PACAP for Retinal Health: Model for Cellular Aging and Rescue
Abstract
1. Introduction
2. Results
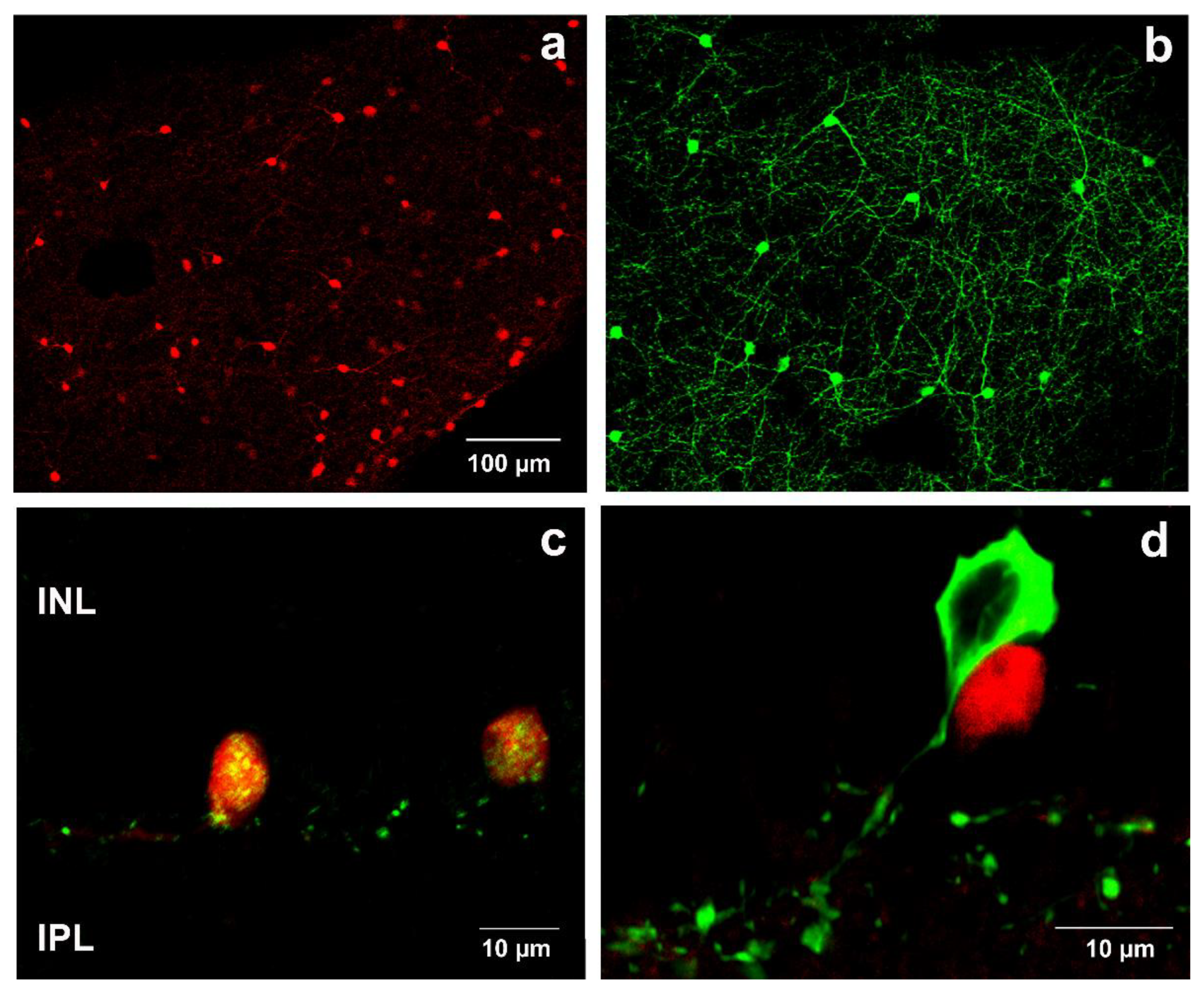
2.1. Validation of SST-tdTomato Mouse Retina Engineered to Detect SST-Expressing Cells
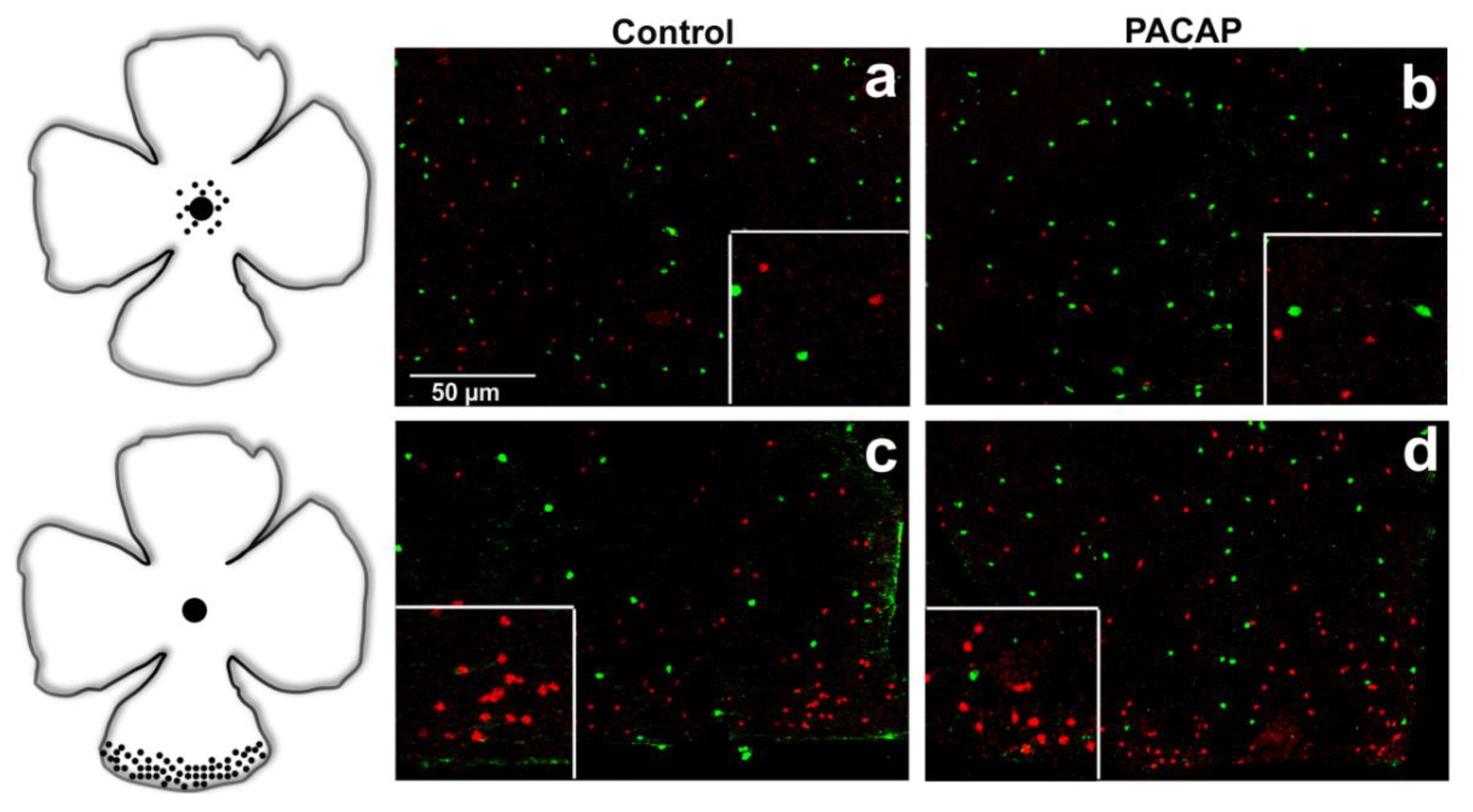
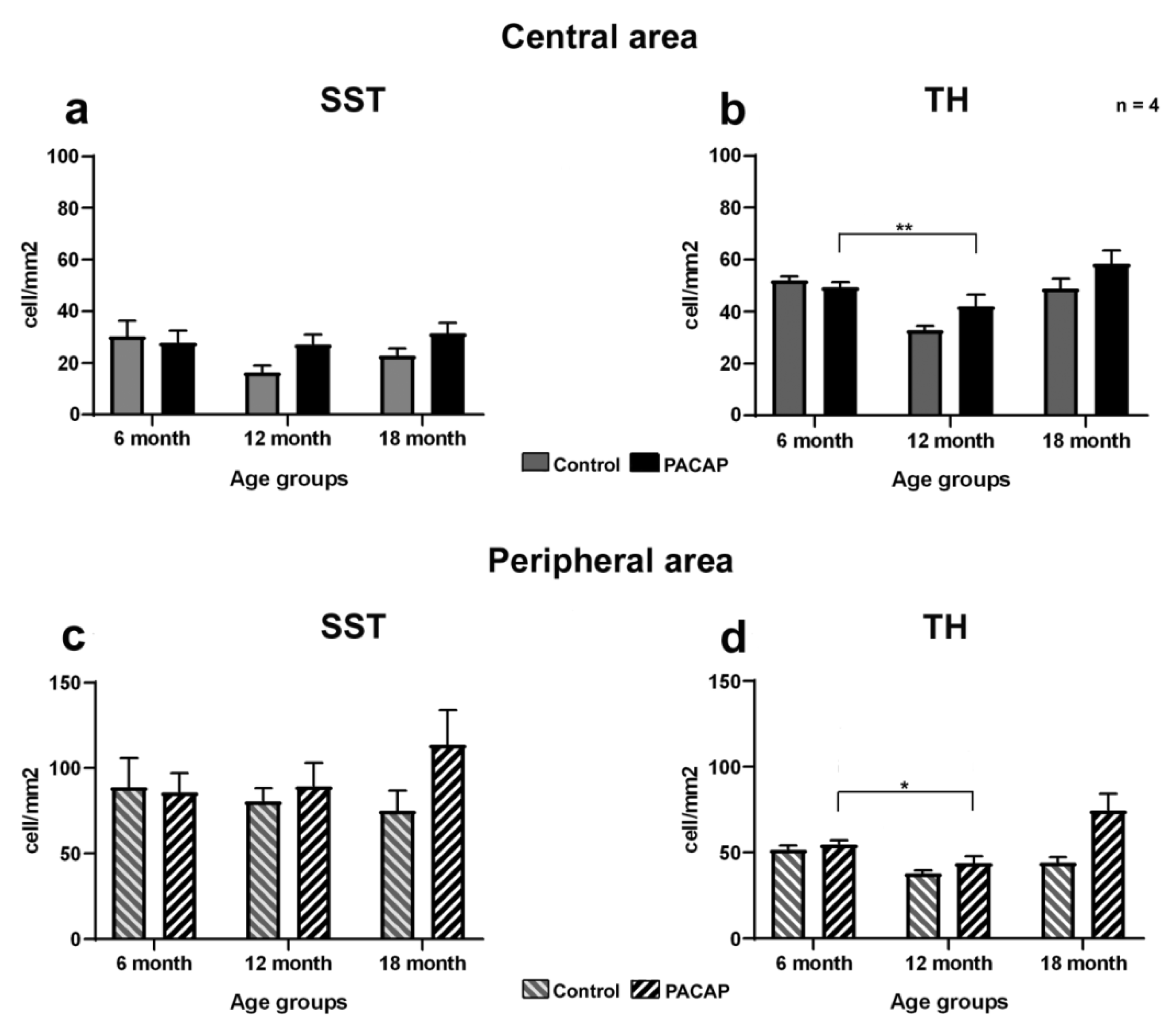
2.2. Quantitative Analysis: PACAP Treatment Caused an Increase in Cell Density
3. Discussion
4. Materials and Methods
4.1. Animals and PACAP Treatment
4.2. Immunohistochemistry on Retinal Whole Mounts
4.3. Immunohistochemistry on Retinal Sections
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SST | somatostatin |
| PACAP | pituitary adenylate cyclase-activating polypeptide |
| INL | inner nuclear layer |
| GCL | ganglion cell layer |
| VEGF | vascular endothelial growth factor |
| IPL | inner plexiform layer |
| OCT | octreotide |
| TH | tyrosine hydroxylase |
| cAMP | cyclic adenosine monophosphate |
| CREB | cAMP response element-binding protein |
| p38 MAPK | p38 mitogen-activated protein kinases |
| ERK | extracellular-signal-regulated kinase |
| PKC | protein kinase C |
References
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Guzik, T.J.; Cosentino, F. Epigenetics and Immunometabolism in Diabetes and Aging. Antioxid. Redox Signal. 2018, 29, 257–274. [Google Scholar] [CrossRef]
- Shakeri, H.; Lemmens, K.; Gevaert, A.B.; De Meyer, G.R.Y.; Segers, V.F.M. Cellular senescence links aging and diabetes in cardiovascular disease. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H448–H462. [Google Scholar] [CrossRef]
- Bagnoli, P.; Dal Monte, M.; Casini, G. Expression of neuropeptides and their receptors in the developing retina of mammals. Histol. Histopathol. 2003, 18, 1219–1242. [Google Scholar] [CrossRef]
- Cervia, D.; Casini, G.; Bagnoli, P. Physiology and pathology of somatostatin in the mammalian retina: A current view. Mol. Cell. Endocrinol. 2008, 286, 112–122. [Google Scholar] [CrossRef]
- Casini, G. Neuropeptides and retinal development. Arch. Ital. Biol 2005, 143, 191–198. [Google Scholar]
