Melatonin as an Oncostatic Molecule Based on Its Anti-Aromatase Role in Breast Cancer
Abstract
1. Introduction
2. The Role of Estrogen and Aromatase in Breast Cancer Development
2.1. Estrogen Promotes the Growth of Breast Cancers
2.2. Aromatase: The Major Estrogen Synthase Is Involved in Breast Cancer Development
3. Aromatase-Inhibitory Role of Melatonin
3.1. Melatonin Modulates the Estrogenic Effects in Breast Cancer
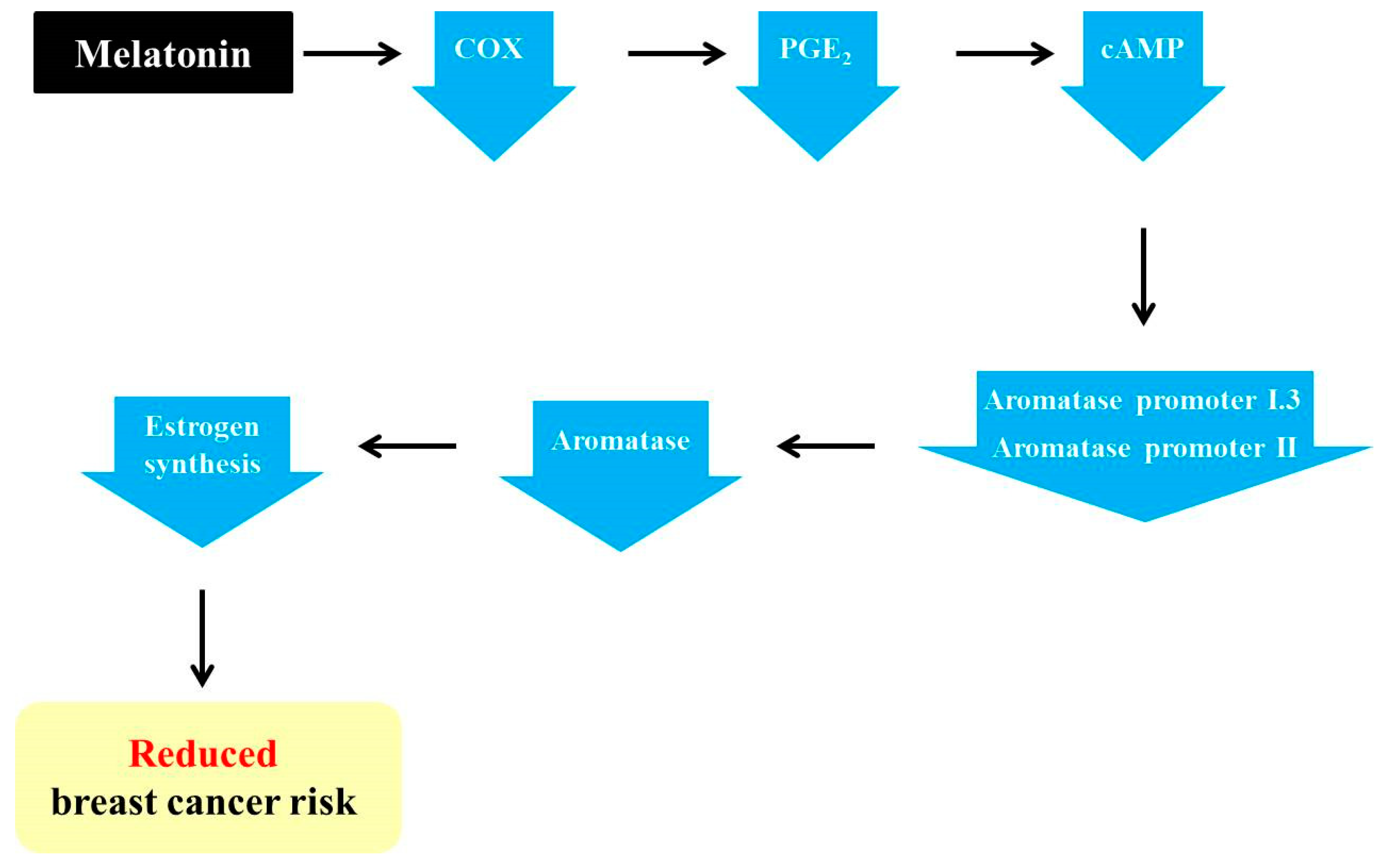
3.2. Melatonin Exerts Anti-Aromatase Roles via Regulating Cyclooxygenase (COX) Gene Activity
3.3. Melatonin Increases the Efficiency of Conventional SEEMs and SERMs
3.4. Melatonin Potentiates the Anti-Aromatase Effect of Radiation and Its Presumable Link to p53
3.5. Melatonin Enhances Anti-Angiogenic Function and Suppresses the Disadvantages of Chemotherapeutic Agents
3.6. Overexpression of the MT1 Melatonin Receptor Has an Aromatase-Suppressive Role and Mediates Oncostatic Action of Melatonin in the MCF7 Human Breast Cancer Cell Line
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clemons, M.; Goss, P. Estrogen and the risk of breast cancer. N. Engl. J. Med. 2001, 344, 276–285. [Google Scholar] [CrossRef]
- Saha, T.; Makar, S.; Swetha, R.; Gutti, G.; Singh, S. Estrogen signaling: An emanating therapeutic target for breast cancer treatment. Eur. J. Med. Chem. 2019, 177, 116–143. [Google Scholar] [CrossRef] [PubMed]
- Patani, N.; Martin, L.-A. Understanding response and resistance to oestrogen deprivation in ER-positive breast cancer. Mol. Cell. Endocrinol. 2014, 382, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Fusi, C.; Materazzi, S.; Benemei, S.; Coppi, E.; Trevisan, G.; Marone, I.M.; Minocci, D.; De Logu, F.; Tuccinardi, T.; Di Tommaso, M.R.; et al. Steroidal and non-steroidal third-generation aromatase inhibitors induce pain-like symptoms via TRPA1. Nat. Commun. 2014, 5, 5736. [Google Scholar] [CrossRef] [PubMed]
- Goss, P.E.; Strasser, K. Aromatase inhibitors in the treatment and prevention of breast cancer. J. Clin. Oncol. 2001, 19, 881–894. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Yu, B.; Sherman, M.; Yuan, Y.-C.; Zhou, D.; Chen, S. Molecular basis for the aromatization reaction and exemestane-mediated irreversible inhibition of human aromatase. Mol. Endocrinol. 2007, 21, 401–414. [Google Scholar] [CrossRef]
- Miller, W.R. Aromatase inhibitors: Mechanism of action and role in the treatment of breast cancer. Semin. Oncol. 2003, 30, 3–11. [Google Scholar] [CrossRef]
- Wardell, S.E.; Norris, J.D.; McDonnell, D.P. Targeting mutant estrogen receptor. eLife 2019, 8, e44181. [Google Scholar] [CrossRef]
- Higgins, M.J.; Liedke, P.E.R.; Goss, P.E. Extended adjuvant endocrine therapy in hormone dependent breast cancer: The paradigm of the NCIC-CTG MA. 17/BIG 1–97 trial. Crit. Rev. Oncol. Hematol. 2013, 86, 23–32. [Google Scholar] [CrossRef]
- Lumachi, F.; Brunello, A.; Maruzzo, M.; Basso, U.; Basso, S.M.M. Treatment of estrogen receptor-positive breast cancer. Curr. Med. Chem. 2013, 20, 596–604. [Google Scholar] [CrossRef]
- Schmidberger, H.; Hermann, R.M.; Hess, C.F.; Emons, G. Interactions between radiation and endocrine therapy in breast cancer. Endocr. Relat. Cancer 2003, 10, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.; Costantino, J.P.; Redmond, C.K.; Fisher, E.R.; Wickerham, D.L.; Cronin, W.M. Endometrial cancer in tamoxifen-treated breast cancer patients: Findings from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14. J. Natl. Cancer Inst. 1994, 86, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Jordan, V.C. Tamoxifen: Toxicities and drug resistance during the treatment and prevention of breast cancer. Annu. Rev. Pharmacol. Toxicol. 1995, 35, 195–211. [Google Scholar] [CrossRef] [PubMed]
