Ouabain Promotes Gap Junctional Intercellular Communication in Cancer Cells
Abstract
1. Introduction
2. Results
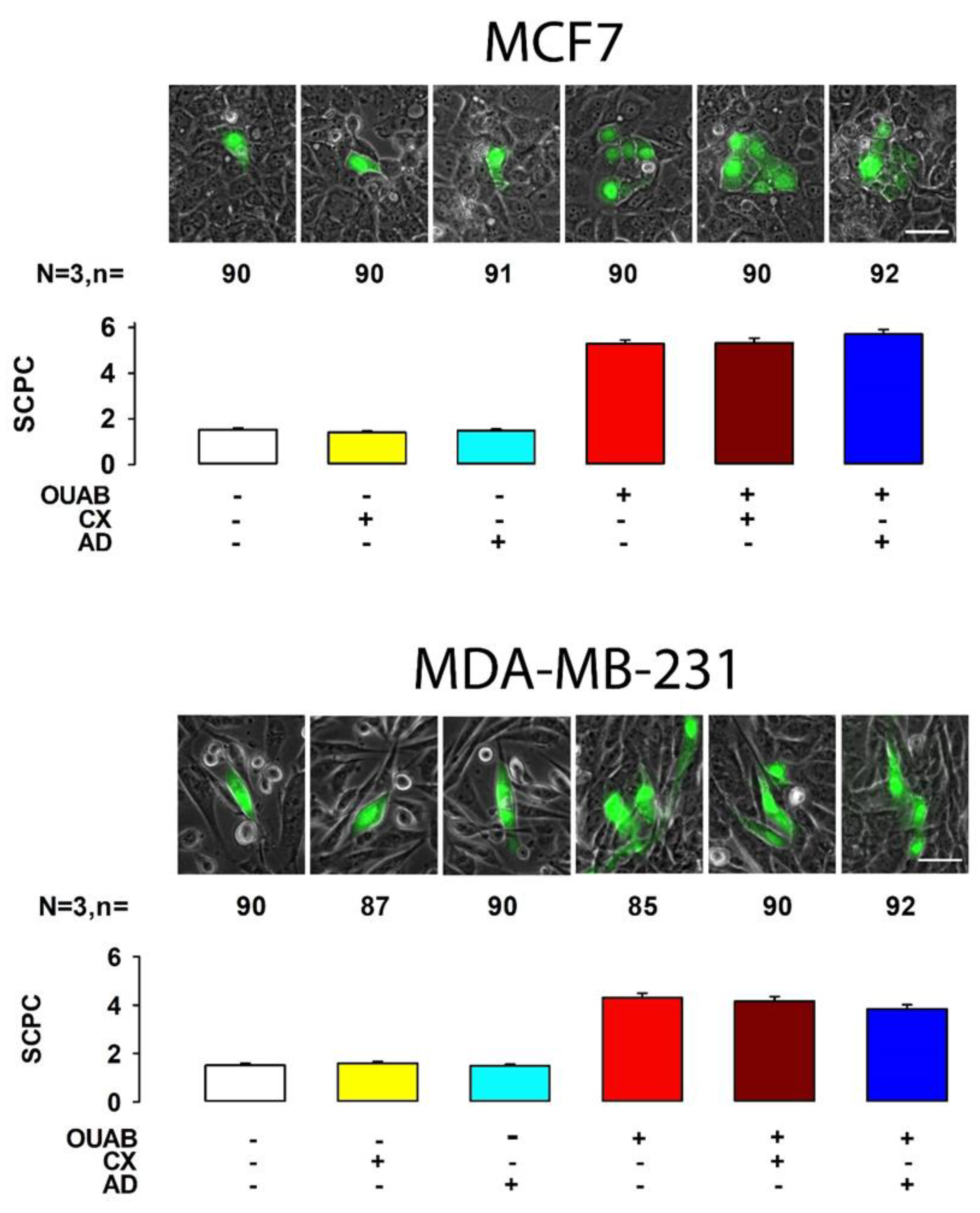
2.1. Octanol Suppresses the Enhancement of GJIC Induced by Ouabain
2.2. No Synthesis of New Units of Proteins or mRNA Is Required for Ouabain to Induce the Change of GJIC
2.3. Involvement of c-Src, ERK1/2 and Rho-ROCK in Ouabain-Induced GJIC
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Measurement of Gap Junctional Intercellular Communication by Dye Transfer Assays
4.3. Chemicals
4.4. Statistical Analyses
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hollman, A. Plants and cardiac glycosides. Br. Heart J. 1985, 54, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Haustein, K.O. Therapeutic range of cardiac glycosides. In Cardiac Glycoside Receptors and Positive Inotropy; Erdmann, E., Ed.; Steinkopff: Heidelberg, Germany, 1984. [Google Scholar]
- Hamlyn, J.M.; Lu, Z.R.; Manunta, P.; Ludens, J.H.; Kimura, K.; Shah, J.R.; Laredo, J.; Hamilton, J.P.; Hamilton, M.J.; Hamilton, B.P. Observations on the nature, biosynthesis, secretion and significance of endogenous ouabain. Clin. Exp. Hypertens. 1998, 20, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Hamlyn, J.M. Natriuretic hormones, endogenous ouabain, and related sodium transport inhibitors. Front. Endocrinol. 2014, 5, 199. [Google Scholar] [CrossRef] [PubMed]
- Schoner, W.; Bauer, N.; Müller-Ehmsen, J.; Krämer, U.; Hambarchian, N.; Schwinger, R.; Moeller, H.; Kost, H.; Weitkamp, C.; Schweitzer, T.; et al. Ouabain as a mammalian hormone. Ann. N. Y. Acad. Sci. 2003, 986, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Bagrov, A.Y.; Agalakova, N.I.; Kashkin, V.A.; Fedorova, O.V. Endogenous cardiotonic steroids and differential patterns of sodium pump inhibition in NaCl-loaded salt-sensitive and normotensive rats. Am. J. Hypertens. 2009, 22, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z. Molecular mechanisms of Na/K-ATPase-mediated signal transduction. Ann. N. Y. Acad. Sci. 2003, 986, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xie, Z. The Na/K-ATPase/Src complex and cardiotonic steroid-activated protein kinase cascades. Pflugers Arch. 2009, 457, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Contreras, R.G.; Flores-Beni Tez, D.; Flores-Maldonado, C.; Larre, I.; Shoshani, L.; Cereijido, M. Na+,K+-ATPase and hormone ouabain:new roles for an old enzyme and an old inhibitor. Cell. Mol. Biol. 2006, 52, 31–40. [Google Scholar]
- Aperia, A. New roles for an old enzyme: Na, K-ATPase emerges as an interesting drug target. J. Intern. Med. 2007, 261, 44–52. [Google Scholar] [CrossRef]
- Al-Ghoul, M.; Valdes, R., Jr. Mammalian cardenolides in cancer prevention and therapeutics. Ther. Drug Monit. 2008, 30, 234–238. [Google Scholar] [CrossRef]