- Gabriel, R.; Postyeni, E.; Denes, V. Neuroprotective Potential of Pituitary Adenylate Cyclase Activating Polypeptide in Retinal Degenerations of Metabolic Origin. Front. Neurosci. 2019, 13, 1031. [Google Scholar] [CrossRef]
- Van Hagen, P.M.; Baarsma, G.S.; Mooy, C.M.; Ercoskan, E.M.; ter Averst, E.; Hofland, L.J.; Lamberts, S.W.; Kuijpers, R.W. Somatostatin and somatostatin receptors in retinal diseases. Eur. J. Endocrinol. 2000, 143, S43–S51. [Google Scholar] [CrossRef]
- Cristiani, R.; Petrucci, C.; Dal Monte, M.; Bagnoli, P. Somatostatin (SRIF) and SRIF receptors in the mouse retina. Brain Res. 2002, 936, 1–14. [Google Scholar] [CrossRef]
- Brazeau, P.; Vale, W.; Burgus, R.; Ling, N.; Butcher, M.; Rivier, J.; Guillemin, R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science 1973, 179, 77–79. [Google Scholar] [CrossRef]
- Miyata, A.; Arimura, A.; Dahl, R.R.; Minamino, N.; Uehara, A.; Jiang, L.; Culler, M.D.; Coy, D.H. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem. Biophys. Res. Commun. 1989, 164, 567–574. [Google Scholar] [CrossRef]
- Olias, G.; Viollet, C.; Kusserow, H.; Epelbaum, J.; Meyerhof, W. Regulation and function of somatostatin receptors. J. Neurochem. 2004, 89, 1057–1091. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, D.; Bell, G.I.; Berelowitz, M.; Epelbaum, J.; Feniuk, W.; Humphrey, P.P.; O’Carroll, A.M.; Patel, Y.C.; Schonbrunn, A.; Taylor, J.E.; et al. Classification and nomenclature of somatostatin receptors. Trends Pharmacol. Sci. 1995, 16, 86–88. [Google Scholar] [CrossRef]
- Gunther, T.; Tulipano, G.; Dournaud, P.; Bousquet, C.; Csaba, Z.; Kreienkamp, H.J.; Lupp, A.; Korbonits, M.; Castano, J.P.; Wester, H.J.; et al. International Union of Basic and Clinical Pharmacology. CV. Somatostatin Receptors: Structure, Function, Ligands, and New Nomenclature. Pharmacol. Rev. 2018, 70, 763–835. [Google Scholar] [CrossRef]
- Arimura, A.; Shioda, S. Pituitary adenylate cyclase activating polypeptide (PACAP) and its receptors: Neuroendocrine and endocrine interaction. Front. Neuroendocrinol. 1995, 16, 53–88. [Google Scholar] [CrossRef] [PubMed]
