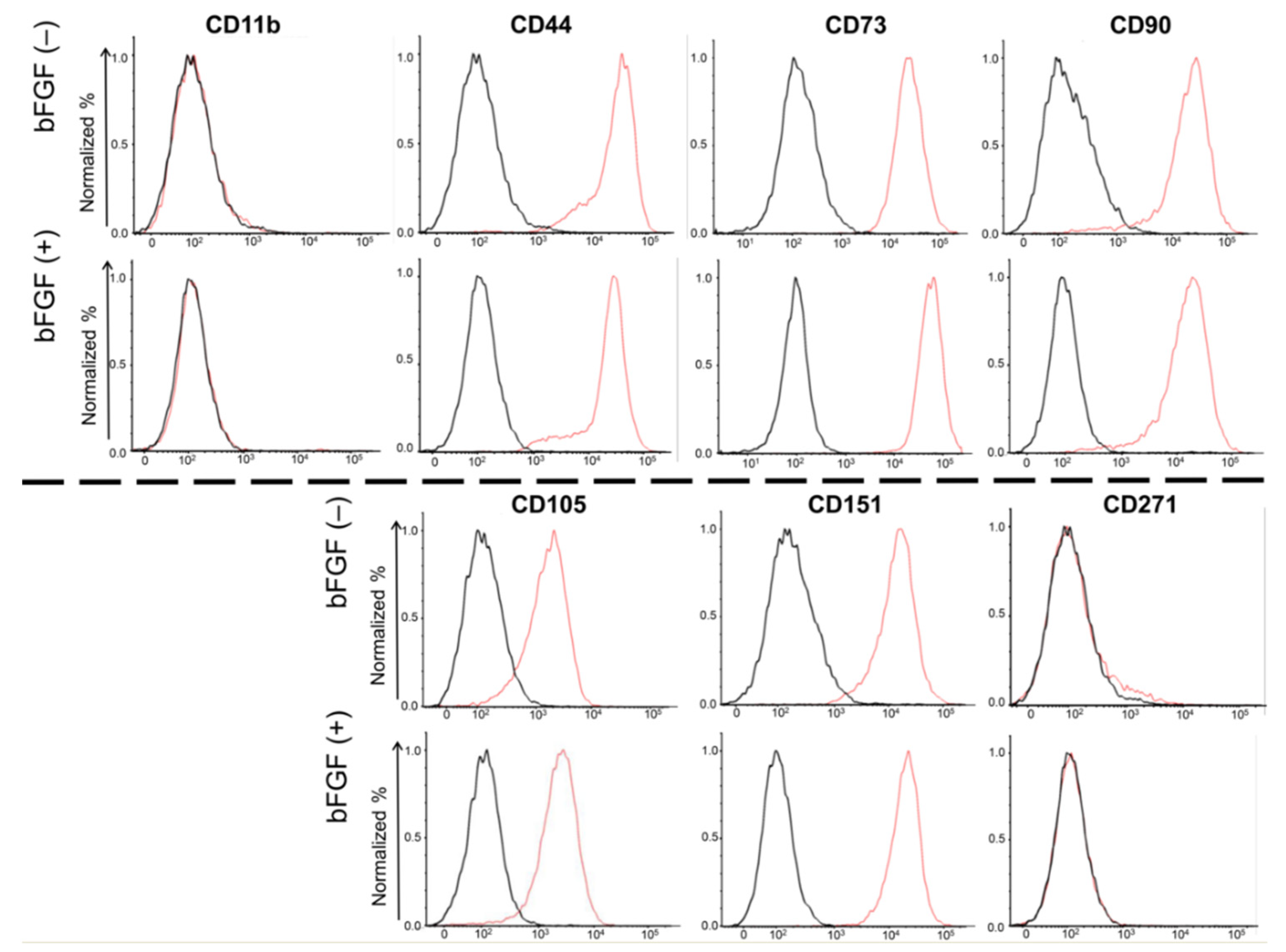
To evaluate the effects of bFGF on the expression of cell surface markers in SMSCs, fluorescence-activated cell sorting (FACS) was conducted for four mesenchymal surface markers (CD44, CD73, CD90, and CD105), two mesenchymal negative markers (CD11b, CD271), and one chondrocyte surface marker (CD151) (Figure 3). Almost 100% of the SMSCs expressed all four mesenchymal surface markers and one chondrocyte marker, and almost none of SMSCs expressed mesenchymal negative markers. Moreover, their expression levels were not affected by bFGF.
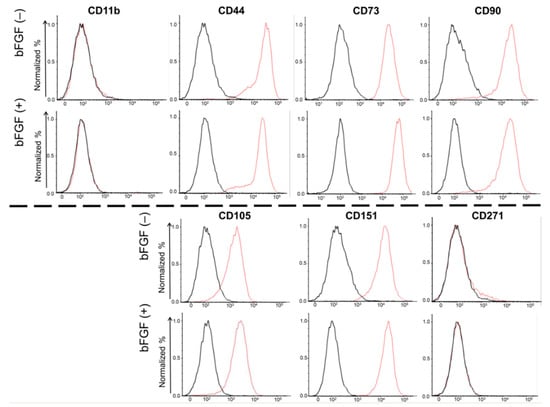
Figure 3.
Cell surface expression of four mesenchymal surface markers (CD44, CD73, CD90, and CD105), two mesenchymal negative markers (CD11b, CD271), and one chondrocyte surface marker (CD151). SMSCs were isolated from patient #5. The results of the FACS analysis of synovial SMSCs are shown. The red line indicates the binding of the specific antibody. The black line indicates the isotypic control. SMSCs, synovial mesenchymal stem cells; FACS, fluorescence-activated cell sorting.
2.4. The Effect of bFGF on the Chondrogenesis of SMSCs in a Three-Dimensional (3D) Chondrogenic Culture
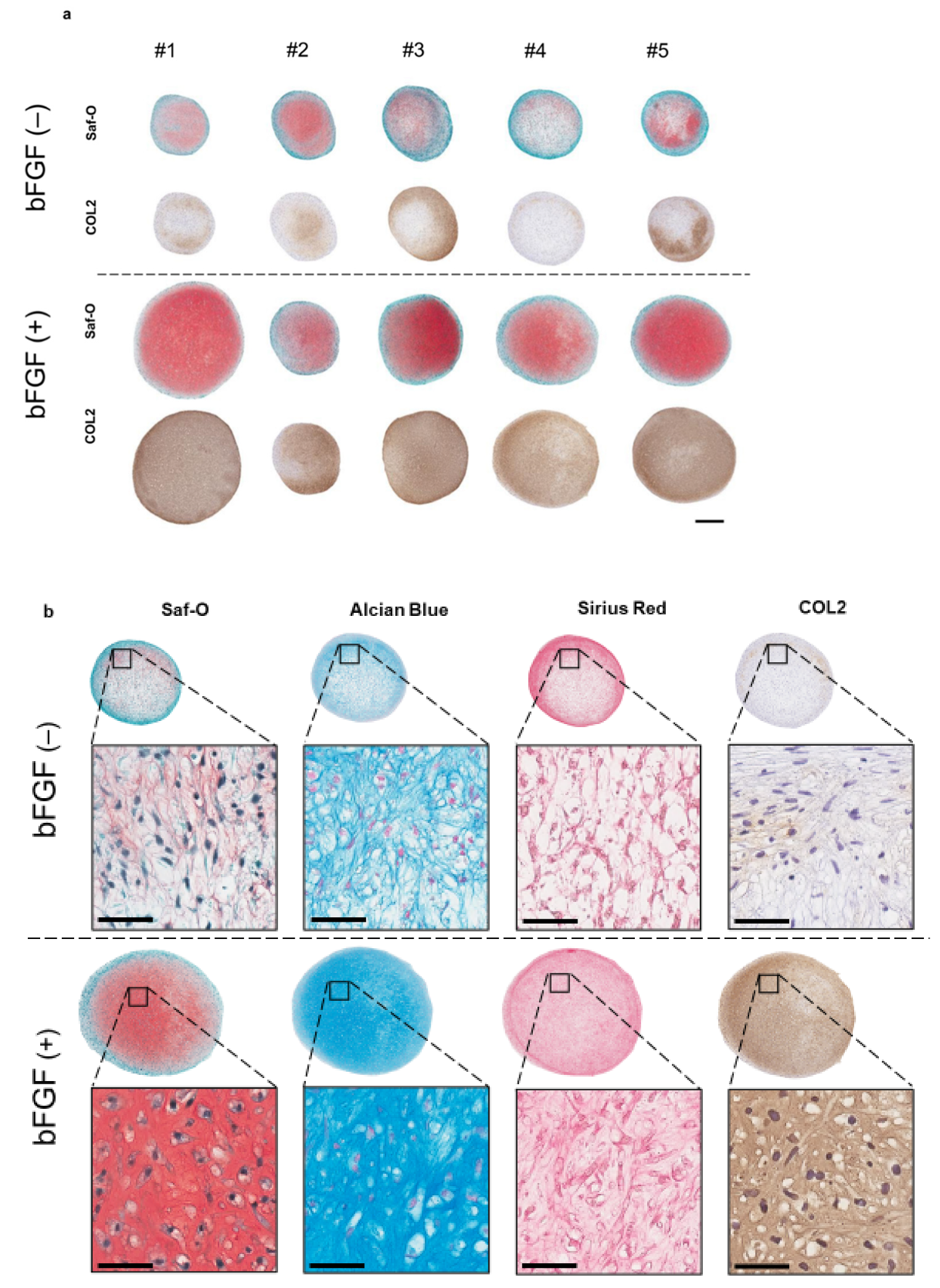
To assess the effect of the addition of bFGF in the cell-proliferation phase on cartilaginous tissue formation, a 3D pellet culture was established. After four weeks of chondrogenic induction, histology, the expression of cartilage differentiation-related genes, and extracellular matrix (ECM) production, were evaluated. Synovial pellets of the bFGF (+) group grew larger and were more strongly stained with Safranin-O (Saf-O) and immunostained for collagen type II (COL2) than those of the bFGF (−) group (n = 5 per group) (Figure 4a). A similar tendency was observed in all patients. Moreover, pellets of the bFGF (+) group showed hyaline cartilage-like differentiation with chondrocyte-like round-shaped cells in the lacuna, abundant collagen stained with Sirius Red and abundant ECM stained with Saf-O and Alcian Blue (Figure 4b).
