Radon Exposure—Therapeutic Effect and Cancer Risk
Abstract
:1. Introduction
2. Intake and Distribution of Radon in the Human Organism
2.1. Inhalation
2.2. Incorporation via Skin
2.3. Distribution
3. Cancer Risk
4. Radon as a Therapeutic Agent
| Musculoskeletal disorders and chronic pain diseases | Ankylosing spondylitis and other spondylarthropathies (AS) |
| Chronic polyarthritis (rheumatoid arthritis, RA) | |
| Chronic arthritis urica | |
| Psoriasis arthropathy | |
| Polymyalgia rheumatic | |
| Arthrosis and osteoarthritis (OA) | |
| Degenerative diseases of the spinal column | |
| Auxiliary treatment consecutive to intervertebral disc operations | |
| Osteoporosis | |
| Non-inflammatory soft tissue rheumatism (e.g., fibromyalgia) | |
| Chronic consequences of casualty or sporting injuries | |
| Auxiliary treatment consecutive to orthopedic operations | |
| Neuralgia, neuritis, polyneuropathy | |
| Multiple Sclerosis (MS) | |
| Cutaneous disorders and diseases | Insufficiently healing wounds (e.g., ulcus cruris) |
| Atopic dermatitis (neurodermatitis) | |
| Psoriasis | |
| Scleroderma | |
| Low grade circulatory problems of the skin | |
| Pulmonary diseases | Asthma bronchiale |
| Chronic-obstructive pulmonary diseases (COPD) | |
| Rhinitis allergica | |
| Chronic sinusitis | |
| Gynaecological diseases | Praeclimacteric and climacteric disorders |
| Pelvipethia spastica |
4.1. Clinical Trials
| First Author Year of Publication | Trial Design | Patient Number Indication | Dose | Type of Exposure Frequency Duration | Endpoints and Timepoints | Most Important Findings | Ref. |
|---|---|---|---|---|---|---|---|
| Franke et al., 2000 | Prospective; blinded; randomized | 60 patients RA | Radon group: 1.3 kBq/L, 1.6 g/L CO2 Placebo group: 1.6 g/L CO2 | Bath 20 min 15 times 4 weeks T = 35 °C Additional: Physiotherapy Occupational therapy Galvanic bathes (3/week) Classic massage | Endpoints: Pain intensity (VAS) Keitel functional Test (KFI) Arthritis Impact Measurement (AIMS) Timepoints: Before and directly after therapy, as well as, 3 and 6 months after therapy. | Pain intensity decreased in both groups, radon treatment results in a significant and longer lasting benefit from pain relief; KFI more in radon group; AIMS score was significantly increased in radon treated patients up to 6 months; KFI score shows a not significant benefit in radon treated patients | [98] |
| Van Tubergen et al., 2001 | Prospective; different treatment groups at different places. | 120 patients AS (40 spa with radon, 40 spa w/o radon 40 physical therapy at home) | Radon group: 0.536 WLM Placebo group: Thermal water + sauna Hydrotherapy Bathing Sports | Gallery/ inhalation Each 1 h 16 times 3 weeks T = 38–41 °C Additional: Physical exercise Postural correction therapy | Endpoints: BASFI Well-being VAS Pain intensity VAS Morning stiffness Timepoints: Before therapy After therapy week 4, 16, 28, and 40 | Primary goals borderline significant; pooled index of change shows highly significant differences as well as long-lasting effects of radon compared to conventional treatment | [105] |
| Yamaoka et al., 2004 | Prospective | 15 people (putative healthy individuals) | Radon group: 2080 Bq/m3 Sauna Group: 54 Bq/m3 Control Group: 54 Bq/m3 | Inhalation 40 min 5 times TRadon = 36 °C TSauna = 48 °C TControl = 36 °C | Endpoints: SOD AOC lipid metabolism CD4/CD8 immune cells vasoactive substances diabetes-associated markers Timepoints: Blood draw before and at 2 h after each treatment. In addition, 5 and 10 days after treatment. | Significant increase in SOD as well as decrease of lipid metabolism and cholesterol at 10 days for radon and sauna group; radon enhances T cell activity significantly, while sauna has similar effects, only significant at 10 days after treatment; radon enhances the CD4 T cell amount significantly after treatment, while CD8 T cells were decreased, respectively; radon group shows significantly more endorphin and a reduced vasopression | [112] |
| Yamaoka et al., 2004 | Prospective | 20 patients OA | Radon group: 2080 Bq/m3 non-controlled | Inhalation 40 min each Every 2 days T = 42 °C | Endpoints: SOD, catalase, lipid peroxide, total cholesterol, GSH, β-endorphin, ACTH, uric acid, ANP, and vasopressin levels in blood Timepoints: Before therapy, 2 h, 2, 4 and 6 weeks after therapy | SOD activity is significantly and long-lasting increased; Catalase activity is significantly increased after 4 and 6 weeks; T cells of CD4 type are increased, while CD8 T cells are decreased from 2 to 4 weeks after therapy; β-endorphin and anti-ANP levels were significantly and long-lasting increased after therapy; Vasopressin was significantly and long-lasting decreased; Cholesterol and lipid peroxide levels were significantly and long-lasting decreased | [113] |
| Shehata et al., 2006 | Retrospective | 83 patients AS (radon treatment) 10 patients AS (conv. Treatment) 10 patients LBP | Radon group: ~4.5 nCi/l Placebo groups: Convent. Therapy | Gallery/ inhalation 1 h each T = 38–41 °C 9–12 times 3–4 weeks Additional: Physiotherapy Hydrotherapy Massage Exercises | Endpoint: TGF-β (total and active form) Timepoint: Before, during and after the treatment (~0, 2 and 4 weeks)- | Total TGF-β level increased significantly in radon exposed patients compared to conventional treated patients or LBP controls; active TGF-β increased strongly and significantly in radon exposed patients compared to conventional treated patients or LBP controls; therapy responders show an inverse correlation with CRP concentration | [107] |
| Franke et al., 2007 | Prospective; blinded; randomized | 134 patients RA (67 patients per group) | Radon group: 1.1 kBq/L, 1.3 g/L CO2 Placebo group: 1.6 g/L CO2 | Baths 20 min 15 times 3 weeks T = 35 °C Additional: Physiotherapy Occupational therapy Galvanic bathes (3/week) Swedish massage | Endpoints: pain intensity, pain frequency, morning stiffness, functional capacity (all VAS), Drug intake Timepoints: Before and after last treatment, 3, 6, 9, and 12 months after treatment | Drug intake was significantly reduced from month 9 on; both groups had treatment effects, most not significant; repeated measurement ANCOVA revealed significant and long-lasting enhanced quality of life due to fewer limitations induced by pain | [99] |
| Moder et al., 2010 | Prospective | 33 AS patients | Radon group: ~4.5 nCi/L non-controlled | Gallery/ inhalation 90 min each 10 times 3 weeks 37–40.5 °C | Endpoints: Disease activity, BASDAI. BASFI, BASMI serum concentration of RANKL, OPG, TNFα, TGF-β, IL-17, IL-6 Timepoints: Before and after therapy (3 weeks) | Disease-associated scores BASDAI. BASFI, BASMI decreased significantly after therapy; serum conc. of TGF-β1, IL-6, TNF-α, RANKL, OPG, as well as OPG/RANKL ratio was significantly increased; active form of TGF-β, IL-6, TNFα. | [106] |
| Franke et al., 2013 (IMuRa Trial) | Prospective; blinded; randomized; multicentric | 652 Patients BP 437 pts. OA 230 pts. RA 98 pts. AS 39 pts. Multi 146 pts. | Radon group (332 patients) Radon bathes according to specific center (with or without CO2) or Radon Speleotherapy Control group: (320 patients) Placebo bathes according to specific center (either tap water or non-radon-containing fountain, with or without CO2) | Bath 20 min 12 times 3–4 weeks T = 36–38 °C | Endpoints: Pain intensity (VAS) Pain Questionnaire Functional capacity (FFbH) Western Ontario questionnaire (WOMAC) Health assessment questionnaire (HAQ) BASFI Drug intake Timepoints: Before and after last treatment, 3, 6, and 9 month after treatment | Radon treatment leading to significant and long-lasting relieve of self-assessed pain (VAS); OA and BP patients have the strongest and most lasting benefit from radon treatment, while OA patients seem to additionally have an enhanced quality of living up to 6 months after treatment | [76] |