- Newman, R.A.; Yang, P.; Pawlus, A.D.; Block, K.I. Cardiac glycosides as novel cancer therapeutic agents. Mol. Interv. 2008, 8, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Willecke, K.; Eiberger, J.; Degen, J.; Eckardt, D.; Romualdi, A.; Güldenagel, M.; Deutsch, U.; Söhl, G. Structural and functional diversity of connexin genes in the mouse and human genome. Biol. Chem. 2002, 383, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Sáez, J.C.; Berthoud, V.M.; Brañes, M.C.; Martínez, A.D.; Beyer, E.C. Plasma membrane channels formed by connexins: Their regulation and functions. Physiol. Rev. 2003, 83, 1359–1400. [Google Scholar] [CrossRef] [PubMed]
- Loewenstein, W.R.; Kanno, Y. Intercellular communication and tissue growth. I. Cancerous growth. J. Cell Biol. 1967, 33, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Loewenstein, W.R.; Kanno, Y. Intercellular communication and the control of tissue growth: Lack of communication between cancer cells. Nature 1966, 209, 1248–1249. [Google Scholar] [CrossRef] [PubMed]
- Cronier, L.; Crespin, S.; Strale, P.O.; Defamie, N.; Mesnil, M. Gap junctions and cancer: New functions for an old story. Antioxid. Redox Signal. 2009, 11, 323–338. [Google Scholar] [CrossRef]
- Aasen, T.; Mesnil, M.; Naus, C.C.; Lampe, P.D.; Laird, D.W. Gap junctions and cancer: Communicating for 50 years. Nat. Rev. Cancer 2017, 17, 74. [Google Scholar] [CrossRef]
- Leithe, E.; Sirnes, S.; Omori, Y.; Rivedal, E. Downregulation of gap junctions in cancer cells. Crit. Rev. Oncog. 2006, 12, 225–256. [Google Scholar] [CrossRef]
- Kandouz, M.; Batist, G. Gap junctions and connexins as therapeutic targets in cancer. Expert Opin. Ther. Targets 2010, 14, 681–692. [Google Scholar] [CrossRef]
- Czyz, J. The stage-specific function of gap junctions during tumourigenesis. Cell. Mol. Biol. Lett. 2008, 13, 92–102. [Google Scholar] [CrossRef]
- Naus, C.C.; Laird, D.W. Implications and challenges of connexin connections to cancer. Nat. Rev. Cancer 2010, 10, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Defamie, N.; Chepied, A.; Mesnil, M. Connexins, gap junctions and tissue invasion. FEBS Lett. 2014, 588, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Larre, I.; Lazaro, A.; Contreras, R.G.; Balda, M.S.; Matter, K.; Flores-Maldonado, C.; Ponce, A.; Flores-Benitez, D.; Rincon-Heredia, R.; Padilla-Benavides, T.; et al. Ouabain modulates epithelial cell tight junction. Proc. Natl. Acad. Sci. USA 2010, 107, 11387–11392. [Google Scholar] [CrossRef] [PubMed]
- Castillo, A.; Ortuño-Pineda, C.; Flores-Maldonado, C.; Larre, I.; Martínez Rendón, J.; Hinojosa, L.; Ponce, A.; Ogazón, A.; Serrano, M.; Valdes, J.; et al. Ouabain Modulates the Adherens Junction in Renal Epithelial Cells. Cell Physiol. Biochem. 2019, 52, 1381–1397. [Google Scholar] [CrossRef]
- Larre, I.; Castillo, A.; Flores-Maldonado, C.; Contreras, R.G.; Galvan, I.; Muñoz-Estrada, J.; Cereijido, M. Ouabain modulates ciliogenesis in epithelial cells. Proc. Natl. Acad. Sci. USA 2011, 108, 20591–20596. [Google Scholar] [CrossRef]
- Ponce, A.; Larre, I.; Castillo, A.; García-Villegas, R.; Romero, A.; Flores-Maldonado, C.; Martinez-Rendón, J.; Contreras, R.G.; Cereijido, M. Ouabain increases gap junctional communication in epithelial cells. Cell. Physiol. Biochem. 2014, 34, 2081–2090. [Google Scholar] [CrossRef]