- Chumsri, S.; Howes, T.; Bao, T.; Sabnis, G.; Brodi, A. Aromatase, aromatase inhibitors, and breast cancer. J. Steroid Biochem. Mol. Biol. 2011, 125, 13–22. [Google Scholar] [CrossRef]
- Nelson, H.D.; Smith, M.E.B.; Griffin, J.C.; Fu, R. Use of medications to reduce risk for primary breast cancer: A systematic review for the U.S. preventive services task force. Ann. Intern. Med. 2013, 158, 604–614. [Google Scholar] [CrossRef]
- Mocellin, S.; Goodwin, A.; Pasquali, S. Risk-reducing medications for primary breast cancer: A network meta-analysis. Cochr. Database Syst. Rev. 2019, 4, CD012191. [Google Scholar] [CrossRef]
- Harderland, R.; Cardinali, D.P.; Srinivasan, V.; Spence, D.W.; Brown, G.M.; Pandi-Perumal, S.R. Melatonin-A Pleiotropic, orchestrating regulator molecule. Prog. Neurobiol. 2011, 93, 350–384. [Google Scholar] [CrossRef]
- Sarlak, G.; Jenwitheewuk, A.; Chetsawang, B.; Govitrapong, P. Effects of melatonin on nervous system aging: Neurogenesis and neurodegeneration. J. Pharmacol. Sci. 2013, 123, 9–24. [Google Scholar] [CrossRef]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal. Res. 2016, 61, 253–278. [Google Scholar] [CrossRef]
- Calvo, J.R.; Maldonado, M.D. The role of melatonin in autoimmune and atopic diseases. AIMS Mol. Sci. 2016, 3, 158. [Google Scholar] [CrossRef]
- Jin, Y.; Hong, Y.; Park, C.Y.; Hong, Y. Molecular interactions of autophagy with the immune system and cancer. Int. J. Mol. Sci. 2017, 18, 1694. [Google Scholar] [CrossRef] [PubMed]
- Griffin, F.; Marignol, L. Therapeutic potential of melatonin for breast cancer radiation therapy patients. Int. J. Radiat. Biol. 2018, 94, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gonzalez, A.; Gonzalez, A.; Rueda, N.; Alonso-Gonzalez, C.; Menendez-Menendez, J.; Martinez-Campa, C.; Mitola, S.; Cos, S. Usefulness of melatonin as complementary to chemotherapeutic agents at different stages of the angiogenic process. Sci. Rep. 2020, 10, 4790. [Google Scholar] [CrossRef] [PubMed]
- Amini, P.; Mirtavoos-Mahyari, H.; Motevasli, E.; Shabeeb, D.; Musa, A.E.; Cheki, M.; Farhood, B.; Yahyapour, R.; Shirazi, A.; Goushbolagh, N.A.; et al. Mechanisms for radioprotection by melatonin; Can it be used as a radiation countermeasure? Curr. Mol. Pharmacol. 2019, 12, 2–11. [Google Scholar] [CrossRef]
- Fernandez-Gil, B.I.; Guerra-Librero, A.; Shen, Y.-Q.; Florido, J.; Martinez-Ruiz, L.; Garcia-Lopz, S.; Adan, C.; Rodriguez-Santana, C.; Acuna-Castroviejo, D.; Quinones-Hinojosa, A.; et al. Melatonin enhances cisplatin and radiation cytotoxicity in head and neck squamous cell carcinoma by stimulating mitochondrial ROS generation, apoptosis, and autophagy. Oxid. Med. Cell. Longev. 2019, 2019, 718128. [Google Scholar] [CrossRef]
- Cos, S.; Martinez-Campa, C.; Mediavilla, M.D.; Sanchez-Barcelo, E.J. Melatonin modulates aromatase activity in MCF-7 human breast cancer cells. J. Pineal Res. 2005, 38, 136–142. [Google Scholar] [CrossRef]
- Martinez-Campa, C.; Gonzalez, A.; Mediavilla, M.D.; Alonso-Gonzalez, C.; Alvarez-Garcia, V.; Sanchez-Barcel, E.J.; Cos, S. Melatonin inhibits aromatase promoter expression by regulating cyclooxygenases expression and activity in breast cancer cells. Br. J. Cancer. 2009, 101, 1613–1619. [Google Scholar] [CrossRef]
