Global Genome Conformational Programming during Neuronal Development Is Associated with CTCF and Nuclear FGFR1—The Genome Archipelago Model
Abstract
:1. Introduction
2. Results
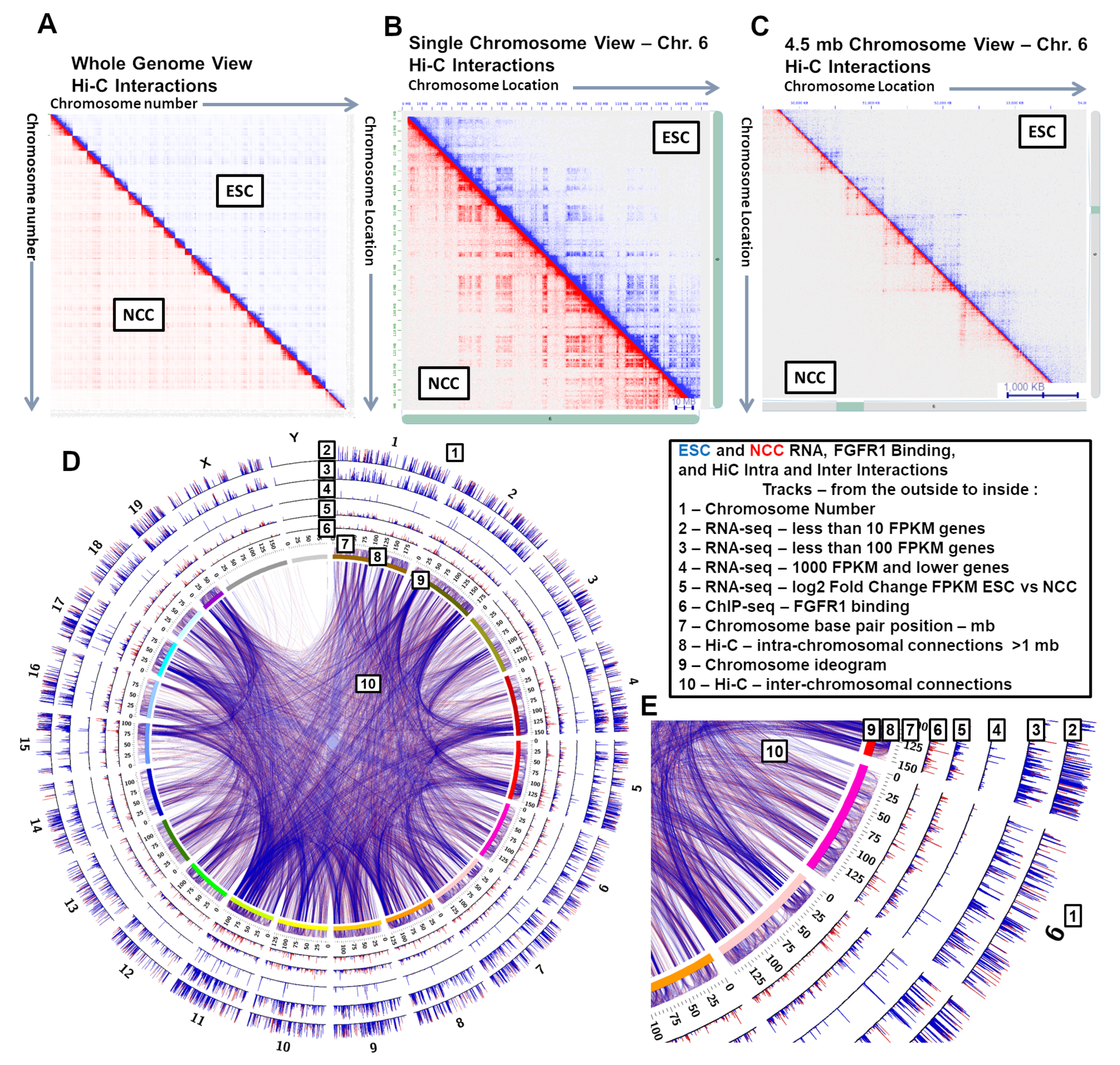
2.1. High-Throughput Interaction Analysis Points to a Vast Complexity of Genome Organization and Remodeling during Neuronal Development
2.2. Inter- and Intra-Chromosomal Interactions Change between ESC and NCC. Close Range Intra-Chromosomal Interactions Are Favored in ESC and Longer Range in NCC
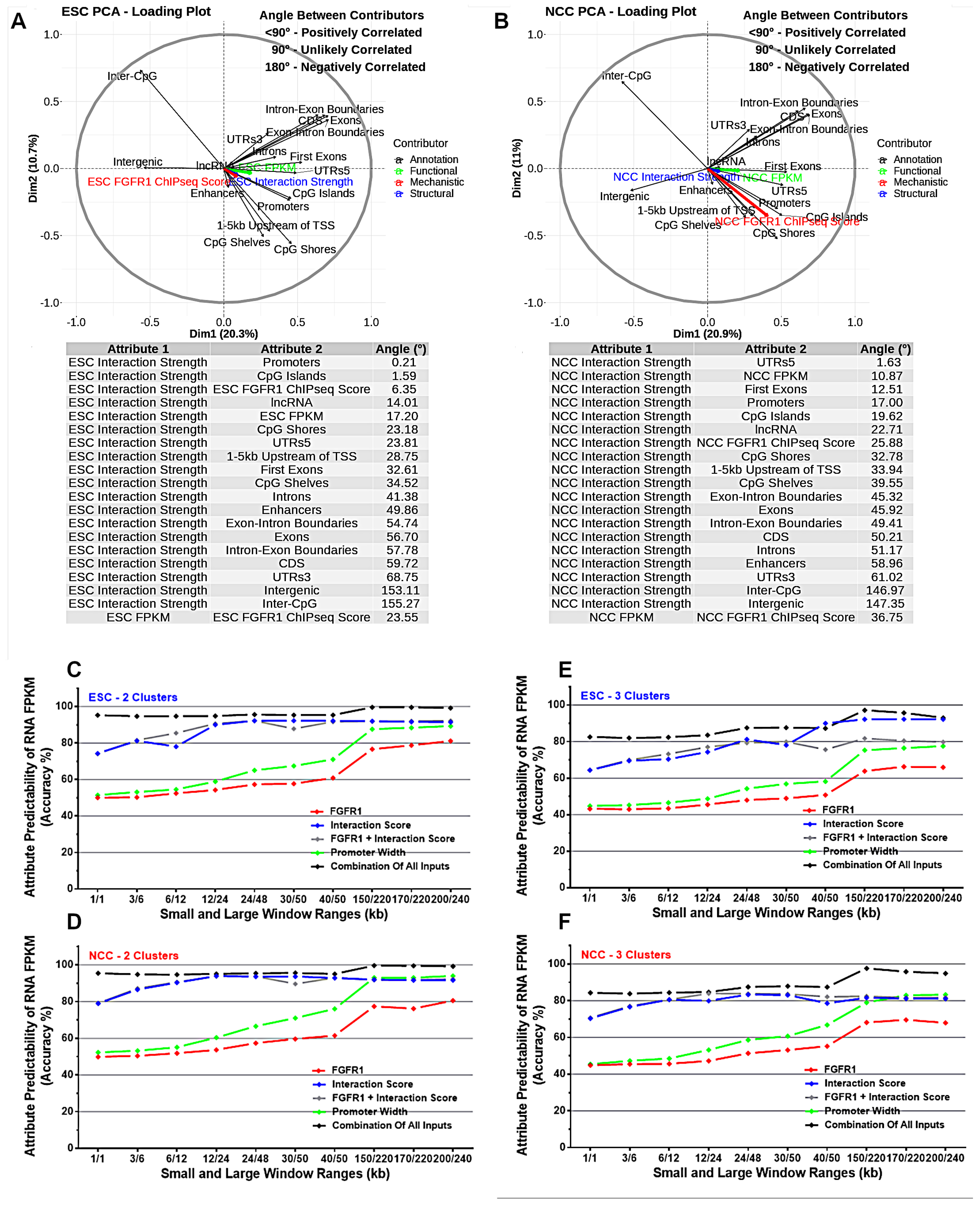
2.3. Chromatin Interaction Strength Correlates with Genome Regulatory and Coding Features, with nFGFR1 Binding and with Gene Expression Levels
2.4. Machine Learning Indicates That Genome Regulatory Features and Interaction Anchor Strength Predict Gene Expression FPKM
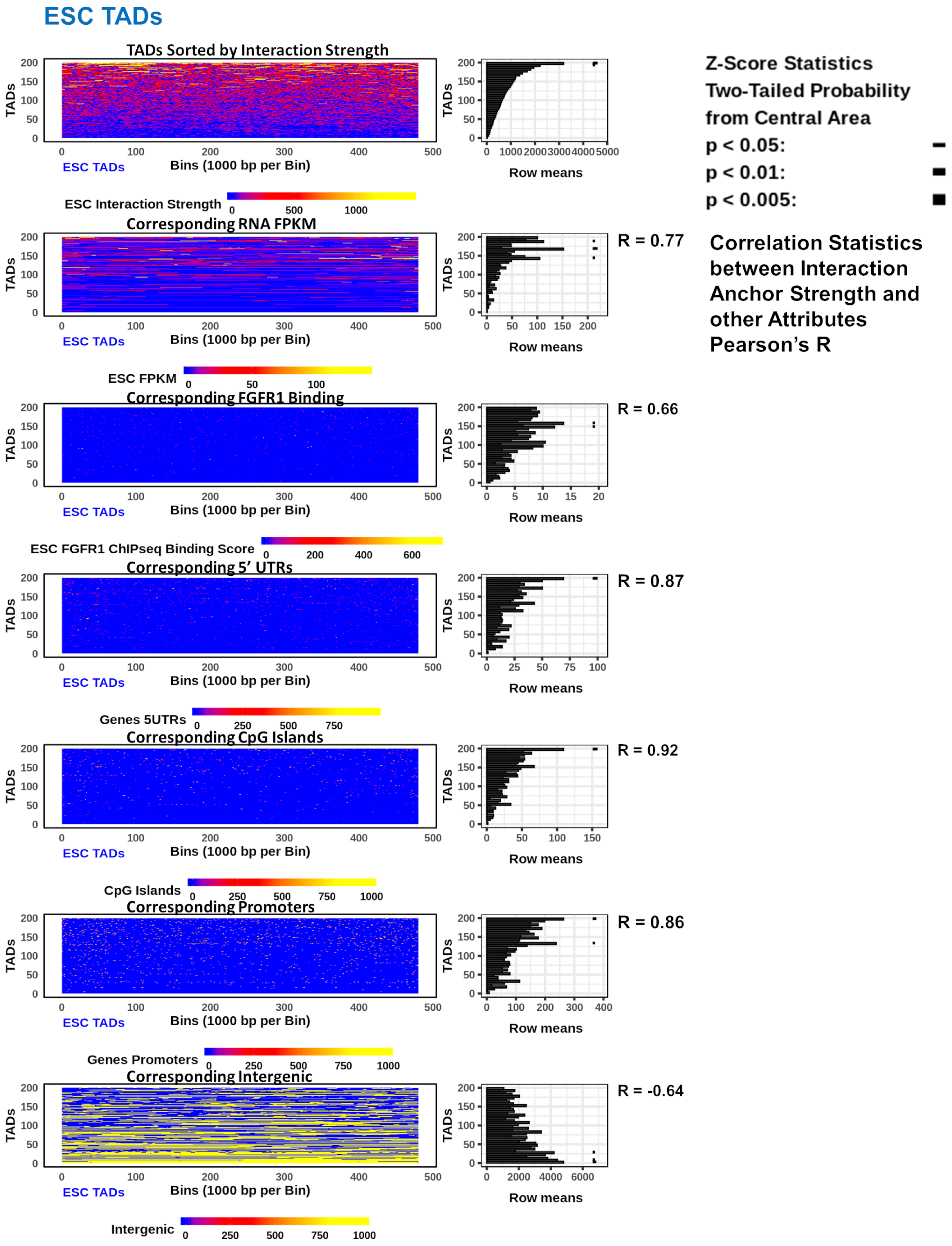
2.5. In ESC and NCC TADs Interaction Anchors Coincide with Gene Promoter and Coding Regions, Increase with nFGFR1 Binding, but Are Fewer in Intergenic Regions
2.6. Interacting Genes Concentrate within TADs, Regulate Together, and Share Ontological Functions
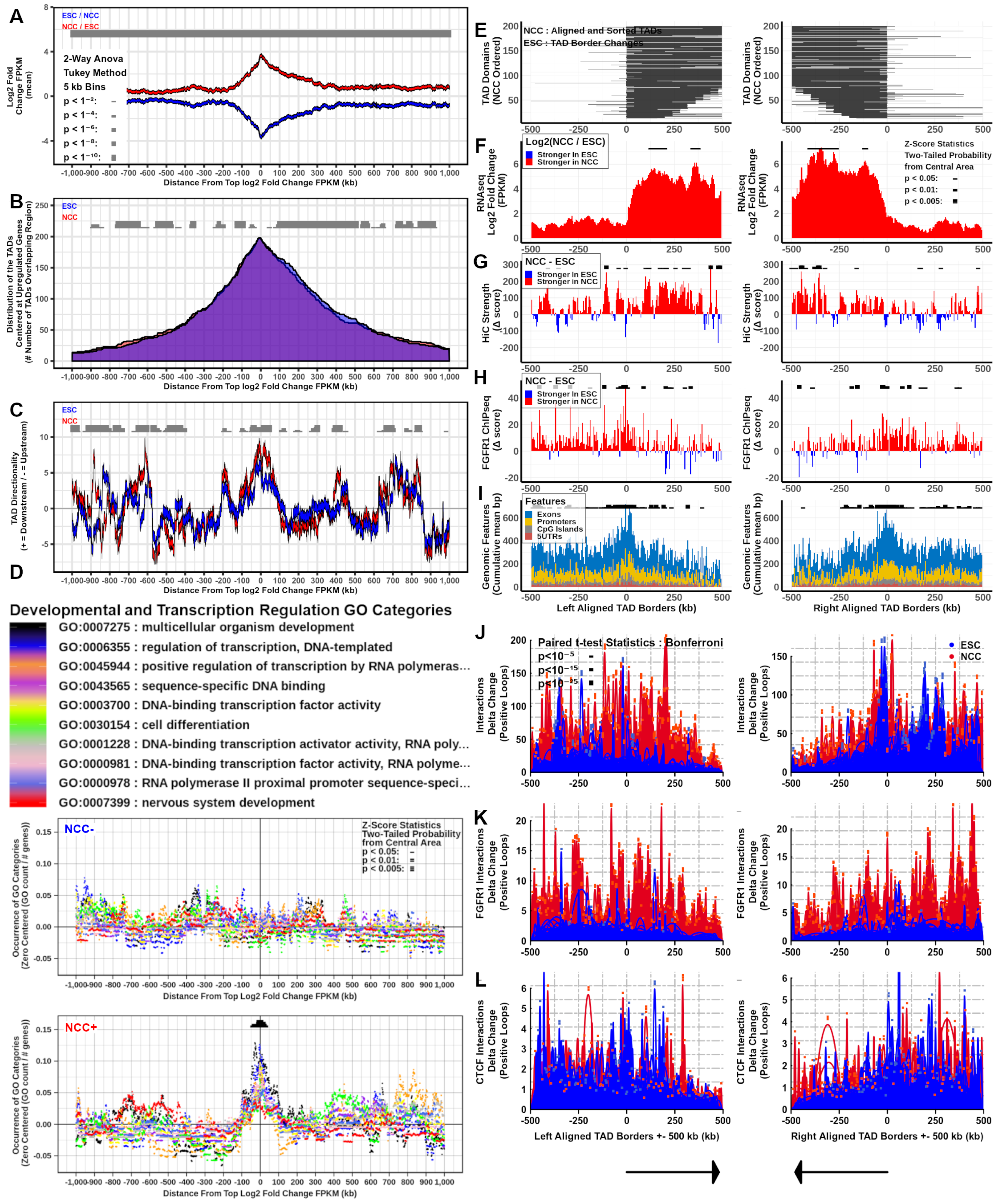
2.7. TAD Boundaries, Interaction Strength, and nFGFR1 Binding Change as TADs Genes Are Co-Regulated during Neuronal Development
2.8. nFGFR1 Bound Loops Are Enriched in NCC and CTCF Bound Loops Are Enriched in ESC
2.9. CTCF, MYC, MAX, NFIC, NFKB1, Pdx1, Spz1, and ZEB1 Binding Motifs Are Overrepresented on All TAD Borders, Other TFs Motifs Are Enriched Specifically on NCC+ or NCC− DiffTADs and Several Are Targeted by nFGFR1
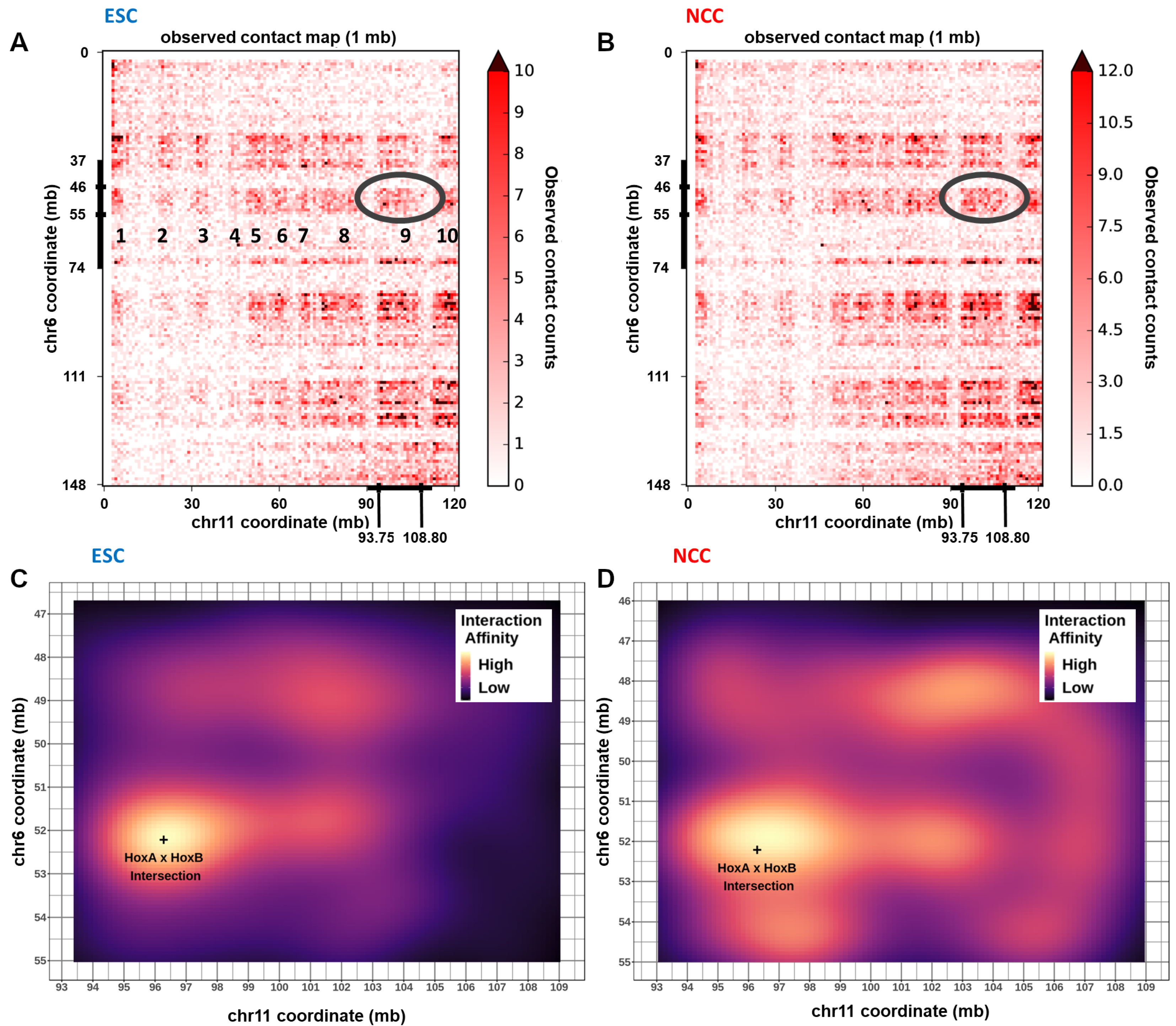