- Vaudry, D.; Gonzalez, B.J.; Basille, M.; Yon, L.; Fournier, A.; Vaudry, H. Pituitary adenylate cyclase-activating polypeptide and its receptors: From structure to functions. Pharmacol. Rev. 2000, 52, 269–324. [Google Scholar]
- Vaudry, D.; Falluel-Morel, A.; Bourgault, S.; Basille, M.; Burel, D.; Wurtz, O.; Fournier, A.; Chow, B.K.; Hashimoto, H.; Galas, L.; et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol. Rev. 2009, 61, 283–357. [Google Scholar] [CrossRef]
- Laburthe, M.; Couvineau, A.; Tan, V. Class II G protein-coupled receptors for VIP and PACAP: Structure, models of activation and pharmacology. Peptides 2007, 28, 1631–1639. [Google Scholar] [CrossRef]
- Izumi, S.; Seki, T.; Shioda, S.; Zhou, C.J.; Arimura, A.; Koide, R. Ultrastructural localization of PACAP immunoreactivity in the rat retina. Ann. N. Y. Acad. Sci. 2000, 921, 317–320. [Google Scholar] [CrossRef]
- Atlasz, T.; Babai, N.; Kiss, P.; Reglodi, D.; Tamas, A.; Szabadfi, K.; Toth, G.; Hegyi, O.; Lubics, A.; Gabriel, R. Pituitary adenylate cyclase activating polypeptide is protective in bilateral carotid occlusion-induced retinal lesion in rats. Gen. Comp. Endocrinol. 2007, 153, 108–114. [Google Scholar] [CrossRef]
- Szabadfi, K.; Atlasz, T.; Kiss, P.; Reglodi, D.; Szabo, A.; Kovacs, K.; Szalontai, B.; Setalo, G., Jr.; Banki, E.; Csanaky, K.; et al. Protective effects of the neuropeptide PACAP in diabetic retinopathy. Cell Tissue Res. 2012, 348, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Kovacs-Valasek, A.; Szabadfi, K.; Denes, V.; Szalontai, B.; Tamas, A.; Kiss, P.; Szabo, A.; Setalo, G., Jr.; Reglodi, D.; Gabriel, R. Accelerated retinal aging in PACAP knock-out mice. Neuroscience 2017, 348, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Danyadi, B.; Szabadfi, K.; Reglodi, D.; Mihalik, A.; Danyadi, T.; Kovacs, Z.; Batai, I.; Tamas, A.; Kiss, P.; Toth, G.; et al. PACAP application improves functional outcome of chronic retinal ischemic injury in rats-evidence from electroretinographic measurements. J. Mol. Neurosci. 