CK2 Down-Regulation Increases the Expression of Senescence-Associated Secretory Phenotype Factors through NF-κB Activation
Abstract
1. Introduction
2. Results
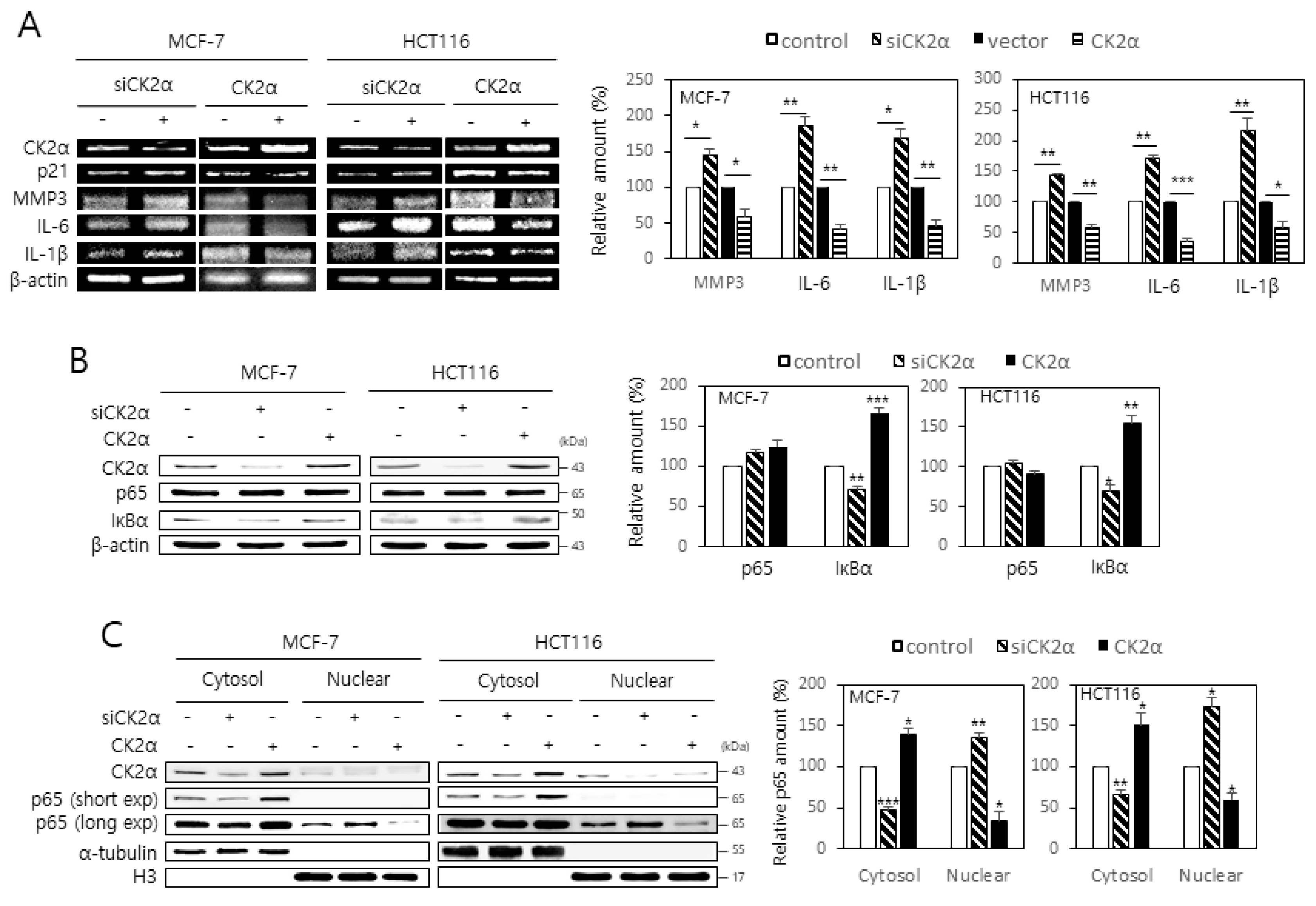
2.1. CK2 Down-Regulation Stimulates the Expression of SASP Factors by Enhancing the Nuclear Localization of NF-κB in Human Cancer Cells
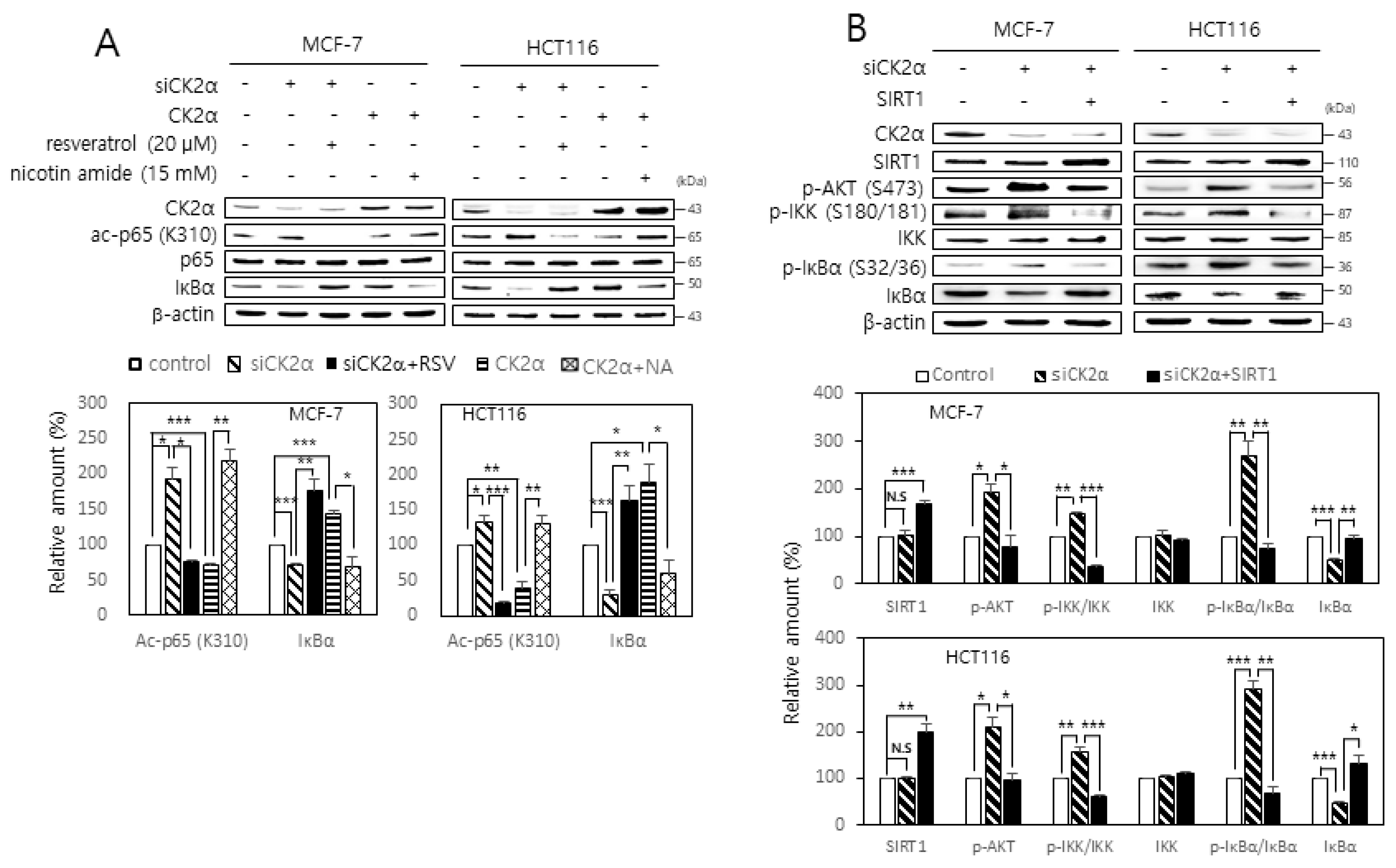
2.2. Activation of the AKT-IKK-IκB Pathway Is Associated with the CK2 Down-Regulation-Mediated Nuclear Import of NF-κB
2.3. SIRT1 Attenuates both RelA/p65 Acetylation and Activation of the AKT-IKK-IκB Axis Mediated by CK2 Down-Regulation
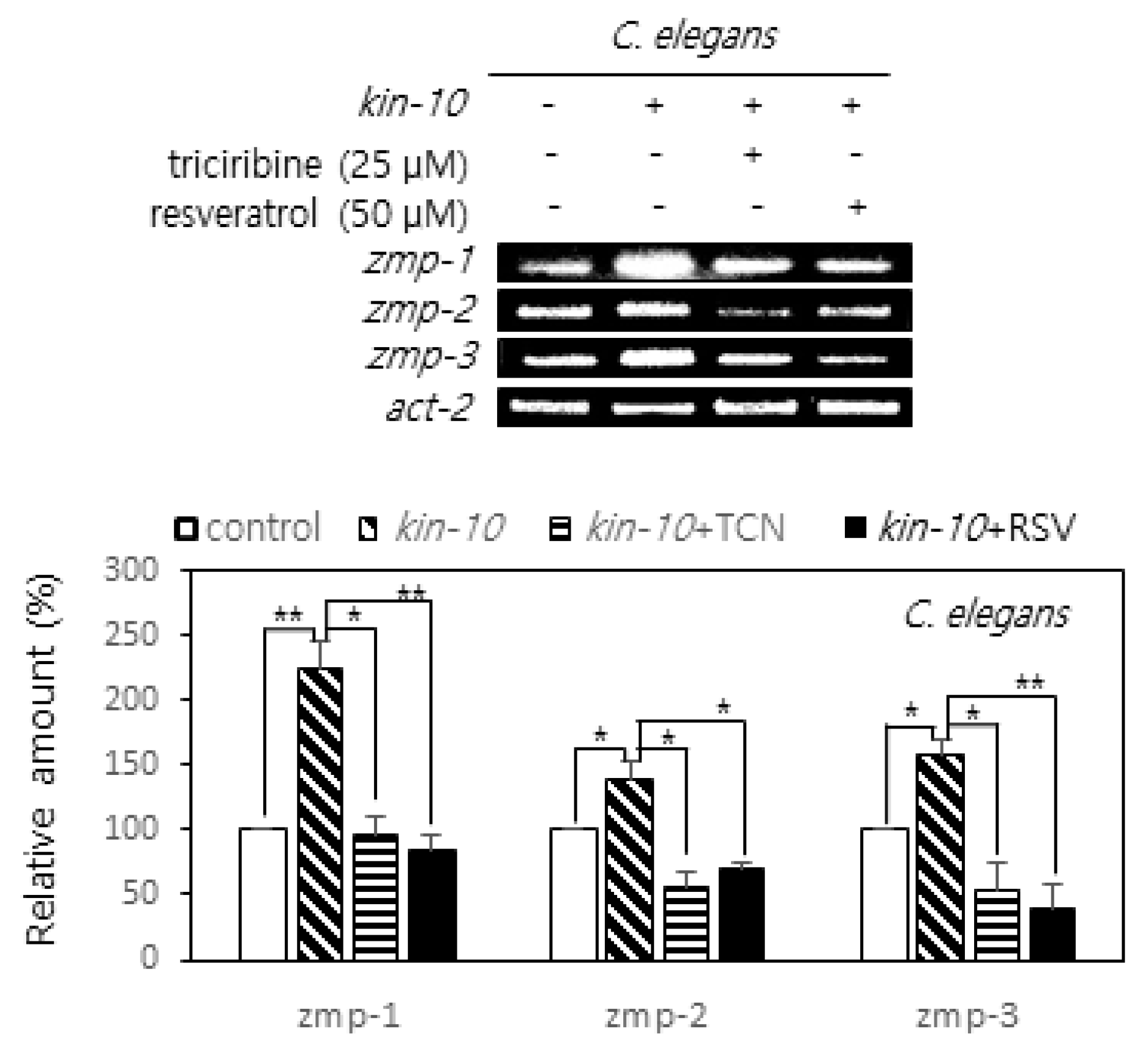
2.4. Triciribine and Resvzeratrol Attenuate the Expression of MMP Orthologs Induced by CK2 Down-Regulation in Nematodes
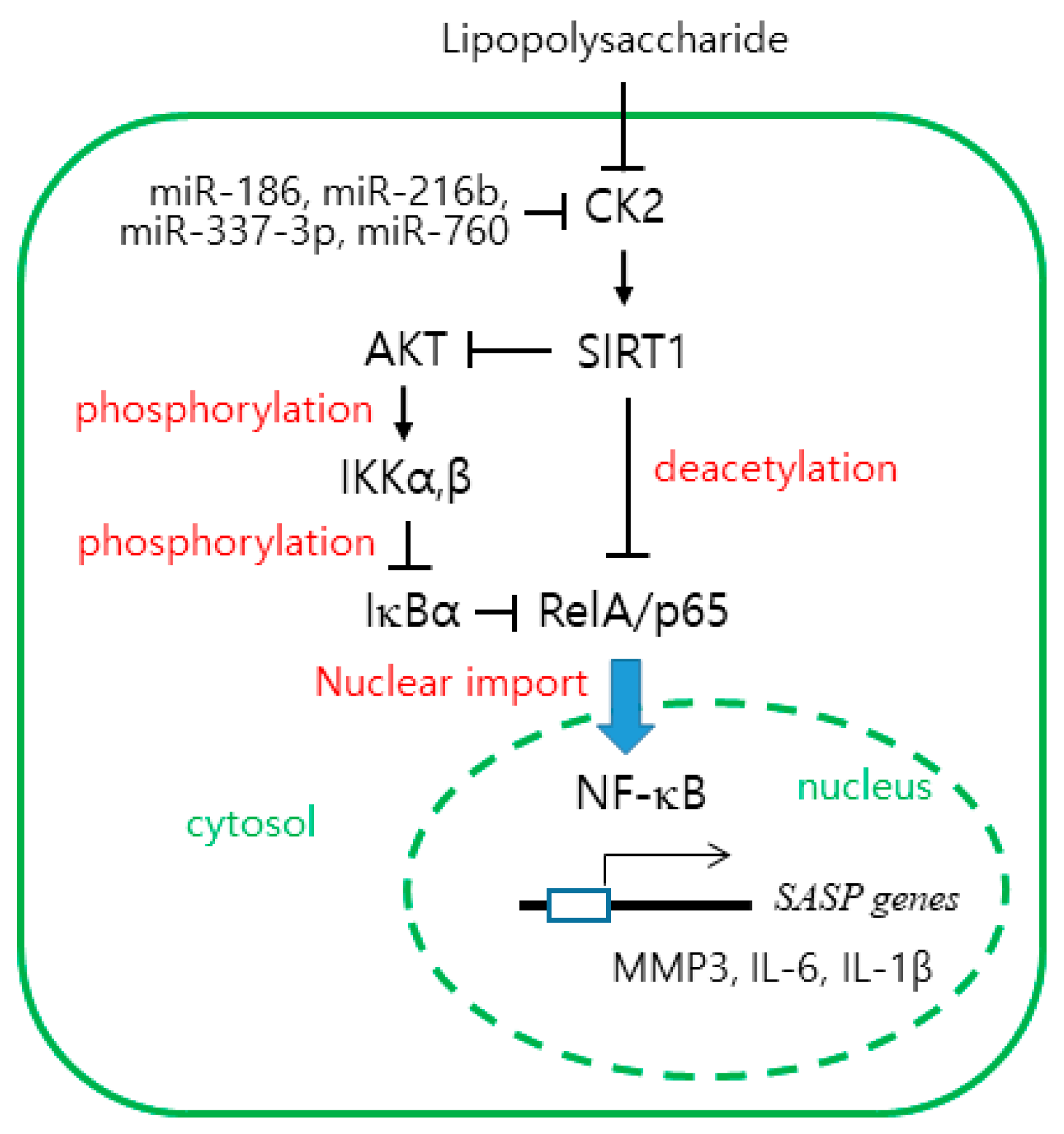
2.5. Antisense Inhibitors of miR-186, miR-216b, miR-337-3p, and miR-760 Attenuate SASP Gene Expression Induced by Lipopolysaccharide (LPS)
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture, RNAi, and DNA Transfection
4.3. Immunoblotting
4.4. RT-PCR
4.5. Isolation of Nuclear and Cytoplasmic Extracts
4.6. Culture of Nematodes and RNAi Experiment
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CK2 | protein kinase CK2 |
| HMG | high mobility group |
| IκB | inhibitors of NF-κB |
| IKK | IκB kinase |
| IL | interleukin |
| LPS | lipopolysaccharide |
| mTOR | mammalian target of rapamycin |
| MMP | matrix metalloproteinase |
| NF-κB | nuclear factor-κB |
| RT-PCR | reverse transcription-polymerase chain reaction |
| PI3K | phosphatidylinositol 3-kinase |
| ROS | reactive oxygen species |
| SA-β-gal | senescence-associated β-galactosidase |