- Alonso-Gonzalez, C.; Gonzalez, A.; Menendez-Menendez, J.; Martinez-Campa, C.; Cos, S. Melatonin as a Radio-Sensitizer in Cancer. Boimedicines 2020, 8, 247. [Google Scholar] [CrossRef]
- Ram, P.T.; Dai, J.; Yuan, L.; Dong, C.; Kiefer, T.L.; Lai, L.; Hill, S.M. Involvement of the mt1 melatonin receptor in human breast cancer. Cancer Lett. 2002, 179, 141–150. [Google Scholar] [CrossRef]
- Goyal, R.; Gupta, T.; Bal, A.; Sahni, D.; Singh, G. Role of melatonin in breast carcinoma: Correlation of expression patterns of melatonin-1 receptor with estrogen, progesterone, and HER2 receptors. Appl. Immunohistochem. Mol. Morphol. 2020, 28, 518–523. [Google Scholar] [CrossRef]
- Cos, S.; Martinez-Campa, C.; Gonzalez, A.V.; Alvarez-Garcia, V.; Alonso-Gonzalez, C.; Mediavilla, M.D.; Sanchez-Barcelo, E.J. Melatonin and aromatase in breast cancer. Clin. Cancer Drugs 2014, 1, 54–64. [Google Scholar] [CrossRef]
- Deroo, B.J.; Korach, K.S. Estrogen receptors and human disease. J. Clin. Investig. 2006, 116, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Russo, I.H.; Russo, J. Role of hormones in mammary cancer initiation and progression. J. Mammary Gland Biol. Neoplasia 1998, 3, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Endogenous Hormones and Breast Cancer Collaborative Group. Sex hormones and risk of breast cancer in premenopausal women: A collaborative reanalysis of individual participant data from seven prospective studies. Lancet Oncol. 2013, 14, 1009–1019. [Google Scholar] [CrossRef]
- Preston-Martin, S.; Pike, M.C.; Ross, R.K.; Jones, P.A.P.; Henderson, B.E. Increased cell division as a cause of human cancer. Cancer Res. 1990, 50, 7415–7421. [Google Scholar]
- Yue, W.; Wang, J.P.; Li, Y.; Fan, P.; Liu, G.; Zhang, N.; Conaway, M.; Wang, H.; Korach, K.S.; Bocchinfuso, W.; et al. Effects of estrogen on breast cancer development: Role of estrogen receptor independent mechanisms. Int. J. Cancer. 2010, 127, 1748–1757. [Google Scholar] [CrossRef]
- Han, R.; Gu, S.; Zhang, Y.; Luo, A.; Jing, X.; Zhao, L.; Zhao, X.; Zhang, L. Estrogen promotes progression of hormone-dependent breast cancer through CCL2-CCR2 axis by upregulation of Twist via PI3K/NF-κB signaling. Sci. Rep. 2018, 8, 9575. [Google Scholar] [CrossRef]
- Pinsky, P.F.; Miller, E.; Heckman-Stoddard, B.; Minasian, L. Use of raloxifene and tamoxifen by breast cancer risk level in a Medicare-eligible cohort. Am. J. Obstet. Gynecol. 2018, 218, 606. [Google Scholar] [CrossRef]
- Tian, J.-M.; Ran, B.; Zhang, C.-L.; Yan, D.-M.; Li, X.-H. Estrogen and progesterone promote breast cancer cell proliferation by inducing cyclin G1 expression. Braz. J. Med. Biol. Res. 2018, 51, 1–7. [Google Scholar] [CrossRef]
- Chen, S.A.; Besman, M.J.; Sparkes, R.S.; Zollman, S.; Klisak, I.; Mohandas, T.; Hall, P.F.; Shively, J.E. Human Aromatase: cDNA Cloning, Southern Blot Analysis, and Assignment of the Gene to Chromosome 15. DNA 1988, 7, 27–38. [Google Scholar] [CrossRef]
- Conley, A.; Hinshelwood, M. Mammalian aromatases. Reproduction 2001, 121, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Haynes, B.P.; Dowsett, M.; Miller, W.R.; Dixon, J.M.; Bhatnagar, A.S. The pharmacology of letrozole. J. Steroid Biochem. Mol. Biol. 2003, 87, 35–45. [Google Scholar] [CrossRef]
- Avendario, C.; Menendez, J.C. Medicinal Chemistry of Anticancer Drugs. Chapter 3-Anticancer Drugs that Modulate Hormone Action; Elsevier: Amsterdam, The Netherlands, 2015; pp. 82–122. [Google Scholar]