2014, 54, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Amato, R.; Biagioni, M.; Cammalleri, M.; Dal Monte, M.; Casini, G. VEGF as a Survival Factor in Ex Vivo Models of Early Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3066–3076. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, E.; Hernandez, C.; Miralles, A.; Huguet, P.; Farres, J.; Simo, R. Lower somatostatin expression is an early event in diabetic retinopathy and is associated with retinal neurodegeneration. Diabetes Care 2007, 30, 2902–2908. [Google Scholar] [CrossRef]
- Hernandez, C.; Carrasco, E.; Casamitjana, R.; Deulofeu, R.; Garcia-Arumi, J.; Simo, R. Somatostatin molecular variants in the vitreous fluid: A comparative study between diabetic patients with proliferative diabetic retinopathy and nondiabetic control subjects. Diabetes Care 2005, 28, 1941–1947. [Google Scholar] [CrossRef][Green Version]
- Simo, R.; Lecube, A.; Sararols, L.; Garcia-Arumi, J.; Segura, R.M.; Casamitjana, R.; Hernandez, C. Deficit of somatostatin-like immunoreactivity in the vitreous fluid of diabetic patients: Possible role in the development of proliferative diabetic retinopathy. Diabetes Care 2002, 25, 2282–2286. [Google Scholar] [CrossRef][Green Version]
- Mehnert, H. Diabetes mellitus as a clinical model of the process of aging. Eur. J. Med. Res. 1997, 2, 441–444. [Google Scholar]
- D’Alessandro, A.; Cervia, D.; Catalani, E.; Gevi, F.; Zolla, L.; Casini, G. Protective effects of the neuropeptides PACAP, substance P and the somatostatin analogue octreotide in retinal ischemia: A metabolomic analysis. Mol. Biosyst. 2014, 10, 1290–1304. [Google Scholar] [CrossRef]
- Kuijpers, R.W.; Baarsma, S.; van Hagen, P.M. Treatment of cystoid macular edema with octreotide. N. Engl. J. Med. 1998, 338, 624–626. [Google Scholar] [CrossRef]
- Minhas, G.; Morishita, R.; Anand, A. Preclinical models to investigate retinal ischemia: Advances and drawbacks. Front. Neurol. 2012, 3, 75. [Google Scholar] [CrossRef] [PubMed]