| Dischereit et al., 2014 (Article in german) | Prospective | 24 patients RA 24 patients OA | Radon group 44 kBq/m3 non-controlled | Gallery/ inhalation 60 min each 12 times 3 weeks T = 37.5–41 °C | Endpoints: Pain intensity and duration Disease activity and functional score (BASDAI; BAS-G) Serum levels of RANKL, OPG, and TNF-α Timepoints: Directly before and after therapy, as well as 3 months after therapy | Pain was relieved after therapy and after 3 months in AS patients and after 3 months in OA patients; BASDAI was reduced significantly, and long-lasting in AS patients; TNF-α level was decreased in both groups, significantly in OA; RANKL level was significantly decreased in both groups, OPG increased only in AS; RANKL/OPG ratio decreased only AS significantly | [108] |
| Winklmayr et al., 2015 | Prospective; blinded; randomized | 64 healthy individuals Married partners | Radon group 412–900 Bq/L, Placebo: thermal water | Bath 20 min 5 times + 3 times brush up T = 36–39 °C Additional: Mountain hiking 3–4 h daily | Endpoints: Serum conc. OPG, RANKL, OPG/RANKL ratio Timepoints: Day 0, 6, 60, and 63 and 6 months after last treatment | Treatment benefits were seen in both groups in OPG, RANKL, and OPG/RANKL ratio; detected borderline significant trend towards bigger effect in Radon treated group | [114] |
| Lange et al., 2016 and 2012 | Prospective | 25 patients RA 24 patients OA | Radon group 4.5 nCi/l non-controlled | Gallery/ inhalation 60 min each 12 times 3 weeks T = 37.5–41 °C | Endpoints: serum conc. RANKL, OPG, TNF-α, and ACPA Timepoints: Directly before and after therapy | The serum conc. of TNFα and RANKL levels decreased in both groups; only in RA patients, OPG level increased, leading to a decreased RANKL/OPG ratio; ACPA titers decreased only in RA patients | [115,116] |
| Lange et al., 2017 | Endpoints: Pain VAS FFbH questionnaire ESR Serum CRP, RANKL, OPG, TNF-α, IL-10, and ACPA Timepoints: Directly before and after therapy, as well as 3 months after therapy | RA patients have significant immediate and lasting effect in pain relief, while health status (FFbH) is increasing; OA patients have significantly lasting pain relief effect; serum concentration of IL-10 is significantly increased directly after treatment in RA patients | [117] | ||||
| Rühle et al., 2017 (RAD-ON01) | Prospective Blinded Randomized | 100 patients with musculoskeletal disorders 50 patients per group Ambulant patients | Radon group 1200 Bq/L, Radon water only group); Radon/CO2 group 600 Bq/L and 1 g/l CO2; Radon-CO2-group Covered bath-tube | Bath 20 min each 9 times 3 weeks T = 35 °C | Endpoints: Immune modulation via DIoB [100] method Pain relief (VAS and questionnaire) Pain sensitivity (dolorimetry, pressure point measurement) Timepoints: Directly before as well as 6, 12, and 30 weeks after therapy | Long-lasting and significant pain reduction until end of observation period in whole trial population; significant and long-lasting increase in T cells and monocytes; significant temporarily increase of dendritic cells and regulatory T cells; significant and long-lasting reduction of the expression of the activation marker CD69 on T, B, and NK cells | [104] |
| Cucu et al., 2017 (RAD-ON01) | Endpoints: Amount of regulatory T cells Serum markers of bone and lipid metabolism | significant and long-lasting decrease of collagen fragments (CTX-I) and reduced levels of visfatin. Both factors are correlating significantly with pain intensity (VAS); regulatory T cells increase significantly and long-lasting after treatment | [102] | ||||
| Rühle et al., 2018 (RAD-ON01) | Endpoints: Pain relief (VAS and questionnaire) Pain sensitivity (dolorimetry, pressure point measurement) Blood pressure Antioxidative capacity (AOC) Superoxiddismutase (SOD) | Long-lasting and significant pain reduction until the end of the observation period in whole trial population, Radon CO2 bathes show a trend to be less effective (n.s.); lowered blood pressure in both groups, nightly measured systolic and diastolic blood pressure significantly decreased in Radon/CO2 treated patients; SD-VLF decreased significantly after radon therapy; SOD2 reduced significantly 6 weeks after treatment and increased significantly long-lasting | [103] | ||||
| Kullmann et al., 2018 (RAD-ON01) | Endpoints: Detection of inflammatory and anti-inflammatory cytokines in serum of patients. | No significant effects found for TNFα, IL-1β, IFNγ, IL-18, IL-1Ra, IL-10 concentration in serum of the patients; TGF-β concentration was significantly increased after treatment and significantly correlates with pain sensitivity; IL-18 level corresponds with lowered pain perception | [101] |
4.2. Biomedical Investigations in Patients
4.3. Animal Studies
5. Discussion: What Do We Know So Far about the Dose Distribution and Mechanism of Action Originating from Radon Exposure and Where Are Limitations?
- (I)
- The RAD-ON02 trial (EudraCT: 2016-002085-31; DRKS00016019) according to the German drug law was started in 2018 and covers molecular and osteoimmunological analyses correlated to pain relief as well as safety issues of the patients treated in radon bathes. The final analysis of this placebo-controlled, blinded, and randomized trial is anticipated for late 2021 [151].
- (II)
- The radon register trial of Austria was started in 2017 to cover the procedures and effects of many patients as a European basis for upcoming multicenter trials [152].
- (1)
- Trigger of the antioxidative defence by increased superoxide dismutase (SOD) and catalase activities.
- (2)
- Inhibition of the local and systemic inflammatory processes by increased release of TGF-β1 along with reduced TNF- α levels.
- (3)
- Decreased activation of immune cells and shift of the ratio of immune cells towards a more anti-inflammatory state.
- (4)
- Alterations in bone metabolism resulting in diminished bone erosion.
- (5)
- Enhanced bone formation and pain release are mediated by hormones.
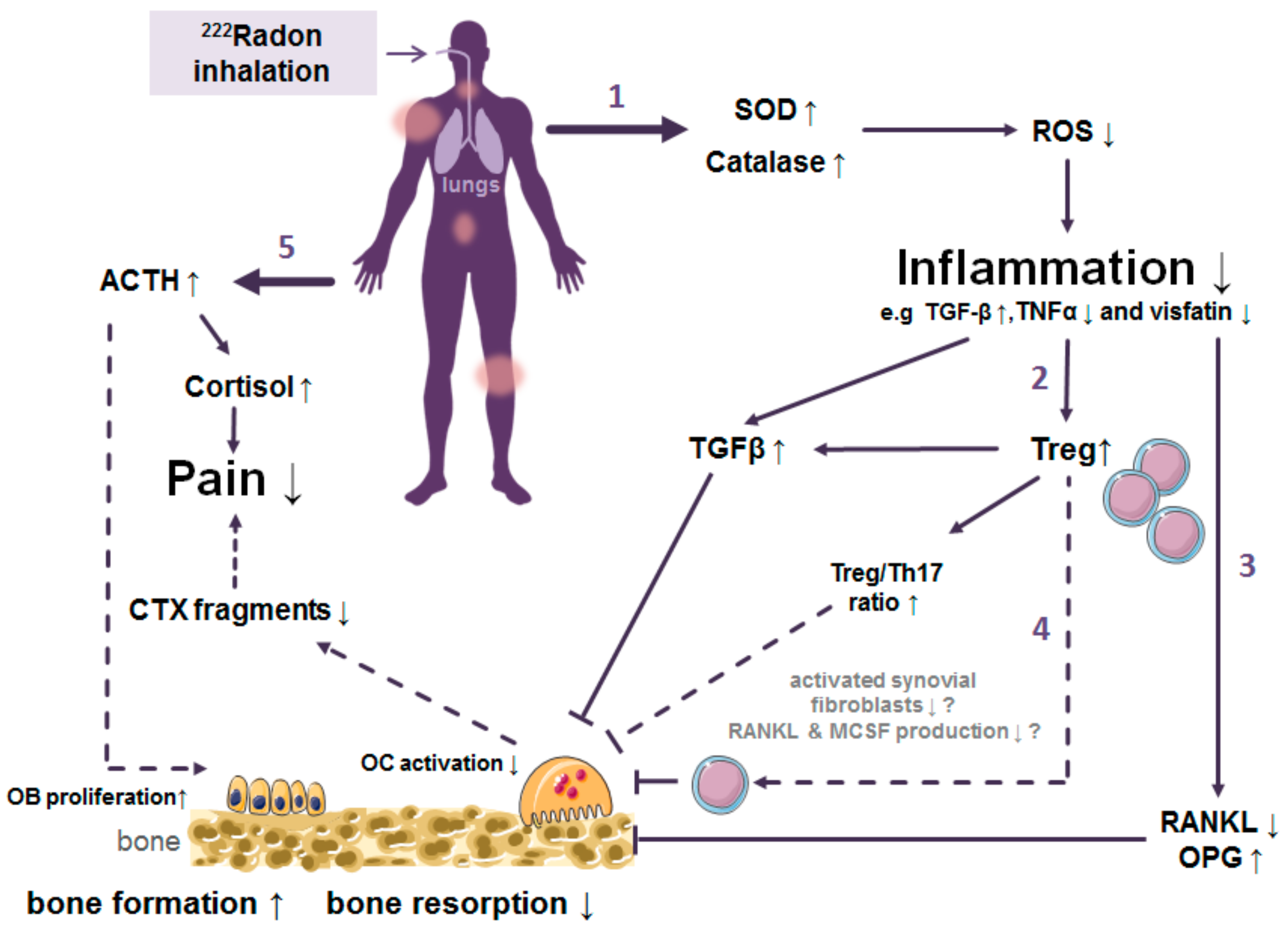
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Country | Place (City) |
|---|---|
| Austria | Bad Gastein, Bad Hofgastein, Bad Zell, Gasteiner Heilstollen |
| Bulgaria | Hisarja |
| Czech Republic | Jáchymov |
| Chile | Jahucl Hot Springs |
| China | Nanshui, Taishan |
| France | Plombiers |
| Germany | Bad Brambach, Bad Kreuznach, Bad Münster am Stein, Bad Schlema, Bad Steben, Sibyllenbad, Menzenschwand St. Blasien, Weissenstadt |
| Greece | Ikaria, Polichnitos, Eftalou |
| Hungary | Abaliget Cave, Budapest, Beke Cave, Eger, lstván Cave, Tapolca Hospital Cave, Szemlöhegy Cave |
| Italy | Ischia, Meran |
| Japan | Misasa |
| Poland | Długopole-Zdrój, Ladek-Zdrój, Świeradów-Zdrój, Szczawno-Zdrój, Przerzeczyn-Zdrój |
| Romania | Felix Spa |
| Russia | Pyatigorsk (Caucasus). Belokuriha (Altai, Siberia) and Yangan Tau (Ural) |
| Ukraine | Khmelnik |
| USA | Boulder (Montana) |
References
- ICRP. Occupational intake of radionuclides: Part 3. ICRP Publication 137. Ann. ICRP 2017, 46, 1–486. [Google Scholar] [CrossRef]
- Avrorin, V.V.; Krasikova, R.N.; Nefedov, V.D.; Toropova, M.A. The chemistry of radon. Russ. Chem. Rev. 1982, 51, 12–20. [Google Scholar] [CrossRef]
- Lederer, C.M.; Shirley, V.S. Table of Isotopes, 7th ed.; John Wiley & Sons: New York, NY, USA, 1978. [Google Scholar]
- Seilnacht, T.; Binder, H. Lexikon der Chemischen Elemente; Hirzel Verlag: Stuttgart/Leipzig, Germany, 1999. [Google Scholar]
- Rutherford, E.; Owens, R.B. Thorium and uranium radiation. Trans. R. Soc. Can. 1899, 2, 9–12. [Google Scholar]
- Rutherford, E. A radioactive substabce emitted from thorium compounds. Philos. Mag. 1900, 5, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Curie, P.; Curie, M. Sur La Radioactivité Provoquée Par Les Rayons De Becquerel; Comptes Rendus Hebdomadaires des Séances de l’Académie des Sciences; Gauthier-Villars: Paris, France, 1899; pp. 714–716. [Google Scholar]
- Dorn, E. Über die von radioaktiven Substanzen ausgesandte Emanation. Abhandel Naturforsch. Ges. 1901, 23, 1–15. [Google Scholar]