- Ponce, A.; Larre, I.; Castillo, A.; Flores-Maldonado, C.; Verdejo-Torres, O.; Contreras, R.G.; Cereijido, M. Ouabain Modulates the Distribution of Connexin 43 in Epithelial Cells. Cell. Physiol. Biochem. 2016, 39, 1329–1338. [Google Scholar] [CrossRef]
- Weingart, R.; Bukauskas, F.F. Long-chain n-alkanols and arachidonic acid interfere with the Vm-sensitive gating mechanism of gap junction channels. Pflugers Arch. 1998, 435, 310–319. [Google Scholar] [CrossRef]
- Manjarrez-Marmolejo, J.; Franco-Pérez, J. Gap Junction Blockers: An Overview of their Effects on Induced Seizures in Animal Models. Curr. Neuropharmacol. 2016, 14, 759–771. [Google Scholar] [CrossRef]
- Wang, H.; Haas, M.; Liang, M.; Cai, T.; Tian, J.; Li, S.; Xie, Z. Ouabain assembles signaling cascades through the caveolar Na+-K+-ATPase. J. Biol. Chem. 2004, 279, 7250–17259. [Google Scholar] [CrossRef]
- Zhang, S.; Malmersjö, S.; Li, J.; Ando, H.; Aizman, O.; Uhlén, P.; Mikoshiba, K.; Aperia, A. Distinct role of the N-terminal tail of the Na, K-ATPase catalytic subunit as a signal transducer. J. Biol. Chem. 2006, 281, 21954–21962. [Google Scholar] [CrossRef] [PubMed]
- Abramowitz, J.; Dai, C.; Hirschi, K.K.; Dmitrieva, R.I.; Doris, P.A.; Liu, L.; Allen, J.C. Ouabain- and marinobufagenin-induced proliferation of human umbilical vein smooth muscle cells and a rat vascular smooth muscle cell line, A7r5. Circulation 2003, 108, 3048–3053. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Q.; Yu, S.P. Novel regulation of Na, K-ATPase by Src tyrosine kinases in cortical neurons. J. Neurochem. 2005, 93, 1515–1523. [Google Scholar] [CrossRef]
- Barwe, S.P.; Anilkumar, G.; Moon, S.Y.; Zheng, Y.; Whitelegge, J.P.; Rajasekaran, S.A.; Rajasekaran, A.K. Novel role for Na,K-ATPase in phosphatidylinositol 3-kinase signaling and suppression of cell motility. Mol. Biol. Cell. 2005, 16, 1082–1094. [Google Scholar] [CrossRef] [PubMed]
- Heasman, S.J.; Ridley, A.J. Mammalian Rho GTPases: New insights into their functions from in vivo studies. Nat. Rev. Mol. Cell. Biol. 2008, 9, 690–701. [Google Scholar] [CrossRef] [PubMed]
- Mack, N.A.; Georgiou, M. The interdependence of the Rho GTPases and apicobasal cell polarity. Small GTPases 2014, 5, 10. [Google Scholar] [CrossRef]
- Vasioukhin, V.; Bauer, C.; Yin, M.; Fuchs, E. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell 2000, 100, 209–219. [Google Scholar] [CrossRef]
- Kovacs, E.M.; Goodwin, M.; Ali, R.G.; Paterson, A.D.; Yap, A.S. Cadherin-directed actin assembly: E-cadherin physically associates with the Arp23 complex to direct actin assembly in nascent adhesive contacts. Curr. Biol. 2002, 12, 379–382. [Google Scholar] [CrossRef]
- Wojciak-Stothard, B.; Ridley, A.J. Rho GTPases and the regulation of endothelial permeability. Vascul. Pharmacol. 2002, 39, 187–199. [Google Scholar] [CrossRef]
- Hanke, J.H.; Gardner, J.P.; Dow, R.L.; Changelian, P.S.; Brissette, W.H.; Weringer, E.J.; Pollok, B.A.; Connelly, P.A. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J. Biol. Chem. 1996, 271, 695–701. [Google Scholar] [CrossRef]