- Tatton, W.G.; Kwan, M.M.; Verrier, M.C.; Seniuk, N.A.; Theriault, E. MPTP produces reversible disappearance of tyrosine hydroxylase-containing retinal amacrine cells. Brain Res. 1990, 527, 21–31. [Google Scholar] [CrossRef]
- Versaux-Botteri, C.; Nguyen-Legros, J.; Vigny, A.; Raoux, N. Morphology, density and distribution of tyrosine hydroxylase-like immunoreactive cells in the retina of mice. Brain Res. 1984, 301, 192–197. [Google Scholar] [CrossRef]
- Tornqvist, K.; Uddman, R.; Sundler, F.; Ehinger, B. Somatostatin and VIP neurons in the retina of different species. Histochemistry 1982, 76, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Hollyfield, J.G. Aging of the human retina. Differential loss of neurons and retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 1992, 33, 1–17. [Google Scholar] [PubMed]
- Shapiro, B.; Kronheim, S.; Pimstone, B. The presence of immunoreactive somatostatin in rat retina. Horm. Metab. Res. 1979, 11, 79–80. [Google Scholar] [CrossRef]
- Rorstad, O.P.; Brownstein, M.J.; Martin, J.B. Immunoreactive and biologically active somatostatin-like material in rat retina. Proc. Natl. Acad. Sci. USA 1979, 76, 3019–3023. [Google Scholar] [CrossRef]
- Kirsch, B.; Leonhardt, H. Demonstration of a somatostatin-like activity in retinal cells of the rat. Cell Tissue Res. 1979, 204, 127–140. [Google Scholar] [CrossRef]
- Rorstad, O.P.; Senterman, M.K.; Hoyte, K.M.; Martin, J.B. Immunoreactive and biologically active somatostatin-like material in the human retina. Brain Res. 1980, 199, 488–492. [Google Scholar] [CrossRef]
- Olianas, M.C.; Ingianni, A.; Sogos, V.; Onali, P. Expression of pituitary adenylate cyclase-activating polypeptide (PACAP) receptors and PACAP in human fetal retina. J. Neurochem. 1997, 69, 1213–1218. [Google Scholar] [CrossRef]
- Mathis, U.; Schaeffel, F. Glucagon-related peptides in the mouse retina and the effects of deprivation of form vision. Graefes Arch. Clin. Exp. Ophthalmol. 2007, 245, 267–275. [Google Scholar] [CrossRef]