- Debierne, A. Sur La Radioactivité Induite Provoquée Par Les Sels D’actinium; Comptes Rendus Hebdomadaires des Séances de l’Académie des Sciences; Gauthier-Villars: Paris, France, 1903; pp. 446–449. [Google Scholar]
- Ramola, R.C.; Prasad, M.; Kandari, T.; Pant, P.; Bossew, P.; Mishra, R.; Tokonami, S. Dose estimation derived from the exposure to radon, thoron and their progeny in the indoor environment. Sci. Rep. 2016, 6, 31061. [Google Scholar] [CrossRef]
- Committee on the Biological Effects of Ionizing Radiation (BEIR V). Health Effects of Exposure to Low Levels of Ionizing Radiation: BEIR V; Acadamies Press: Washington, DC, USA, 1990; Volume 5. [Google Scholar]
- Tollefsen, T.; Cinelli, G.; Bossew, P.; Gruber, V.; De Cort, M. From the European indoor radon map towards an atlas of natural radiation. Radiat. Prot. Dosim. 2014, 162, 129–134. [Google Scholar] [CrossRef] [Green Version]
- Doi, K.; Tokonami, S.; Yonehara, H.; Yoshinaga, S. A simulation study of radon and thoron discrimination problem in case-control studies. J. Radiat. Res. 2009, 50, 495–506. [Google Scholar] [CrossRef]
- World Health Organization. WHO Handbook on Indoor Radon: A Public Health Perspective; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). Sources and Effects of Ionizing Radiation: Sources; United Nations Publications: New York, NY, USA, 2000; Volume 1. [Google Scholar]
- Harley, J.H. Radioactive emissions and radon. Bull. N. Y. Acad. Med. 1981, 57, 883. [Google Scholar]
- Amanat, B.; Kardan, M.; Faghihi, R.; Pooya, S.H. Comparative Measurements of Radon Concentration in Soil Using Passive and Active Methods in High Level Natural Radiation Area (HLNRA) of Ramsar. J. Biomed. Phys. Eng. 2013, 3, 139. [Google Scholar]
- Andelman, J.B. Human exposures to volatile halogenated organic chemicals in indoor and outdoor air. Environ. Health Perspect. 1985, 62, 313. [Google Scholar] [CrossRef]
- Vogiannis, E.; Niaounakis, M.; Halvadakis, C. Contribution of 222 Rn-bearing water to the occupational exposure in thermal baths. Environ. Int. 2004, 30, 621–629. [Google Scholar] [CrossRef]
- Lettner, H.; Hubmer, A.; Rolle, R.; Steinhäusler, F. Occupational exposure to radon in treatment facilities of the radon-spa Badgastein, Austria. Environ. Int. 1996, 22, 399–407. [Google Scholar] [CrossRef]
- Council, N.R. Risk Assessment of Radon in Drinking Water; National Academies Press: Washington, DC, USA, 1999. [Google Scholar]
- Sarenio, O. Leitfaden zur Messung von Radon, Thoron und ihren Zerfallsprodukten, Veröffentlichungen der Strahlenschutzkommission. Bundesminist. für Umw. Nat. Reakt. 2002. Available online: https://www.ssk.de/SharedDocs/Publikationen/VeroeffentlichungenderSSK/Band_47.html (accessed on 23 October 2020).
- Porstendörfer, J.; Reineking, A. Indoor behaviour and characteristics of radon progeny. Radiat. Prot. Dosim. 1992, 45, 303–311. [Google Scholar] [CrossRef]
- Castleman, A., Jr. Consideration of the chemistry of radon progeny. Environ. Sci. Technol. 1991, 25, 730–735. [Google Scholar] [CrossRef]
- Porstendörfer, J. Physical parameters and dose factors of the radon and thoron decay products. Radiat. Prot. Dosim. 2001, 94, 365–373. [Google Scholar] [CrossRef]
- Porstendörfer, J.; Röbig, G.; Ahmed, A. Experimental determination of the attachment coefficients of atoms and ions on monodisperse aerosols. J. Aerosol Sci. 1979, 10, 21–28. [Google Scholar] [CrossRef]
- Smerajec, M.; Vaupotič, J. Nanoaerosols including radon decay products in outdoor and indoor air at a suburban site. J. Toxicol. 2012, 2012, 510876. [Google Scholar] [CrossRef]
- Kendall, G.; Smith, T. Doses to organs and tissues from radon and its decay products. J. Radiol. Prot. 2002, 22, 389. [Google Scholar] [CrossRef]
- Islam, G.; Mazumdar, S.; Ashraf, M. Influence of various room parameters upon radon daughter equilibrium indoors. Radiat. Meas. 1996, 26, 193–201. [Google Scholar] [CrossRef]
- Grosskopf, A.; Irlweck, K. Radon Exposure and Urinary 210Po Excretion of Austrian Spa Workers. Radiat. Prot. Dosim. 1985, 12, 39–43. [Google Scholar] [CrossRef]
- Khursheed, A. Doses to systemic tissues from radon gas. Radiat. Prot. Dosim. 2000, 88, 171–181. [Google Scholar] [CrossRef]
- Sakoda, A.; Ishimori, Y.; Fukao, K.; Yamaoka, K.; Kataoka, T.; Mitsunobu, F. Lung dosimetry of inhaled radon progeny in mice. Radiat. Environ. Biophys. 2012, 51, 425–442. [Google Scholar] [CrossRef]
- Sakoda, A.; Ishimori, Y.; Yamaoka, K.; Kataoka, T.; Mitsunobu, F. Absorbed doses of lungs from radon retained in airway lumens of mice and rats. Radiat. Environ. Biophys. 2013, 52, 389–395. [Google Scholar] [CrossRef]
- Sakoda, A.; Ishimori, Y.; Kawabe, A.; Kataoka, T.; Hanamoto, K.; Yamaoka, K. Physiologically Based Pharmacokinetic Modeling of Inhaled Radon to Calculate Absorbed Doses in Mice, Rats, and Humans. J. Nucl. Sci. Technol. 2010, 47, 731–738. [Google Scholar] [CrossRef]
- Stuart, B.O. Deposition and clearance of inhaled particles. Environ. Health Perspect. 1984, 55, 369–390. [Google Scholar] [CrossRef]
- Carvalho, T.C.; Peters, J.I.; Williams, R.O., III. Influence of particle size on regional lung deposition—What evidence is there? Int. J. Pharm. 2011, 406, 1–10. [Google Scholar] [CrossRef]
- Hofmann, W. Modelling inhaled particle deposition in the human lung—A review. J. Aerosol Sci. 2011, 42, 693–724. [Google Scholar] [CrossRef]
- Harley, N.; Robbins, E. 222Rn alpha dose to organs other than lung. Radiat. Prot. Dosim. 1992, 45, 619–622. [Google Scholar] [CrossRef]
- Balásházy, I.; Farkas, Á.; Madas, B.G.; Hofmann, W. Non-linear relationship of cell hit and transformation probabilities in a low dose of inhaled radon progenies. J. Radiol. Prot. 2009, 29, 147. [Google Scholar] [CrossRef]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef]
- Ferin, J.; Oberdorster, G.; Penney, D. Pulmonary retention of ultrafine and fine particles in rats. Am. J. Respir. Cell Mol. Biol. 1992, 6, 535–542. [Google Scholar] [CrossRef]
- Ferin, J.; Oberdörster, G. Translocation of particles from pulmonary alveoli into the interstitium. J. Aerosol Med. 1992, 5, 179–187. [Google Scholar] [CrossRef]
- Paquet, F.; Etherington, G.; Bailey, M.; Leggett, R.; Lipsztein, J.; Bolch, W.; Eckerman, K.; Harrison, J. ICRP publication 130: Occupational intakes of radionuclides: Part 1. Ann. ICRP 2015, 44, 5–188. [Google Scholar] [CrossRef]
- Hofmann, W.; Winkler-Heil, R.; Lettner, H.; Hubmer, A.; Gaisberger, M. Radon transfer from thermal water to human organs in radon therapy: Exhalation measurements and model simulations. Radiat. Environ. Biophys. 2019, 58, 513–529. [Google Scholar] [CrossRef] [Green Version]
- Sakoda, A.; Ishimori, Y.; Tschiersch, J. Evaluation of the intake of radon through skin from thermal water. J. Radiat. Res. 2016, 57, 336–342. [Google Scholar] [CrossRef] [Green Version]
- Lettner, H.; Hubmer, A.; Hofmann, W.; Landrichinger, J.; Gaisberger, M.; Winkler-Heil, R. Radon in the Exhaled Air of Patients in Radon Therapy. Radiat. Prot. Dosim. 2017, 177, 78–82. [Google Scholar] [CrossRef]
- Kávási, N.; Kovács, T.; Somlai, J.; Jobbágy, V.; Nagy, K.; Deák, E.; Berhés, I.; Bender, T.; Ishikawa, T.; Tokonami, S. Comparison of urinary excretion of radon from the human body before and after radon bath therapy. Radiat. Prot. Dosim. 2011, 146, 27–30. [Google Scholar] [CrossRef]
- Tempfer, H.; Hofmann, W.; Schober, A.; Lettner, H.; Dinu, A. Deposition of radon progeny on skin surfaces and resulting radiation doses in radon therapy. Radiat. Environ. Biophys. 2010, 49, 249–259. [Google Scholar] [CrossRef]
- Falkenbach, A.; Kleinschmidt, J.; Soto, J.; Just, G. Radon progeny activity on skin and hair after speleotherapeutic radon exposure. J. Environ. Radioact. 2002, 62, 217–223. [Google Scholar] [CrossRef]
- Tobias, C.; Jones, H.; Lawrence, J.; Hamilton, J. The uptake and elimination of krypton and other inert gases by the human body. J. Clin. Investig. 1949, 28, 1375. [Google Scholar] [CrossRef] [Green Version]
- Harley, J.H.; Jetter, E.S.; Nelson, N. Elimination of radon from the body. Environ. Int. 1994, 20, 573–584. [Google Scholar] [CrossRef]