2.10. Chromatin Interactions, nFGFR1 Binding, and Gene Expression Change at Hox Gene Clusters during ESC to NCC Development
2.11. HoxA Cluster Quantitative Analysis: RNA Expression and Chromatin Interactions Are Delineated by FGFR1 and CTCF Binding Domains during ESC to NCC Differentiation
2.12. PD173074 Inhibition of FGFR1 Reduces nFGFR1 and CTCF Binding in the HoxA Cluster Accompanied by Altered Chromatin Interactions and Gene Dysregulation
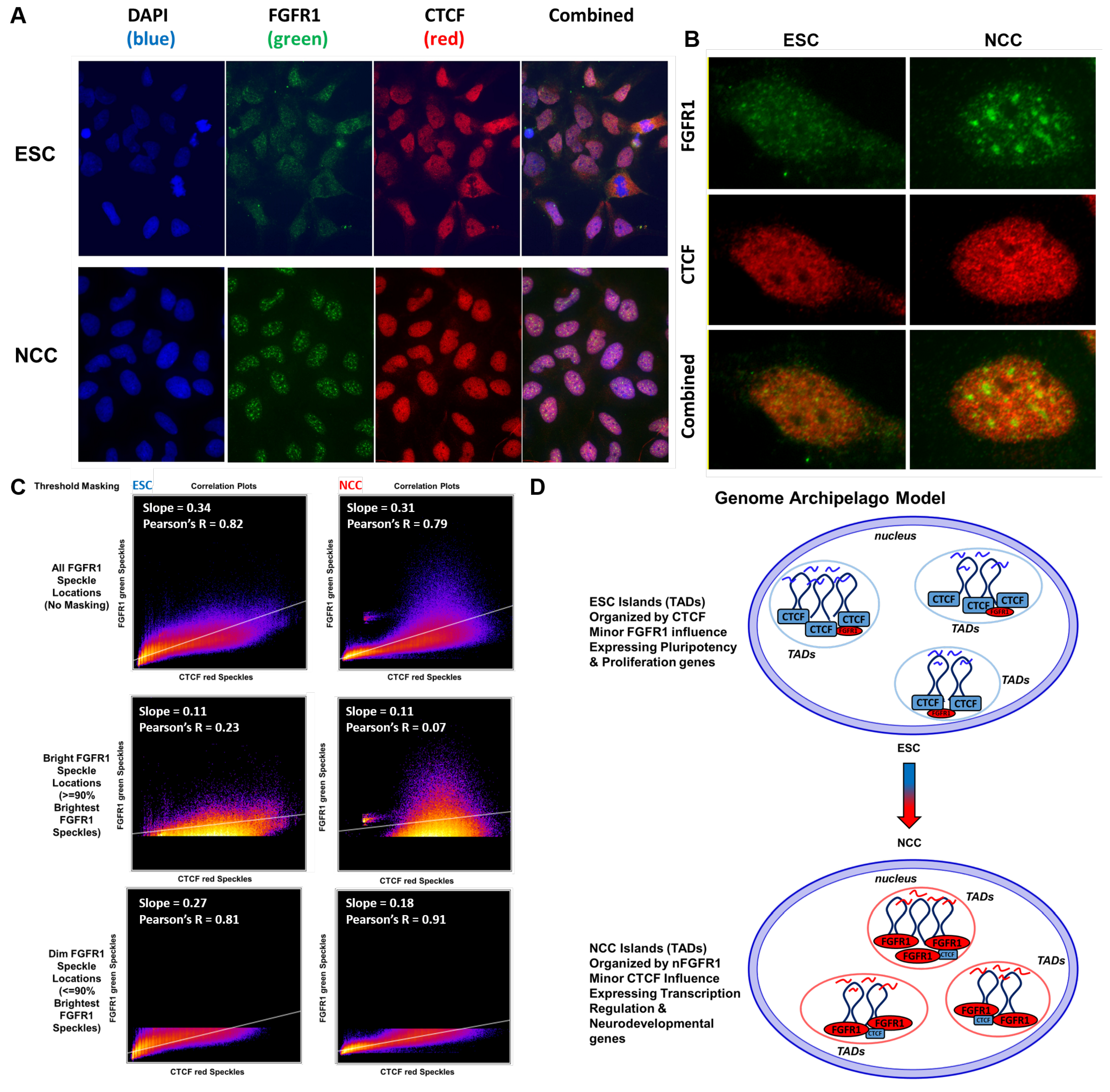
2.13. nFGFR1 and CTCF Occupy Colocalized as Well as Adjacent Loci in 3D Nuclear Chromatin Space
3. Discussion
3.1. Structure and Reorganization of Genome TADs Are Associated with nFGFR1 Binding
3.2. nFGFR1 May Construct TADs by Targeting TAD Border Enriched TF Motifs
3.3. Hox Clusters Exemplify Global Gene Inter and Intra-Chromosomal Interactions and Their NCC Development Remodeling
3.4. Genome Archipelago Model
4. Materials and Methods
4.1. Experimental Design
4.2. ChIP-seq, RNA-seq, HiC, and HiChIP Datasets
4.3. Hi-C/HiChIP Data Processing and Combined Analyses
4.3.1. Identifying Chromatin Loops, Interaction Anchor Strength, and TADs
4.3.2. Visualization of Data Analyses
4.3.3. Uniform Comparison Matrix-1 kb Binned Genome and Other Binning
4.3.4. All against All Binned Interactions Full Genome Paired t-Tests
4.3.5. Location Specific Binned Interactions Paired t-Tests
 ; p < 10−15
; p < 10−15  ; p < 10−25