- Bulun, S.E.; Lin, Z.; Imir, G.; Amin, S.; Demura, M.; Yilmaz, B.; Martin, R.; Utsunomiya, H.; Thung, S.; Gurates, B. Regulation of aromatase expression in estrogen-responsive breast and uterine disease: From bench to treatment. Pharmacol. Rev. 2005, 57, 359–383. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Reierstad, S.; Lu, M.; Lin, Z.; Ishikawa, H.; Bulun, S.E. Regulation of breast cancer-associated aromatase promoters. Cancer Lett. 2009, 273, 15–27. [Google Scholar] [CrossRef]
- Alvarez-Garcia, V.; Gonzalez, A.; Alonso-Gonzalez, C.; Martinez-Campa, C.; Cos, S. Melatonin interferes in the desmoplastic reaction in breast cancer by regulating cytokine production. J. Pineal Res. 2012, 52, 282–290. [Google Scholar] [CrossRef]
- Guerrero, J.; Tobar, N.; Caceres, M.; Espinoza, L.; Escobar, P.; Dotor, J.; Smith, P.C.; Martinez, J. Soluble factors derived from tumor mammary cell lines induce a stromal mammary adipose reversion in human and mice adipose cells. Possible role of TGF-beta1 and TNF-alpha. Breast Cancer Res. Treat. 2010, 119, 497–508. [Google Scholar] [CrossRef]
- Cos, S.; Alvarez-García, V.; González, A.; Alonso-González, C.; Martínez-Campa, C. Melatonin modulation of crosstalk among malignant epithelial, endothelial and adipose cells in breast cancer. Oncol. Lett. 2014, 8, 487–492. [Google Scholar] [CrossRef]
- Gonzalez-Gonzalez, A.; Nieto, E.G.; Gonzalez, A.; Sanchez-Fernandez, C.; Alonso-Gonzalez, C.; Menendez-Menendez, J.; Gomez-Arozamena, J.; Cos, S.; Martinez-Campa, C. Melatonin Modulation of Radiation and Chemotherapeutics-induced Changes on Differentiation of Breast Fibroblasts. Int. J. Mol. Sci. 2019, 20, 3935. [Google Scholar] [CrossRef]
- Meng, L.; Zhou, J.; Sasano, H.; Suzuki, T.; Zeitoun, K.M.; Bulun, S.E. Tumor necrosis factor α and interleukin 11 secreted by malignant breast epithelial cells inhibit adipocyte differentiation by selectively down-regulating CCAAT/enhancer binding protein α and peroxisome proliferator-activated receptor γ: Mechanism of desmoplastic reaction. Cancer Res. 2001, 61, 2250–2255. [Google Scholar]
- Bulun, S.E.; Chen, D.; Lu, M.; Zhao, H.; Cheng, Y.; Demura, M.; Yilmaz, B.; Martin, R.; Utsunomiya, H.; Thung, S.; et al. Aromatase excess in cancers of breast, endometrium and ovary. J. Steroid Biochem. Mol. Biol. 2007, 106, 81–96. [Google Scholar] [CrossRef]
- Anderson, W.F.; Chatterjee, N.; Ershler, W.B.; Brawley, O.W. Estrogen receptor breast cancer phenotypes in the Surveillance, Epidemiology, and End Results database. Breast Cancer Res. Treat. 2002, 76, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.F.R. Fulvestrant (Faslodex®)-how to make a good drug better. Oncologist 2007, 12, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Burstein, H.J.; Prestrud, A.A.; Seidenfeld, J.; Anderson, H.; Buchholz, T.A.; Davidson, N.E.; Gelmon, K.E.; Giordano, S.H.; Hudis, C.A.; Malin, J.; et al. American society of clinical oncology clinical practice guideline update on adjuvant endocrine therapy for women with hormone receptor–positive breast cancer. J. Clin. Oncol. 2010, 28, 3784–3796. [Google Scholar] [CrossRef] [PubMed]
- Harada, N. Aberrant expression of aromatase in breast cancer. J. Steroid Biochem. Mol. Biol. 1997, 61, 175–184. [Google Scholar] [CrossRef]
