Wisteria floribunda Agglutinin-Positive Mac-2 Binding Protein as a Screening Tool for Significant Liver Fibrosis in Health Checkup
Abstract
1. Introduction
2. Materials and Methods
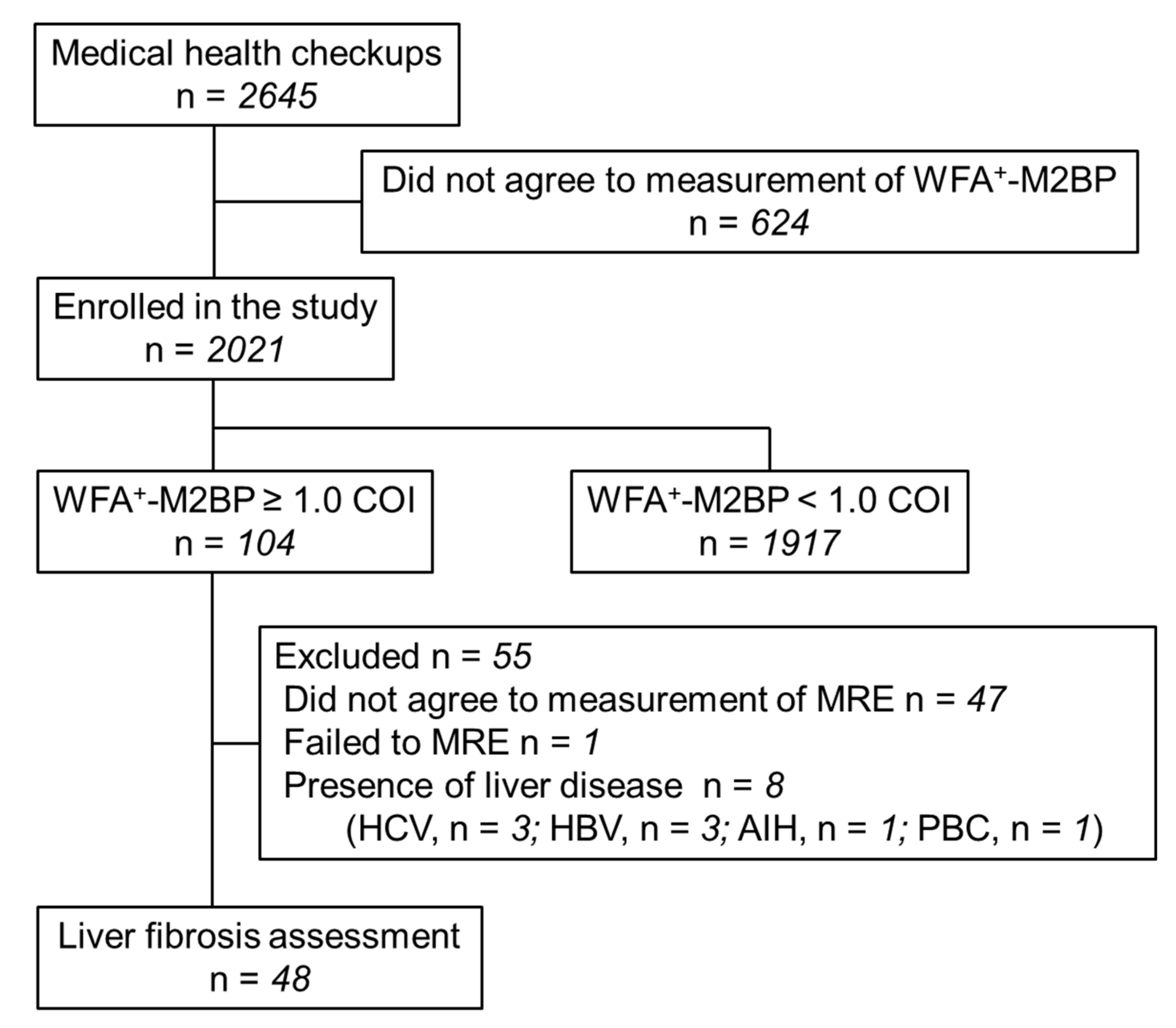
2.1. Study Protocol
2.2. Clinical and Laboratory Data
2.3. Definition of Comorbidity Status
2.4. Ultrasound Diagnostics
2.5. Measurement of Liver Stiffness by MRE
2.6. Assessment of Liver Fibrosis
2.7. Statistical Analysis
3. Results
3.1. The Characteristics of the Study Subjects
3.2. Distribution of WFA+-M2BP
3.3. Diagnostic Accuracy of WFA+-M2BP for Significant Liver Fibrosis
3.4. Characteristics of Newly Diagnosed Subjects with Significant Fibrosis
3.5. Comparison the Diagnostic Accuracy of Serum Fibrosis Markers
3.6. Factors Associated with Significant Fibrosis
4. Discussion
4.1. Main Findings
4.2. Context with Published Literature
4.3. Strengths and Limitations
4.4. Future Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| CLD | chronic liver disease |
| NAFLD | nonalcoholic fatty liver disease |
| WFA+-M2BP | Wisteria floribunda agglutinin-positive mac-2 binding protein |
| HSC | hepatic stellate cell |
| HCC | hepatocellular carcinoma |
| AST | aspartate aminotransferase |
| ALT | alanine aminotransferase |
| MRE | magnetic resonance elastography |
| COI | cut-off index |
| PBC | primary biliary cholangitis |
| ROC | receiver operating characteristic curve |
| BMI | body mass index |
| PPV | positive predictive value |
| NPV | negative predictive value |
| OR | odds ratio |
| CI | confidence interval |
| TE | transient elastography |
| HCC | hepatocellular carcinoma |
| APRI | aspartate aminotransferase-to-platelet ratio index |
References
- Dulai, P.S.; Singh, S.; Patel, J.; Soni, M.; Prokop, L.J.; Younossi, Z.; Sebastiani, G.; Ekstedt, M.; Hagstrom, H.; Nasr, P.; et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology 2017, 65, 1557–1565. [Google Scholar] [CrossRef]
- Yasui, Y.; Abe, T.; Kurosaki, M.; Higuchi, M.; Komiyama, Y.; Yoshida, T.; Hayashi, T.; Kuwabara, K.; Takaura, K.; Nakakuki, N.; et al. Elastin Fiber Accumulation in Liver Correlates with the Development of Hepatocellular Carcinoma. PLoS ONE 2016, 11, e0154558. [Google Scholar]
- Loomba, R.; Sanyal, A.J. The global NAFLD epidemic. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Sumida, Y.; Shima, T.; Mitsumoto, Y.; Katayama, T.; Umemura, A.; Yamaguchi, K.; Itoh, Y.; Yoneda, M.; Okanoue, T. Epidemiology: Pathogenesis, and Diagnostic Strategy of Diabetic Liver Disease in Japan. Int. J. Mol. Sci. 2020, 21, 4337. [Google Scholar] [CrossRef]