; p < 10−25  (Figure 9A,B and Figure S15E,F) (Statistics are shown only for one loop, the strongest delta change, per midpoint for better visualization clarity). Log2 fold change FPKM, nFGFR1 binding, and Delta Change Interaction Strength values from the 1 kb binned genome data structure are shown with the chromatin looping changes to show the relationship between chromatin structure changes, gene expression changes, nFGFR1 binding, and interaction anchor site changes (Figure 9C–H and Figure S15G–L).
(Figure 9A,B and Figure S15E,F) (Statistics are shown only for one loop, the strongest delta change, per midpoint for better visualization clarity). Log2 fold change FPKM, nFGFR1 binding, and Delta Change Interaction Strength values from the 1 kb binned genome data structure are shown with the chromatin looping changes to show the relationship between chromatin structure changes, gene expression changes, nFGFR1 binding, and interaction anchor site changes (Figure 9C–H and Figure S15G–L).4.3.6. Alignment Comparisons
 ; p < 0.01
; p < 0.01  ; p < 0.005
; p < 0.005  .
.4.3.7. PCA Analysis
4.3.8. Machine Learning
4.3.9. All TADs Analysis
 ; p < 0.01
; p < 0.01  ; p < 0.005
; p < 0.005  .
.4.3.10. Regulated Genes-Chromatin Structure Analysis
 ; p < 1−4
; p < 1−4  ; p < 1−6
; p < 1−6  ; p < 1−8
; p < 1−8  ; p < 1−10
; p < 1−10  .
.4.3.11. Regulated Genes-Gene Ontology Analysis
 ; p < 0.01
; p < 0.01  ; p < 0.005
; p < 0.005  .
.4.3.12. Regulated Genes—Aligned TAD Analysis
 ; p < 0.01
; p < 0.01  ; p < 0.005
; p < 0.005  .
.4.3.13. Regulated Genes—Aligned TAD Analysis—Motifs
 ; p < 0.01:
; p < 0.01:  ; p < 0.005:
; p < 0.005:  . Additionally, Bonferroni correction was applied to the Z-Score derived p values based on the number of proteins analyzed to mark the highest confidence motifs in each location adjusted p < 0.05: +.