- Brown, K.A.; Lyengar, N.M.; Zhou, X.K.; Gucalp, A.; Subbaramaiah, K.; Wang, H.; Giri, D.D.; Morrow, M.; Falcone, D.J.; Wendel, N.K.; et al. Menopause is a determinant of breast aromatase expression and its associations with BMI, inflammation, and systemic markers. J. Clin. Endocrinol. Metab. 2017, 102, 1692–1701. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Cos, S.; Martinez-Campa, C.; Alonso-Gonzalez, C.; Sanchez-Mateos, S.; Mediavilla, M.D.; Sanchez-Barcel, E.J. Selective estrogen enzyme modulator actions of melatonin in human breast cancer cells. J. Pineal Res. 2008, 45, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Alvarez-Garcia, V.; Martinez-Campa, C.; Mediavilla, M.D.; Alonso-Gonzalez, C.; Sanchez-Barcelo, E.J.; Cos, S. In vivo inhibition of the estrogen sulfatase enzyme and growth of DMBA-induced mammary tumors by melatonin. Curr. Cancer Drug Targets 2010, 10, 279–286. [Google Scholar] [CrossRef]
- Blask, D.E.; Sauer, L.A.; Dauch, R.T. Melatonin as a chronobiotic/anticancer agent: Cellular, biochemical and molecular mechanisms of action and their implications for circadian-based cancer therapy. Curr. Top. Med. Chem. 2002, 2, 113–132. [Google Scholar] [CrossRef]
- Cos, S.; Gonzalez, A.; Martınez-Campa, C.; Mediavilla, M.D.; Alonso-Gonzalez, C.; Sanchez-Barcelom, E.J. Melatonin as a selective estrogen enzyme modulator. Curr. Cancer Drug. Targets 2008, 8, 691–702. [Google Scholar] [CrossRef]
- Menendez-Menendez, J.; Martinez-Campa, C. Melatonin: An anti-tumor agent in hormone-dependent cancers. Int. J. Endocrinol. 2018, 2018, 3271948. [Google Scholar] [CrossRef]
- Brueggemeier, R.W.; Richards, J.A.; Petrel, T.A. Aromatase and cyclooxygenases: Enzymes in breast cancer. J. Steroid Biochem. Mol. Biol. 2003, 86, 501–507. [Google Scholar] [CrossRef]
- Gonzalez, A.; Alvarez-Garcia, V.; Martinez-Campa, C.; Alonso-Gonzalez, C.; Cos, S. Melatonin promotes differentiation of 3T3-L1 fibroblasts. J. Pineal Res. 2012, 52, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Knower, K.C.; To, S.Q.; Takagi, K.; Miki, Y.; Sasano, H.; Simpson, E.R.; Clyne, C.D. Melatonin suppresses aromatase expression and activity in breast cancer associated fibroblasts. Breast Cancer Res. Treat. 2012, 132, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Liu, X.; Zhou, Z.; Xiang, Y.; Yuan, S.; Xie, W.; Zhou, M.; Hu, Z.; Li, Y.; Ji, A.; et al. Melatonin attenuates expression of cyclooxygenase-2 (COX-2) in activated microglia induced by lipopolysaccharide (LPS). J. Toxicol. Environ. Health A 2019, 82, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Dubois, R.N. Prostaglandins and cancer. Gut 2006, 55, 115–122. [Google Scholar] [CrossRef]
- Amin, N.; Shafabakhsh, R.; Reiter, R.J.; Asemi, Z. Melatonin is an appropriate candidate for breast cancer treatment: Based on known molecular mechanisms. J. Cell. Biochem. 2019, 120, 12208–12215. [Google Scholar] [CrossRef]
- Miettinen, M.M.; Mustonen, M.V.; Poutanen, M.H.; Isomaa, V.V.; Vihko, R.K. Human 17 β-hydroxysteroid dehydrogenase type 1 and type 2 isoenzymes have opposite activities in cultured cells and characteristic cell-and tissue-specific expression. Biochem. J. 1996, 314, 839–845. [Google Scholar] [CrossRef]
- Hilborn, E.; Stål, O.; Jansson, A. Estrogen and androgen-converting enzymes 17β-hydroxysteroid dehydrogenase and their involvement in cancer: With a special focus on 17β-hydroxysteroid dehydrogenase type 1, 2, and breast cancer. Oncotarget 2017, 8, 30552–30562. [Google Scholar] [CrossRef]
- Pasqualini, J.R.; Chetrite, G.S. Recent insight on the control of enzymes involved in estrogen formation and transformation in human breast cancer. J. Steroid Biochem. Mol. Biol. 2005, 93, 221–236. [Google Scholar] [CrossRef]