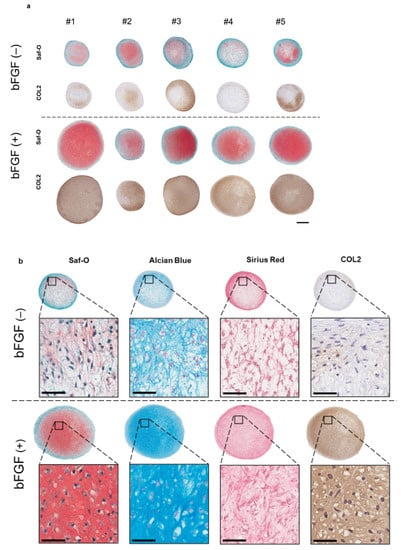
Figure 4.
Effect of bFGF on the chondrogenesis of SMSCs in 3D chondrogenic culture: histological evaluation. Synovial pellets from both the bFGF (+) and bFGF (−) groups cultured in chondrogenic medium (without bFGF) for 4 weeks. (a) Images of Saf-O staining and immunostaining for COL2 (patients #1 to #5) (bar = 500 µm). (b) High-magnification images of Saf-O, Arcian Blue, Sirius Red, and COL2 staining (patient #4) (bar = 50 µm). bFGF, basic fibroblast growth factor; SMSCs, synovial mesenchymal stem cells; 3D, three-dimensional; Saf-O, Safranin-O; COL2, collagen type II.
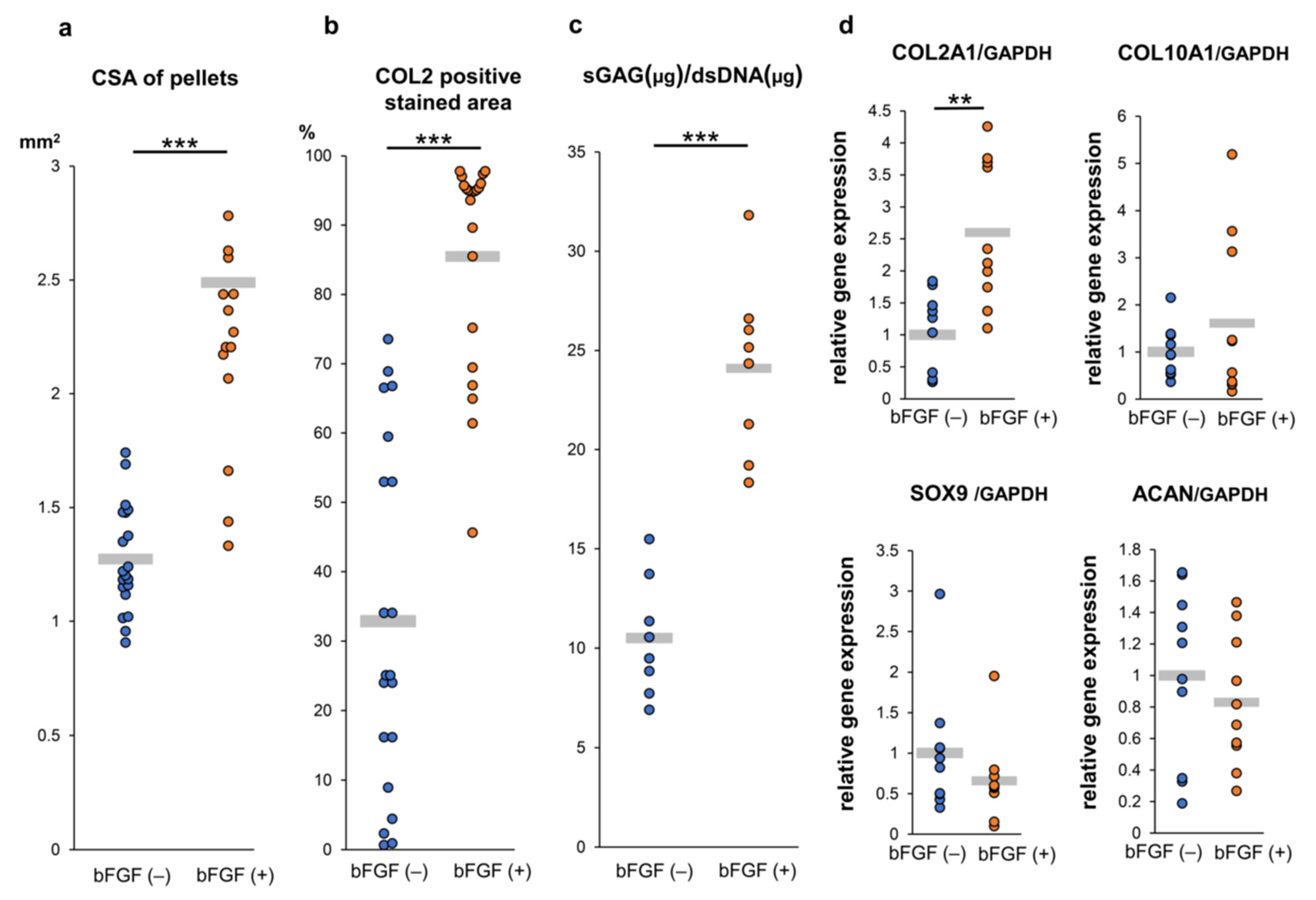
To assess the effect of pretreatment with bFGF on cartilaginous tissue formation of SMSC in vitro, a quantitative evaluation of the cross-section area (CSA) of pellets demonstrated that pellets of the bFGF (+) group grew significantly larger than those of the bFGF (−) group (n = 20 per group) [bFGF (−) vs. bFGF (+), 1.3 ± 0.2 vs. 2.5 ± 0.6 mm2; p = 0.00000368] (Figure 5a). A semi-quantitative evaluation of COL2 staining revealed that the bFGF (+) group had a significantly larger COL2-positive area than the bFGF (−) group (n = 20 per group) [bFGF (−) vs. bFGF (+), 32.9 ± 24.4% vs. 85.5 ± 14.9%; p = 0.0000237] (Figure 5b). In addition, the bFGF (+) group had a significantly higher sulfated glycosaminoglycan (sGAG) content than the bFGF (−) group (n = 8 per group) [bFGF (−) vs. bFGF (+), 10.5 ± 2.7 vs. 23.3 ± 4.5 μg/dsDNA (μg); p = 0.0000823] (Figure 5c). The effects of bFGF on the expression of cartilage differentiation-related genes [collagen type II alpha 1 (COL2A1), collagen type X alpha 1 (COL10A1), sex-determining region Y-box 9 (SOX9), and aggrecan (ACAN)] were analyzed by quantitative real-time polymerase chain reaction (qRT-PCR) analysis. COL2A1 gene expression in pellets from the bFGF (+) group was significantly higher than that detected in pellets from the bFGF (−) group [bFGF (−) vs. bFGF (+), 1.0 ± 0.6 vs. 2.6 ± 1.1-fold; p = 0.0015]. Conversely, the expression of other related genes (COL10A1, SOX9, and ACAN) was not significantly affected by bFGF administration (Figure 5d).
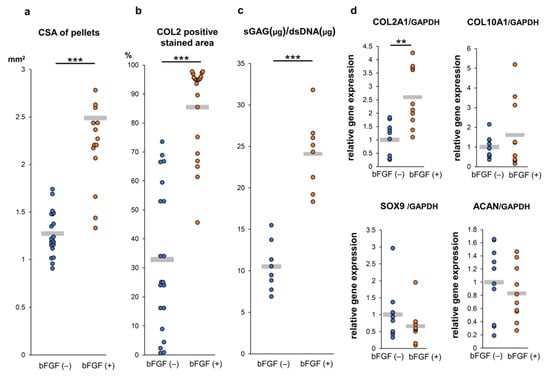
Figure 5.