| SAHF | senescence-associated heterochromatin foci |
| SASP | senescence-associated secretory phenotype |
| siRNA | small interfering RNA |
References
- Muñoz-Espín, D.; Serrano, M. Cellular senescence: From physiology to pathology. Nat. Rev. Mol. Cell. Biol. 2014, 15, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Childs, B.G.; Durik, M.; Baker, D.J.; van Deursen, J.M. Cellular senescence in aging and age-related disease: From mechanisms to therapy. Nat. Med. 2015, 21, 1424–1435. [Google Scholar] [CrossRef] [PubMed]
- McHugh, D.; Gil, J. Senescence and aging: Causes, consequences, and therapeutic avenues. J. Cell Biol. 2018, 217, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Coppe, J.P.; Desprez, P.Y.; Krtolica, A.; Campisi, J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. 2010, 5, 99–118. [Google Scholar] [CrossRef]
- Kuilman, T.; Peeper, D.S. Senescence-messaging secretome: SMS-ing cellular stress. Nat. Rev. Cancer 2009, 9, 81–94. [Google Scholar] [CrossRef]
- Acosta, J.C.; Banito, A.; Wuestefeld, T.; Georgilis, A.; Janich, P.; Morton, J.P.; Athineos, D.; Kang, T.W.; Lasitschka, F.; Andrulis, M.; et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol. 2013, 15, 978–990. [Google Scholar] [CrossRef]
- Chapman, J.; Fielder, E.; Passos, J.F. Mitochondrial dysfunction and cell senescence: Deciphering a complex relationship. FEBS Lett. 2019, 593, 1566–1579. [Google Scholar] [CrossRef]
- Salminen, A.; Kauppinen, A.; Kaarniranta, K. Emerging role of NF-κB signaling in the induction of senescence-associated secretory phenotype (SASP). Cell Signal. 2012, 24, 835–845. [Google Scholar] [CrossRef]