- González-González, A.; Mediavilla, M.D.; Sánchez-Barceló, E.J. Melatonin: A molecule for reducing breast cancer risk. Molecules 2018, 23, 336. [Google Scholar] [CrossRef]
- Sanchez-Barcelo, E.J.; Mediavilla, M.D.; Alonso-Gonzalez, C.; Rueda, N. Breast cancer therapy based on melatonin. Recent Pat. Endocr. Metab. Immune Drug Discov. 2012, 6, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Barceló, E.J.; Cos, S.; Mediavilla, D.; Martinez-Campa, C.; Gonzalez, A.; Alonso-Gonzalez, C. Melatonin–estrogen interactions in breast cancer. J. Pineal Res. 2005, 38, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Treeck, O.; Haldar, C.; Ortmann, O. Antiestrogens modulate MT1 melatonin receptor expression in breast and ovarian cancer cell lines. Oncol. Rep. 2006, 15, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Collins, A.R.; Dai, J.; Dubocovich, M.L.; Hill, S.M. MT1 melatonin receptor overexpression enhances the growth suppressive effect of melatonin in human breast cancer cells. Mol. Cell. Endocrinol. 2002, 192, 147–156. [Google Scholar] [CrossRef]
- Clemens, J.W.; Jarzynka, M.J.; Witt-Enderby, P.A. Down-regulation of mt1 melatonin receptors in rat ovary following estrogen exposure. Life Sci. 2001, 69, 27–35. [Google Scholar] [CrossRef]
- Wilson, S.T.; Blask, D.E.; Lemus-Wilson, A.M. Melatonin augments the sensitivity of MCF-7 human breast cancer cells to tamoxifen in vitro. J. Clin. Endocrinol. Metab. 1992, 75, 669–670. [Google Scholar]
- Martínez-Campa, C.; Gonzalez, A.; Mediavilla, M.D.; Alonso-Gonzalez, C.; Sanchez-Barcelo, E.J.; Cos, S. Melatonin enhances the inhibitory effect of aminoglutethimide on aromatase activity in MCF-7 human breast cancer cells. Breast Cancer Res. Treat. 2005, 94, 249–254. [Google Scholar] [CrossRef]
- Aydin, M.; Oktar, S.; Ozkan, O.V.; Alçin, E.; Oztürk, O.H.; Nacar, A. Letrozole induces hepatotoxicity without causing oxidative stress: The protective effect of melatonin. Gynecol. Endocrinol. 2011, 27, 209–215. [Google Scholar] [CrossRef]
- Choi, J.; Cha, Y.J.; Koo, J.S. Adipocyte biology in breast cancer: From silent bystander to active facilitator. Prog. Lipid Res. 2018, 69, 11–20. [Google Scholar] [CrossRef]
- Goto, H.; Shimono, Y.; Funakoshi, Y.; Imamura, Y.; Toyoda, M.; Kiyota, N.; Kono, S.; Takao, S.; Mukohara, T.; Minami, H. Adipose-derived stem cells enhance human breast cancer growth and cancer stem cell-like properties through adipsin. Oncogene 2019, 38, 767–779. [Google Scholar] [CrossRef]
- Bindhu, J.; Arunava, D. An edible fungi Pleurotus ostreatus inhibits adipogenesis via suppressing expression of PPAR γ and C/EBP α in 3T3-L1 cells: In vitro validation of gene knock out of RNAs in PPAR γ using CRISPR spcas9. Biomed. Pharmacother. 2019, 116, 109030. [Google Scholar]
- Dong, X.R.; Wang, J.N.; Liu, L.; Chen, X.; Chen, M.-S.; Chen, J.; Ren, J.-H.; Li, Q.; Han, J. Modulation of radiation-induced tumour necrosis factor-α and transforming growth factor β1 expression in the lung tissue by Shengqi Fuzheng injection. Mol. Med. Rep. 2010, 3, 621–627. [Google Scholar]