Effect of bFGF on the chondrogenesis of SMSCs in 3D chondrogenic culture: quantitative evaluation. Synovial pellets from both the bFGF (+) and bFGF (−) groups cultured in chondrogenic medium (without bFGF) for 4 weeks. (a) The cross-section area (CSA) of pellets (20 pellets from patients #1 to #5). (b) Quantitative area analysis of immunostaining for COL2 (20 pellets from patients #1 to #5). (c) The content of sGAG normalized to the dsDNA on day 28 (eight pellets from patients #1 and #5). (d) Gene expression levels in cell pellets for COL2A1, COL10A1, SOX9, and ACAN normalized to GAPDH (10 pellets from patients #3 to #5). ** p < 0.01; *** p < 0.001. bFGF, basic fibroblast growth factor; SMSCs, synovial mesenchymal stem cells; 3D, three-dimensional; CSA, cross-section area; COL2, collagen type II; sGAG, sulfated glycosaminoglycan; dsDNA, double-stranded DNA; COL2A1, collagen type II alpha 1; COL10A1, collagen type X alpha 1; SOX9, sex-determining region Y-box 9; ACAN, aggrecan; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
2.5. In vivo Osteochondral Repair in the Mouse Model Using Pellets Generated From the SMSCs From the bFGF (−) and bFGF (+) Groups
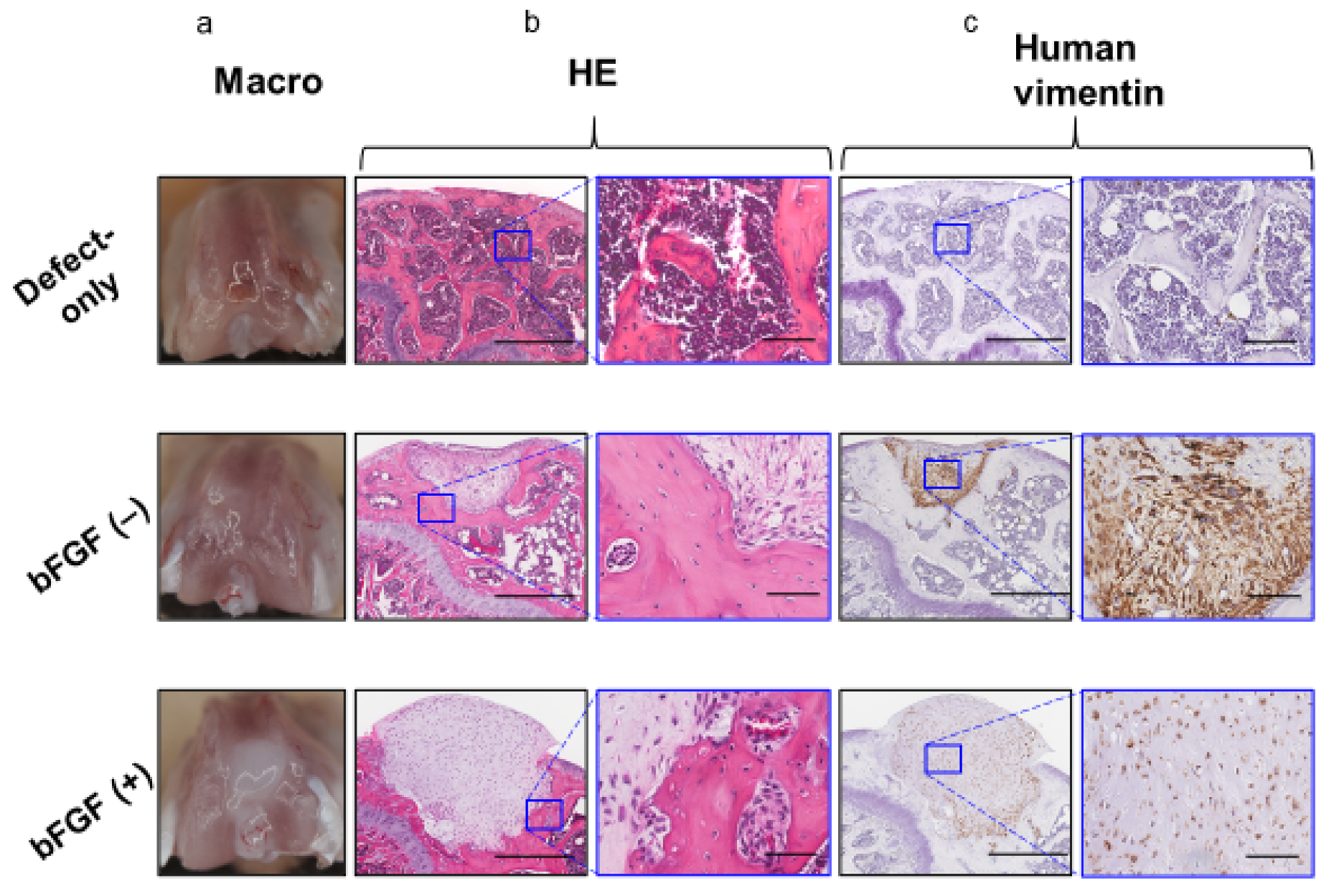
To assess the effect of the pretreatment with bFGF on cartilaginous regeneration of SMSC in vivo, synovial pellets were implanted into the osteochondral defects created in the knee of SCID mice. Eight weeks after implantation, no grossly visible synovitis or inflammation (Figure 6a) was observed in mouse knees in all groups, and no apparent infiltration of inflammatory cells into the repair cartilage was detected by hematoxylin and eosin (HE) staining in the two groups (Figure 6b). In both pellet-treated groups, the implanted cell aggregates were retained, and a little bony repair and a sclerotic rim formed around the implanted pellets were observed in the defects of the subchondral region.
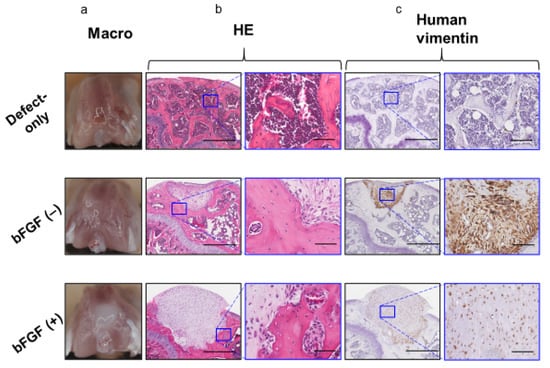
Figure 6.
In vivo osteochondral repair in a mouse model (8 weeks after operation) (a) Macroscopic view of the knees at sacrifice. (b) Histology of the tissue stained with HE (left columns; bar = 500 µm) and the corresponding high-magnification images (right columns; bar = 100 µm). (c) Immunohistochemical analysis of repair tissue stained for human vimentin (left columns; bar = 500 µm) and the corresponding high-magnification images (right columns; bar = 100 µm). bFGF, basic fibroblast growth factor; HE, hematoxylin and eosin.
However, the defects of the subchondral bone region were filled with repaired bone in the defect-only group. Human-vimentin-positive cells were found in the pellet implantation site in both the bFGF (−) and bFGF (+) groups, suggesting the existence of implanted human SMSCs (Figure 6c).