- Susskind, H.; Atkins, H.L.; Cohn, S.H.; Ellis, K.J.; Richards, P. Whole-body retention of radioxenon. J. Nucl. Med. 1977, 18, 462–471. [Google Scholar]
- Conn, J.R.H.L. Equilibrium distribution of radioxenon in tissue: Xenon-hemoglobin association curve. J. Appl. Physiol. 1961, 16, 1065–1070. [Google Scholar] [CrossRef]
- Kirk, W.P.I. In Vivo Behavior and Effects of Krypton-85 in Guinea Pigs; The University of Rochester: Rochester, NY, USA, 1975. [Google Scholar]
- Ishimori, Y.; Tanaka, H.; Sakoda, A.; Kataoka, T.; Yamaoka, K.; Mitsunobu, F. Measurements of radon activity concentration in mouse tissues and organs. Radiat. Environ. Biophys. 2017, 56, 161–165. [Google Scholar] [CrossRef]
- Nussbaum, E.; Hursh, J. Radon solubility in rat tissues. Science 1957, 125, 552–553. [Google Scholar] [CrossRef]
- Henshaw, D.L.; Eatough, J.P.; Richardson, R.B. Radon as a causative factor in induction of myeloid leukaemia and other cancers. Lancet 1990, 335, 1008–1012. [Google Scholar] [CrossRef]
- Nussbaum, E.; Harsh, J.B. Radon solubility in fatty acids and triglycerides. J. Phys. Chem. 1958, 62, 81–84. [Google Scholar] [CrossRef]
- Schubert, M.; Paschke, A.; Lieberman, E.; Burnett, W.C. Air–water partitioning of 222Rn and its dependence on water temperature and salinity. Environ. Sci. Technol. 2012, 46, 3905–3911. [Google Scholar] [CrossRef]
- Sanjon, E.P.; Maier, A.; Hinrichs, A.; Kraft, G.; Drossel, B.; Fournier, C. A combined experimental and theoretical study of radon solubility in fat and water. Sci. Rep. 2019, 9, 10768. [Google Scholar] [CrossRef]
- Breustedt, B.; Giussani, A.; Noßke, D. Internal dose assessments—Concepts, models and uncertainties. Radiat. Meas. 2018, 115, 49–54. [Google Scholar] [CrossRef]
- Peterman, B.; Perkins, C. Dynamics of radioactive chemically inert gases in the human body. Radiat. Prot. Dosim. 1988, 22, 5–12. [Google Scholar]
- Leggett, R.; Marsh, J.; Gregoratto, D.; Blanchardon, E. A generic biokinetic model for noble gases with application to radon. J. Radiol. Prot. 2013, 33, 413. [Google Scholar] [CrossRef] [PubMed]
- Harley, N.H. Effect of residential radon decay product dose factor variability on reporting of dose. Health Phys. 2018, 114, 398–407. [Google Scholar] [CrossRef]
- Mirsch, J.; Hintz, L.; Maier, A.; Fournier, C.; Löbrich, M. An assessment of radiation doses from radon exposures using a mouse model system. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, 770–778. [Google Scholar] [CrossRef]
- United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). Sources and Effects of Ionizing Radiation: Sources; United Nations Publications: New York, NY, USA, 2010; Volume 1. [Google Scholar]
- Radford, E.P. Potential health effects of indoor radon exposure. Environ. Health Perspect. 1985, 62, 281. [Google Scholar] [CrossRef]
- Kreuzer, M.; Sobotzki, C.; Schnelzer, M.; Fenske, N. Factors Modifying the Radon-Related Lung Cancer Risk at Low Exposures and Exposure Rates among German Uranium Miners. Radiat. Res. 2018, 189, 165–176. [Google Scholar] [CrossRef]
- Lubin, J.H. Models for the analysis of radon-exposed populations. Yale J. Biol. Med. 1988, 61, 195. [Google Scholar]
- Zhang, Z.-L.; Sun, J.; Dong, J.-Y.; Tian, H.-L.; Xue, L.; Qin, L.-Q.; Tong, J. Residential Radon and Lung Cancer Risk: An Updated Meta-analysis of Case-control Studies. Asian Pac. J. Cancer Prev. 2012, 13, 2459–2465. [Google Scholar] [CrossRef] [Green Version]
- Krewski, D.; Lubin, J.H.; Zielinski, J.M.; Alavanja, M.; Catalan, V.S.; Field, R.W.; Klotz, J.B.; Letourneau, E.G.; Lynch, C.F.; Lyon, J.I.; et al. Residential radon and risk of lung cancer: A combined analysis of 7 North American case-control studies. Epidemiology 2005, 16, 137–145. [Google Scholar] [CrossRef]
- Chen, J. Lifetime lung cancer risks associated with indoor radon exposure based on various radon risk models for canadian population. Radiat. Prot. Dosim. 2016, 173, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Darby, S.; Hill, D.; Auvinen, A.; Barros-Dios, J.M.; Baysson, H.; Bochicchio, F.; Deo, H.; Falk, R.; Forastiere, F.; Hakama, M.; et al. Radon in homes and risk of lung cancer: Collaborative analysis of individual data from 13 European case-control studies. BMJ 2005, 330, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kreuzer, M.; Fenske, N.; Schnelzer, M.; Walsh, L. Lung cancer risk at low radon exposure rates in German uranium miners. Br. J. Cancer 2015, 113, 1367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Little, J.B. What are the risks of low-level exposure to α radiation from radon? Proc. Natl. Acad. Sci. USA 1997, 94, 5996–5997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franke, A.; Franke, T. Long-term benefits of radon spa therapy in rheumatic diseases: Results of the randomised, multi-centre IMuRa trial. Rheumatol. Int. 2013, 33, 2839–2850. [Google Scholar]
- Brooks, A.L.; Hoel, D.G.; Preston, R.J. The role of dose rate in radiation cancer risk: Evaluating the effect of dose rate at the molecular, cellular and tissue levels using key events in critical pathways following exposure to low LET radiation. Int. J. Radiat. Biol. 2016, 92, 405–426. [Google Scholar] [CrossRef] [Green Version]
- Charles, M. Radon exposure of the skin: I. Biological effects. J. Radiol. Prot. 2007, 27, 231. [Google Scholar] [CrossRef]
- Kristbjornsdottir, A.; Rafnsson, V. Incidence of cancer among residents of high temperature geothermal areas in Iceland: A census based study 1981 to 2010. Environ. Health 2012, 11, 73. [Google Scholar] [CrossRef] [Green Version]
- Bräuner, E.V.; Loft, S.; Sørensen, M.; Jensen, A.; Andersen, C.E.; Ulbak, K.; Hertel, O.; Pedersen, C.; Tjønneland, A.; Kjær, S.K. Residential radon exposure and skin cancer incidence in a prospective Danish cohort. PLoS ONE 2015, 10, e0135642. [Google Scholar] [CrossRef] [Green Version]
- Ruano-Ravina, A.; Aragonés, N.; Kelsey, K.T.; Pérez-Ríos, M.; Piñeiro-Lamas, M.; López-Abente, G.; Barros-Dios, J.M. Residential radon exposure and brain cancer: An ecological study in a radon prone area (Galicia, Spain). Sci. Rep. 2017, 7, 3595. [Google Scholar] [CrossRef] [Green Version]
- Momcilovic, B.; Alkhatib, H.; Duerre, J.; Cooley, M.; Long, W.; Harris, T.; Lykken, G. Environmental lead-210 and bismuth-210 accrue selectively in the brain proteins in Alzheimer disease and brain lipids in Parkinson disease. Alzheimer Dis. Assoc. Disord. 2001, 15, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Momčilović, B.; Lykken, G.I.; Cooley, M. Natural distribution of environmental radon daughters in the different brain areas of an Alzheimer Disease victim. Mol. Neurodegener. 2006, 1, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evrard, A.-S.; Hémon, D.; Billon, S.; Laurier, D.; Jougla, E.; Tirmarche, M.; Clavel, J. Childhood leukemia incidence and exposure to indoor radon, terrestrial and cosmic gamma radiation. Health Phys. 2006, 90, 569–579. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Vol. 100D. A Review of Human Carcinogens: Part D. Radiation; International Agency for Research on Cancer: Lyon, France, 2012. [Google Scholar]
- Hall, E.J.; Giaccia, A.J. Radiobiology for the Radiologist; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006; Volume 6. [Google Scholar]
- Deetjen, P.; Falkenbach, A.; Harder, D.; Jöckel, H.; Kaul, A.; von Philippsborn, H. Radon as a Medicine; Verlag Dr. Kovac: Hamburg, Germany, 2014. [Google Scholar]
- Becker, K. One century of radon therapy. Int. J. Low Radiat. 2004, 1, 333–357. [Google Scholar] [CrossRef]
- Santos, I.; Cantista, P.; Vasconcelos, C. Balneotherapy in rheumatoid arthritis—A systematic review. Int. J. Biometeorol. 2016, 60, 1287–1301. [Google Scholar] [CrossRef]
- Zdrojewicz, Z.; Strzelczyk, J. Radon treatment controversy. Dose Response 2006, 4, 106–118. [Google Scholar] [CrossRef] [Green Version]
- EURADON. Indikationsliste/Konsensusliste der Badeärzte des Vereins EURADON. Available online: https://www.euradon.de/fragen/indikationsliste-der-arge (accessed on 15 June 2020).