- Esquivel-Velazquez, M.; Ostoa-Saloma, P.; Palacios-Arreola, M.I.; Nava-Castro, K.E.; Castro, J.I.; Morales-Montor, J. The role of cytokines in breast cancer development and progression. J. Interferon. Cytokine Res. 2015, 35, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Gonzalez, C.; Gonzalez, A.; Martinez-Campa, C.; Menendez-Menendez, J.; Gomez-Arozamena, J.; Garcia-Vidal, A.; Cos, S. Melatonin enhancement of the radiosensitivity of human breast cancer cells is associated with the modulation of proteins involved in estrogen biosynthesis. Cancer Lett. 2016, 370, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Docanto, M.M.; Sasano, H.; Lo, C.; Simpson, E.R.; Brown, K.A.; Kathleen Cuningham Foundation Consortium for Research into Familial Breast Cancer. Prostaglandin E2 inhibits p53 in human breast adipose stromal cells: A novel mechanism for the regulation of aromatase in obesity and breast cancer. Cancer Res. 2015, 75, 645–655. [Google Scholar] [CrossRef]
- Speidel, D. The role of DNA damage responses in p53 biology. Arch. Toxikol. 2015, 89, 501–517. [Google Scholar] [CrossRef] [PubMed]
- Berger, C.; Qian, Y.; Chen, X. The p53-estrogen receptor loop in cancer. Curr. Mol. Med. 2013, 13, 1229–1240. [Google Scholar] [CrossRef]
- Garvin, S.; Nilsson, U.W.; Dabrosin, C. Effects of oestradiol and tamoxifen on VEGF, soluble VEGFR-1, and VEGFR-2 in breast cancer and endothelial cells. Br. J. Cancer. 2005, 93, 1005–1010. [Google Scholar] [CrossRef]
- Turner, H.E.; Harris, A.L.; Melmed, S.; Wass, J.A.H. Angiogenesis in endocrine tumors. Endocr. Rev. 2003, 24, 600–632. [Google Scholar] [CrossRef]
- Ma, Q.; Reiter, R.J.; Chen, Y. Role of melatonin in controlling angiogenesis under physiological and pathological conditions. Angiogenesis 2020, 23, 91–104. [Google Scholar] [CrossRef]
- Cheng, J.; Yang, H.-L.; Gu, C.-J.; Liu, Y.-K.; Shao, J.; Zhu, R.; He, Y.-Y.; Zhu, X.-Y.; Li, M.-Q. Melatonin restricts the viability and angiogenesis of vascular endothelial cells by suppressing HIF-1α/ROS/VEGF. Int. J. Mol. Med. 2019, 43, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1995, 1, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Goradel, N.H.; Asghari, M.H.; Moloudizargari, M.; Negahdari, B.; Haghi-Aminjan, H.; Abdollahi, M. Melatonin as an angiogenesis inhibitor to combat cancer: Mechanistic evidence. Toxicol. Appl. Pharmacol. 2017, 335, 56–63. [Google Scholar] [CrossRef]
- Harada, N.; Sasano, H.; Murakami, H.; Ohkuma, T.; Nagura, H.; Takagi, Y. Localized expression of aromatase in human vascular tissues. Circ. Res. 1999, 84, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-García, V.; Gonzalez, A.; Martinez-Campa, C.; Alonso-Gonzalez, C.; Cos, S. Melatonin modulates aromatase activity and expression in endothelial cells. Oncol. Rep. 2013, 29, 2058–2064. [Google Scholar] [CrossRef] [PubMed]
- Irahara, N.; Miyoshi, Y.; Taguchi, T.; Tamaki, Y.; Noguchi, S. Quantitative analysis of aromatase mRNA expression derived from various promoters (I. 4, I. 3, PII and I. 7) and its association with expression of TNF-α, IL-6 and COX-2 mRNAs in human breast cancer. Int. J. Cancer 2006, 118, 1915–1921. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-García, V.; Gonzalez, A.; Alonso-Gonzalez, C.; Martinez-Campa, C.; Cos, S. Antiangiogenic effects of melatonin in endothelial cell cultures. Microvasc. Res. 2013, 87, 25–33. [Google Scholar] [CrossRef]
- Cos, S.; Gonzalez, A.; Guezmes, A.; Mediavilla, M.D.; Martinez-Campa, C.; Alonso-Gonzalez, C.; Sanchez-Barcelo, E.J. Melatonin inhibits the growth of DMBA-induced mammary tumors by decreasing the local biosynthesis of estrogens through the modulation of aromatase activity. Int. J. Cancer 2006, 118, 274–278. [Google Scholar] [CrossRef]