. Additionally, Bonferroni correction was applied to the Z-Score derived p values based on the number of proteins analyzed to mark the highest confidence motifs in each location adjusted p < 0.05: +. ; p < 0.01:
; p < 0.01:  ; p < 0.005:
; p < 0.005:  ; and Bonferroni correction was applied to the Z-Score derived p values based on the number of proteins analyzed to mark the highest confidence motifs in each location adjusted p < 0.05: +. In addition, 500,000 samplings of 20 bp sequences in each 10 kb bin, +/−195 kb surrounding the ESC and NCC DiffTAD borders, we re taken to determine short DNA sequences contributing to regulated TAD formations. Table S1 Sheets 7–10 shows sequences overrepresented using Z-Score Statistics: Two-Tailed Probability from the Central area, filtered by Bonferroni adjusted p < 0.05.
; and Bonferroni correction was applied to the Z-Score derived p values based on the number of proteins analyzed to mark the highest confidence motifs in each location adjusted p < 0.05: +. In addition, 500,000 samplings of 20 bp sequences in each 10 kb bin, +/−195 kb surrounding the ESC and NCC DiffTAD borders, we re taken to determine short DNA sequences contributing to regulated TAD formations. Table S1 Sheets 7–10 shows sequences overrepresented using Z-Score Statistics: Two-Tailed Probability from the Central area, filtered by Bonferroni adjusted p < 0.05. .
.4.3.14. Regulated Gene Containing TADs Differential Looping
 ; p < 10−15
; p < 10−15  ; p < 10−25
; p < 10−25  . (Statistics are shown only for one loop, the strongest delta change, per midpoint for better visualization clarity). Hi-C chromatin looping, nFGFR1 chromatin looping, and CTCF chromatin looping are shown together to show the main changes in chromatin organization in differential gene expression containing TAD borders, and how nFGFR1 and CTCF are involved in the chromatin looping changes (Figure 6J–L and Figure 7J–L ).
. (Statistics are shown only for one loop, the strongest delta change, per midpoint for better visualization clarity). Hi-C chromatin looping, nFGFR1 chromatin looping, and CTCF chromatin looping are shown together to show the main changes in chromatin organization in differential gene expression containing TAD borders, and how nFGFR1 and CTCF are involved in the chromatin looping changes (Figure 6J–L and Figure 7J–L ).4.4. 3C-qPCR, ChIP-qPCR, and RT-qPCR Sample Preparations and Quantification
4.5. Immunocytochemistry and Microscopy
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ESC | Embryonic Stem Cell |
| NCC | Neuronal Committed Cells |
| INFS | Integrative Nuclear FGFR1 Signaling |
| nFGFR1 | Nuclear Fibroblast Growth Factor Receptor 1 |
| TAD | Topologically Associating Domains |
| RA | Retinoic Acid |
| TF | Transcription Factor |
| CC | Clustering Coefficients |
| DD | Degree Distributions |
| PCA | Principle Component Analysis |
| Diffgene | Differentially Regulated Interacting Gene |
| DiffTAD | Differentially Regulated Gene Containing TAD |
| GO | Gene Ontology |
| PD | PD173074 |
| 3C | Chromosome Conformation Capture |
| ChIP | Chromatin Immunoprecipitation |
References
- Stachowiak, M.K.; Stachowiak, E.K. Evidence-Based Theory for Integrated Genome Regulation of Ontogeny—An Unprecedented Role of Nuclear FGFR1 Signaling. J. Cell. Physiol. 2016, 231, 1199–1218. [Google Scholar] [CrossRef] [Green Version]
- Terranova, C.; Narla, S.T.; Lee, Y.W.; Bard, J.; Parikh, A.; Stachowiak, E.K.; Tzanakakis, E.S.; Buck, M.J.; Birkaya, B.; Stachowiak, M.K. Global Developmental Gene Programing Involves a Nuclear Form of Fibroblast Growth Factor Receptor-1 (FGFR1). PLoS ONE 2015, 10, e0123380. [Google Scholar] [CrossRef] [Green Version]
- Stachowiak, E.K.; Benson, C.A.; Narla, S.T.; Dimitri, A.; Chuye, L.E.B.; Dhiman, S.; Harikrishnan, K.; Elahi, S.; Freedman, D.; Brennand, K.J.; et al. Cerebral organoids reveal early cortical maldevelopment in schizophrenia—Computational anatomy and genomics, role of FGFR1. Transl. Psychiatry 2017, 7, 6. [Google Scholar] [CrossRef] [Green Version]
- Narla, S.T.; Lee, Y.W.; Benson, C.A.; Sarder, P.; Brennand, K.J.; Stachowiak, E.K.; Stachowiak, M.K. Common developmental genome deprogramming in schizophrenia - Role of Integrative Nuclear FGFR1 Signaling (INFS). Schizophr. Res. 2017, 185, 17–32. [Google Scholar] [CrossRef]
- Dekker, J.; Rippe, K.; Dekker, M.; Kleckner, N. Capturing chromosome conformation. Science 2002, 295, 1306–1311. [Google Scholar] [CrossRef] [Green Version]
- Dixon, J.R.; Selvaraj, S.; Yue, F.; Kim, A.; Li, Y.; Shen, Y.; Hu, M.; Liu, J.S.; Ren, B. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature 2012, 485, 376. [Google Scholar] [CrossRef] [Green Version]
- Rao, S.S.; Huntley, M.H.; Durand, N.C.; Stamenova, E.K.; Bochkov, I.D.; Robinson, J.T.; Sanborn, A.L.; Machol, I.; Omer, A.D.; Lander, E.S.; et al. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell 2014, 159, 1665–1680. [Google Scholar] [CrossRef] [Green Version]
- Sanborn, A.L.; Rao, S.S.P.; Huang, S.C.; Durand, N.C.; Huntley, M.H.; Jewett, A.I.; Bochkov, I.D.; Chinnappan, D.; Cutkosky, A.; Li, J.; et al. Chromatin extrusion explains key features of loop and domain formation in wild-type and engineered genomes. Proc. Natl. Acad. Sci. USA 2015, 112, E6456–E6465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ke, Y.; Xu, Y.; Chen, X.; Feng, S.; Liu, Z.; Sun, Y.; Yao, X.; Li, F.; Zhu, W.; Gao, L.; et al. 3D Chromatin Structures of Mature Gametes and Structural Reprogramming during Mammalian Embryogenesis. Cell 2017, 170, 367–381.e20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, Z.; Zheng, H.; Huang, B.; Ma, R.; Wu, J.; Zhang, X.; He, J.; Xiang, Y.; Wang, Q.; Li, Y.; et al. Allelic reprogramming of 3D chromatin architecture during early mammalian development. Nature 2017, 547, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Stachowiak, M.; Maher, P.; Joy, A.; Mordechai, E.; Stachowiak, E. Nuclear accumulation of fibroblast growth factor receptors is regulated by multiple signals in adrenal medullary cells. Mol. Biol. Cell 1996, 7, 1299–1317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stachowiak, E.K.; Maher, P.A.; Tucholski, J.; Mordechai, E.; Joy, A.; Moffett, J.; Coons, S.; Stachowiak, M.K. Nuclear accumulation of fibroblast growth factor receptors in human glial cells–association with cell proliferation. Oncogene 1997, 14, 2201–2211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reilly, J.F.; Maher, P.A. Importin β–mediated nuclear import of fibroblast growth factor receptor: Role in cell proliferation. J. Cell Biol. 2001, 152, 1307–1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porębska, N.; Latko, M.; Kucińska, M.; Zakrzewska, M.; Otlewski, J.; Opaliński, Ł. Targeting cellular trafficking of fibroblast growth factor receptors as a strategy for selective cancer treatment. J. Clin. Med. 2019, 8, 7. [Google Scholar]
- Bobbs, A.S.; Saarela, A.V.; Yatskievych, T.A.; Antin, P.B. Fibroblast growth factor (FGF) signaling during gastrulation negatively modulates the abundance of microRNAs that regulate proteins required for cell migration and embryo patterning. J. Biol. Chem. 2012, 287, 38505–38514. [Google Scholar] [CrossRef] [Green Version]
- Jornet, J.M.; Bae, Y.; Handelmann, C.R.; Decker, B.; Balcerak, A.; Sangwan, A.; Miao, P.; Desai, A.; Feng, L.; Stachowiak, E.K.; et al. Optogenomic Interfaces: Bridging Biological Networks With the Electronic Digital World. Proc. IEEE 2019, 107, 1387–1401. [Google Scholar] [CrossRef]
- Formisano, L.; Stauffer, K.M.; Young, C.D.; Bhola, N.E.; Guerrero-Zotano, A.L.; Jansen, V.M.; Estrada, M.M.; Hutchinson, K.E.; Giltnane, J.M.; Schwarz, L.J.; et al. Association of FGFR1 with ERα Maintains Ligand-Independent ER Transcription and Mediates Resistance to Estrogen Deprivation in ER(+) Breast Cancer. Clin. Cancer Res. 2017, 23, 6138–6150. [Google Scholar] [CrossRef] [Green Version]