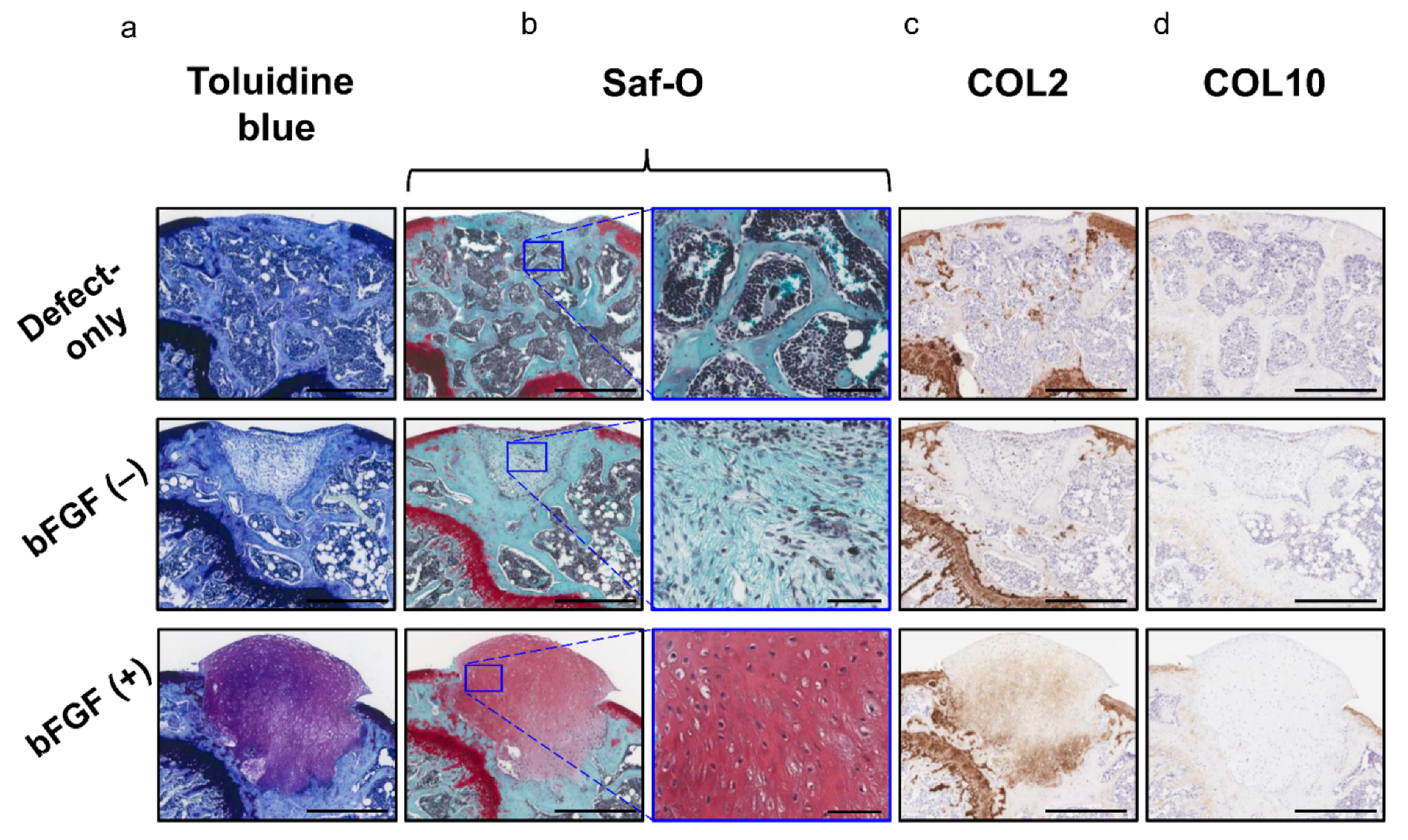
Moreover, the bFGF (+) group exhibited hyaline cartilage-like repair with chondrocyte-like round-shaped cells in the lacuna and abundant ECM stained with toluidine blue (Figure 7a) and Saf-O (Figure 7b); these cells also expressed COL 2 (Figure 7c). Collagen type X (COL10) expression was not observed in any of the three groups (Figure 7d). The pellet-treated groups showed significantly higher scores in the cartilage repair than the defect-only group [defect-only vs. bFGF (−), 8.8 ± 2.6 vs. 11.2 ± 1.5; p = 0.044; defect-only vs. bFGF (+), 8.8 ± 2.6 vs. 14.5 ± 2.6; p = 0.00023]. Moreover, the bFGF (+) group exhibited significantly higher scores than the bFGF (−) group (bFGF (−) vs. bFGF (+), 11.2 ± 1.5 vs. 14.5 ± 2.6; p = 0.0015). On the other hand, the pellet-treated groups showed significantly lower scores in the subchondral bone repair than the defect-only group [defect-only vs. bFGF (−), 7.6 ± 1.1 vs. 2.7 ± 0.5; p = 0.00003; defect-only vs. bFGF (+), 7.6 ± 1.1 vs. 2.8 ± 0.4; p = 0.00003]. In overall assessment (cartilage + subchondral bone), the bFGF (+) group exhibited significantly higher scores than the bFGF (−) group [bFGF (−) vs. bFGF (+), 13.9 ± 1.5 vs. 17.3 ± 2.6; p = 0.003] (Table 1).
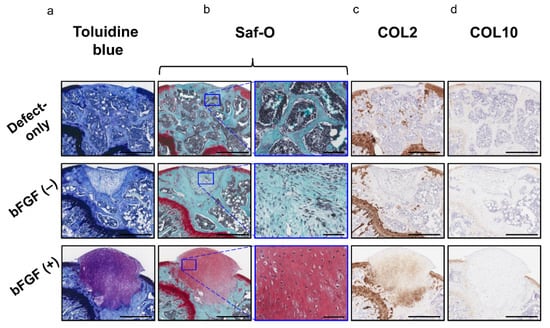
Figure 7.
In vivo osteochondral repair in a mouse model: evaluation of chondrogenic repair (a) Histology of the tissue stained with toluidine blue (bar = 500 µm). (b) Histology of the tissue stained with Saf-O (left columns; bar = 500 µm) and the corresponding high-magnification images (right columns; bar = 100 µm). (c) Immunohistochemical analysis of repair cartilage stained for COL2 (bar = 500 µm). (d) Immunohistochemical analysis of repair tissue stained for COL10 (bar = 500 µm). bFGF, basic fibroblast growth factor; Saf-O, Safranin-O; COL2, collagen type II; COL10, collagen type X.

Table 1.
Histological evaluation for osteochondral repair.
3. Discussion
Fibroblast growth factors (FGFs) are expressed widely in various tissues and display various biological properties [21]. FGFs control the migration, proliferation, differentiation, and survival of various cells and affect the expression of other factors involved in the regenerative response [22]. The human–mouse FGF family comprises 22 members [23], and the bFGF (also known as FGF2) is a member of the FGF family. Most FGFs bind to heparin-sulfate proteoglycan for signaling through the fibroblast growth factor receptor (FGFR) [24] and FGFR1–4 has been identified in both humans and mice [21]. As mentioned, FGFR3 is one of the markers of mesenchymal pre-cartilaginous stem cells, and bFGF regulates undifferentiated state and multipotent differentiation of stem cells through FGFR3-mediated signaling [14,17,18]. In the present study, the FGFR3 protein was expressed in the SMSCs of all donors assessed by Western blotting, and the addition of bFGF to the growth medium changed the morphology of SMSCs and promoted its proliferation rate without altering the expression of mesenchymal surface markers (CD44, CD73, CD90, and CD105), and the chondrocyte marker (CD151). These results imply bFGF/FGFR3 could promote chondrogenic differentiation of MSCs without changing their population. SOX9 is known as a master chondrogenic factor [25] and involved in the early phase of chondrogenesis and chondrocyte proliferation [26]. bFGF upregulates SOX9 gene expression for at least first 24 h [27] and induces chondrocyte proliferation [28]. In the present study, however, SOX9 was not significantly affected by bFGF administration. This may be due to the timing of evaluation, because SOX9 gene expression was assessed at 28 days after bFGF stimulation, which may be too late to detect SOX9 upregulation by bFGF. On the other hand, bFGF controlled the maintenance of stemness in MSCs [29,30], and suppressed the cellular senescence of MSCs by downregulating transforming growth factor β2 (TGF-β2) [31]. Cellular senescence downregulates the therapeutic potential of human MSCs by reducing their migratory, homing, and immunoregulatory abilities in vivo [32,33]. The binding interaction between chondrocytes and the ECM is critical for maintaining chondrocyte activity [34]. Altogether, these findings suggest that suppressed cellular senescence and enhanced ECM production by bFGF may lead to enhanced cartilage regeneration in vivo.
Regarding the mechanism underlying the in vivo tissue repair provided by MSCs, studies have demonstrated that transplanted MSCs differentiate directly into repaired tissue [35,36]. However, other reports concluded that MSCs lead to enhanced reparative response via the secretion of growth factors, cell–cell interactions, and the release of extracellular vesicles, which contain peptides/proteins, mRNA, and microRNAs [37,38,39]. Likewise, it is unclear whether MSCs differentiate into chondrocytes or promote host-side cartilage repair [40]. Ozeki et al. reported that synovial MSCs from a green fluorescent protein-expressing rat injected into the rat’s knee joint were detectable three weeks after injection [41]. In contrast, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate-labeled pig synovial MSCs implanted into osteochondral defects of the knee were detectable at one week but could not be found at one month, after implantation [42]. In the present study, human synovial MSCs were clearly detectable 8 weeks after the operation, as assessed by human vimentin immunostaining. Moreover, synovial pellets of the bFGF (+) group exhibited a higher cartilage differentiation potential in vivo than those of the bFGF (−) group. It has been previously reported that chondrocytes overexpressing bFGF are effective in repairing osteochondral defects [43,44]. However, to the best of our knowledge, there are no reports demonstrating that adding bFGF in the culture medium enhanced the cartilage tissue repair ability of SMSCs, which is by far a simple method. Regarding tissue engineering, we developed a scaffold-free three-dimensional tissue-engineered construct (TEC) with SMSCs, which produces rich ECM [45], and reported its cartilage repair efficacy in nude rats [8] and humans [9]. In the present study, instead of TEC, we used simple synovial pellet to simplify the methods. However, unlike our previous reports using TEC [7,8], the cartilage repair ability using simple synovial pellet without bFGF was generally low. In addition, an osteosclerotic image at the boundary with the subchondral bone as well as bone remodeling delay was observed in both bFGF(−) and bFGF(+) groups. These results suggest that synovial pellet transplantation may have prevented the repair of the host-side subchondral bone in the process of producing ECM and differentiating into cartilage tissue, and further improvement in transplantation methods may be required.