- Bain, J.; Plater, L.; Elliott, M.; Shpiro, N.; Hastie, C.J.; McLauchlan, H.; Klevernic, I.; Arthur, J.S.; Alessi, D.R.; Cohen, P. The selectivity of protein kinase inhibitors: A further update. Biochem. J. 2007, 408, 297–315. [Google Scholar] [CrossRef] [PubMed]
- Brandvold, K.R.; Steffey, M.E.; Fox, C.C.; Soellner, M.B. Development of a highly selective c-Src kinase inhibitor. ACS Chem. Biol. 2012, 7, 1393–1398. [Google Scholar] [CrossRef] [PubMed]
- Reiners, J.J., Jr.; Lee, J.-Y.; Clift, R.E.; Dudley, D.T.; Myrand, S.P. PD98059 is an equipotent antagonist of the aryl hydrocarbon receptor and inhibitor of mitogen-activated protein kinase kinase. Mol. Pharmacol. 1998, 53, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Alessi, D.R.; Cuenda, A.; Cohen, P.; Dudley, D.T.; Saltiel, A.R. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J. Biol. Chem. 1995, 270, 27489–27494. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, R.; Galuppo, M.; Mazzon, E.; Paterniti, I.; Bramanti, P.; Cuzzocrea, S. PD98059, a specific MAP kinase inhibitor, attenuates multiple organ dysfunction syndrome/failure (MODS) induced by zymosan in mice. Pharmacol. Res. 2010, 61, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Rojewska, E.; Popiolek-Barczyk, K.; Kolosowska, N.; Piotrowska, A.; Zychowska, M.; Makuch, W.; Przewlocka, B.; Mika, J. PD98059 Influences Immune Factors and Enhances Opioid Analgesia in Model of Neuropathy. PLoS ONE 2015, 10, e0138583. [Google Scholar] [CrossRef]
- Huang, P.; Sun, Y.; Yang, J.; Chen, S.; Liu, A.D.; Holmberg, L.; Huang, X.; Tang, C.; Du, J.; Jin, H. The ERK1/2 Signaling Pathway Is Involved in Sulfur Dioxide Preconditioning-Induced Protection against Cardiac Dysfunction in Isolated Perfused Rat Heart Subjected to Myocardial Ischemia/Reperfusion. Int. J. Mol. Sci. 2013, 14, 22190–22201. [Google Scholar] [CrossRef]
- Ishizaki, T.; Uehata, M.; Tamechika, I.; Keel, J.; Nonomura, K.; Maekawa, M.; Narumiya, S. Pharmacological properties of Y-27632, a specific inhibitor of Rho-associated kinase. Mol. Pharmacol. 2000, 57, 976–983. [Google Scholar]
- Saunders, M.; Seraj, M.J.; Li, Z.; Zhou, Z.; Winter, C.R.; Welch, D.R.; Donahue, H.J. Breast cancer metastatic potential correlates with a breakdown in homospecific and heterospecific gap junctional intercellular communication. Cancer Res. 2001, 61, 1765–1767. [Google Scholar]
- Yano, T.; Hernandez-Blazquez, F.-J.; Omori, Y.; Yamasaki, H. Reduction of malignant phenotype of HEPG2 cell is associated with the expression of connexin 26 but not connexin 32. Carcinogenesis 2001, 22, 1593–1600. [Google Scholar] [CrossRef]
- Aasen, T.; Hodgins, M.; Edward, M.; Graham, S.V. The relationship between connexins, gap junctions, tissue architecture and tumour invasion, as studied in a novel in vitro model of HPV-16-associated cervical cancer progression. Oncogene 2003, 22, 7969–7980. [Google Scholar] [CrossRef]
- Toler, C.R.; Taylor, D.D.; Gercel-Taylor, C. Loss of communication in ovarian cancer. Am. J. Obstet. Gynecol. 2006, 194, e27–e31. [Google Scholar] [CrossRef]