- Rhinn, M.; Dollé, P. Retinoic acid signalling during development. Development 2012, 139, 843–858. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.W.; Terranova, C.; Birkaya, B.; Narla, S.; Kehoe, D.; Parikh, A.; Dong, S.; Ratzka, A.; Brinkmann, H.; Aletta, J.M.; et al. A novel nuclear FGF Receptor-1 partnership with retinoid and Nur receptors during developmental gene programming of embryonic stem cells. J. Cell. Biochem. 2012, 113, 2920–2936. [Google Scholar] [CrossRef]
- Förthmann, B.; Aletta, J.M.; Lee, Y.W.; Terranova, C.; Birkaya, B.; Stachowiak, E.K.; Stachowiak, M.K.; Claus, P. Coalition of nuclear receptors in the nervous system. J. Cell. Physiol. 2015, 230, 2875–2880. [Google Scholar] [CrossRef]
- Coleman, S.J.; Chioni, A.M.; Ghallab, M.; Anderson, R.K.; Lemoine, N.R.; Kocher, H.M.; Grose, R.P. Nuclear translocation of FGFR1 and FGF2 in pancreatic stellate cells facilitates pancreatic cancer cell invasion. EMBO Mol. Med. 2014, 6, 467–481. [Google Scholar] [CrossRef] [PubMed]
- Stachowiak, E.; Fang, X.; Myers, J.; Dunham, S.; Stachowiak, M. cAMP-induced differentiation of human neuronal progenitor cells is mediated by nuclear fibroblast growth factor receptor-1 (FGFR1). J. Neurochem. 2003, 84, 1296–1312. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Stachowiak, E.K.; Dunham-Ems, S.M.; Klejbor, I.; Stachowiak, M.K. Control of CREB-binding protein signaling by nuclear fibroblast growth factor receptor-1: A novel mechanism of gene regulation. J. Biol. Chem. 2005, 280, 28451–28462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Droit, A.; Gottardo, R.; Robertson, G.; Li, L. rGADEM: De Novo Motif Discovery, R package version 2.30.0; The R Foundation: Vienna, Austria, 2018. [Google Scholar]
- Mercier, E.; Gottardo, R. MotIV: Motif Identification and Validation; R package 1.38.0; The R Foundation: Vienna, Austria, 2018. [Google Scholar]
- RG Cavalcante, M.S. Annotatr: Genomic regions in context. Bioinformatics 2017, 33, 2381–2383. [Google Scholar] [CrossRef]
- Durand, N.C.; Robinson, J.T.; Shamim, M.S.; Machol, I.; Mesirov, J.P.; Lander, E.S.; Aiden, E.L. Juicebox Provides a Visualization System for Hi-C Contact Maps with Unlimited Zoom. Cell Syst. 2016, 3, 99–101. [Google Scholar] [CrossRef] [Green Version]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Calandrelli, R.; Wu, Q.; Guan, J.; Zhong, S. GITAR: An Open Source Tool for Analysis and Visualization of Hi-C Data. Genom. Proteom. Bioinform. 2018, 16, 365–372. [Google Scholar] [CrossRef]
- Mumbach, M.R.; Rubin, A.J.; Flynn, R.A.; Dai, C.; Khavari, P.A.; Greenleaf, W.J.; Chang, H.Y. HiChIP: Efficient and sensitive analysis of protein-directed genome architecture. Nat. Methods 2016, 13, 919. [Google Scholar] [CrossRef] [Green Version]
- Wutz, G.; Varnai, C.; Nagasaka, K.; Cisneros, D.A.; Stocsits, R.R.; Tang, W.; Schoenfelder, S.; Jessberger, G.; Muhar, M.; Hossain, M.J.; et al. Topologically associating domains and chromatin loops depend on cohesin and are regulated by CTCF, WAPL, and PDS5 proteins. EMBO J. 2017, 36, 3573–3599. [Google Scholar] [CrossRef]
- Andrey, G.; Mundlos, S. The three-dimensional genome: Regulating gene expression during pluripotency and development. Development 2017, 144, 3646–3658. [Google Scholar] [CrossRef] [Green Version]
- Lipkowitz, S.; Göbel, V.; Varterasian, M.L.; Nakahara, K.; Tchorz, K.; Kirsch, I.R. A comparative structural characterization of the human NSCL-1 and NSCL-2 genes. Two basic helix-loop-helix genes expressed in the developing nervous system. J. Biol. Chem. 1992, 267, 21065–21071. [Google Scholar] [PubMed]
- Adams, B.; Dörfler, P.; Aguzzi, A.; Kozmik, Z.; Urbanek, P.; Maurer-Fogy, I.; Busslinger, M. Pax-5 encodes the transcription factor BSAP and is expressed in B lymphocytes, the developing CNS, and adult testis. Genes Dev. 1992, 6, 1589–1607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakamoto, K.; Karelina, K.; Obrietan, K. CREB: A multifaceted regulator of neuronal plasticity and protection. J. Neurochem. 2011, 116, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Stachowiak, M.K.; Birkaya, B.; Aletta, J.M.; Narla, S.T.; Benson, C.A.; Decker, B.; Stachowiak, E.K. “Nuclear FGF receptor-1 and CREB binding protein: An integrative signaling module”. J. Cell. Physiol. 2015, 230, 989–1002. [Google Scholar] [CrossRef] [PubMed]
- Narla, S.T.; Decker, B.; Sarder, P.; Stachowiak, E.K.; Stachowiak, M.K. Induced Pluripotent Stem Cells Reveal Common Neurodevelopmental Genome Deprograming in Schizophrenia. Results Probl. Cell Differ. 2018, 66, 137–162. [Google Scholar] [CrossRef] [PubMed]
- Akam, M. The molecular basis for metameric pattern in the Drosophila embryo. Development 1987, 101, 1–22. [Google Scholar] [PubMed]
- Harding, K.; Wedeen, C.; McGinnis, W.; Levine, M. Spatially regulated expression of homeotic genes in Drosophila. Science 1985, 229, 1236–1242. [Google Scholar] [CrossRef]
- Mohammadi, M.; Froum, S.; Hamby, J.M.; Schroeder, M.C.; Panek, R.L.; Lu, G.H.; Eliseenkova, A.V.; Green, D.; Schlessinger, J.; Hubbard, S.R. Crystal structure of an angiogenesis inhibitor bound to the FGF receptor tyrosine kinase domain. EMBO J. 1998, 17, 5896–5904. [Google Scholar] [CrossRef] [Green Version]
- Giacomini, A.; Chiodelli, P.; Matarazzo, S.; Rusnati, M.; Presta, M.; Ronca, R. Blocking the FGF/FGFR system as a “two-compartment” antiangiogenic/antitumor approach in cancer therapy. Pharmacol. Res. 2016, 107, 172–185. [Google Scholar] [CrossRef]
- Skaper, S.D.; Kee, W.J.; Facci, L.; Macdonald, G.; Doherty, P.; Walsh, F.S. The FGFR1 inhibitor PD 173074 selectively and potently antagonizes FGF-2 neurotrophic and neurotropic effects. J. Neurochem. 2000, 75, 1520–1527. [Google Scholar] [CrossRef]
- Gudernova, I.; Vesela, I.; Balek, L.; Buchtova, M.; Dosedelova, H.; Kunova, M.; Pivnicka, J.; Jelinkova, I.; Roubalova, L.; Kozubik, A.; et al. Multikinase activity of fibroblast growth factor receptor (FGFR) inhibitors SU5402, PD173074, AZD1480, AZD4547 and BGJ398 compromises the use of small chemicals targeting FGFR catalytic activity for therapy of short-stature syndromes. Hum. Mol. Genet. 2016, 25, 9–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chudasama, P.; Renner, M.; Straub, M.; Mughal, S.S.; Hutter, B.; Kosaloglu, Z.; Schwessinger, R.; Scheffler, M.; Alldinger, I.; Schimmack, S.; et al. Targeting Fibroblast Growth Factor Receptor 1 for Treatment of Soft-Tissue Sarcoma. Clin. Cancer Res. 2017, 23, 962–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonev, B.; Mendelson Cohen, N.; Szabo, Q.; Fritsch, L.; Papadopoulos, G.L.; Lubling, Y.; Xu, X.; Lv, X.; Hugnot, J.P.; Tanay, A.; et al. Multiscale 3D Genome Rewiring during Mouse Neural Development. Cell 2017, 171, 557–572.e24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hug, C.B.; Grimaldi, A.G.; Kruse, K.; Vaquerizas, J.M. Chromatin Architecture Emerges during Zygotic Genome Activation Independent of Transcription. Cell 2017, 169, 216–228.e19. [Google Scholar] [CrossRef] [Green Version]
- Stadhouders, R.; Vidal, E.; Serra, F.; Di Stefano, B.; Le Dily, F.; Quilez, J.; Gomez, A.; Collombet, S.; Berenguer, C.; Cuartero, Y.; et al. Transcription factors orchestrate dynamic interplay between genome topology and gene regulation during cell reprogramming. Nat. Genet. 2018, 50, 238–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramirez, F.; Bhardwaj, V.; Arrigoni, L.; Lam, K.C.; Gruning, B.A.; Villaveces, J.; Habermann, B.; Akhtar, A.; Manke, T. High-resolution TADs reveal DNA sequences underlying genome organization in flies. Nat. Commun. 2018, 9, 189. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Sun, Q.; Czajkowsky, D.M.; Shao, Z. Sub-kb Hi-C in D. melanogaster reveals conserved characteristics of TADs between insect and mammalian cells. Nat. Commun. 2018, 9, 188. [Google Scholar] [CrossRef] [Green Version]
- Kerpedjiev, P.; Abdennur, N.; Lekschas, F.; McCallum, C.; Dinkla, K.; Strobelt, H.; Luber, J.M.; Ouellette, S.B.; Azhir, A.; Kumar, N.; et al. HiGlass: Web-based visual exploration and analysis of genome interaction maps. Genome Biol. 2018, 19, 125. [Google Scholar] [CrossRef]
- Wolff, J.; Rabbani, L.; Gilsbach, R.; Richard, G.; Manke, T.; Backofen, R.; Grüning, B.A. Galaxy HiCExplorer 3: A web server for reproducible Hi-C, capture Hi-C and single-cell Hi-C data analysis, quality control and visualization. Nucleic Acids Res. 2020, 48, W177–W184. [Google Scholar] [CrossRef] [Green Version]