- Erickson, B.E. The therapeutic use of radon: A biomedical treatment in Europe; an “alternative” remedy in the United States. Dose Response 2006, 5, 48–62. [Google Scholar] [CrossRef] [Green Version]
- Verhagen, A.; Bierma-Zeinstra, S.; Boers, M.; Cardoso, J.; Lambeck, J.; De Bie, R.; De Vet, H.C. Balneotherapy (or spa therapy) for rheumatoid arthritis. An abridged version of Cochrane Systematic Review. Eur. J. Phys. Rehabil. Med. 2015, 51, 833–847. [Google Scholar]
- Lind-Albrecht, G. Einfluss der Radonstollentherapie Auf Schmerzen und Verlauf Bei Spondylitis Ankylosans; Johannes Gutenberg-Universität: Mainz, Germany, 1994. [Google Scholar]
- Pratzel, H.; Legler, B.; Aurand, K.; Baumann, K.; Franke, T. Wirksamkeitsnachweis von Radonbädern im Rahmen einer kurortmedizinischen Behandlung des zervikalen Schmerzsyndroms. Phys. Med. Rehabil. Kurortmed. 1993, 3, 76–82. [Google Scholar] [CrossRef]
- Pratzel, H. Schmerzstillen-der Langzeiteffekt durch Radonbader bei nicht entzundlichen rheumatischen Erkrankungen. Radon Und Gesundh. Radon Health 1999, 163–182. [Google Scholar]
- Bellomo, R.; Bagshaw, S.M. Evidence-based medicine: Classifying the evidence from clinical trials—The need to consider other dimensions. Crit. Care 2006, 10, 232. [Google Scholar] [CrossRef] [Green Version]
- Franke, A.; Reiner, L.; Pratzel, H.; Franke, T.; Resch, K. Long-term efficacy of radon spa therapy in rheumatoid arthritis—A randomized, sham-controlled study and follow-up. Rheumatology 2000, 39, 894–902. [Google Scholar] [CrossRef] [Green Version]
- Franke, A.; Reiner, L.; Resch, K.L. Long-term benefit of radon spa therapy in the rehabilitation of rheumatoid arthritis: A randomised, double-blinded trial. Rheumatol Int. 2007, 27, 703–713. [Google Scholar] [CrossRef]
- Ruhle, P.F.; Fietkau, R.; Gaipl, U.S.; Frey, B. Development of a Modular Assay for Detailed Immunophenotyping of Peripheral Human Whole Blood Samples by Multicolor Flow Cytometry. Int. J. Mol. Sci. 2016, 17, 1316. [Google Scholar] [CrossRef] [Green Version]
- Kullmann, M.; Rühle, P.F.; Harrer, A.; Donaubauer, A.; Becker, I.; Sieber, R.; Klein, G.; Fournier, C.; Fietkau, R.; Gaipl, U.S. Temporarily increased TGFβ following radon spa correlates with reduced pain while serum IL-18 is a general predictive marker for pain sensitivity. Radiat. Environ. Biophys. 2019, 58, 129–135. [Google Scholar] [CrossRef]
- Cucu, A.; Shreder, K.; Kraft, D.; Rühle, P.F.; Klein, G.; Thiel, G.; Frey, B.; Gaipl, U.S.; Fournier, C. Decrease of Markers related to Bone erosion in serum of Patients with Musculoskeletal Disorders after serial low-Dose radon spa Therapy. Front. Immunol. 2017, 8, 882. [Google Scholar] [CrossRef]
- Rühle, P.F.; Klein, G.; Rung, T.; Tiep Phan, H.; Fournier, C.; Fietkau, R.; Gaipl, U.S.; Frey, B. Impact of radon and combinatory radon/carbon dioxide spa on pain and hypertension: Results from the explorative RAD-ON01 study. Mod. Rheumatol. 2018, 29, 165–172. [Google Scholar] [CrossRef]
- Rühle, P.F.; Wunderlich, R.; Deloch, L.; Fournier, C.; Maier, A.; Klein, G.; Fietkau, R.; Gaipl, U.S.; Frey, B. Modulation of the peripheral immune system after low-dose radon spa therapy: Detailed longitudinal immune monitoring of patients within the RAD-ON01 study. Autoimmunity 2017, 50, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Van Tubergen, A.; Landewé, R.; Van Der Heijde, D.; Hidding, A.; Wolter, N.; Asscher, M.; Falkenbach, A.; Genth, E.; Thè, H.G.; van der Linden, S. Combined spa—Exercise therapy is effective in patients with ankylosing spondylitis: A randomized controlled trial. Arthritis Care Res. 2001, 45, 430–438. [Google Scholar] [CrossRef]
- Moder, A.; Hufnagl, C.; Lind-Albrecht, G.; Hitzl, W.; Hartl, A.; Jakab, M.; Ritter, M. Effect of combined Low-Dose Radon-and Hyperthermia Treatment (LDRnHT) of patients with ankylosing spondylitis on serum levels of cytokines and bone metabolism markers: A pilot study. Int. J. Low Radiat. 2010, 7, 423–435. [Google Scholar] [CrossRef] [Green Version]
- Shehata, M.; Schwarzmeier, J.D.; Hilgarth, M.; Demirtas, D.; Richter, D.; Hubmann, R.; Boeck, P.; Leiner, G.; Falkenbach, A. Effect of combined spa-exercise therapy on circulating TGF-β1 levels in patients with ankylosing spondylitis. Wien. Klin. Wochenschr. 2006, 118, 266–272. [Google Scholar] [CrossRef]
- Dischereit, G.; Neumann, N.; Müller-Ladner, U.; Kürten, B.; Lange, U. Einfluss einer seriellen niedrig-dosierten Radonstollen-Hyperthermie auf Schmerz, Krankheitsaktivität und zentrale Zytokine des Knochenmetabolismus bei ankylosierender Spondylitis—Eine Prospektivstudie. Aktuelle Rheumatol. 2014, 39, 304–309. [Google Scholar] [CrossRef]
- Lange, U.; Muller-Ladner, U.; Dischereit, G. Rheumatic Diseases and Molecular Physical Medicine—New Aspects. Phys. Med. Rehab Kuror 2017, 27, 205–210. [Google Scholar] [CrossRef] [Green Version]
- Kazandjieva, J.; Grozdev, I.; Darlenski, R.; Tsankov, N. Climatotherapy of psoriasis. Clin. Dermatol. 2008, 26, 477–485. [Google Scholar] [CrossRef]
- Naumann, J.; Sadaghiani, C. Therapeutic benefit of balneotherapy and hydrotherapy in the management of fibromyalgia syndrome: A qualitative systematic review and meta-analysis of randomized controlled trials. Arthritis Res. Ther. 2014, 16, R141. [Google Scholar] [CrossRef] [Green Version]
- Yamaoka, K.; Mitsunobu, F.; Hanamoto, K.; Shibuya, K.; Mori, S.; Tanizaki, Y.; Sugita, K. Biochemical comparison between radon effects and thermal effects on humans in radon hot spring therapy. J. Radiat. Res. 2004, 45, 83–88. [Google Scholar] [CrossRef]
- Yamaoka, K.; Mitsunobu, F.; Hanamoto, K.; Mori, S.; Tanizaki, Y.; Sugita, K. Study on biologic effects of radon and thermal therapy on osteoarthritis. J. Pain 2004, 5, 20–25. [Google Scholar] [CrossRef]
- Winklmayr, M.; Kluge, C.; Winklmayr, W.; Küchenhoff, H.; Steiner, M.; Ritter, M.; Hartl, A. Radon balneotherapy and physical activity for osteoporosis prevention: A randomized, placebo-controlled intervention study. Radiat. Environ. Biophys. 2015, 54, 123–136. [Google Scholar] [CrossRef]
- Lange, U.; Neumann, N.; Kürten, B.; Müller-Ladner, U.; Tarner, I. Einfluss einer seriellen niedrig dosierten Radonstollen-Hyperthermie auf zentrale Zytokine des Knochen-metabolismus bei ankylosierender Spondylitis. Phys. Med. Rehabil. Kurortmed. 2012, 22, 203–206. [Google Scholar] [CrossRef]
- Lange, U.; Dischereit, G.; Tarner, I.; Frommer, K.; Neumann, E.; Müller-Ladner, U.; Kürten, B. The impact of serial radon and hyperthermia exposure in a therapeutic adit on pivotal cytokines of bone metabolism in rheumatoid arthritis and osteoarthritis. Clin. Rheumatol. 2016, 35, 2783–2788. [Google Scholar] [CrossRef]
- Lange, U.; Dischereit, G.; Müller-Ladner, U.; Tarner, I.H.; Kürten, B. Einfluss einer kombinierten seriellen Radonstollen-Hyperthermie auf klinische Parameter und ausgewählte Zytokine bei rheumatoider Arthritis und Osteoarthrose. Phys. Med. Rehabil. Kurortmed. 2017, 27, 87–94. [Google Scholar] [CrossRef]
- Nagy, K.; Berhes, I.; Kovacs, T.; Kavasi, N.; Somlai, J.; Kovacs, L.; Barna, I.; Bender, T. Study on endocronological effects of radon speleotherapy on respiratory diseases. Int. J. Radiat. Biol. 2009, 85, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Schett, G.; Gravallese, E. Bone erosion in rheumatoid arthritis: Mechanisms, diagnosis and treatment. Nat. Rev. Rheumatol. 2012, 8, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Guermazi, A.; Niu, J.; Hayashi, D.; Roemer, F.W.; Englund, M.; Neogi, T.; Aliabadi, P.; McLennan, C.E.; Felson, D.T. Prevalence of abnormalities in knees detected by MRI in adults without knee osteoarthritis: Population based observational study (Framingham Osteoarthritis Study). BMJ 2012, 345, e5339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neumann, E.; Junker, S.; Schett, G.; Frommer, K.; Müller-Ladner, U. Adipokines in bone disease. Nat. Rev. Rheumatol. 2016, 12, 296. [Google Scholar] [CrossRef] [PubMed]