- Castera, L.; Friedrich-Rust, M.; Loomba, R. Noninvasive Assessment of Liver Disease in Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1264–1281.e1264. [Google Scholar] [CrossRef]
- Loomba, R.; Adams, L.A. Advances in non-invasive assessment of hepatic fibrosis. Gut 2020, 69, 1343–1352. [Google Scholar] [CrossRef]
- Kuno, A.; Ikehara, Y.; Tanaka, Y.; Ito, K.; Matsuda, A.; Sekiya, S.; Hige, S.; Sakamoto, M.; Kage, M.; Mizokami, M.; et al. A serum “sweet-doughnut” protein facilitates fibrosis evaluation and therapy assessment in patients with viral hepatitis. Sci. Rep. 2013, 3, 1065. [Google Scholar] [CrossRef]
- Tamaki, N.; Kurosaki, M.; Loomba, R.; Izumi, N. Clinical Utility of Mac-2 Binding Protein Glycosylation Isomer in Chronic Liver Diseases. Ann. Lab. Med. 2021, 41, 16–24. [Google Scholar] [CrossRef]
- Shirabe, K.; Bekki, Y.; Gantumur, D.; Araki, K.; Ishii, N.; Kuno, A.; Narimatsu, H.; Mizokami, M. Mac-2 binding protein glycan isomer (M2BPGi) is a new serum biomarker for assessing liver fibrosis: More than a biomarker of liver fibrosis. J. Gastroenterol. 2018, 53, 819–826. [Google Scholar] [CrossRef]
- Ogawa, Y.; Honda, Y.; Kessoku, T.; Tomeno, W.; Imajo, K.; Yoneda, M.; Kawanaka, M.; Kirikoshi, H.; Ono, M.; Taguri, M.; et al. Wisteria floribunda agglutinin-positive Mac-2-binding protein and type 4 collagen 7S: Useful markers for the diagnosis of significant fibrosis in patients with non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2018, 33, 1795–1803. [Google Scholar] [CrossRef]
- Nishikawa, H.; Enomoto, H.; Iwata, Y.; Kishino, K.; Shimono, Y.; Hasegawa, K.; Nakano, C.; Takata, R.; Yoh, K.; Nishimura, T.; et al. Clinical significance of serum Wisteria floribunda agglutinin positive Mac-2-binding protein level in non-alcoholic steatohepatitis. Hepatol. Res. 2016, 46, 1194–1202. [Google Scholar] [CrossRef]
- Kim, M.; Jun, D.W.; Park, H.; Kang, B.K.; Sumida, Y. Sequential Combination of FIB-4 Followed by M2BPGi Enhanced Diagnostic Performance for Advanced Hepatic Fibrosis in an Average Risk Population. J. Clin. Med. 2020, 9, 1119. [Google Scholar] [CrossRef]
- Bekki, Y.; Yoshizumi, T.; Shimoda, S.; Itoh, S.; Harimoto, N.; Ikegami, T.; Kuno, A.; Narimatsu, H.; Shirabe, K.; Maehara, Y. Hepatic stellate cells secreting WFA(+) -M2BP: Its role in biological interactions with Kupffer cells. J. Gastroenterol. Hepatol. 2017, 32, 1387–1393. [Google Scholar] [CrossRef]