This study had several limitations. First, because of the necessity of using human SMSCs, we had to employ an in vivo xenograft model using a severe combined immunodeficient (scid) mouse, in which immunological reactions may be excessively suppressed. Second, the establishment of a full cartilage defect model using a large animal model and a longer follow-up is required to evaluate the mechanical loading and long-term maintenance of the regenerated cartilage. However, to the best of our knowledge, the results of this study demonstrated for the first time that addition of bFGF to SMSC growth culture may be a simple and useful treatment option to promote cartilage regeneration both in in vitro and in vivo.
4. Materials and Methods
All procedures were performed in accordance with the Declaration of Helsinki. All experiments carried out were approved by the Ethical Review Board of the Osaka University Hospital (number 15409-2, approval date April 12, 2016) and the Animal Experiment Regulations of our institution. Written informed consent was obtained from all patients.
4.1. Patient Demographics
The human synovium was obtained from five patients (one male and four female patients; average age, 17.4 years; range, 13–22 years) at the time of arthroscopic surgery of the knee joint. Among them, four patients underwent anterior cruciate ligament reconstruction with arthroscopy and one patient underwent a second look with arthroscopy after internal fixation of osteochondritis dissecans. The average waiting period until surgery was 13.2 weeks (Table 2).

Table 2.
Patient demographics.
4.2. Isolation and Culture of Human SMSCs
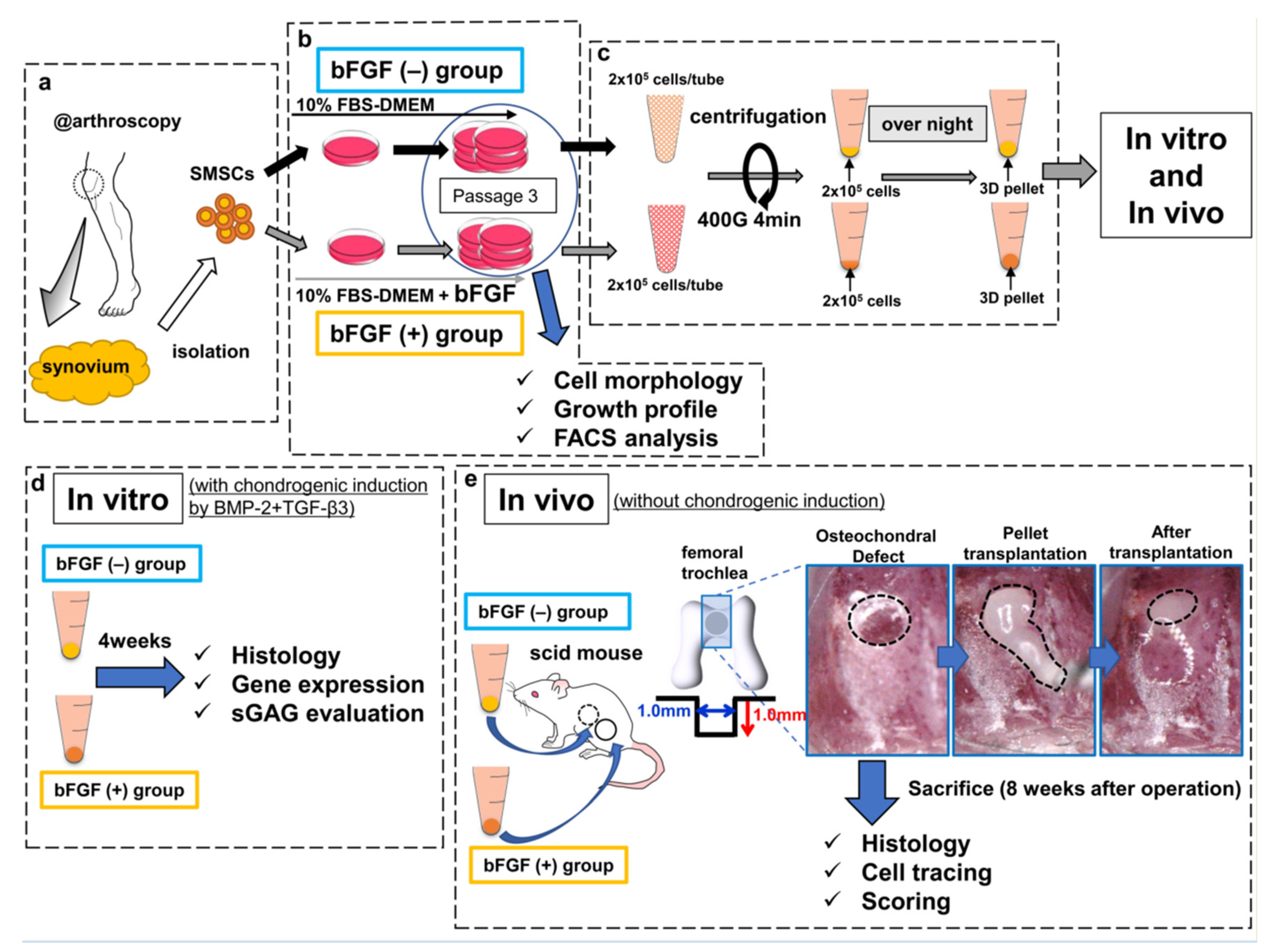
The harvested synovium (average wet weight, 483 mg; range, 400–590 mg) was stored in the 0.9% sodium chloride irrigation (Baxter, Deerfield, IL, USA) at room temperature and SMSCs were isolated within at least 3 h (Figure 8a). The synovium was sheared into small fragments and digested with 0.25% trypsin/EDTA (Gibco, Carlsbad, CA, USA) for 30 min, followed by extraction of floating adipose tissues after centrifugation [46]. Non-floating tissues were resuspended in Dulbecco’s modified Eagle medium (DMEM) (Gibco) containing 10% fetal bovine serum (FBS) (Sigma-Aldrich, St. Louis, MO, USA) and supplemented with 400 U/mL of collagenase type A (Worthington, Lakewood, NJ, USA), followed by incubation at 37 °C. Three hours later, dissociated cells were collected, washed by centrifugation, and cultured as described below. Isolated SMSCs were separated into two groups: the bFGF (−) group and the bFGF (+) group. SMSCs of the bFGF (−) group were cultured in high-glucose DMEM containing 10% FBS and 1% antibiotic–antimitotic (Sigma-Aldrich) growth medium (GM), and SMSCs of the bFGF (+) group were cultured in GM with 5 ng/mL bFGF (Wako, Osaka, Japan) at 37 °C with humidified 5% CO2 (Figure 8b). Then, 5 ng/mL bFGF addition was adopted with reference to previous reports [16,19,47]. SMSCs were expanded as described previously and used for experiments (analyses of cell morphology, growth profile, and FACS) at passage 3 [46].
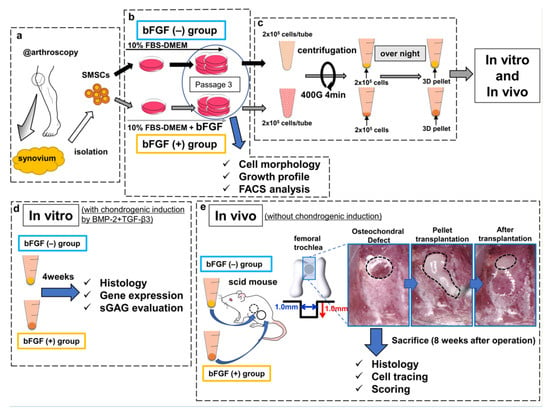
Figure 8.
Schematic diagram of the study design. (a) SMSCs were isolated from the synovium obtained from human knee joints. (b) SMSCs were separated into the bFGF (−) and bFGF (+) groups. (c) For 3D pellet culture and transplantation, SMSCs harvested from passage 3 were aggregated by centrifugation. (d) In vitro, the pellets were cultured for 4 weeks in chondrogenic basal medium with BMP2 (50 ng/mL) and TGF-β3 (10 ng/mL), to induce chondrogenic differentiation. The histology of pellets, the expression of cartilage-differentiation-related genes, and ECM production were evaluated. (e) In vivo, osteochondral defects (the black dotted line in the left picture) were created in the femoral trochlea of scid mice. Synovial pellets from both groups were implanted into each knee (the black dotted line in the middle and right pictures), followed by histological evaluation (including cell tracing and scoring) 8 weeks after the operation. SMSCs, synovial mesenchymal stem cells; bFGF, basic fibroblast growth factor; 3D, three-dimensional; FBS, fetal bovine serum; DMEM, Dulbecco’s modified Eagle medium; FACS, fluorescence-activated cell sorting; BMP2, bone morphogenetic protein-2; TGF-β3, transforming growth factor-β3; ECM, extracellular matrix; sGAG, sulfated glycosaminoglycan; scid, severe combined immunodeficiency.