- González, A.; Martinez-Campa, C.; Mediavilla, M.D.; Alonso-Gonzalez, C.; Sanchez-Mateos, S.; Hill, S.M.; Sanchez-Barcelo, E.J.; Cos, S. Effects of MT1 melatonin receptor overexpression on the aromatase-suppressive effect of melatonin in MCF-7 human breast cancer cells. Oncol. Rep. 2007, 17, 947–953. [Google Scholar] [CrossRef]
- Jockers, R.; Delagrange, P.; Dubocovich, M.L.; Markus, R.P.; Renault, N.; Tosini, G.; Cecon, E.; Zlotos, D.P. Update on melatonin receptors: IUPHAR Review 20. Br. J. Pharmacol. 2016, 173, 2702–2725. [Google Scholar] [CrossRef]
- Stauch, B.; Johansson, L.C.; Cherezov, V. Structural insights into melatonin receptors. FEBS J. 2020, 287, 1496–1510. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Wang, J.; Chen, E.; Murai, J.; Siiteri, P.K.; Chen, S. Aromatase gene is amplified in MCF-7 human breast cancer cells. J. Steroid Biochem. Mol. Biol. 1993, 46, 147–153. [Google Scholar] [CrossRef]
- Sonne-Hansen, K.; Lykkesfeldt, A.E. Endogenous aromatization of testosterone results in growth stimulation of the human MCF-7 breast cancer cell line. J. Steroid Biochem. Mol. Biol. 2005, 93, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Ram, P.T.; Kiefer, T.; Silverman, M.; Song, Y.; Brown, G.M.; Hill, S.M. Estrogen receptor transactivation in MCF-7 breast cancer cells by melatonin and growth factors. Mol. Cell. Endocrinol. 1998, 141, 53–64. [Google Scholar] [CrossRef]
- Kiefer, T.; Ram, P.T.; Yuan, L.; Hill, S.M. Melatonin inhibits estrogen receptor transactivation and cAMP levels in breast cancer cells. Breast Cancer Res. Treat. 2002, 71, 37–45. [Google Scholar] [CrossRef]
- Zhao, Y.; Agarwal, V.R.; Mendelson, C.R.; Simpson, E.R. Estrogen biosynthesis proximal to a breast tumor is stimulated by PGE2 via cyclic AMP, leading to activation of promoter II of the CYP19 (aromatase) gene. Endocrinology 1996, 137, 5739–5742. [Google Scholar] [CrossRef]
- Zhou, D.; Clarke, P.; Wang, J.; Chen, S. Identification of a promoter that controls aromatase expression in human breast cancer and adipose stromal cells. J. Biol. Chem. 1996, 271, 15194–15202. [Google Scholar] [CrossRef]
- Bulun, S.E.; Sebastian, S.; Takayama, K.; Suzuki, T.; Sasano, H.; Shozu, M. The human CYP19 (aromatase P450) gene: Update on physiologic roles and genomic organization of promoters. J. Steroid Biochem. Mol. Biol. 2003, 86, 219–224. [Google Scholar] [CrossRef]
- Collins, A.; Yuan, L.; Kiefer, T.L.; Cheng, Q.; Lai, L.; Hill, S.M. Overexpression of the MT1 melatonin receptor in MCF-7 human breast cancer cells inhibits mammary tumor formation in nude mice. Cancer Lett. 2003, 189, 49–57. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Y.; Choi, Y.J.; Heo, K.; Park, S.J. Melatonin as an Oncostatic Molecule Based on Its Anti-Aromatase Role in Breast Cancer. Int. J. Mol. Sci. 2021, 22, 438. https://doi.org/10.3390/ijms22010438
Jin Y, Choi YJ, Heo K, Park SJ. Melatonin as an Oncostatic Molecule Based on Its Anti-Aromatase Role in Breast Cancer. International Journal of Molecular Sciences. 2021; 22(1):438. https://doi.org/10.3390/ijms22010438
Chicago/Turabian StyleJin, Yunho, Yoo Jin Choi, Kyu Heo, and Seong Joon Park. 2021. "Melatonin as an Oncostatic Molecule Based on Its Anti-Aromatase Role in Breast Cancer" International Journal of Molecular Sciences 22, no. 1: 438. https://doi.org/10.3390/ijms22010438
APA StyleJin, Y., Choi, Y. J., Heo, K., & Park, S. J. (2021). Melatonin as an Oncostatic Molecule Based on Its Anti-Aromatase Role in Breast Cancer. International Journal of Molecular Sciences, 22(1), 438. https://doi.org/10.3390/ijms22010438