- Tamaki, N.; Kurosaki, M.; Kuno, A.; Korenaga, M.; Togayachi, A.; Gotoh, M.; Nakakuki, N.; Takada, H.; Matsuda, S.; Hattori, N.; et al. Wisteria floribunda agglutinin positive human Mac-2-binding protein as a predictor of hepatocellular carcinoma development in chronic hepatitis C patients. Hepatol. Res. 2015, 45, E82–E88. [Google Scholar] [CrossRef]
- Yasui, Y.; Kurosaki, M.; Komiyama, Y.; Takada, H.; Tamaki, N.; Watakabe, K.; Okada, M.; Wang, W.; Shimizu, T.; Kubota, Y.; et al. Wisteria floribunda agglutinin-positive Mac-2 binding protein predicts early occurrence of hepatocellular carcinoma after sustained virologic response by direct-acting antivirals for hepatitis C virus. Hepatol. Res. 2018, 48, 1131–1139. [Google Scholar] [CrossRef]
- Hayashi, T.; Tamaki, N.; Kurosaki, M.; Wang, W.; Okada, M.; Higuchi, M.; Takaura, K.; Takada, H.; Yasui, Y.; Tsuchiya, K.; et al. Use of the Serum Wisteria floribunda Agglutinin-Positive Mac2 Binding Protein as a Marker of Gastroesophageal Varices and Liver-Related Events in Chronic Hepatitis C Patients. Diagnostics 2020, 10, 173. [Google Scholar] [CrossRef] [PubMed]
- Osawa, L.; Tamaki, N.; Kurosaki, M.; Kirino, S.; Watakabe, K.; Wang, W.; Okada, M.; Shimizu, T.; Higuchi, M.; Takaura, K.; et al. Wisteria floribunda Agglutinin-Positive Mac-2 Binding Protein but not α-fetoprotein as a Long-Term Hepatocellular Carcinoma Predictor. Int. J. Mol. Sci. 2020, 21, 3640. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Takata, R.; Nishikawa, H.; Enomoto, H.; Ishii, A.; Iwata, Y.; Miyamoto, Y.; Ishii, N.; Yuri, Y.; Nakano, C.; et al. Impact of Wisteria floribunda Agglutinin-Positive Mac-2-Binding Protein in Patients with Hepatitis C Virus-Related Compensated Liver Cirrhosis. Int. J. Mol. Sci. 2016, 17, 1500. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Enomoto, H.; Yoh, K.; Iwata, Y.; Sakai, Y.; Kishino, K.; Ikeda, N.; Takashima, T.; Aizawa, N.; Takata, R.; et al. Combined albumin-bilirubin grade and Mac-2 binding protein glycosylation isomer as a useful predictor in compensated liver cirrhosis. Medicine (Baltimore) 2019, 98, e18366. [Google Scholar] [CrossRef]
- Eguchi, Y.; Wong, G.; Akhtar, O.; Sumida, Y. Non-invasive diagnosis of non-alcoholic steatohepatitis and advanced fibrosis in Japan: A targeted literature review. Hepatol. Res. 2020, 50, 645–655. [Google Scholar] [CrossRef]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Sulkowski, M.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, N.; Kurosaki, M.; Tanaka, K.; Suzuki, Y.; Hoshioka, Y.; Kato, T.; Yasui, Y.; Hosokawa, T.; Ueda, K.; Tsuchiya, K.; et al. Noninvasive estimation of fibrosis progression overtime using the FIB-4 index in chronic hepatitis C. J. Viral. Hepat. 