- Flyamer, I.M.; Illingworth, R.S.; Bickmore, W.A. Coolpup.py: Versatile pile-up analysis of Hi-C data. Bioinformatics 2020, 36, 2980–2985. [Google Scholar] [CrossRef] [Green Version]
- Ulianov, S.V.; Khrameeva, E.E.; Gavrilov, A.A.; Flyamer, I.M.; Kos, P.; Mikhaleva, E.A.; Penin, A.A.; Logacheva, M.D.; Imakaev, M.V.; Chertovich, A.; et al. Active chromatin and transcription play a key role in chromosome partitioning into topologically associating domains. Genome Res. 2016, 26, 70–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, C.; Li, L.; Qin, Z.S.; Corces, V.G. Gene density, transcription, and insulators contribute to the partition of the Drosophila genome into physical domains. Mol. Cell 2012, 48, 471–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peric-Hupkes, D.; Meuleman, W.; Pagie, L.; Bruggeman, S.W.; Solovei, I.; Brugman, W.; Gräf, S.; Flicek, P.; Kerkhoven, R.M.; van Lohuizen, M.; et al. Molecular maps of the reorganization of genome-nuclear lamina interactions during differentiation. Mol. Cell 2010, 38, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Martello, G.; Smith, A. The nature of embryonic stem cells. Annu. Rev. Cell Dev. Biol. 2014, 30, 647–675. [Google Scholar] [CrossRef]
- Chambeyron, S.; Bickmore, W.A. Chromatin decondensation and nuclear reorganization of the HoxB locus upon induction of transcription. Genes Dev. 2004, 18, 1119–1130. [Google Scholar] [CrossRef] [Green Version]
- Nagano, T.; Lubling, Y.; Stevens, T.J.; Schoenfelder, S.; Yaffe, E.; Dean, W.; Laue, E.D.; Tanay, A.; Fraser, P. Single-cell Hi-C reveals cell-to-cell variability in chromosome structure. Nature 2013, 502, 59–64. [Google Scholar] [CrossRef] [Green Version]
- Sutherland, H.; Bickmore, W.A. Transcription factories: Gene expression in unions? Nat. Rev. Genet. 2009, 10, 457–466. [Google Scholar] [CrossRef]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef] [Green Version]
- Servant, N.; Varoquaux, N.; Lajoie, B.R.; Viara, E.; Chen, C.J.; Vert, J.P.; Heard, E.; Dekker, J.; Barillot, E. HiC-Pro: An optimized and flexible pipeline for Hi-C data processing. Genome Biol. 2015, 16, 259. [Google Scholar] [CrossRef] [Green Version]
- Lareau, C.A.; Aryee, M.J. hichipper: A preprocessing pipeline for calling DNA loops from HiChIP data. Nat. Methods 2018, 15, 155–156. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Core Team. R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Bailey, J.A.; Eichler, E.E. Primate segmental duplications: Crucibles of evolution, diversity and disease. Nat. Rev. Genet. 2006, 7, 552–564. [Google Scholar] [CrossRef] [PubMed]
- Schoenfelder, S.; Sugar, R.; Dimond, A.; Javierre, B.M.; Armstrong, H.; Mifsud, B.; Dimitrova, E.; Matheson, L.; Tavares-Cadete, F.; Furlan-Magaril, M.; et al. Polycomb repressive complex PRC1 spatially constrains the mouse embryonic stem cell genome. Nat. Genet. 2015, 47, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Krzywinski, M.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An information aesthetic for comparative genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef] [Green Version]
- Phanstiel, D.H. Sushi: Tools for Visualizing Genomics Data, R package version 1.20.0; The R Foundation: Vienna, Austria, 2018. [Google Scholar]
- Csardi, G.; Nepusz, T. The igraph software package for complex network research. Int. J. Complex Syst. 2006, 1695, 1–9. [Google Scholar]
- Lawrence, M.; Huber, W.; Pages, H.; Aboyoun, P.; Carlson, M.; Gentleman, R.; Morgan, M.T.; Carey, V.J. Software for computing and annotating genomic ranges. PLoS Comput. Biol. 2013, 9, e1003118. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Multiple significance tests: The Bonferroni method. BMJ 1995, 310, 170. [Google Scholar] [CrossRef] [Green Version]
- Hagege, H.; Klous, P.; Braem, C.; Splinter, E.; Dekker, J.; Cathala, G.; de Laat, W.; Forne, T. Quantitative analysis of chromosome conformation capture assays (3C-qPCR). Nat. Protoc. 2007, 2, 1722–1733. [Google Scholar] [CrossRef] [Green Version]

 ; p < 0.01
; p < 0.01  ; p < 0.005
; p < 0.005  .
.
 ; p < 0.01
; p < 0.01  ; p < 0.005
; p < 0.005  .
.
 ; p < 0.01
; p < 0.01  ; p < 0.005
; p < 0.005  .
.
 ; p < 0.01
; p < 0.01  ; p < 0.005
; p < 0.005  .
.
 ; p < 1−4
; p < 1−4  ; p < 1−6
; p < 1−6  ; p < 1−8
; p < 1−8  ; p < 1−10
; p < 1−10  . At TAD borders aligned left and right based on regulated gene directionality (adjusted p < 0.05): (E) TAD reorganization. (F) Differential gene expression. (G) Differential interaction anchor strength. (H) Differential nFGFR1 binding. (I) Differential gene coding and regulator feature enrichment. Z-Score statistics indicate bins which are outside the mean of all bins p < 0.05
. At TAD borders aligned left and right based on regulated gene directionality (adjusted p < 0.05): (E) TAD reorganization. (F) Differential gene expression. (G) Differential interaction anchor strength. (H) Differential nFGFR1 binding. (I) Differential gene coding and regulator feature enrichment. Z-Score statistics indicate bins which are outside the mean of all bins p < 0.05  ; p < 0.01
; p < 0.01  ; p < 0.005
; p < 0.005  . (J) Differential chromatin looping. (K) Differential nFGFR1 looping. (L) Differential CTCF looping. Paired T-Test Bonferroni adjusted p < 10−5
. (J) Differential chromatin looping. (K) Differential nFGFR1 looping. (L) Differential CTCF looping. Paired T-Test Bonferroni adjusted p < 10−5  ; p < 10−15
; p < 10−15  ; p < 10−25
; p < 10−25  .
.
 ; p < 1−4
; p < 1−4  ; p < 1−6
; p < 1−6  ; p < 1−8
; p < 1−8  ; p < 1−10
; p < 1−10  . At TAD borders aligned left and right based on regulated gene directionality (adjusted p < 0.05): (E) TAD reorganization. (F) Differential gene expression. (G) Differential interaction anchor strength. (H) Differential nFGFR1 binding. (I) Differential gene coding and regulator feature enrichment. Z-Score statistics indicate bins which are outside the mean of all bins p < 0.05
. At TAD borders aligned left and right based on regulated gene directionality (adjusted p < 0.05): (E) TAD reorganization. (F) Differential gene expression. (G) Differential interaction anchor strength. (H) Differential nFGFR1 binding. (I) Differential gene coding and regulator feature enrichment. Z-Score statistics indicate bins which are outside the mean of all bins p < 0.05  ; p < 0.01
; p < 0.01  ; p < 0.005
; p < 0.005  . (J) Differential chromatin looping. (K) Differential nFGFR1 looping. (L) Differential CTCF looping. Paired T-Test Bonferroni adjusted p < 10−5
. (J) Differential chromatin looping. (K) Differential nFGFR1 looping. (L) Differential CTCF looping. Paired T-Test Bonferroni adjusted p < 10−5  ; p < 10−15
; p < 10−15  ; p < 10−25
; p < 10−25  .
.
 ; p < 1−4
; p < 1−4  ; p < 1−6
; p < 1−6  ; p < 1−8
; p < 1−8  ; p < 1−10
; p < 1−10  . At TAD borders aligned left and right based on regulated gene directionality (adjusted p < 0.05): (E) TAD reorganization. (F) Differential gene expression. (G) Differential interaction anchor strength. (H) Differential nFGFR1 binding. (I) Differential gene coding and regulator feature enrichment. Z-Score statistics indicate bins which are outside the mean of all bins p < 0.05
. At TAD borders aligned left and right based on regulated gene directionality (adjusted p < 0.05): (E) TAD reorganization. (F) Differential gene expression. (G) Differential interaction anchor strength. (H) Differential nFGFR1 binding. (I) Differential gene coding and regulator feature enrichment. Z-Score statistics indicate bins which are outside the mean of all bins p < 0.05  ; p < 0.01
; p < 0.01  ; p < 0.005
; p < 0.005  . (J) Differential chromatin looping. (K) Differential nFGFR1 looping. (L) Differential CTCF looping. Paired T-Test Bonferroni adjusted p < 10−5
. (J) Differential chromatin looping. (K) Differential nFGFR1 looping. (L) Differential CTCF looping. Paired T-Test Bonferroni adjusted p < 10−5  ; p < 10−15
; p < 10−15  ; p < 10−25
; p < 10−25  .
.