- Karin, M.; Lin, A. NF-κB at the crossroads of life and death. Nat. Immunol. 2002, 3, 221–227. [Google Scholar] [CrossRef]
- Agarwal, A.; Das, K.; Lerner, N.; Sathe, S.; Cicek, M.; Casey, G.; Sizemore, N. The AKT/IkB kinase pathway promotes angiogenic/metastatic gene expression in colorectal cancer by activating nuclear factor-κB and β-catenin. Oncogene 2005, 24, 1021–1031. [Google Scholar] [CrossRef]
- Yeung, F.; Hoberg, J.E.; Ramsey, C.S.; Keller, M.D.; Jones, D.R.; Frye, R.A.; Mayo, M.W. Modulation of NF-κB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004, 23, 2369–2380. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.D.; Tajkhorshid, E.; Chen, L.F. Functional interplay between acetylation and methylation of the RelA subunit of NF-κB. Mol. Cell. Biol. 2010, 30, 2170–2180. [Google Scholar] [CrossRef] [PubMed]
- Allende, J.E.; Allende, C.C. Protein kinases. 4. Protein kinase CK2: An enzyme with multiple substrates and a puzzling regulation. FASEB J. 1995, 9, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Litchfield, D.W. Protein kinase CK2: Structure, regulation and role in cellular decisions of life and death. Biochem. J. 2003, 369, 1–15. [Google Scholar] [CrossRef]
- Ryu, S.W.; Woo, J.H.; Kim, Y.H.; Lee, Y.S.; Park, J.W.; Bae, Y.S. Downregulation of protein kinase CKII is associated with cellular senescence. FEBS Lett. 2006, 580, 988–994. [Google Scholar] [CrossRef]
- Kang, J.Y.; Kim, J.J.; Jang, S.Y.; Bae, Y.S. The p53-p21Cip1/WAF1 pathway is necessary for cellular senescence induced by the inhibition of protein kinase CKII in human colon cancer cells. Mol. Cells 2009, 28, 489–494. [Google Scholar] [CrossRef]