2013, 20, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, N.; Kurosaki, M.; Matsuda, S.; Muraoka, M.; Yasui, Y.; Suzuki, S.; Hosokawa, T.; Ueda, K.; Tsuchiya, K.; Nakanishi, H.; et al. Non-invasive prediction of hepatocellular carcinoma development using serum fibrosis marker in chronic hepatitis C patients. J. Gastroenterol. 2014, 49, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Kurosaki, M.; Tamaki, N.; Yasui, Y.; Hosokawa, T.; Tsuchiya, K.; Nakanishi, H.; Itakura, J.; Izumi, N. Non-alcoholic fatty liver disease fibrosis score and FIB-4 scoring system could identify patients at risk of systemic complications. Hepatol. Res. 2015, 45, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Sumida, Y.; Yoneda, M.; Hyogo, H.; Itoh, Y.; Ono, M.; Fujii, H.; Eguchi, Y.; Suzuki, Y.; Aoki, N.; Kanemasa, K.; et al. Validation of the FIB4 index in a Japanese nonalcoholic fatty liver disease population. BMC Gastroenterol. 2012, 12, 2. [Google Scholar] [CrossRef] [PubMed]
- McPherson, S.; Hardy, T.; Dufour, J.F.; Petta, S.; Romero-Gomez, M.; Allison, M.; Oliveira, C.P.; Francque, S.; Van Gaal, L.; Schattenberg, J.M.; et al. Age as a Confounding Factor for the Accurate Non-Invasive Diagnosis of Advanced NAFLD Fibrosis. Am. J. Gastroenterol. 2017, 112, 740–751. [Google Scholar] [CrossRef] [PubMed]
- Ishiba, H.; Sumida, Y.; Tanaka, S.; Yoneda, M.; Hyogo, H.; Ono, M.; Fujii, H.; Eguchi, Y.; Suzuki, Y.; Yoneda, M.; et al. The novel cutoff points for the FIB4 index categorized by age increase the diagnostic accuracy in NAFLD: A multi-center study. J. Gastroenterol. 2018, 53, 1216–1224. [Google Scholar] [CrossRef]
- Tamaki, N.; Higuchi, M.; Kurosaki, M.; Kirino, S.; Osawa, L.; Watakabe, K.; Wang, W.; Okada, M.; Shimizu, T.; Takaura, K.; et al. Wisteria floribunda agglutinin-positive mac-2 binding protein as an age-independent fibrosis marker in nonalcoholic fatty liver disease. Sci. Rep. 2019, 9, 10109. [Google Scholar] [CrossRef]
- Kuno, A.; Sato, T.; Shimazaki, H.; Unno, S.; Saitou, K.; Kiyohara, K.; Sogabe, M.; Tsuruno, C.; Takahama, Y.; Ikehara, Y.; et al. Reconstruction of a robust glycodiagnostic agent supported by multiple lectin-assisted glycan profiling. Proteom. Clin. Appl. 2013, 7, 642–647. [Google Scholar] [CrossRef]
- Shah, A.G.; Lydecker, A.; Murray, K.; Tetri, B.N.; Contos, M.J.; Sanyal, A.J. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2009, 7, 1104–1112. [Google Scholar] [CrossRef]