 ; p < 1−4
; p < 1−4  ; p < 1−6
; p < 1−6  ; p < 1−8
; p < 1−8  ; p < 1−10
; p < 1−10  . At TAD borders aligned left and right based on regulated gene directionality (adjusted p < 0.05): (E) TAD reorganization. (F) Differential gene expression. (G) Differential interaction anchor strength. (H) Differential nFGFR1 binding. (I) Differential gene coding and regulator feature enrichment. Z-Score statistics indicate bins which are outside the mean of all bins p < 0.05
. At TAD borders aligned left and right based on regulated gene directionality (adjusted p < 0.05): (E) TAD reorganization. (F) Differential gene expression. (G) Differential interaction anchor strength. (H) Differential nFGFR1 binding. (I) Differential gene coding and regulator feature enrichment. Z-Score statistics indicate bins which are outside the mean of all bins p < 0.05  ; p < 0.01
; p < 0.01  ; p < 0.005
; p < 0.005  . (J) Differential chromatin looping. (K) Differential nFGFR1 looping. (L) Differential CTCF looping. Paired T-Test Bonferroni adjusted p < 10−5
. (J) Differential chromatin looping. (K) Differential nFGFR1 looping. (L) Differential CTCF looping. Paired T-Test Bonferroni adjusted p < 10−5  ; p < 10−15
; p < 10−15  ; p < 10−25
; p < 10−25  .
.
 ; p < 10−15
; p < 10−15  ; p < 10−25
; p < 10−25  . (C,D) Differential interaction anchor strength. (E,F) nFGFR1 binding. (G,H) Differential gene expression. (I) Interactions of HoxA1 with downstream HoxA2-A13. (J) nFGFR1 binding. (K) CTCF binding. (L) Gene expression. (M,P) PD fold change of HoxA1:HoxA2-A13 interactions. (N,Q) PD fold change of nFGFR1 binding. (O,R) PD fold change of CTCF binding. (S) PD fold change of gene expression. (T) ESC to NCC fold change with and without PD. Two-way ANOVA Fisher’s LSD Test: p < 0.05 —p < 0.01 =, p < 0.005 ≡, p < 0.001 ≡.
. (C,D) Differential interaction anchor strength. (E,F) nFGFR1 binding. (G,H) Differential gene expression. (I) Interactions of HoxA1 with downstream HoxA2-A13. (J) nFGFR1 binding. (K) CTCF binding. (L) Gene expression. (M,P) PD fold change of HoxA1:HoxA2-A13 interactions. (N,Q) PD fold change of nFGFR1 binding. (O,R) PD fold change of CTCF binding. (S) PD fold change of gene expression. (T) ESC to NCC fold change with and without PD. Two-way ANOVA Fisher’s LSD Test: p < 0.05 —p < 0.01 =, p < 0.005 ≡, p < 0.001 ≡.
 ; p < 10−15
; p < 10−15  ; p < 10−25
; p < 10−25  . (C,D) Differential interaction anchor strength. (E,F) nFGFR1 binding. (G,H) Differential gene expression. (I) Interactions of HoxA1 with downstream HoxA2-A13. (J) nFGFR1 binding. (K) CTCF binding. (L) Gene expression. (M,P) PD fold change of HoxA1:HoxA2-A13 interactions. (N,Q) PD fold change of nFGFR1 binding. (O,R) PD fold change of CTCF binding. (S) PD fold change of gene expression. (T) ESC to NCC fold change with and without PD. Two-way ANOVA Fisher’s LSD Test: p < 0.05 —p < 0.01 =, p < 0.005 ≡, p < 0.001 ≡.
. (C,D) Differential interaction anchor strength. (E,F) nFGFR1 binding. (G,H) Differential gene expression. (I) Interactions of HoxA1 with downstream HoxA2-A13. (J) nFGFR1 binding. (K) CTCF binding. (L) Gene expression. (M,P) PD fold change of HoxA1:HoxA2-A13 interactions. (N,Q) PD fold change of nFGFR1 binding. (O,R) PD fold change of CTCF binding. (S) PD fold change of gene expression. (T) ESC to NCC fold change with and without PD. Two-way ANOVA Fisher’s LSD Test: p < 0.05 —p < 0.01 =, p < 0.005 ≡, p < 0.001 ≡.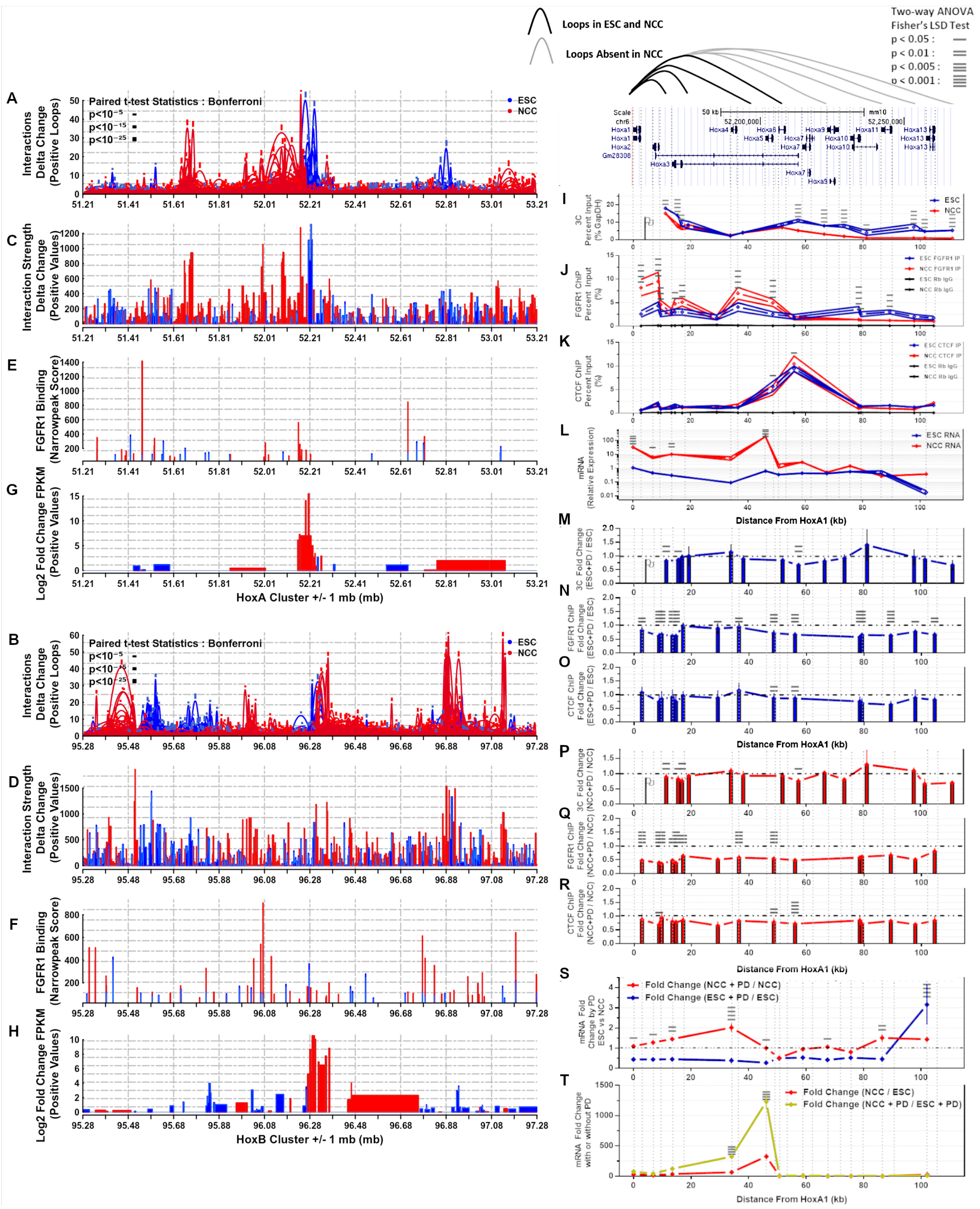
| NCC− | NCC− | NCC+ | NCC+ | ESC | NCC | NCC− | NCC− | NCC+ | NCC+ | ESC | NCC | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| # | Motif | 5’ | 3’ | 5’ | 3’ | FGFR1 | FGFR1 | # | Motif | 5’ | 3’ | 5’ | 3’ | FGFR1 | FGFR1 |
| 1 | CTCF | p = 0.000419 + | p = 0.016 | p = 0.0000406 + | p = 0.000155 + | 53 | E2F1 | p = 0.0000996 + | p = 0.00389 | ⏹ | |||||
| 2 | MYC..MAX | p = 0.00319 | p = 0.000136 + | p = 0.0000245 + | p = 0.00282 | ⏹ | 54 | Foxa2 | p = 0.00224 | p = 0.00302 | |||||
| 3 | NFIC | p = 0.0472 | p = 0.02 | p = 0.0286 | p = 0.00977 | 55 | FOXO3 | p = 0.0227 | p = 0.0302 | ||||||
| 4 | NFKB1 | p = 0.00632 | p = 0.0284 | p = 0.0252 | p = 0.0309 | 56 | Nkx2.5 | p = 0.000182 + | p = 0.0391 | ||||||
| 5 | Pdx1 | p = 0.0299 | p = 0.0415 | p = 0.0175 | p = 0.000532 | 57 | SRY | p = 0.0259 | p = 0.0192 | ||||||
| 6 | Spz1 | p = 0.0193 | p = 0.0059 | p = 0.0327 | p = 0.00452 | 58 | ESR1 | p = 0.0274 | p = 0.0311 | ||||||
| 7 | ZEB1 | p = 0.00383 | p = 0.0284 | p = 0.00849 | p = 0.0178 | ⏹ | 59 | FOXD1 | p = 0.0368 | p = 0.0465 | |||||
| 8 | ARID3A | p = 0.0343 | p = 0.0114 | p = 0.012 | 60 | HNF1B | p = 0.000173 + | p = 0.00459 | |||||||
| 9 | Mycn | p = 0.0126 | p = 0.0493 | p = 0.000000141 + | 61 | MEF2A | p = 0.000206 + | p = 0.00314 | |||||||
| 10 | PLAG1 | p = 0.0278 | p = 0.0181 | p = 0.00189 | ⏹ | 62 | PPARG | p = 0.00667 | p = 0.00881 | ||||||
| 11 | Ddit3..Cebpa | p = 0.0446 | p = 0.0394 | p = 0.000392 + | 63 | PPARG..RXRA | p = 0.0023 | p = 0.0238 | ⏹ | ⏹ | |||||
| 12 | ELK4 | p = 0.0359 | p = 0.0026 | p = 0.014 | 64 | SOX9 | p = 0.00105 | p = 0.0000956 + | |||||||
| 13 | Foxq1 | p = 0.00347 | p = 0.0172 | p = 0.0045 | 65 | SP1 | p = 0.0409 | p = 0.0304 | ⏹ | ⏹ | |||||