4.3. Cell-Proliferation Assay
The cell-proliferation capacity of SMSCs from patients #1 to #5 was assessed from passages 1 to 6 by evaluating the population doubling level (PDL). For this, 3 × 105 cells/dish were seeded onto 150-mm dishes and cultured to reach approximately 90% confluence, followed by passaging after cell counting. The PDL was calculated using the following formula: PDL = log(N/N0)/log2, where N0 is the number of plated cells and N is the number of harvested cells at the time of passage. The cumulative PDL was the total PDL = Σ(PDL)n, where n is the passage number [48].
4.4. Western Blotting
Western blotting was conducted as previously described [49]. The primary antibodies were as follow: Anti-FGF Receptor 3 antibody (1:1000) (Cell Signaling Technology, Danvers, MA, USA, Cat# 4574) and anti-β-actin antibody (1:1000) (Cell Signaling Technology, Cat# 4967).
4.5. FACS Analysis
FACS was performed to evaluate the expression of four mesenchymal surface markers (CD44, CD73, CD90, and CD105), two mesenchymal negative markers (CD11b, CD271), and one chondrocyte surface marker (CD151) using a BD FACSVerse™ flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA), according to the manufacturer’s protocol. SMSCs were dissociated and resuspended in 0.1% bovine serum albumin (Sigma-Aldrich) in phosphate-buffered saline (PBS (0.1% BSA–PBS)) and incubated for 30 min at room temperature with fluorescence-conjugated antibodies. The antibodies used for FACS were as follows: anti-CD11b (BioLegend, San Diego, CA, USA, Cat# 301405), anti-CD44 (BD Biosciences, Cat# 550989), anti-CD73 (BioLegend, Cat# 344004), anti-CD90 (BioLegend, Cat# 328108), anti-CD105 (BioLegend, Cat# 323208), anti-CD151 (BD Biosciences, Cat# 556057), and anti-CD271 (BD Biosciences, Cat# 557196). Isotype-specific controls and antibodies were used according to the manufacturer’s protocol. The cells were washed with 0.1% BSA–PBS twice and suspended in 0.5 mL of 0.1% BSA–PBS for analysis on a BD FACSVerse (BD Biosciences). Data retrieved from the sorting were analyzed using the BD FACSuite Software (BD Biosciences).
4.6. Chondrogenesis Assays
For three-dimensional pellet culture, 2 × 105 cells harvested from passage 3 were centrifuged in 96 deep-well polypropylene plates (Evergreen Scientific, Vernon, CA, USA) and cultured in GM (Figure 8c). The next day, the medium was changed to 0.5 mL of chondrogenic basal medium (high-glucose DMEM (Gibco), 110 µg/mL sodium pyruvate (Gibco), 1% ITS + premix (Corning Incorporated, NY, USA), 50 µg/mL ascorbic acid-2-phosphate (Sigma-Aldrich), 40 µg/mL l-proline (Wako, Osaka, Japan), and 1% antibiotic–antimitotic) supplemented with 50 ng/mL BMP2 (Medtronic, Dublin, Ireland) [50], and 10 ng/mL TGF-β3 (Peprotech, Rocky Hill, NJ, USA). The medium was replaced twice per week and cells were maintained at 37 °C with humidified 5% CO2. The synovial pellets were cultured in chondrogenic basal medium for 4 weeks to induce chondrogenic differentiation and used in experiments (histological analysis, qRT-PCR analysis, and sGAG evaluation) (Figure 8d). The effects of bFGF on the expression of cartilage differentiation-related genes (COL2A1, COL10A1, SOX9, and ACAN) were analyzed by qRT-PCR. The total RNA of pellets was extracted using a Direct-ZolTM RNA kit (Zymo Research, Irvine, CA, USA) and reverse transcribed into complementary DNA using the ReverTra Ace® qPCR RT Master Mix (TOYOBO, Osaka, Japan), according to the manufacturer’s protocol. qRT-PCR was performed as described previously [51]. The transcriptional levels of target genes were normalized to the level of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene expression. The expression levels of each target gene were calculated using the 2–ΔCt method [51]. The TaqMan assays were performed as follows: COL2A1, Hs00264051_m1; COL10A1, Hs00166657_m1; ACAN, Hs00153936_m1; SOX9, Hs01001343_g1; and GAPDH, Hs02758991_g1. sGAG in the pellets was measured using the Blyscan Glycosaminoglycan Assay Kit (Biocolor, Westbury, NY, USA) after lysis with papain. sGAG production was normalized to the double-stranded DNA (dsDNA) content, which was measured on a Qubit 3.0 Fluorometer (Thermo Fisher Scientific, Waltham, MA, USA) using a QubitTM dsDNA HS Assay Kit (Thermo Fisher Scientific).
4.7. Implantation of Synovial Pellets onto Osteochondral Defects
For implantation, 2 × 105 cells harvested from passage 3 were centrifuged in 96 deep-well polypropylene plates and cultured in GM (Figure 8c), and surgery was performed on the following day. Ten-week-old male scid mice (C.B-17/Icr-scid/scidJcl; CLEA Japan, Fujinomiya, Japan) were anesthetized with an intraperitoneal injection of 0.3 mg/kg medetomidine (Nippon Zenyaku Kogyo Co., Ltd., Fukushima, Tokyo, Japan), 4.0 mg/kg midazolam (Astellas Pharma, Inc., Tokyo, Japan), and 5.0 mg/kg butorphanol (Meiji Seika Pharma Co., Ltd., Tokyo, Japan) [52]. In both knees, the femoral trochlear grooves were exposed via a medial parapatellar incision with lateral patellar dislocation, and an osteochondral defect of 1.0 mm diameter and depth was created in each knee using a micro drill (Kiso power tool, Osaka, Japan, Cat# 28854, 28855), and a microscope. A line was marked 1.0 mm from the tip of the micro drill. Subsequently, 12 knees were left empty as a defect-only group, and 26 knees were implanted with synovial pellets using a microscope. Pellets from the bFGF (−) and bFGF (+) groups were implanted randomly, and one pellet was implanted in each defect.
Eight weeks after surgery, the scid mice were sacrificed and their knees were harvested for analyses (Figure 8e).
4.8. Histology and Immunohistochemistry
All samples (pellets and distal femurs) were fixed in 4% paraformaldehyde and embedded in paraffin wax, followed by dehydration with an ethanol series and clearance with xylene. To evaluate osteochondral tissues, the dissected femoral ends were decalcified with 10% ethylenediaminetetraacetic acid (pH 7.4) before paraffin embedding. The samples were cut into 5-µm-thick sections and used for HE staining, toluidine blue staining, Saf-O staining, and immunohistochemistry, as described previously [51,53]. The following antibodies were used: anti-COL2 (Kyowa Pharma Chemical, Toyama, Japan, Cat# F-57, 1:500 dilution), anti-COL10 (Thermo Fisher Scientific, Cat# 14-9771-82, 1:500 dilution), and anti-human vimentin (Abcam, Cambridge, UK, Cat# ab16700, 1:100 dilution). The CSA of pellets and COL2 staining were measured automatically using an Aperio Image Scope (Leica Biosystems, Wetzlar, Germany). The sections stained with HE and Saf-O were used for histological evaluation using the O’Driscoll scoring system for cartilage repair, as described previously [54].
4.9. Statistical Analysis
All data were expressed as the mean ± standard deviation. Differences between two groups were assessed using the Mann–Whitney U test. Differences between three groups were assessed using the Kruskal–Wallis test. Significance was set at p < 0.05. Statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan) [55].
Author Contributions
G.O. and K.E. take responsibility for the integrity of the work as a whole, from inception to the completed manuscript. conception and design, G.O., K.E., M.H. (Makoto Hirao), R.C., H.Y., and K.N.; analysis and interpretation of the data, statistical expertise, G.O., K.E., and M.H. (Makoto Hirao), R.C., Y.E., A.M., K.T., A.G., T.K., and K.N.; administrative, technical, or logistic support:, G.O., K.E., and M.H. (Makoto Hirao), R.C., Y.Y., H.Y., T.I., T.N., and M.H. (Masayuki Hamada), and K.N; drafting the manuscript, G.O., K.E., M.H. (Makoto Hirao), and K.N.; collection and assembly of data, G.O., K.E., M.H. (Makoto Hirao), and K.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Health and Labor Sciences Research Grant of Japan (grant number: 20K09410), AbbVie, Astellas, Eisai, Eli Lilly, Mitsubishi Tanabe, and Ono. The funders had no role in the study design, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethical Review Board of Osaka University Hospital (number 15409-2, approval date April 12, 2016).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data available from the corresponding author on reasonable request.