- Park, J.W.; Kim, J.J.; Bae, Y.S. CK2 downregulation induces senescence-associated heterochromatic foci formation through activating SUV39h1 and inactivating G9a. Biochem. Biophys. Res. Commun. 2018, 505, 67–73. [Google Scholar] [CrossRef]
- Jeon, S.M.; Lee, S.J.; Kwon, T.K.; Bae, Y.S. NADPH oxidase is involved in protein kinase CKII down-regulation-mediated senescence through elevation of the level of reactive oxygen species in human colon cancer cells. FEBS Lett. 2010, 584, 3137–3142. [Google Scholar] [CrossRef]
- Jang, S.Y.; Kim, S.Y.; Bae, Y.S. p53 deacetylation by SIRT1 decreases during protein kinase CK2 downregulation-mediated cellular senescence. FEBS Lett. 2011, 585, 3360–3366. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, J.J.; Bae, Y.S. Involvement of PI3K-AKT-mTOR pathway in protein kinase CKII inhibition-mediated senescence in human colon cancer cells. Biochem. Biophys. Res. Commun. 2013, 433, 420–425. [Google Scholar] [CrossRef]
- Park, S.Y.; Bae, Y.S. Inactivation of the FoxO3a transcription factor is associated with the production of reactive oxygen species during protein kinase CK2 downregulation-mediated senescence in human colon cancer and breast cancer cells. Biochem. Biophys. Res. Commun. 2016, 478, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.E.; Song, J.; Park, J.W.; Yoon, S.H.; Bae, Y.H. Protein kinase CK2 activates Nrf2 via autophagic degradation of Keap1 and activation of AMPK in human cancer cells. BMB Rep. 2020, 53, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Lee, Y.H.; Bae, Y.S. miR-186, miR-216b, miR-337-3p, and miR-760 cooperatively induce cellular senescence by targeting α subunit of protein kinase CKII in human colorectal cancer cells. Biochem. Biophys. Res. Commun. 