- Rosenkranz, H.S.; Pollack, N.; Cunningham, A.R. Exploring the relationship between the inhibition of gap junctional intercellular communication and other biological phenomena. Carcinogenesis 2000, 21, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- Avanzo, J.L.; Mesnil, M.; Hernandez-Blazquez, F.J.; Mackowiak, I.I.; Mori, C.M.C.; Da Silva, T.C.; Oloris, S.C.S.; Gárate, A.P.; Massironi, S.M.G.; Yamasaki, H.; et al. Increased susceptibility to urethane-induced lung tumors in mice with decreased expression of connexin43. Carcinogenesis 2004, 25, 1973–1982. [Google Scholar] [CrossRef]
- Dagli, M.; Yamasaki, H.; Krutovskikh, V.; Omori, Y. Delayed liver regeneration and increased susceptibility to chemical hepatocarcinogenesis in transgenic mice expressing a dominant-negative mutant of connexin32 only in the liver. Carcinogenesis 2004, 25, 483–492. [Google Scholar] [CrossRef] [PubMed]
- King, T.J.; Lampe, P.D. Mice deficient for the gap junction protein Connexin32 exhibit increased radiation-induced tumorigenesis associated with elevated mitogenactivated protein kinase (p44/Erk1, p42/Erk2) activation. Carcinogenesis 2004, 25, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Bigelow, K.; Nguyen, T.A. Increase of gap junction activities in SW480 human colorectal cancer cells. BMC Cancer 2014, 14, 502. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, T.; Yamasaki, H. Lack of intercellular communication between chemically transformed and surrounding nontransformed BALB/c 3T3 cells. Cancer Res. 1984, 44, 5200–5203. [Google Scholar]
- Yamasaki, H.; Hollstein, M.; Mesnil, M.; Martel, N.; Aguelon, A.M. Selective lack of intercellular communication between transformed and nontransformed cells as a common property of chemical and oncogene transformation of BALB/c 3T3 cells. Cancer Res. 1987, 47, 5658–5664. [Google Scholar]
- Qin, H.; Shao, Q.; Curtis, H.; Galipeau, J.; Belliveau, D.J.; Wang, T.; Alaoui-Jamali, M.A.; Laird, D.W. Retroviral delivery of connexin genes to human breast tumor cells inhibits in vivo tumor growth by a mechanism that is independent of significant gap junctional intercellular communication. J. Biol. Chem. 2002, 277, 29132–29138. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Kaneda, M.; Morita, I. The gap junction-independent tumor-suppressing effect of connexin 43. J. Biol. Chem. 2003, 278, 44852–44856. [Google Scholar] [CrossRef] [PubMed]
- Piwowarczyk, K.; Paw, M.; Ryszawy, D.; Rutkowska-Zapała, M.; Madeja, Z.; Siedlar, M.; Czyż, J. Connexin43 high prostate cancer cells induce endothelial connexin43 up-regulation through the activation of intercellular ERK1/2-dependent signaling axis. Eur. J. Cell Biol. 2017, 96, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Pollmann, M.A.; Shao, Q.; Laird, D.W.; Sandig, M. Connexin 43 mediated gap junctional communication enhances breast tumor cell diapedesis in culture. Breast Cancer Res. 2005, 7, R522–R534. [Google Scholar] [CrossRef] [PubMed]
- Sohl, G.; Willecke, K. An update on connexin genes and their nomenclature in mouse and man. Cell Commun. Adhes. 2003, 10, 173–180. [Google Scholar] [CrossRef]
- Sosinsky, G.E.; Nicholson, B.J. Structural organization of gap junction channels. Biochim. Biophys. Acta 2005, 1711, 99–125. [Google Scholar] [CrossRef]