Acknowledgments
We thank Akira Tsujii, Yukiko Eguchi, Fumiko Hirayama, and Mari Shinkawa for excellent assistance. We thank all the members of Yoshikawa’s laboratory for the helpful discussion and comments.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ACI | autologous chondrocyte implantation |
| ACAN | aggrecan |
| ACLR | anterior cruciate ligament reconstruction |
| bFGF | basic fibroblast growth factor |
| BMP | bone morphogenetic protein |
| COL10 | collagen type X |
| COL10A1 | collagen type X alpha 1 |
| COL2 | collagen type II |
| COL2A1 | collagen type II alpha 1 |
| CSA | cross-section area |
| ECM | extracellular matrix |
| FACS | fluorescence-activated cell sorting |
| FGF | Fibroblast growth factor |
| FGFR | fibroblast growth factor receptor |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase |
| GM | growth medium |
| HE | hematoxylin and eosin |
| MSC | mesenchymal stem cell |
| OCD | osteochondritis dissecans |
| PDL | population doubling level |
| qRT-PCR | quantitative real-time polymerase chain reaction |
| Saf-O | Safranin-O |
| scid | severe combined immunodeficient |
| sGAG | sulfated glycosaminoglycan |
| SMSC | Synovial mesenchymal stem cell |
| SOX9 | sex-determining region Y-box 9 |
| TEC | tissue-engineered construct |
| TGF-β | transforming growth factor β |
References
- Newman, A.P. Articular Cartilage Repair. Am. J. Sports Med. 1998, 26, 309–324. [Google Scholar] [CrossRef]
- Wakitani, S.; Mitsuoka, T.; Nakamura, N.; Toritsuka, Y.; Nakamura, Y.; Horibe, S. Autologous Bone Marrow Stromal Cell Transplantation for Repair of Full-Thickness Articular Cartilage Defects in Human Patellae: Two Case Reports. Cell Transplant. 2004, 13, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Jo, C.H.; Gil Lee, Y.; Shin, W.H.; Kim, H.; Chai, J.W.; Jeong, E.C.; Kim, J.E.; Shim, H.; Shin, J.S.; Shin, I.S.; et al. Intra-Articular Injection of Mesenchymal Stem Cells for the Treatment of Osteoarthritis of the Knee: A Proof-of-Concept Clinical Trial. Stem Cells 2014, 32, 1254–1266. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, Y.; Sekiya, I.; Yagishita, K.; Muneta, T. Comparison of human stem cells derived from various mesenchymal tissues: Superiority of synovium as a cell source. Arthritis Rheum. 2005, 52, 2521–2529. [Google Scholar] [CrossRef]
- Segawa, Y.; Muneta, T.; Makino, H.; Nimura, A.; Mochizuki, T.; Ju, Y.J.; Ezura, Y.; Umezawa, A.; Sekiya, I. Mesenchymal stem cells derived from synovium, meniscus, anterior cruciate ligament, and articular chondrocytes share similar gene expression profiles. J. Orthop. Res. 2009, 27, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Koga, H.; Muneta, T.; Nagase, T.; Nimura, A.; Ju, Y.J.; Mochizuki, T.; Sekiya, I. Comparison of mesenchymal tissues-derived stem cells for in vivo chondrogenesis: Suitable conditions for cell therapy of cartilage defects in rabbit. Cell Tissue Res. 2008, 333, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, K.; Ando, W.; Tateishi, K.; Nansai, R.; Fujie, H.; Hart, D.A.; Kohda, H.; Kita, K.; Kanamoto, T.; Mae, T.; et al. The influence of skeletal maturity on allogenic synovial mesenchymal stem cell-based repair of cartilage in a large animal model. Biomaterials 2010, 31, 8004–8011. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, K.; Ebina, K.; Hart, D.A.; Hirao, M.; Noguchi, T.; Sugita, N.; Yasui, Y.; Chijimatsu, R.; Yoshikawa, H.; Nakamura, N. Synovial mesenchymal stem cells from osteo- or rheumatoid arthritis joints exhibit good potential for cartilage repair using a scaffold-free tissue engineering approach. Osteoarthr. Cartil. 2016, 24, 1413–1422. [Google Scholar] [CrossRef]
- Shimomura, K.; Yasui, Y.; Koizumi, K.; Chijimatsu, R.; Hart, D.A.; Yonetani, Y.; Ando, W.; Nishii, T.; Kanamoto, T.; Horibe, S.; et al. First-in-Human Pilot Study of Implantation of a Scaf-fold-Free Tissue-Engineered Construct Generated From Autologous Synovial Mesenchymal Stem Cells for Repair of Knee Chondral Lesions. Am. J. Sports Med. 2018, 46, 2384–2393. [Google Scholar] [CrossRef]
- Narcisi, R.; Cleary, M.A.; Brama, P.A.; Hoogduijn, M.J.; Tüysüz, N.; Berge, D.T.; Van Osch, G.J.V.M. Long-Term Expansion, Enhanced Chondrogenic Potential, and Suppression of Endochondral Ossification of Adult Human MSCs via WNT Signaling Modulation. Stem Cell Rep. 2015, 4, 459–472. [Google Scholar] [CrossRef]
- Chang, C.; Han, S.; Kim, E.; Lee, S.; Seong, S.; Lee, M.C. Chondrogenic potentials of human synovium-derived cells sorted by specific surface markers. Osteoarthr. Cartil. 2013, 21, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, J.H.; Jo, C.H.; Seong, S.C.; Lee, J.C.; Lee, M.C. Effect of serum and growth factors on chondrogenic differen-tiation of synovium-derived stromal cells. Tissue Eng. Part A 2009, 15, 3401–3415. [Google Scholar] [CrossRef] [PubMed]
- Yasui, Y.; Chijimatsu, R.; Hart, D.A.; Koizumi, K.; Sugita, N.; Shimomura, K.; Myoui, A.; Yoshikawa, H.; Nakamura, N. Preparation of Scaffold-Free Tissue-Engineered Constructs Derived from Human Synovial Mesenchymal Stem Cells Under Low Oxygen Tension Enhances Their Chondrogenic Differentiation Capacity. Tissue Eng. Part A 2016, 22, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, S.; Shimazu, A.; Miyazaki, K.; Pan, H.; Koike, C.; Yoshida, E.; Takagishi, K.; Kato, Y. Retention of Multilineage Differentiation Potential of Mesenchymal Cells during Proliferation in Response to FGF. Biochem. Biophys. Res. Commun. 2001, 288, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Solchaga, L.A.; Penick, K.; Porter, J.D.; Goldberg, V.M.; Caplan, A.I.; Welter, J.F. FGF-2 enhances the mitotic and chon-drogenic potentials of human adult bone marrow-derived mesenchymal stem cells. J. Cell Physiol. 2005, 203, 398–409. [Google Scholar] [CrossRef]
- Buckley, C.; Kelly, D.J. Expansion in the presence of FGF-2 enhances the functional development of cartilaginous tissues engineered using infrapatellar fat pad derived MSCs. J. Mech. Behav. Biomed. Mater. 2012, 11, 102–111. [Google Scholar] [CrossRef]
- Dvořak, P.; Hampl, A. Basic fibroblast growth factor and its receptors in human embryonic stem cells. Folia Histochem. Cytobiol. 2005, 43, 203–208. [Google Scholar]
- Maric, D.; Pla, A.F.; Chang, Y.H.; Barker, J.L. Self-Renewing and Differentiating Properties of Cortical Neural Stem Cells Are Selectively Regulated by Basic Fibroblast Growth Factor (FGF) Signaling via Specific FGF Receptors. J. Neurosci. 2007, 27, 1836–1852. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, M.C.; Seong, S.C.; Park, K.H.; Lee, S. Enhanced Proliferation and Chondrogenic Differentiation of Human Synovium-Derived Stem Cells Expanded with Basic Fibroblast Growth Factor. Tissue Eng. Part A 2011, 17, 991–1002. [Google Scholar] [CrossRef]
- Tojyo, I.; Yamaguti, A.; Ozaki, H.; Yoshida, H.; Fujita, S. The expression of fibroblast growth factor receptor-3 in synovial osteochondromatosis of the temporomandibular joint. Arch. Oral Biol. 2004, 49, 591–594. [Google Scholar] [CrossRef]
- Itoh, N.; Ornitz, D.M. Evolution of the Fgf and Fgfr gene families. Trends Genet. 2004, 20, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Maddaluno, L.; Urwyler, C.; Werner, S. Fibroblast growth factors: Key players in regeneration and tissue repair. Development 2017, 144, 4047–4060. [Google Scholar] [CrossRef] [PubMed]
- Ornitz, D.; Itoh, N.M. Fibroblast growth factors. Genome Biol. 2001, 2. [Google Scholar] [CrossRef] [PubMed]
- Katoh, M. FGFR inhibitors: Effects on cancer cells, tumor microenvironment and whole-body homeostasis (Review). Int. J. Mol. Med. 2016, 38, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.; Deng, J.M.; Zhang, Z.; Behringer, R.R.; De Crombrugghe, B. Sox9 is required for cartilage formation. Nat. Genet. 1999, 22, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B.; Tsuchimochi, K.; Ijiri, K. The control of chondrogenesis. J. Cell. Biochem. 2005, 97, 33–44. [Google Scholar] [CrossRef]