2012, 429, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Kim, S.Y.; Bae, Y.S. Upregulation of miR-760 and miR-186 is associated with replicative senescence in human lung fibroblast cells. Mol. Cells 2014, 37, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, J.H.; Park, J.W.; Kim, D.Y.; Hahm, J.H.; Nam, H.G.; Bae, Y.S. Downregulation of protein kinase CK2 activity induces age-related biomarkers in C. elegans. Oncotarget 2017, 8, 36950–36963. [Google Scholar] [CrossRef] [PubMed]
- Ham, H.J.; Park, J.W.; Bae, Y.S. Defect of SIRT1-FoxO3a axis is associated with the production of reactive oxygen species during protein kinase CK2 downregulation-mediated cellular senescence and nematode aging. BMB Rep. 2019, 52, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Feng, G.; Xing, J.; Shen, B.; Tan, W.; Huang, D.; Lu, X.; Tao, T.; Zhang, J.; Li, L.; et al. Repeated lipopolysaccharide stimulation promotes cellular senescence in human dental pulp stem cells (DPSCs). Cell Tissue Res. 2014, 356, 369–380. [Google Scholar] [CrossRef]
- Kim, C.O.; Huh, A.J.; Han, S.H.; Kim, J.M. Analysis of cellular senescence induced by lipopolysaccharide in pulmonary alveolar epithelial cells. Arch. Gerontol. Geriatr. 2012, 54, e35–e41. [Google Scholar] [CrossRef]
- Chai, R.; Fu, H.; Zheng, Z.; Liu, T.; Ji, S.; Li, G. Resveratrol inhibits proliferation and migration through SIRT1 mediated post‑translational modification of PI3K/AKT signaling in hepatocellular carcinoma cells. Mol. Med. Rep. 2017, 16, 8037–8044. [Google Scholar] [CrossRef]
- Romieu-Mourez, R.; Landesman-Bollag, E.; Seldin, D.C.; Sonenshein, G.E. Protein kinase CK2 promotes aberrant activation of NF-κB, transformed phenotype, and survival of breast cancer cells. Cancer Res. 2002, 62, 6770–6778. [Google Scholar]