- Koval, M. Sharing signals: Connecting lung epithelial cells with gap junction channels. Am. J. Physiol. Lung Cell Mol. Physiol. 2002, 283, L875–L893. [Google Scholar] [CrossRef]
- Monaghan, P.; Clarke, C.; Perusinghe, N.P.; Moss, D.W.; Chen, X.-Y.; Evans, W. Gap junction distribution and connexin expression in human breast. Exp. Cell Res. 1996, 223, 29–38. [Google Scholar] [CrossRef]
- Jamieson, S.; Going, J.J.; D’Arcy, R.; George, W.D. Expression of gap junction proteins connexin 26 and connexin 43 in normal human breast and in breast tumours. J. Pathol. 1998, 184, 37–43. [Google Scholar] [CrossRef]
- Aasen, T.; Graham, S.V.; Edward, M.; Hodgins, M. Reduced expression of multiple gap junction proteins is a feature of cervical dysplasia. Mol. Cancer 2005, 4, 31. [Google Scholar] [CrossRef]
- El-Alfy, M.; Pelletier, G.; Hermo, L.; Labrie, F. Unique features of the basal cells of human prostate epithelium. Microsc. Res. Tech. 2000, 51, 436–446. [Google Scholar] [CrossRef]
- Habermann, H.; Ray, V.; Habermann, W.; Prins, G.S. Alterations in gap junction protein expression in human benign prostatic hyperplasia and prostate cancer. J. Urol. 2002, 167, 655–660. [Google Scholar] [CrossRef]
- Bonacquisti, E.E.; Nguyen, J. Connexin 43 (Cx43) in cancer: Implications for therapeutic approaches via gap junctions. Cancer Lett. 2019, 442, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Naser Al Deen, N.; AbouHaidar, M.; Talhouk, R. Connexin43 as a Tumor Suppressor: Proposed Connexin43 mRNA-circularRNAs-microRNAs Axis towards Prevention and Early Detection in Breast Cancer. Front. Med. 2019, 6, 192. [Google Scholar] [CrossRef] [PubMed]
- Lombaerts, M.; Van Wezel, T.; Philippo, K.; Dierssen, J.W.F.; Zimmerman, R.M.E.; Oosting, J.; Van Eijk, R.; Eilers, P.H.; Van De Water, B.; Cornelisse, C.J.; et al. E-cadherin transcriptional downregulation by promoter methylation but not mutation is related to epithelial-tomesenchymal transition in breast cancer cell lines. Br. J. Cancer 2006, 94, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Tabaries, S.; Dong, Z.; Annis, M.G.; Omeroglu, A.; Pepin, F.; Ouellet, V.; Russo, C.; Hassanain, M.; Metrakos, P.; Diaz, Z.H.; et al. Claudin-2 is selectively enriched in and promotes the formation of breast cancer liver metastases through engagement of integrin complexes. Oncogene 2011, 30, 1318–1328. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, K.; Nagafuchi, A.; Tsukita, S.; Kuraoka, A.; Ohokuma, A.; Shibata, Y. Dynamics of connexins, E-cadherin and alpha-catenin on cell membranes during gap junction formation. J. Cell Sci. 1997, 110 Pt. 3, 311–322. [Google Scholar]
- Jongen, W.M.; Fitzgerald, D.J.; Asamoto, M.; Piccoli, C.; Slaga, T.J.; Gros, D.; Takeichi, M.; Yamasaki, H. Regulation of connexin 43-mediated gap junctional intercellular communication by Ca2+ in mouse epidermal cells is controlled by E-cadherin. J. Cell Biol. 1991, 114, 545–555. [Google Scholar] [CrossRef]
- Kanno, Y.; Sasaki, Y.; Shiba, Y.; Yoshida-Noro, C.; Takeichi, M. Monoclonal antibody ECCD-1 inhibits intercellular communication in teratocarcinoma PCC3 cells. Exp. Cell Res. 1984, 152, 270–274. [Google Scholar] [CrossRef]