- Murakami, S.; Kan, M.; McKeehan, W.L.; De Crombrugghe, B. Up-regulation of the chondrogenic Sox9 gene by fibroblast growth factors is mediated by the mitogen-activated protein kinase pathway. Proc. Natl. Acad. Sci. USA 2000, 97, 1113–1118. [Google Scholar] [CrossRef]
- Shi, S.; Wang, C.; Acton, A.J.; Eckert, G.J.; Trippel, S.B. Role of Sox9 in Growth Factor Regulation of Articular Chondrocytes. J. Cell. Biochem. 2015, 116, 1391–1400. [Google Scholar] [CrossRef]
- Osathanon, T.; Nowwarote, N.; Pavasant, P. Basic fibroblast growth factor inhibits mineralization but induces neuronal dif-ferentiation by human dental pulp stem cells through a FGFR and PLCgamma signaling pathway. J. Cell. Biochem. 2011, 112, 1807–1816. [Google Scholar] [CrossRef]
- Nowwarote, N.; Sukarawan, W.; Pavasant, P.; Foster, B.L.; Osathanon, T. Basic fibroblast growth factor regulates phos-phate/pyrophosphate regulatory genes in stem cells isolated from human exfoliated deciduous teeth. Stem Cell Res 2018, 9, 345. [Google Scholar]
- Ito, T.; Sawada, R.; Fujiwara, Y.; Seyama, Y.; Tsuchiya, T. FGF-2 suppresses cellular senescence of human mesenchymal stem cells by down-regulation of TGF-beta2. Biochem. Biophys. Res. Commun. 2007, 359, 108–114. [Google Scholar] [CrossRef]
- Rombouts, W.J.C.; Ploemacher, R.E. Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia 2003, 17, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda, J.C.; Tomé, M.; Fernández, M.E.; Delgado, M.; Campisi, J.; Bernad, A.; González, M.A. Cell senescence abrogates the therapeutic potential of human mesenchymal stem cells in the lethal endotoxemia model. Steam Cells 2014, 32, 1865–1877. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, A.; Tamamura, Y.; Morioka, M.; Karagiannis, P.; Shima, N.; Tsumaki, N. Considerations in hiPSC-derived cartilage for articular cartilage repair. Inflamm. Regen. 2018, 38, 17. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I.; Bruder, S.P. Mesenchymal stem cells: Building blocks for molecular medicine in the 21st century. Trends Mol. Med. 2001, 7, 259–264. [Google Scholar] [CrossRef]
- Horwitz, E.M.; Gordon, P.L.; Koo, W.K.K.; Marx, J.C.; Neel, M.D.; McNall, R.Y.; Muul, L.; Hofmann, T. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc. Natl. Acad. Sci. USA 2002, 99, 8932–8937. [Google Scholar] [CrossRef]
- Sacchetti, B.; Funari, A.; Michienzi, S.; Di Cesare, S.; Piersanti, S.; Saggio, I.; Tagliafico, E.; Ferrari, S.; Robey, P.G.; Riminucci, M.; et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 2007, 131, 324–336. [Google Scholar] [CrossRef]
- Önfelt, B.; Nedvetzki, S.; Benninger, R.K.P.; Purbhoo, M.A.; Sowinski, S.; Hume, A.N.; Seabra, M.C.; Neil, M.A.A.; French, P.M.W.; Davis, D.M. Structurally Distinct Membrane Nanotubes between Human Macrophages Support Long-Distance Vesicular Traffic or Surfing of Bacteria. J. Immunol. 2006, 177, 8476–8483. [Google Scholar] [CrossRef]
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Exosome-mediated transfer of mRNAs and mi-croRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Fellows, C.R.; Matta, C.; Zakany, R.; Khan, I.M.; Mobasheri, A. Adipose, Bone Marrow and Synovial Joint-Derived Mes-enchymal Stem Cells for Cartilage Repair. Front. Genet. 2016, 7, 213. [Google Scholar] [CrossRef]
- Ozeki, N.; Muneta, T.; Koga, H.; Nakagawa, Y.; Mizuno, M.; Tsuji, K.; Mabuchi, Y.; Akazawa, C.; Kobayashi, E.; Matsumoto, K.; et al. Not single but periodic injections of synovial mesenchymal stem cells maintain viable cells in knees and inhibit osteoarthritis progression in rats. Osteoarthr. Cartil. 2016, 24, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Sekiya, I.; Muneta, T.; Hatsushika, D.; Horie, M.; Tsuji, K.; Kawarasaki, T.; Watanabe, A.; Hishikawa, S.; Fujimoto, Y.; et al. Arthroscopic, histological and MRI analyses of cartilage repair after a minimally invasive method of transplantation of allogeneic synovial mesenchymal stromal cells into cartilage defects in pigs. Cytotherapy 2012, 14, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Filová, E.; Rampichová, M.; Litvinec, A.; Držík, M.; Míčková, A.; Buzgo, M.; Košťáková, E.; Martinová, L.; Usvald, D.; Prosecká, E.; et al. A cell-free nanofiber composite scaffold regenerated osteochondral de-fects in miniature pigs. Int. J. Pharm. 2013, 447, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Cucchiarini, M.; Madry, H.; Ma, C.; Thurn, T.; Zurakowski, D.; Menger, M.D.; Kohn, D.; Trippel, S.B.; Terwilliger, E.F. Improved tissue repair in articular cartilage defects in vivo by rAAV-mediated overexpression of human fibroblast growth factor 2. Mol. Ther. J. Am. Soc. Gene Ther. 2005, 12, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Ando, W.; Tateishi, K.; Hart, D.A.; Katakai, D.; Tanaka, Y.; Nakata, K.; Hashimoto, J.; Fujie, H.; Shino, K.; Yoshikawa, H.; et al. Cartilage repair using an in vitro generated scaffold-free tissue-engineered construct derived from porcine synovial mesenchymal stem cells. Biomaterials 2007, 28, 5462–5470. [Google Scholar] [CrossRef]
- Chijimatsu, R.; Kobayashi, M.; Ebina, K.; Iwahashi, T.; Okuno, Y.; Hirao, M.; Fukuhara, A.; Nakamura, N.; Yoshikawa, H. Impact of dexamethasone concentration on cartilage tissue formation from human synovial derived stem cells in vitro. Cytotechnology 2018, 70, 819–829. [Google Scholar] [CrossRef]
- Cheng, T.; Yang, C.; Weber, N.; Kim, H.T.; Kuo, A.C. Fibroblast growth factor 2 enhances the kinetics of mesenchymal stem cell chondrogenesis. Biochem. Biophys. Res. Commun. 2012, 426, 544–550. [Google Scholar] [CrossRef]
- Toh, W.S.; Guo, X.M.; Choo, A.B.; Lu, K.; Lee, E.H.; Cao, T. Differentiation and enrichment of expandable chondrogenic cells from human embryonic stem cells in vitro. J. Cell. Mol. Med. 2009, 13, 3570–3590. [Google Scholar] [CrossRef]
- Noguchi, T.; Ebina, K.; Hirao, M.; Morimoto, T.; Koizumi, K.; Kitaguchi, K.; Matsuoka, H.; Iwahashi, T.; Yoshikawa, H. Oxygen ultrafine bubbles water administration prevents bone loss of glucocorticoid-induced osteoporosis in mice by suppressing osteoclast differentiation. Osteoporos Int. 2017, 28, 1063–1075. [Google Scholar] [CrossRef]
- Shirasawa, S.; Sekiya, I.; Sakaguchi, Y.; Yagishita, K.; Ichinose, S.; Muneta, T. In vitro chondrogenesis of human synovi-um-derived mesenchymal stem cells: Optimal condition and comparison with bone marrow-derived cells. J. Cell. Biochem. 2006, 97, 84–97. [Google Scholar] [CrossRef]
- Chijimatsu, R.; Ikeya, M.; Yasui, Y.; Ikeda, Y.; Ebina, K.; Moriguchi, Y.; Shimomura, K.; Hart, D.A.; Yoshikawa, H.; Nakamura, N. Characterization of Mesenchymal Stem Cell-Like Cells Derived From Human iPSCs via Neural Crest Development and Their Application for Osteochondral Repair. Stem Cells Int. 2017, 2017, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kitaguchi, K.; Kashii, M.; Ebina, K.; Kaito, T.; Okada, R.; Makino, T.; Noguchi, T.; Ishimoto, T.; Nakano, T.; Yoshikawa, H. Effects of single or combination therapy of teriparatide and anti-RANKL monoclonal antibody on bone defect regeneration in mice. Bone 2018, 106, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, K.; Moriguchi, Y.; Ando, W.; Nansai, R.; Fujie, H.; Hart, D.A.; Gobbi, A.; Kita, K.; Horibe, S.; Shino, K.; et al. Osteochondral repair using a scaffold-free tissue-engineered construct derived from synovial mes-enchymal stem cells and a hydroxyapatite-based artificial bone. Tissue Eng. Part A 2014, 20, 2291–2304. [Google Scholar] [CrossRef] [PubMed]
- O’Driscoll, S.W.; Keeley, F.W.; Salter, R.B. Durability of regenerated articular cartilage produced by free autogenous periosteal grafts in major full-thickness defects in joint surfaces under the influence of continuous passive motion. A follow-up report at one year. J. Bone Jt. Surg. Am. Vol. 1988, 70, 595–606. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2012, 48, 452–458. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).