- Xiao, G.; Zhu, S.; Xiao, X.; Yan, L.; Yang, J.; Wu, G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: A meta-analysis. Hepatology 2017, 66, 1486–1501. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P.; Hui, J.M.; Marchesini, G.; Bugianesi, E.; George, J.; Farrell, G.C.; Enders, F.; Saksena, S.; Burt, A.D.; Bida, J.P.; et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007, 45, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Wai, C.T.; Greenson, J.K.; Fontana, R.J.; Kalbfleisch, J.D.; Marrero, J.A.; Conjeevaram, H.S.; Lok, A.S. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003, 38, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Hernaez, R.; Lazo, M.; Bonekamp, S.; Kamel, I.; Brancati, F.L.; Guallar, E.; Clark, J.M. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: A meta-analysis. Hepatology 2011, 54, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Talwalkar, J.A.; Glaser, K.J.; Manduca, A.; Grimm, R.C.; Rossman, P.J.; Fidler, J.L.; Ehman, R.L. Assessment of hepatic fibrosis with magnetic resonance elastography. Clin. Gastroenterol. Hepatol. 2007, 5, 1207–1213.e1202. [Google Scholar] [CrossRef]
- Loomba, R.; Sirlin, C.B.; Ang, B.; Bettencourt, R.; Jain, R.; Salotti, J.; Soaft, L.; Hooker, J.; Kono, Y.; Bhatt, A.; et al. Ezetimibe for the treatment of nonalcoholic steatohepatitis: Assessment by novel magnetic resonance imaging and magnetic resonance elastography in a randomized trial (MOZART trial). Hepatology 2015, 61, 1239–1250. [Google Scholar] [CrossRef]
- Tamaki, N.; Higuchi, M.; Kurosaki, M.; Kirino, S.; Osawa, L.; Watakabe, K.; Wang, W.; Okada, M.; Shimizu, T.; Takaura, K.; et al. Risk assessment of hepatocellular carcinoma development by magnetic resonance elastography in chronic hepatitis C patients who achieved sustained virological responses by direct-acting antivirals. J. Viral. Hepat. 2019, 26, 893–899. [Google Scholar] [CrossRef]
- Hsu, C.; Caussy, C.; Imajo, K.; Chen, J.; Singh, S.; Kaulback, K.; Le, M.D.; Hooker, J.; Tu, X.; Bettencourt, R.; et al. Magnetic Resonance vs Transient Elastography Analysis of Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review and Pooled Analysis of Individual Participants. Clin. Gastroenterol. Hepatol. 2019, 17, 630–637.e638. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Tamaki, N.; Kurosaki, M.; Matsuda, S.; Nakata, T.; Muraoka, M.; Suzuki, Y.; Yasui, Y.; Suzuki, S.; Hosokawa, T.; Nishimura, T.; et al. Prospective comparison of real-time tissue elastography and serum fibrosis markers for the estimation of liver fibrosis in chronic hepatitis C patients. Hepatol. Res. 2014, 44, 720–727. [Google Scholar] [CrossRef]