- Reglodi, D.; Jungling, A.; Longuespee, R.; Kriegsmann, J.; Casadonte, R.; Kriegsmann, M.; Juhasz, T.; Bardosi, S.; Tamas, A.; Fulop, B.D.; et al. Accelerated pre-senile systemic amyloidosis in PACAP knockout mice—A protective role of PACAP in age-related degenerative processes. J. Pathol. 2018, 245, 478–490. [Google Scholar] [CrossRef] [PubMed]
- Szegeczki, V.; Bauer, B.; Jungling, A.; Fulop, B.D.; Vago, J.; Perenyi, H.; Tarantini, S.; Tamas, A.; Zakany, R.; Reglodi, D.; et al. Age-related alterations of articular cartilage in pituitary adenylate cyclase-activating polypeptide (PACAP) gene-deficient mice. Geroscience 2019, 41, 775–793. [Google Scholar] [CrossRef]
- Samuel, M.A.; Zhang, Y.; Meister, M.; Sanes, J.R. Age-related alterations in neurons of the mouse retina. J. Neurosci. 2011, 31, 16033–16044. [Google Scholar] [CrossRef]
- Troilo, D.; Xiong, M.; Crowley, J.C.; Finlay, B.L. Factors controlling the dendritic arborization of retinal ganglion cells. Vis. Neurosci. 1996, 13, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Hanisch, U.K.; Kettenmann, H. Microglia: Active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 2007, 10, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Rashid, K.; Akhtar-Schaefer, I.; Langmann, T. Microglia in Retinal Degeneration. Front. Immunol. 2019, 10, 1975. [Google Scholar] [CrossRef] [PubMed]
- Abad, C.; Tan, Y.V. VIP and PACAP as Regulators of Immunity: New Perspectives from a Receptor Point of View. J. Clin. Exp. Neuroimmunol. 2016, 1, 104. [Google Scholar]
- Tropepe, V.; Coles, B.L.; Chiasson, B.J.; Horsford, D.J.; Elia, A.J.; McInnes, R.R.; van der Kooy, D. Retinal stem cells in the adult mammalian eye. Science 2000, 287, 2032–2036. [Google Scholar] [CrossRef]
- Fischer, A.J.; Reh, T.A. Identification of a proliferating marginal zone of retinal progenitors in postnatal chickens. Dev. Biol. 2000, 220, 197–210. [Google Scholar] [CrossRef]