- Tennant, F. The physiologic effects of pain on the endocrine system. Pain Ther. 2013, 2, 75–86. [Google Scholar] [CrossRef] [Green Version]
- Sabatine, M.S.; Morrow, D.A.; de Lemos, J.A.; Omland, T.; Sloan, S.; Jarolim, P.; Solomon, S.D.; Pfeffer, M.A.; Braunwald, E. Evaluation of multiple biomarkers of cardiovascular stress for risk prediction and guiding medical therapy in patients with stable coronary disease. Circulation 2012, 125, 233–240. [Google Scholar] [CrossRef] [Green Version]
- Xu, N.-Y.; Zhang, S.-P.; Dong, L.; Nie, J.-H.; Tong, J. Proteomic analysis of lung tissue of rats exposed to cigarette smoke and radon. J. Toxicol. Environ. Health Part A 2009, 72, 752–758. [Google Scholar] [CrossRef]
- Kataoka, T.; Nishiyama, Y.; Toyota, T.; Yoshimoto, M.; Sakoda, A.; Ishimori, Y.; Aoyama, Y.; Taguchi, T.; Yamaoka, K. Radon inhalation protects mice from carbon-tetrachloride-induced hepatic and renal damage. Inflammation 2011, 34, 559–567. [Google Scholar] [CrossRef] [Green Version]
- Kataoka, T.; Sakoda, A.; Yoshimoto, M.; Nakagawa, S.; Toyota, T.; Nishiyama, Y.; Yamato, K.; Ishimori, Y.; Kawabe, A.; Hanamoto, K. Studies on possibility for alleviation of lifestyle diseases by low-dose irradiation or radon inhalation. Radiat. Prot. Dosim. 2011, 146, 360–363. [Google Scholar] [CrossRef] [Green Version]
- Kataoka, T.; Nishiyama, Y.; Yamato, K.; Teraoka, J.; Morii, Y.; Sakoda, A.; Ishimori, Y.; Taguchi, T.; Yamaoka, K. Comparative study on the inhibitory effects of antioxidant vitamins and radon on carbon tetrachloride-induced hepatopathy. J. Radiat. Res. 2012, 53, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yonehara, H.; Ikebuchi, M.; Aoyama, T. Effect of radon exposure on superoxide dismutase (SOD) activity in rats. J. Radiat. Res. 1996, 37, 12–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kataoka, T.; Sakoda, A.; Ishimori, Y.; Toyota, T.; Nishiyama, Y.; Tanaka, H.; Mitsunobu, F.; Yamaoka, K. Study of the response of superoxide dismutase in mouse organs to radon using a new large-scale facility for exposing small animals to radon. J. Radiat. Res. 2011, 52, 775–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kataoka, T.; Etani, R.; Kanzaki, N.; Kobashi, Y.; Yunoki, Y.; Ishida, T.; Sakoda, A.; Ishimori, Y.; Yamaoka, K. Radon inhalation induces manganese-superoxide dismutase in mouse brain via nuclear factor-κB activation. J. Radiat. Res. 2017, 58, 887–893. [Google Scholar] [CrossRef] [Green Version]
- Deloch, L.; Derer, A.; Hueber, A.J.; Herrmann, M.; Schett, G.A.; Wölfelschneider, J.; Hahn, J.; Rühle, P.-F.; Stillkrieg, W.; Fuchs, J. Low-dose radiotherapy ameliorates advanced arthritis in hTNF-α tg mice by particularly positively impacting on bone metabolism. Front. Immunol. 2018, 9, 1834. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, M.; Kojima, S. Suppression of atopic dermatitis and tumor metastasis in mice by small amounts of radon. Radiat. Res. 2006, 165, 337–342. [Google Scholar] [CrossRef]
- Kataoka, T.; Yamato, K.; Nishiyama, Y.; Morii, Y.; Etani, R.; Takata, Y.; Hanamoto, K.; Kawabe, A.; Sakoda, A.; Ishimori, Y. Comparative study on the inhibitory effects of α-tocopherol and radon on carbon tetrachloride-induced renal damage. Ren. Fail. 2012, 34, 1181–1187. [Google Scholar] [CrossRef] [Green Version]
- Kataoka, T.; Teraoka, J.; Sakoda, A.; Nishiyama, Y.; Yamato, K.; Monden, M.; Ishimori, Y.; Nomura, T.; Taguchi, T.; Yamaoka, K. Protective effects of radon inhalation on carrageenan-induced inflammatory paw edema in mice. Inflammation 2012, 35, 713–722. [Google Scholar] [CrossRef] [Green Version]
- Nishiyama, Y.; Kataoka, T.; Yamato, K.; Taguchi, T.; Yamaoka, K. Suppression of dextran sulfate sodium-induced colitis in mice by radon inhalation. Mediat. Inflamm. 2012, 2012, 239617. [Google Scholar] [CrossRef] [Green Version]
- Toyota, T.; Kataoka, T.; Nishiyama, Y.; Taguchi, T.; Yamaoka, K. Inhibitory effects of pretreatment with radon on acute alcohol-induced hepatopathy in mice. Mediat. Inflamm. 2012, 2012, 382801. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Kataoka, T.; Teraoka, J.; Sakoda, A.; Tanaka, H.; Ishimori, Y.; Mitsunobu, F.; Taguchi, T.; Yamaoka, K. Suppression of streptozotocin-induced type-1 diabetes in mice by radon inhalation. Physiol. Res. 2013, 62, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Yamato, K.; Kataoka, T.; Nishiyama, Y.; Taguchi, T.; Yamaoka, K. Antinociceptive effects of radon inhalation on formalin-induced inflammatory pain in mice. Inflammation 2013, 36, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Etani, R.; Kataoka, T.; Kanzaki, N.; Sakoda, A.; Tanaka, H.; Ishimori, Y.; Mitsunobu, F.; Yamaoka, K. Difference in the action mechanism of radon inhalation and radon hot spring water drinking in suppression of hyperuricemia in mice. J. Radiat. Res. 2016, 57, 250–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kataoka, T.; Horie, S.; Etani, R.; Kanzaki, N.; Sasaoka, K.; Kobashi, Y.; Hanamoto, K.; Yamaoka, K. Activation of antioxidative functions by radon inhalation enhances the mitigation effects of pregabalin on chronic constriction injury-induced neuropathic pain in mice. Oxidative Med. Cell. Longev. 2016, 2016, 9853692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etani, R.; Kataoka, T.; Kanzaki, N.; Sakoda, A.; Tanaka, H.; Ishimori, Y.; Mitsunobu, F.; Taguchi, T.; Yamaoka, K. Protective effects of hot spring water drinking and radon inhalation on ethanol-induced gastric mucosal injury in mice. J. Radiat. Res. 2017, 58, 614–625. [Google Scholar] [CrossRef] [PubMed]
- Pei, W.; Tao, L.; Zhang, L.W.; Zhang, S.; Cao, J.; Jiao, Y.; Tong, J.; Nie, J. Circular RNA profiles in mouse lung tissue induced by radon. Environ. Health Prev. Med. 2017, 22, 36. [Google Scholar] [CrossRef] [Green Version]
- Paletta, B.; Truppe, W.; Mlekusch, W.; Pohl, E.; Hofmann, W.; Steinhäusler, F. Time function of corticosteroid levels in the blood plasma of rats under the influence of 222 Rn inhalation. Experientia 1976, 32, 652–653. [Google Scholar] [CrossRef]
- Taya, A.; Morgan, A.; Baker, S.T.; Humphreys, J.A.; Bisson, M.; Collier, C.G. Changes in the rat lung after exposure to radon and its progeny: Effects on incorporation of bromodeoxyuridine in epithelial cells and on the incidence of nuclear aberrations in alveolar macrophages. Radiat. Res. 1994, 139, 170–177. [Google Scholar] [CrossRef]
- Collier, C.G.; Bisson, M.; Baker, S.T.; Eldred, T.; Fritsch, P.; Morlier, J.P.; Monchaux, G. Early cellular responses in rats exposed to radon and radon progeny. Ann. Occup. Hyg. 1997, 41, 86–91. [Google Scholar]
- Cui, F.; Fan, S.; Hu, M.; Nie, J.; Li, H.; Tong, J. Micronuclei rate and hypoxanthine phosphoribosyl transferase mutation in radon-exposed rats. Prog. Nat. Sci. 2008, 18, 1305–1308. [Google Scholar] [CrossRef]
- Yamaoka, K.; Komoto, Y.; Suzuka, I.; Edamatsu, R.; Mori, A. Effects of radon inhalation on biological function-lipid peroxide level, superoxide dismutase activity, and membrane fluidity. Arch. Biochem. Biophys. 1993, 302, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, T.; Etani, R.; Takata, Y.; Nishiyama, Y.; Kawabe, A.; Kumashiro, M.; Taguchi, T.; Yamaoka, K. Radon inhalation protects against transient global cerebral ischemic injury in gerbils. Inflammation 2014, 37, 1675–1682. [Google Scholar] [CrossRef] [PubMed]