- Yada, N.; Tamaki, N.; Koizumi, Y.; Hirooka, M.; Nakashima, O.; Hiasa, Y.; Izumi, N.; Kudo, M. Diagnosis of Fibrosis and Activity by a Combined Use of Strain and Shear Wave Imaging in Patients with Liver Disease. Dig. Dis. 2017, 35, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, N.; Koizumi, Y.; Hirooka, M.; Yada, N.; Takada, H.; Nakashima, O.; Kudo, M.; Hiasa, Y.; Izumi, N. Novel quantitative assessment system of liver steatosis using a newly developed attenuation measurement method. Hepatol. Res. 2018, 48, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Roulot, D.; Costes, J.L.; Buyck, J.F.; Warzocha, U.; Gambier, N.; Czernichow, S.; Le Clesiau, H.; Beaugrand, M. Transient elastography as a screening tool for liver fibrosis and cirrhosis in a community-based population aged over 45 years. Gut 2011, 60, 977–984. [Google Scholar] [CrossRef]
- Caballeria, L.; Pera, G.; Arteaga, I.; Rodriguez, L.; Aluma, A.; Morillas, R.M.; de la Ossa, N.; Diaz, A.; Exposito, C.; Miranda, D.; et al. High Prevalence of Liver Fibrosis Among European Adults With Unknown Liver Disease: A Population-Based Study. Clin. Gastroenterol. Hepatol. 2018, 16, 1138–1145.e1135. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Miyake, T.; Kuno, A.; Imai, Y.; Sawai, Y.; Hino, K.; Hara, Y.; Hige, S.; Sakamoto, M.; Yamada, G.; et al. Association between Wisteria floribunda agglutinin-positive Mac-2 binding protein and the fibrosis stage of non-alcoholic fatty liver disease. J. Gastroenterol. 2015, 50, 776–784. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- EASL-EASD-EASO. Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Davison, B.A.; Harrison, S.A.; Cotter, G.; Alkhouri, N.; Sanyal, A.; Edwards, C.; Colca, J.R.; Iwashita, J.; Koch, G.G.; Dittrich, H.C. Suboptimal reliability of liver biopsy evaluation has implications for randomized clinical trials. J. Hepatol. 2020, 73, 1322. [Google Scholar] [CrossRef]
- Kuwashiro, T.; Takahashi, H.; Hyogo, H.; Ogawa, Y.; Imajo, K.; Yoneda, M.; Nakahara, T.; Oeda, S.; Tanaka, K.; Amano, Y.; et al. Discordant pathological diagnosis of non-alcoholic fatty liver disease: A prospective multicenter study. JGH Open 2020, 4, 497–502. [Google Scholar] [CrossRef]
- Loomba, R.; Lawitz, E.; Mantry, P.S.; Jayakumar, S.; Caldwell, S.H.; Arnold, H.; Diehl, A.M.; Djedjos, C.S.; Han, L.; Myers, R.P.; et al. The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis: A randomized, phase 2 trial. Hepatology 2018, 67, 549–559. [Google Scholar] [CrossRef]