- Keane, R.W.; Mehta, P.P.; Rose, B.; Honig, L.S.; Loewenstein, W.R.; Rutishauser, U. Neural differentiation, NCAM-mediated adhesion, and gap junctional communication in neuroectoderm. A study in vitro. J. Cell Biol. 1988, 106, 1307–1319. [Google Scholar] [CrossRef]
- Mege, R.M.; Matsuzaki, F.; Gallin, W.J.; Goldberg, J.I.; Cunningham, B.A.; Edelman, G.M. Construction of epithelioid sheets by transfection of mouse sarcoma cells with cDNAs for chicken cell adhesion molecules. Proc. Natl. Acad. Sci. USA 1988, 85, 7274–7278. [Google Scholar] [CrossRef]
- Meyer, R.A.; Laird, D.W.; Revel, J.P.; Johnson, R.G. Inhibition of gap junction and adherens junction assembly by connexin and A-CAM antibodies. J. Cell Biol. 1992, 119, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.J.; Francis, R.; Xu, X.; Lo, C.W. Connexin43 associated with an N-cadherincontaining multiprotein complex is required for gap junction formation in NIH3T3 cells. J. Biol. Chem. 2005, 280, 19925–19936. [Google Scholar] [CrossRef] [PubMed]
- Pattillo, R.A.; Hussa, R.O.; Story, M.T.; Ruckert, A.C.; Shalaby, M.R.; Mattingly, R.F. Tumor antigen and human chorionic gonadotropin in CaSki cells: A new epidermoid cervical cancer cell line. Science 1977, 196, 1456–1458. [Google Scholar] [CrossRef] [PubMed]
- Friedl, F.; Kimura, I.; Osato, T.; Ito, Y. Studies on a new human cell line (SiHa) derived from carcinoma of uterus. I. Its establishment and morphology. Proc. Soc. Exp. Biol. Med. 1970, 135, 543–545. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.W., Jr.; McKusick, V.A.; Harper, P.S.; Wuu, K.D. George Otto Gey. (1899–1970): The HeLa cell and a reappraisal of its origin. Obstet. Gynecol. 1971, 38, 945–949. [Google Scholar]
- Soule, H.D.; Vazguez, J.; Long, A.; Albert, S.; Brennan, M. A human cell line from a pleural effusion derived from a breast carcinoma. J. Natl. Cancer Inst. 1973, 51, 1409–1416. [Google Scholar] [CrossRef]
- Cailleau, R.; Young, R.; Olivé, M.; Reeves, W.J., Jr. Breast tumor cell lines from pleural effusions. J. Natl. Cancer Inst. 1974, 53, 661–674. [Google Scholar] [CrossRef]
- Foster, K.A.; Oster, C.G.; Mayer, M.M.; Avery, M.L.; Audus, K.L. Characterization of the A549 cell line as a type II pulmonary epithelial cell model for drug metabolism. Exp. Cell Res. 1998, 243, 359–366. [Google Scholar] [CrossRef]
- Leibovitz, A.; Stinson, J.C.; McCombs, W.B., 3rd; McCoy, C.E.; Mazur, K.C.; Mabry, N.D. Classification of human colorectal adenocarcinoma cell lines. Cancer Res. 1976, 36, 4562–4569. [Google Scholar]
- Metzgar, R.S.; Gaillard, M.T.; Levine, S.J.; Tuck, F.L.; Bossen, E.H.; Borowitz, M.J. Antigens of human pancreatic adenocarcinoma cells defined by murine monoclonal antibodies. Cancer Res. 1982, 42, 601–608. [Google Scholar]
- Kim, Y.W.; Kern, H.F.; Mullins, T.D.; Koriwchak, M.J.; Metzgar, R.S. Characterization of clones of a human pancreatic adenocarcinoma cell line representing different stages of differentiation. Pancreas 1989, 4, 353–362. [Google Scholar] [CrossRef] [PubMed]
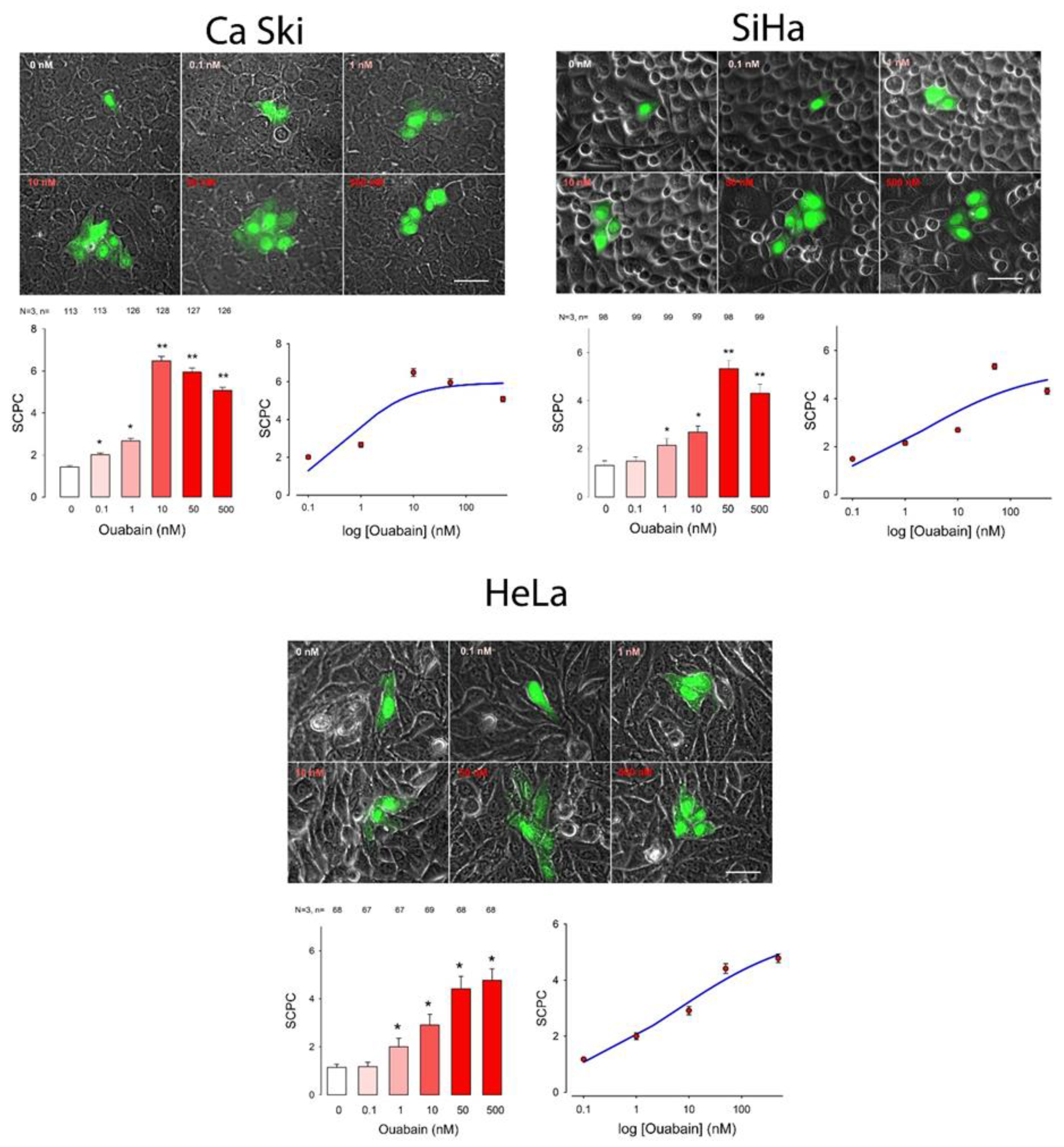