- ICRP. ICRP Publication 66: Human Respiratory Tract Model for Radiological Protection; Elsevier Health Sciences: Amsterdam, The Netherlands, 1995; Volume 66. [Google Scholar]
- Maier, A.; van Beek, P.; Hellmund, J.; Durante, M.; Schardt, D.; Kraft, G.; Fournier, C. Experimental setup for radon exposure and first diffusion studies using gamma spectroscopy. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2015, 362, 187–193. [Google Scholar] [CrossRef]
- Becker, I.; Donaubauer, A.-J.; Klein, G.; Fournier, C.; Fietkau, R.; Frey, B.; Gaipl, U. P150 Impact of radon SPA on pain and the immune system of patients with musculoskeletal disorders. J. Ann. Rheum. Dis. 2019, 78, A66. [Google Scholar] [CrossRef] [Green Version]
- Landrichinger, J.; Holzl, B.; Untner, J.; Foisner, W.; Edtinger, S.; Knapp, M.; Ritter, M.; Gaisberger, M. Radon Registry Study. Acta Physiol. 2017, 221, 108–110. [Google Scholar]
- Shaw, A.T.; Gravallese, E.M. Mediators of inflammation and bone remodeling in rheumatic disease. In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2016; pp. 2–10. [Google Scholar]
- Morgan, W.F. Non-targeted and delayed effects of exposure to ionizing radiation: I. Radiation-induced genomic instability and bystander effects in vitro. Radiat. Res. 2012, 178, AV223–AV236. [Google Scholar] [CrossRef]
- Morgan, W.F. Non-targeted and delayed effects of exposure to ionizing radiation: II. Radiation-induced genomic instability and bystander effects in vivo, clastogenic factors and transgenerational effects. Radiat. Res. 2003, 159, 581–596. [Google Scholar] [CrossRef]
- Zaiss, M.M.; Axmann, R.; Zwerina, J.; Polzer, K.; Gückel, E.; Skapenko, A.; Schulze-Koops, H.; Horwood, N.; Cope, A.; Schett, G. Treg cells suppress osteoclast formation: A new link between the immune system and bone. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2007, 56, 4104–4112. [Google Scholar] [CrossRef]
- Barcellos-Hoff, M.H.; Dix, T.A. Redox-mediated activation of latent transforming growth factor-beta 1. Mol. Endocrinol. 1996, 10, 1077–1083. [Google Scholar] [CrossRef] [Green Version]
- Kotake, S.; Udagawa, N.; Hakoda, M.; Mogi, M.; Yano, K.; Tsuda, E.; Takahashi, K.; Furuya, T.; Ishiyama, S.; Kim, K.J. Activated human T cells directly induce osteoclastogenesis from human monocytes: Possible role of T cells in bone destruction in rheumatoid arthritis patients. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2001, 44, 1003–1012. [Google Scholar] [CrossRef]
- Weitzmann, M.N.; Cenci, S.; Haug, J.; Brown, C.; DiPersio, J.; Pacifici, R. B lymphocytes inhibit human osteoclastogenesis by secretion of TGFbeta. J. Cell Biochem. 2000, 78, 318–324. [Google Scholar] [CrossRef]
- Lee, B.; Oh, Y.; Jo, S.; Kim, T.H.; Ji, J.D. A dual role of TGF-beta in human osteoclast differentiation mediated by Smad1 versus Smad3 signaling. Immunol. Lett. 2019, 206, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Isales, C.M.; Zaidi, M.; Blair, H.C. ACTH is a novel regulator of bone mass. Skelet. Biol. Med. 2010, 1192, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Gillmore, G.K.; Perrier, F.; Crockett, R.G. Radon, Health and Natural Hazards; The Geological Society: London, UK, 2018. [Google Scholar]

| 222Rn | 220Rn | ||||
|---|---|---|---|---|---|
| Nuclide | Half-Life | Decay-Mode | Nuclide | Half-Life | Decay-Mode |
| 222Rn | 3.825 d | α | 220Rn | 55 s | α, γ |
| 218Po | 3.05 min | α | 216Po | 0.15 s | α |
| 214Pb | 26.8 min | β, γ | 212Pb | 10.64 h | β, γ |
| 214Bi | 19.9 min | β, γ | 212Bi | 60.6 min | α, β, γ |
| 214Po | 164 µs | α | 212Po | 304 ns | α |
| 210Pb | 22.3 a | β, γ | 208Tl | 3.05 min | β, γ |
| 210Bi | 5.0 d | β, γ | 208Pb | stable | |
| 210Po | 138.4 d | α | |||
| 206Pb | stable | ||||
| First Author Year of Publication | Species | Group Size | Type of Treatment and Dose | Time of Analysis after Exposure | Disease Model | Endpoints | Most Important Findings | Ref. |
|---|---|---|---|---|---|---|---|---|
| Takahashi et al., 2006 | Mice (SPF NC/Nga, female, 5 weeks) Mice (C57BL/6, male, 6 weeks) | n= 4–9 | Drinking water; 203 Bq/L; approximate amount of radon ingested by each mouse 140–176, 68–85 and 0.86–1.08 Bq/kg week | Up to 4 weeks | Atopic dermatitis model: sensitization with 5% purified picrylchloride Lung metastasis model: injection of B16 melanoma cells (both 2 weeks after start of radon treatment) | Atopic dermatitis: Skin severity score, Plasma IgE Lung metastasis: number of metastasis | Lower skin severity score and lower plasma IgE, only after radon pretreatment, Lower number of lung metastasis only after radon pretreatment and small number of inoculated tumor cells | [132] |
| Kataoka et al., 2011 | Mice (BALB/c, male, 7–8 weeks, 25 g) | n = 5 (Exp.3) n = 4–7 (Exp.4) n = 5–6 (Exp.5) | Exp.3: inhalation for 24 h, 4000 Bq/m3 Exp:4 600 and 3500 Bq/m3 Exp.5: 180 Bq/m3 for 6 h | Exp.3: directly Exp.4: 4 h Exp.5: 24 h | Alcohol-induced oxidative damage; CCl4-induced hepathopathy | SOD activity Catalase activity ALD-activity and t-GSH in brain and liver | Protective effect of radon on oxidative damage | [126] |
| Kataoka et al., 2011 | Mice (BALB/c, male, 7 weeks, 25 g) | n= 4–6 | Inhalation, 18 kBq/m3 for 6 h | 24 h | CCl4-induced hepatic and renal damage | t-GSH content, lipid peroxide levels, and GPx and GR activity in liver and kidney GOT, GPT, ALP activity, CRE, and T-CHO in serum | Radon inhalation inhibits oxidative damage of liver and kidney | [125] |
| Kataoka et al., 2011 | Mice (BALB/c, male, 7 weeks, 25 g) | n = 5 | Inhalation, 250, 500, 1000, 2000, or 4000 Bq/m3 for 0.5, 1, 2, 4, or 8 days | Directly | Healthy | SOD activity in brain, lung, thymus, heart, liver, stomach, pancreas, kidney | Activation of SOD; in plasma, brain, and lung strong and rapid response (enhancement); in liver, heart, pancreas, and small intestine only after low and high concentrations; in thymus and kidney after low concentration; no change in stomach | [129] |
| Kataoka et al., 2012 | Mice (ICR, female, 8 weeks, 28 g) | n = 5–8 | Inhalation, 1000 or 2000 Bq/m3 for 24 h or (L(+)-ascorbic acid injection or DL-α- tocopherol injection | 24 h | CCl4-induced hepathopathy | SOD activity, catalase activity, GPx activity, t-GSH, LP levels and TG in the liver; GOT, GPT activity, TG and T-CHO levels in the serum; and histological examination of liver tissue | Decreased activities of GOT and GPT in serum; decreased TG levels in liver significantly higher SOD, catalase and GPx activity in livers; radon inhalation has an antioxidative effect against CCl4-induced hepatopathy that is comparable to treatment with AA or α-tocopherol | [127] |
| Kataoka et al., 2012 | Mice (ICR, female, 8 weeks, 28 g) | n = 5–8 | Inhalation, 1000 or 2000 Bq/m3 for 24 h or DL-α-tocopherol injection different concentrations) | 24 h | CCl4-induced hepathopathy | SOD, catalase, t-GSH, and LP in kidneys CRE level in serum, | Decrease of CRE an LP levels; radon inhalation has an antioxidative effect comparable to the treatment with α-tocopherol at a dose of 300–500 mg/kg weight | [133] |
| Kataoka et al., 2012 | Mice (ICR, female, 8 weeks, 28 g) | n = 6–7 | Inhalation, 2000 Bq/m3 for 24 h | 2 h | Carrageenan-induced inflammatory paw edema | SOD activity, catalase activity, t-GSH content, LP levels, TNF-α, NO, and paw histology. | Paw volume significantly decreased; lower TNF- α and NO levels; SOD activity increased; fewer infiltrating leukocytes; increased SOD and catalase activities | [134] |