- Nickerson, P.E.; Emsley, J.G.; Myers, T.; Clarke, D.B. Proliferation and expression of progenitor and mature retinal phenotypes in the adult mammalian ciliary body after retinal ganglion cell injury. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5266–5275. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Baxter, P.S.; Martel, M.A.; McMahon, A.; Kind, P.C.; Hardingham, G.E. Pituitary adenylate cyclase-activating peptide induces long-lasting neuroprotection through the induction of activity-dependent signaling via the cyclic AMP response element-binding protein-regulated transcription co-activator 1. J. Neurochem. 2011, 118, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Shi, Y.; Xu, Y.; Huang, J. PACAP Attenuates Optic Nerve Crush-Induced Retinal Ganglion Cell Apoptosis via Activation of the CREB-Bcl-2 Pathway. J. Mol. Neurosci. 2019, 68, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Walton, M.; Woodgate, A.M.; Muravlev, A.; Xu, R.; During, M.J.; Dragunow, M. CREB phosphorylation promotes nerve cell survival. J. Neurochem. 1999, 73, 1836–1842. [Google Scholar]
- Riccio, A.; Ahn, S.; Davenport, C.M.; Blendy, J.A.; Ginty, D.D. Mediation by a CREB family transcription factor of NGF-dependent survival of sympathetic neurons. Science 1999, 286, 2358–2361. [Google Scholar] [CrossRef] [PubMed]
- Mantamadiotis, T.; Lemberger, T.; Bleckmann, S.C.; Kern, H.; Kretz, O.; Martin Villalba, A.; Tronche, F.; Kellendonk, C.; Gau, D.; Kapfhammer, J.; et al. Disruption of CREB function in brain leads to neurodegeneration. Nat. Genet. 2002, 31, 47–54. [Google Scholar] [CrossRef]
- Gabriel, R. Neuropeptides and diabetic retinopathy. Br. J. Clin. Pharmacol. 2013, 75, 1189–1201. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, C.; Garcia-Ramirez, M.; Corraliza, L.; Fernandez-Carneado, J.; Farrera-Sinfreu, J.; Ponsati, B.; Gonzalez-Rodriguez, A.; Valverde, A.M.; Simo, R. Topical administration of somatostatin prevents retinal neurodegeneration in experimental diabetes. Diabetes 2013, 62, 2569–2578. [Google Scholar] [CrossRef]
- Arroba, A.I.; Mazzeo, A.; Cazzoni, D.; Beltramo, E.; Hernandez, C.; Porta, M.; Simo, R.; Valverde, A.M. Somatostatin protects photoreceptor cells against high glucose-induced apoptosis. Mol. Vis. 2016, 22, 1522–1531. [Google Scholar] [PubMed]