| 14 | HNF4A | p = 0.00214 | p = 0.0263 | p = 0.045 | 66 | TEAD1 | p = 0.0228 | p = 0.000765 | ⏹ | ⏹ | |||||
| 15 | NR1H2..RXRA | p = 0.00637 | p = 0.00468 | p = 0.0199 | 67 | Zfp423 | p = 0.0077 | p = 0.0218 | ⏹ | ||||||
| 16 | NR4A2 | p = 0.0227 | p = 0.00945 | p = 0.0194 | 68 | GATA2 | p = 0.0417 | p = 0.0358 | |||||||
| 17 | RXRA..VDR | p = 0.000555 | p = 0.0217 | p = 0.00115 | 69 | MAX | p = 0.00105 | p = 0.00151 | |||||||
| 18 | STAT1 | p = 0.0112 | p = 0.00069 | p = 0.00509 | 70 | Pax6 | p = 0.0327 | p = 0.0407 | ⏹ | ||||||
| 19 | AP1 | p = 0.0207 | p = 0.0125 | p = 0.0229 | 71 | RELA | p = 0.0199 | p = 0.0065 | |||||||
| 20 | Ar | p = 0.00165 | p = 0.00847 | p = 0.0004 + | 72 | SRF | p = 0.0021 | p = 0.0482 | |||||||
| 21 | REL | p = 0.0222 | p = 0.0168 | p = 0.0356 | 73 | YY1 | p = 0.0000987 + | p = 0.000421 + | |||||||
| 22 | RXR..RAR_DR5 | p = 0.016 | p = 0.0158 | p = 0.0087 | 74 | FOXI1 | p = 0.0234 | ⏹ | |||||||
| 23 | TAL1..TCF3 | p = 0.000705 | p = 0.0000833 + | p = 0.0165 | 75 | Hand1..Tcfe2a | p = 0.0165 | ||||||||
| 24 | Arnt | p = 0.00146 | p = 0.0000373 + | p = 0.0000167 + | ⏹ | 76 | Nr2e3 | p = 0.000295 + | |||||||
| 25 | BRCA1 | p = 0.0182 | p = 0.0445 | p = 0.000583 | ⏹ | 77 | SOX10 | p = 0.0436 | ⏹ | ||||||
| 26 | ESR2 | p = 0.0418 | p = 0.00483 | p = 0.00348 | 78 | TFAP2A | p = 0.0396 | ⏹ | |||||||
| 27 | FOXA1 | p = 0.00129 | p = 0.0203 | p = 0.025 | 79 | CREB1 | p = 0.00606 | ||||||||
| 28 | GABPA | p = 0.0000218 + | p = 0.0184 | p = 0.0000239 + | ⏹ | 80 | Evi1 | p = 0.0347 | |||||||
| 29 | GATA3 | p = 0.00391 | p = 0.00418 | p = 0.00013 + | 81 | FEV | p = 0.0112 | ⏹ | |||||||
| 30 | HNF1A | p = 0.00000372 + | p = 0.00921 | p = 0.0154 | 82 | FOXF2 | p = 0.00178 | ||||||||
| 31 | HOXA5 | p = 0.0255 | p = 0.00488 | p = 0.0218 | 83 | Mafb | p = 0.000463 | ||||||||
| 32 | INSM1 | p = 0.0216 | p = 0.0215 | p = 0.0231 | ⏹ | ⏹ | 84 | MZF1_5.13 | p = 0.00631 | ⏹ | ⏹ | ||||
| 33 | Lhx3 | p = 0.000149 + | p = 0.0029 | p = 0.0000534 + | 85 | NFIL3 | p = 0.000132 + | ||||||||
| 34 | NFE2L2 | p = 0.00466 | p = 0.00185 | p = 0.000788 | ⏹ | 86 | NHLH1 | p = 0.000326 + | ⏹ | ||||||
| 35 | Prrx2 | p = 0.0111 | p = 0.00459 | p = 0.00142 | 87 | NR3C1 | p = 0.0185 | ||||||||
| 36 | RUNX1 | p = 0.0419 | p = 0.0281 | p = 0.0000178 + | ⏹ | 88 | Pax4 | p = 0.0243 | ⏹ | ⏹ | |||||
| 37 | Tal1..Gata1 | p = 0.0207 | p = 0.00127 | p = 0.0216 | ⏹ | ⏹ | 89 | Pax5 | p = 0.000701 | ⏹ | ⏹ | ||||
| 38 | Tcfcp2l1 | p = 0.0293 | p = 0.0257 | p = 0.0397 | ⏹ | 90 | RORA_1 | p = 0.00413 | |||||||
| 39 | TLX1..NFIC | p = 0.0342 | p = 0.0015 | p = 0.0269 | ⏹ | 91 | RREB1 | p = 0.0149 | ⏹ | ||||||
| 40 | ZNF354C | p = 0.000762 | p = 0.0303 | p = 0.00751 | ⏹ | ⏹ | 92 | Sox17 | p = 0.00000485 + | ||||||
| 41 | En1 | p = 0.0004 + | p = 0.0447 | ⏹ | 93 | TP53 | p = 0.00666 | ⏹ | |||||||
| 42 | Myc | p = 0.000751 | p = 0.00337 | ⏹ | 94 | NF.kappaB | p = 0.0106 | ||||||||
| 43 | MZF1_1.4 | p = 0.00249 | p = 0.022 | ⏹ | ⏹ | 95 | Pou5f1 | p = 0.00291 | ⏹ | ||||||
| 44 | Zfx | p = 0.0174 | p = 0.00635 | ⏹ | ⏹ | 96 | CEBPA | p = 0.000467 | ⏹ | ||||||
| 45 | Sox5 | p = 0.000105 + | p = 0.00132 | 97 | Foxd3 | p = 0.0281 | ⏹ | ||||||||
| 46 | Stat3 | p = 0.0407 | p = 0.0454 | ⏹ | 98 | Klf4 | p = 0.0115 | ⏹ | ⏹ | ||||||
| 47 | Esrrb | p = 0.0182 | p = 0.0191 | 99 | NFYA | p = 0.00374 | |||||||||
| 48 | Myf | p = 0.0118 | p = 0.00443 | ⏹ | 100 | Nkx3.2 | p = 0.0000242 + | ||||||||
| 49 | NFATC2 | p = 0.00737 | p = 0.0249 | 101 | PBX1 | p = 0.00908 | |||||||||
| 50 | REST | p = 0.0238 | p = 0.00427 | ⏹ | 102 | Sox2 | p = 0.0404 | ⏹ | |||||||
| 51 | znf143 | p = 0.0421 | p = 0.0303 | ⏹ | 103 | T | p = 0.0156 | ⏹ | |||||||
| 52 | Arnt..Ahr | p = 0.00464 | p = 0.0466 | ⏹ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Decker, B.; Liput, M.; Abdellatif, H.; Yergeau, D.; Bae, Y.; Jornet, J.M.; Stachowiak, E.K.; Stachowiak, M.K. Global Genome Conformational Programming during Neuronal Development Is Associated with CTCF and Nuclear FGFR1—The Genome Archipelago Model. Int. J. Mol. Sci. 2021, 22, 347. https://doi.org/10.3390/ijms22010347
Decker B, Liput M, Abdellatif H, Yergeau D, Bae Y, Jornet JM, Stachowiak EK, Stachowiak MK. Global Genome Conformational Programming during Neuronal Development Is Associated with CTCF and Nuclear FGFR1—The Genome Archipelago Model. International Journal of Molecular Sciences. 2021; 22(1):347. https://doi.org/10.3390/ijms22010347
Chicago/Turabian StyleDecker, Brandon, Michal Liput, Hussam Abdellatif, Donald Yergeau, Yongho Bae, Josep M. Jornet, Ewa K. Stachowiak, and Michal K. Stachowiak. 2021. "Global Genome Conformational Programming during Neuronal Development Is Associated with CTCF and Nuclear FGFR1—The Genome Archipelago Model" International Journal of Molecular Sciences 22, no. 1: 347. https://doi.org/10.3390/ijms22010347
APA StyleDecker, B., Liput, M., Abdellatif, H., Yergeau, D., Bae, Y., Jornet, J. M., Stachowiak, E. K., & Stachowiak, M. K. (2021). Global Genome Conformational Programming during Neuronal Development Is Associated with CTCF and Nuclear FGFR1—The Genome Archipelago Model. International Journal of Molecular Sciences, 22(1), 347. https://doi.org/10.3390/ijms22010347