| Nishiyama et al., 2012 | Mice (BALB/c, male, 7 weeks, 23 g) | n = 8 | Inhalation, 2000 Bq/m3 for 8 days | Directly | Dextran sulfate sodium (DSS) model of colitis (while radon exposure) | MPO, NO, TNF-α, SOD, CAT, t-GSH), LPO level, and Histology, DAI and weight gain | Significant lower DAI score; less shortened colon; lower plasma TNF- α and MPO activity in colon; enhanced SOD activity and tGSH content; lower LPO level in the colon and NO level in plasma | [135] |
| Toyota et al., 2012 | Mice (C57BL/6J, male, 8 weeks, 20 g) | n = 4–6 | Inhalation, 4000 Bq/m3 for 24 h | 6 and 24 h | Acute alcohol-induced hepatopathy | SOD, catalase, t-GSH, GPx, GR, TG, and lipid peroxide in liver, GOT and GPT, activity and the TG, T-CHO in serum | Radon treatment activates antioxidative functions and inhibits acute alcohol-induced oxidative damage, hepatopathy and fatty liver in mice | [136] |
| Nishiyama et al., 2013 | Mice, (C57BL/6J, male, 9 weeks, 25–28 g) | n = 5–8 | Inhalation, 1000, 2500, and 5500 Bq/m3 for 24 h | 4 days | Streptozotocin-induced Type-1 Diabetes (after radon exposure) | SOD activity, CAT activity, t-GSH content, LPO, blood glucose, serum insulin, and body weight | Higher SOD activity and t-GSH content, lower LPO levels; significantly suppressed blood glucose elevation and body weight decrease; higher serum insulin; radon inhalation partially suppressed type-1 diabetes induced by STZ administration | [137] |
| Yamato et al., 2013 | Mice (male ICR, 8 weeks, 38 g) | n = 5–10 | Inhalation, 1000 or 2000 Bq/m3 for 24 h | Up to 35 min (licking response), no information for other endpoints | Formalin-induced transient inflammatory pain | licking response (pain), TNF-α, NO, paw histology, SOD and CAT activities, total glutathione (t-GSH) content, and LPO levels | Enhanced SOD-activity, t-GSH content in serum and paws, reduced number of leukocytes, reduced TNF-α and NO level | [138] |
| Etani et al., 2016 | Mice (male, 8 weeks, 32–38 g) | n = 8–9 (drinking treatment) n = 6 (inhalation) | Drinking water: 338 ± 11 Bq/L for 2 weeks Inhalation: 2000 Bq/m3 for 24 h | 3 h | PO model of hyperuricemia (induced after radon treatment) | Activities of XOD, SOD andCAT; levels of t-GSH and proteins in liver and kidney | Radon-inhalation activates antioxidative function and reduces serum uric acid levels | [139] |
| Kataoka et al., 2016 | Mice (ICR, male, 8 weeks; 33–40 g) | n = 5–6 | Inhalation, 1000 Bq/m3 for 24 h and/or pregabalin treatment. | 30 min, 60 min, 90 min, 120 min | CCI—induced neuropathic pain | von Frey Test (pain), SOD activity, catalase activity, t-GSH content, and LP level in paw. | Pregabalin and radon has mitigative effect on pain after CCI due to antioxidative function after radon inhalation | [140] |
| Etani et al., 2017 | Mice (BALB/c, male, 8 weeks, 25–28 g) | n = 8 (drinking treatment) n = 8 (inhalation) | Drinking water: 663 ± 36 Bq/L for 2 weeks Inhalation: 2000 Bq/m3 for 24 h | 1 h | Gastric mucosal injury induced by oral ethanol administration (induced after radon treatment) | UI and HI: SOD and CAT activity, and the levels of t-GSH in stomachs | Lower UI and IHI after radon treatment; activation of antioxidative mechanisms | [141] |
| Kataoka et al., 2017 | Mice (BALB/c, male, 8 weeks, 24–28 g) | n = 7 | Inhalation, 500–2000 Bq/m3 for 24 h | Unclear | Healthy | NF-κB, NIK, IKK-β, ATM; total SOD, Mn-SOD and Cu/Zn-SOD activities and protein levels | Induction of SOD proteins, mainly Mn-SOD; Mn-SOD induced by NF-κB activation stimulated by DNA damage and oxidative stress | [130] |
| Pei et al., 2017 | Mice, (BALB/c, male, 15 g) | n = 6 | Inhalation, 100,000 Bq/m3, 12 h/d, for up to cumulative doses of 60 WLM | Directly | Healthy | circRNA, H&E, Caspase 3 | Enhanced Caspase 3 expression, circRNA profiles are changed | [142] |
| Paletta et al. 1975 | Rat (male, 200 g) | n = 5 | Series 1: Rn 12.5 nCi/L, RaB/Rn 0,25; Series 2: Rn 110 nCi/L, RaB/Rn 0,33 Different doses to organs? | 12 d | Healthy | Corticosteroid level in serum | 2 maxima of corticosteroid after exposure, one after 8 h, one after 5 (low) or 9 h (high concentration) | [143] |
| Taya et al., 1994 | Rat (male, 4–6 months old) | n = 10–25 | 120–990 WLM (dose rate 7–9 WLM/h; 725–770 Bq/m3) | 7–28 d | Healthy | Proliferation in epithelial cells of respiratory tract; binucleate alveolar macrophages (AM) and/or micronuclei | Labelling indices increased after exposure; highest in bronchial epithelial cells; binculeate AM as well as induction of micronuclei was increased after exposure; binucleate AM with micronuclei were only induced in exposed animals; no inflammation | [144] |
| Ma et al., 1996 | Rats (Wistar, male, 30 weeks) | n = 3 | Inhalation, 1000–5000 kBq/m3 or 400–1600 kBq/m3 for 4 or 16 h | Directly | Healthy | SOD activity in blood, kidney, spleen, and liver | Increase after 4 h, decrease after 16 h of exposure | [128] |
| Collier et al., 1997 | Rats (Sprague-Dawley, male, 2–12 month, | n = 2–6 | Inhalation, 200–1600 WLM, 250–7142 WL for 1–27.5 days | 14 d | Healthy | Cell number, nuclear abberations, number of macrophages and macrophage proliferation in lung lavage fluid, H&E and BrdU staining of lung sections | Positive dose-response for most effects | [145] |
| Cui et al., 2008 | Rats (Wistar) | n = 6 | Inhalation; 60, 90, and 120 working level months (WLM) in total; inhalation for 8 h per day, 6 days per week | No information | Healthy | MNR, hprt assay in lymphocytes, and tracheal-bronchial epithelial cells | Dose-dependent increase of MNR, the mutation frequency of hprt is increased with accumulated dose, can be used as biomarkers for genetic changes after radon exposure | [146] |
| Yamaoka et al., 1993 | Rabbits | n = 10–14 | Inhalation of nebulized radon water; 7–10 kBq/L or 14–18 kBq/L | Directly and 2 h | Healthy | Lipid peroxide, SOD, membrane fluidity in brain, spleen, lung, liver and serum | Enhanced SOD activity, reduced lipid peroxide levels | [147] |
| Kataoka et al., 2014 | Mongolian gerbil MGS/sea, (female, 8 weeks, 50 g) | n = 5–7 | Inhalation, 2000 Bq/m3 for 24 h | Directly | Transient global cerebral ischemia induced by bilateral occlusion of the common carotid artery (3 days before radon treatment) | Brain histology, SOD activity, CAT activity, and t-GSH content in the brain and serum. | Number of damaged neurons significantly lower; increased SOD activity; unchanged t-GSH | [148] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maier, A.; Wiedemann, J.; Rapp, F.; Papenfuß, F.; Rödel, F.; Hehlgans, S.; Gaipl, U.S.; Kraft, G.; Fournier, C.; Frey, B. Radon Exposure—Therapeutic Effect and Cancer Risk. Int. J. Mol. Sci. 2021, 22, 316. https://doi.org/10.3390/ijms22010316
Maier A, Wiedemann J, Rapp F, Papenfuß F, Rödel F, Hehlgans S, Gaipl US, Kraft G, Fournier C, Frey B. Radon Exposure—Therapeutic Effect and Cancer Risk. International Journal of Molecular Sciences. 2021; 22(1):316. https://doi.org/10.3390/ijms22010316
Chicago/Turabian StyleMaier, Andreas, Julia Wiedemann, Felicitas Rapp, Franziska Papenfuß, Franz Rödel, Stephanie Hehlgans, Udo S. Gaipl, Gerhard Kraft, Claudia Fournier, and Benjamin Frey. 2021. "Radon Exposure—Therapeutic Effect and Cancer Risk" International Journal of Molecular Sciences 22, no. 1: 316. https://doi.org/10.3390/ijms22010316
APA StyleMaier, A., Wiedemann, J., Rapp, F., Papenfuß, F., Rödel, F., Hehlgans, S., Gaipl, U. S., Kraft, G., Fournier, C., & Frey, B. (2021). Radon Exposure—Therapeutic Effect and Cancer Risk. International Journal of Molecular Sciences, 22(1), 316. https://doi.org/10.3390/ijms22010316