| SST Cell Density (Cell/mm2) | ||||||
|---|---|---|---|---|---|---|
| Central Retina | Peripheral Retina | |||||
| 6 m | 12 m | 18 m | 6 m | 12 m | 18 m | |
| Non-treated group | 46.0 ± 12.3 | 22.5 ± 5.2 | 27.8 ± 6.1 | 64.0 ± 12.5 | 71.2 ± 6.8 | 64.9 ± 16.9 |
| Saline-treated group | 30.4 ± 6.0 | 16.3 ± 2.7 | 22.9 ± 2.7 | 88.8 ± 17.0 | 80.7 ± 7.5 | 75.1 ± 11.9 |
| PACAP-treated group | 27.9 ± 4.5 | 27.3 ± 3.7 | 31.5 ± 3.9 | 85.9 ± 11.3 | 89.5 ± 13.5 | 113.6 ± 20.3 |
| TH Cell Density (Cell/mm2) | ||||||
| Central Retina | Peripheral Retina | |||||
| 6 m | 12 m | 18 m | 6 m | 12 m | 18 m | |
| Non-treated group | 50.1 ± 5.2 | 35.4 ± 2.2 | 43.5 ± 4.2 | 41.2 ± 4.8 | 37.7 ± 1.9 | 43.8 ± 3.2 |
| Saline-treated group | 52.1 ± 1.5 | 32.9 ± 1.5 | 49.0 ± 3.7 | 51.8 ± 2.3 | 37.9 ± 1.7 | 44.3 ± 3.1 |
| PACAP-treated group | 49.4 ± 1.9 | 42.1 ± 4.5 | 58.4 ± 5.1 | 54.9 ± 2.4 | 43.9 ± 3.9 | 74.7 ± 9.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pöstyéni, E.; Kovács-Valasek, A.; Dénes, V.; Mester, A.; Sétáló, G., Jr.; Gábriel, R. PACAP for Retinal Health: Model for Cellular Aging and Rescue. Int. J. Mol. Sci. 2021, 22, 444. https://doi.org/10.3390/ijms22010444
Pöstyéni E, Kovács-Valasek A, Dénes V, Mester A, Sétáló G Jr., Gábriel R. PACAP for Retinal Health: Model for Cellular Aging and Rescue. International Journal of Molecular Sciences. 2021; 22(1):444. https://doi.org/10.3390/ijms22010444
Chicago/Turabian StylePöstyéni, Etelka, Andrea Kovács-Valasek, Viktória Dénes, Adrienn Mester, György Sétáló, Jr., and Róbert Gábriel. 2021. "PACAP for Retinal Health: Model for Cellular Aging and Rescue" International Journal of Molecular Sciences 22, no. 1: 444. https://doi.org/10.3390/ijms22010444
APA StylePöstyéni, E., Kovács-Valasek, A., Dénes, V., Mester, A., Sétáló, G., Jr., & Gábriel, R. (2021). PACAP for Retinal Health: Model for Cellular Aging and Rescue. International Journal of Molecular Sciences, 22(1), 444. https://doi.org/10.3390/ijms22010444