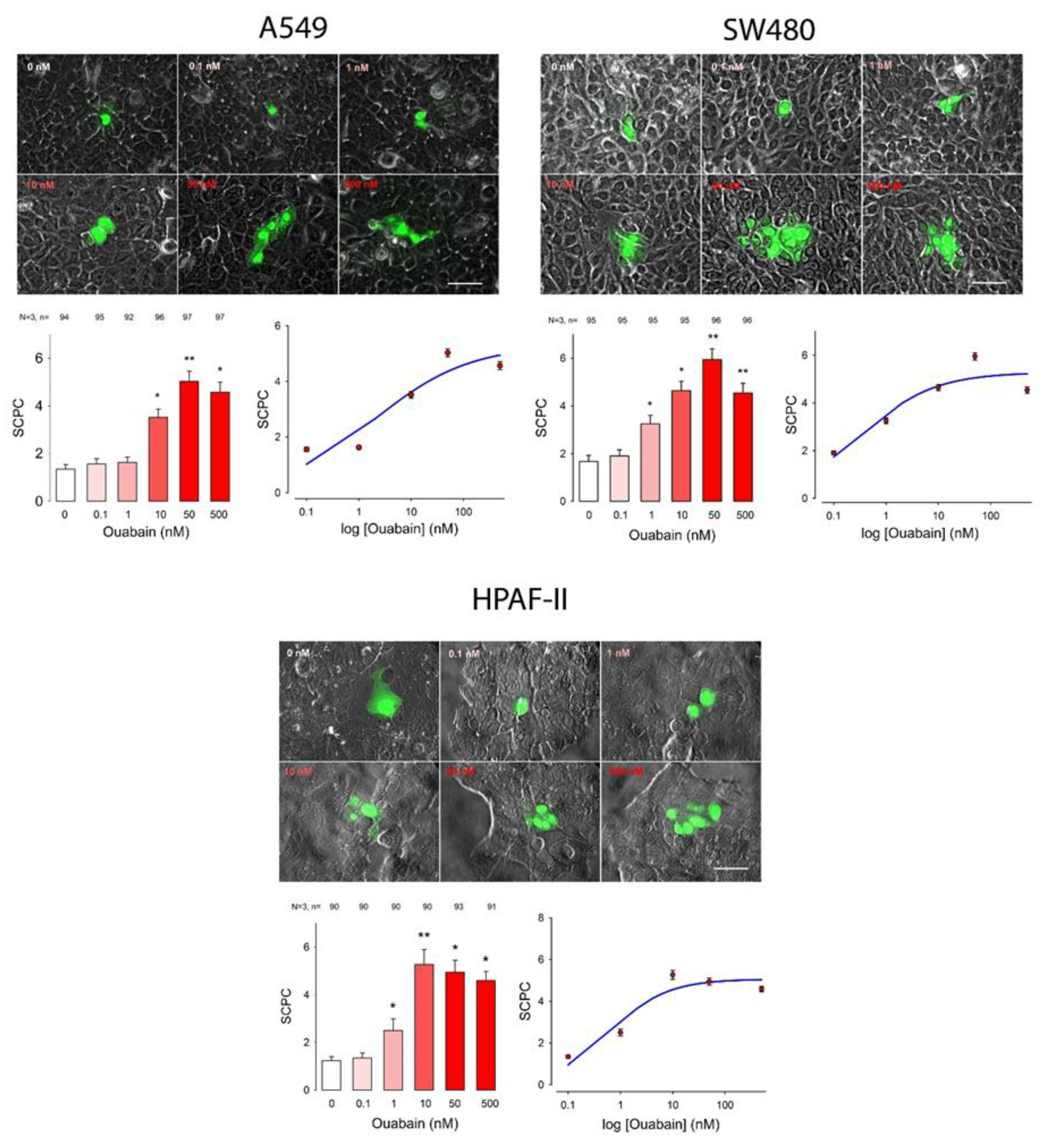


| Cell Line | Ouabain (nM) | ANOVA | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.1 | 1 | 10 | 50 | 500 | |||||||||||||||||||||
| mSCPC | SE | n | mSCPC | SE | n | p < 0.05 | mSCPC | SE | n | p < 0.05 | mSCPC | SE | n | p < 0.05 | mSCPC | SE | n | p < 0.05 | mSCPC | SE | n | p < 0.05 | H | d.f. | p < 0.001 | |
| CaSki | 1.4 | 0.1 | 113 | 2.0 | 0.1 | 113 | No | 2.7 | 0.1 | 126 | Yes | 6.5 | 0.2 | 128 | Yes | 5.9 | 0.2 | 127 | Yes | 5.1 | 0.1 | 126 | Yes | 472.0 | 5 | Yes |
| SiHa | 1.3 | 0.1 | 98 | 1.5 | 0.1 | 99 | No | 2.1 | 0.1 | 99 | Yes | 2.7 | 0.1 | 99 | Yes | 5.3 | 0.2 | 98 | Yes | 4.3 | 0.1 | 99 | Yes | 407.6 | 5 | Yes |
| HeLa | 1.1 | 0.0 | 68 | 1.2 | 0.1 | 67 | No | 2.0 | 0.1 | 67 | Yes | 2.9 | 0.2 | 69 | Yes | 4.4 | 0.2 | 68 | Yes | 4.8 | 0.2 | 68 | Yes | 274.5 | 5 | Yes |
| MDA | 1.7 | 0.1 | 231 | 1.8 | 0.1 | 267 | No | 2.0 | 0.1 | 256 | Yes | 2.1 | 0.1 | 260 | Yes | 2.6 | 0.1 | 278 | Yes | 3.0 | 0.1 | 215 | Yes | 141.1 | 5 | Yes |
| MCF7 | 2.0 | 0.1 | 147 | 3.3 | 0.1 | 137 | Yes | 2.8 | 0.1 | 120 | Yes | 4.3 | 0.1 | 138 | Yes | 4.8 | 0.2 | 71 | Yes | 2.7 | 0.1 | 78 | Yes | 208.3 | 5 | Yes |
| A549 | 1.4 | 0.1 | 94 | 1.6 | 0.1 | 95 | No | 1.6 | 0.1 | 92 | No | 3.5 | 0.1 | 96 | Yes | 5.0 | 0.1 | 97 | Yes | 4.6 | 0.1 | 97 | Yes | 397.9 | 5 | Yes |
| SW480 | 1.7 | 0.1 | 95 | 1.9 | 0.1 | 95 | No | 3.3 | 0.1 | 95 | Yes | 4.6 | 0.1 | 95 | Yes | 5.9 | 0.2 | 96 | Yes | 4.5 | 0.1 | 96 | Yes | 376.7 | 5 | Yes |
| Hpaf1 | 1.1 | 0.1 | 67 | 1.2 | 0.1 | 65 | No | 2.5 | 0.2 | 66 | Yes | 5.4 | 0.3 | 67 | Yes | 5.2 | 0.2 | 67 | Yes | 4.6 | 0.2 | 68 | Yes | 274.0 | 5 | Yes |
| Cell Line | SCPCmax | Hill Coef | EC50 | S0 | R |
|---|---|---|---|---|---|
| CaSki | 6.0 | −0.7 | 0.6 | 0.6 | |
| SiHa | 5.3 | −0.4 | 2.1 | 0.7 | |
| HeLa | 5.9 | −0.4 | 5.8 | 0.8 | |
| MDA | 2.7 | −1.0 | 37.8 | 1.9 | 0.5 |
| MCF7 | 3.8 | −0.4 | 2.1 × 10−3 | 0.3 | |
| A549 | 5.3 | −0.5 | 2.0 | 0.8 | |
| HPAF1 | 5.1 | −0.8 | 0.6 | 0.7 | |
| SW480 | 5.7 | −0.6 | 0.3 | 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrano-Rubi, M.; Jimenez, L.; Martinez-Rendon, J.; Cereijido, M.; Ponce, A. Ouabain Promotes Gap Junctional Intercellular Communication in Cancer Cells. Int. J. Mol. Sci. 2021, 22, 358. https://doi.org/10.3390/ijms22010358
Serrano-Rubi M, Jimenez L, Martinez-Rendon J, Cereijido M, Ponce A. Ouabain Promotes Gap Junctional Intercellular Communication in Cancer Cells. International Journal of Molecular Sciences. 2021; 22(1):358. https://doi.org/10.3390/ijms22010358
Chicago/Turabian StyleSerrano-Rubi, Mauricio, Lidia Jimenez, Jacqueline Martinez-Rendon, Marcelino Cereijido, and Arturo Ponce. 2021. "Ouabain Promotes Gap Junctional Intercellular Communication in Cancer Cells" International Journal of Molecular Sciences 22, no. 1: 358. https://doi.org/10.3390/ijms22010358
APA StyleSerrano-Rubi, M., Jimenez, L., Martinez-Rendon, J., Cereijido, M., & Ponce, A. (2021). Ouabain Promotes Gap Junctional Intercellular Communication in Cancer Cells. International Journal of Molecular Sciences, 22(1), 358. https://doi.org/10.3390/ijms22010358