- Caussy, C.; Bhargava, M.; Villesen, I.F.; Gudmann, N.S.; Leeming, D.J.; Karsdal, M.A.; Faulkner, C.; Bao, D.; Liu, A.; Lo, M.T.; et al. Collagen Formation Assessed by N-Terminal Propeptide of Type 3 Procollagen Is a Heritable Trait and Is Associated With Liver Fibrosis Assessed by Magnetic Resonance Elastography. Hepatology 2019, 70, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Honda, Y.; Imajo, K.; Kobayashi, T.; Kessoku, T.; Ogawa, Y.; Tomeno, W.; Yoneda, M.; Kobayashi, N.; Saito, S.; Nakajima, A. Autotaxin is a valuable biomarker for the prediction of liver fibrosis in patients with non-alcoholic fatty liver disease. Hepatol. Res. 2019, 49, 1136–1146. [Google Scholar] [CrossRef] [PubMed]



| All Subjects | Subjects with WFA+-M2BP < 1.0 COI | Subjects with WFA+-M2BP ≥ 1.0 COI | p Value | |
|---|---|---|---|---|
| (n = 2021) | (n = 1917) | (n = 104) | ||
| Age, years | 57.7 ± 12 | 57.1 ± 12 | 67.1 ± 13 | <0.01 |
| Sex, male/female | 1112/909 | 1059/858 | 53/51 | 0.4 |
| BMI, kg/m2 | 22.6 ± 3.3 | 22.6 ± 3.3 | 23.1 ± 3.2 | 0.1 |
| Abdominal circumference, cm | 84.5 ± 9.7 | 84.4 ± 9.8 | 86.6 ± 8.5 | 0.02 |
| Albumin, g/dL | 4.3 ± 0.26 | 4.3 ± 0.26 | 4.1 ± 0.26 | <0.01 |
| AST, IU/L | 22.8 ± 7.8 | 22.6 ± 7.6 | 26.7 ± 8.9 | <0.01 |
| ALT, IU/L | 21.2 ± 12 | 21.1 ± 12 | 23.0 ± 13 | 0.2 |
| Gamma-glutamyl transpeptidase, IU/L | 33.7 ± 33 | 33.3 ± 32 | 41.6 ± 55 | 0.01 |
| Total cholesterol, mg/dL | 207 ± 32 | 207 ± 32 | 200 ± 34 | 0.03 |
| Triglycerides, mg/dL | 101 ± 67 | 100 ± 68 | 105 ± 61 | 0.5 |
| Platelet counts, × 109/L | 231 ± 52 | 233 ± 51 | 211 ± 58 | <0.01 |
| WFA+-M2BP, COI | 0.53 ± 0.28 | 0.48 ± 0.18 | 1.34 ± 0.45 | <0.01 |
| FIB-4 | 1.38 ± 0.7 | 1.34 ± 0.6 | 2.04 ± 1.0 | <0.01 |
| NAFLD fibrosis score | −1.977 ± 1.2 | −2.027 ± 1.1 | −1.068 ± 1.3 | <0.01 |
| APRI | 0.27 ± 0.12 | 0.27 ± 0.11 | 0.37 ± 0.18 | <0.01 |
| Hemoglobin A1c, % | 5.79 ± 0.48 | 5.78 ± 0.46 | 5.94 ± 0.67 | <0.01 |
| Fatty liver, n (%) | 797 (39.4%) | 749 (39.1%) | 48 (46.2%) | 0.2 |
| Hypertension, n (%) | 432 (21.4%) | 393 (20.5%) | 39 (37.5%) | <0.01 |
| Diabetes mellitus, n (%) | 146 (7.2%) | 129 (6.7%) | 17 (16.3%) | <0.01 |
| Dyslipidemia, n (%) | 359 (17.8%) | 334 (17.4%) | 25 (24.0%) | 0.08 |
| Significant alcohol consumption, n (%) | 418 (20.8%) | 400 (20.9%) | 18 (17.3%) | 0.5 |
| PPV | NPV | Sensitivity | Specificity | |
|---|---|---|---|---|
| WFA+-M2BP ≥ 1.2 COI | 43.5% | 84.0% | 71.4% | 61.8% |
| FIB-4 ≥ 2.67 | 41.2% | 77.4% | 50.0% | 70.6% |
| NAFLD fibrosis score ≥ 0.675 | 60.0% | 74.4% | 21.4% | 94.1% |
| APRI ≥ 1.5 | No subject met the criteria of APRI ≥ 1.5 | |||