- Eddy, S.F.; Guo, S.; Demicco, E.G.; Romieu-Mourez, R.; Landesman-Bollag, E.; Seldin, D.C.; Sonenshein, G.E. Inducible IκB kinase/IκB kinase epsilon expression is induced by CK2 and promotes aberrant NF-κB activation in breast cancer cells. Cancer Res. 2005, 65, 11375–11383. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Yeh, J.; van Waes, C. Protein kinase casein kinase 2 mediates IκB kinase and aberrant NF-κB activation by serum factor(s) in head and neck squamous carcinoma cells. Cancer Res. 2006, 66, 6722–6731. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Westerheide, S.D.; Hanson, J.L.; Baldwin, A.S., Jr. Tumor necrosis factor alpha-induced phosphorylation of RelA/p65 on Ser529 is controlled by casein kinase II. J. Biol. Chem. 2000, 275, 32592–32597. [Google Scholar] [CrossRef] [PubMed]
- Chantôme, A.; Pance, A.; Gauthier, N.; Vandroux, D.; Chenu, J.; Solary, E.; Jeannin, J.F.; Reveneau, S. Casein kinase II-mediated phosphorylation of NF-kappaB p65 subunit enhances inducible nitric-oxide synthase gene transcription in vivo. J. Biol. Chem. 2004, 279, 23953–23960. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, X.; Chen, H.P.; Li, L.; Xie, W.; Lan, G.; Zhao, Z.W.; Zheng, X.L.; Wang, Z.B.; Tang, C.K. MicroRNA-186 promotes macrophage lipid accumulation and secretion of pro-inflammatory cytokines by targeting cystathionine γ-lyase in THP-1 macrophages. Atherosclerosis 2016, 250, 122–132. [Google Scholar] [CrossRef]
- He, J.; Zhang, J.; Wang, D. Down-regulation of microRNA-216b inhibits IL-1β-induced chondrocyte injury by up-regulation of Smad3. Biosci. Rep. 2017, 37, BSR20160588. [Google Scholar] [CrossRef]