| OR | 95% CI | p-Value | |
|---|---|---|---|
| Age (per 10 years) | 0.75 | 0.4–1.3 | 0.3 |
| Sex (female) | 0.71 | 0.2–2.6 | 0.6 |
| BMI, kg/m2 | 1.16 | 0.9–1.5 | 0.2 |
| Albumin < 4.1 g/dL * | 2.44 | 0.7–9.2 | 0.2 |
| AST > 38 IU/L * | 4.4e7 | 0.0–inf | 0.9 |
| ALT > 43 IU/L * | 2.54 | 0.2–43 | 0.5 |
| Gamma-glutamyl transpeptidase >80 IU/L for male and >40 IU/L for female * | 2.59 | 0.6–10 | 0.2 |
| Platelet counts < 160 (109/L) * | 1.54 | 0.4–5.9 | 0.5 |
| Presence of fatty liver | 0.79 | 0.2–2.9 | 0.7 |
| Presence of hypertension | 1.07 | 0.3–3.8 | 0.9 |
| Presence of diabetes mellitus | 2.82 | 0.5–16 | 0.2 |
| Presence of dyslipidemia | 1.30 | 0.3–5.3 | 0.7 |
| Presence of significant alcohol consumption | 4.13 | 0.8–21 | 0.1 |
| WFA+-M2BP ≥ 1.2 COI | 4.04 | 1.1–16 | 0.04 |
| FIB-4 ≥ 2.67 | 2.40 | 0.7–8.6 | 0.2 |
| NAFLD fibrosis score ≥ 0.675 | 4.36 | 0.6–29 | 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamaki, N.; Kurosaki, M.; Takahashi, Y.; Itakura, Y.; Kirino, S.; Inada, K.; Yamashita, K.; Sekiguchi, S.; Hayakawa, Y.; Osawa, L.; et al. Wisteria floribunda Agglutinin-Positive Mac-2 Binding Protein as a Screening Tool for Significant Liver Fibrosis in Health Checkup. Int. J. Mol. Sci. 2021, 22, 40. https://doi.org/10.3390/ijms22010040
Tamaki N, Kurosaki M, Takahashi Y, Itakura Y, Kirino S, Inada K, Yamashita K, Sekiguchi S, Hayakawa Y, Osawa L, et al. Wisteria floribunda Agglutinin-Positive Mac-2 Binding Protein as a Screening Tool for Significant Liver Fibrosis in Health Checkup. International Journal of Molecular Sciences. 2021; 22(1):40. https://doi.org/10.3390/ijms22010040
Chicago/Turabian StyleTamaki, Nobuharu, Masayuki Kurosaki, Yuka Takahashi, Yoshie Itakura, Sakura Kirino, Kento Inada, Koji Yamashita, Shuhei Sekiguchi, Yuka Hayakawa, Leona Osawa, and et al. 2021. "Wisteria floribunda Agglutinin-Positive Mac-2 Binding Protein as a Screening Tool for Significant Liver Fibrosis in Health Checkup" International Journal of Molecular Sciences 22, no. 1: 40. https://doi.org/10.3390/ijms22010040
APA StyleTamaki, N., Kurosaki, M., Takahashi, Y., Itakura, Y., Kirino, S., Inada, K., Yamashita, K., Sekiguchi, S., Hayakawa, Y., Osawa, L., Higuchi, M., Takaura, K., Maeyashiki, C., Kaneko, S., Yasui, Y., Tsuchiya, K., Nakanishi, H., Itakura, J., Loomba, R., & Izumi, N. (2021). Wisteria floribunda Agglutinin-Positive Mac-2 Binding Protein as a Screening Tool for Significant Liver Fibrosis in Health Checkup. International Journal of Molecular Sciences, 22(1), 40. https://doi.org/10.3390/ijms22010040