- Aird, K.M.; Iwasaki, O.; Kossenkov, A.V.; Tanizawa, H.; Fatkhutdinov, N.; Bitler, B.G.; Le, L.; Alicea, G.; Yang, T.L.; Johnson, F.B.; et al. HMGB2 orchestrates the chromatin landscape of senescence-associated secretory phenotype gene loci. J. Cell Biol. 2016, 215, 325–334. [Google Scholar] [CrossRef]
- Pedersen, D.S.; Merkle, T.; Marktl, B.; Lildballe, D.L.; Antosch, M.; Bergmann, T.; Tönsing, K.; Anselmetti, D.; Grasser, K.D. Nucleocytoplasmic distribution of the Arabidopsis chromatin-associated HMGB2/3 and HMGB4 proteins. Plant Physiol. 2010, 154, 1831–1841. [Google Scholar] [CrossRef][Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.; Bae, Y.-S. CK2 Down-Regulation Increases the Expression of Senescence-Associated Secretory Phenotype Factors through NF-κB Activation. Int. J. Mol. Sci. 2021, 22, 406. https://doi.org/10.3390/ijms22010406
Song J, Bae Y-S. CK2 Down-Regulation Increases the Expression of Senescence-Associated Secretory Phenotype Factors through NF-κB Activation. International Journal of Molecular Sciences. 2021; 22(1):406. https://doi.org/10.3390/ijms22010406
Chicago/Turabian StyleSong, Junbin, and Young-Seuk Bae. 2021. "CK2 Down-Regulation Increases the Expression of Senescence-Associated Secretory Phenotype Factors through NF-κB Activation" International Journal of Molecular Sciences 22, no. 1: 406. https://doi.org/10.3390/ijms22010406
APA StyleSong, J., & Bae, Y.-S. (2021). CK2 Down-Regulation Increases the Expression of Senescence-Associated Secretory Phenotype Factors through NF-κB Activation. International Journal of Molecular Sciences, 22(1), 406. https://doi.org/10.3390/ijms22010406




