Emerging Options for the Diagnosis of Bacterial Infections and the Characterization of Antimicrobial Resistance
Abstract
1. Introduction
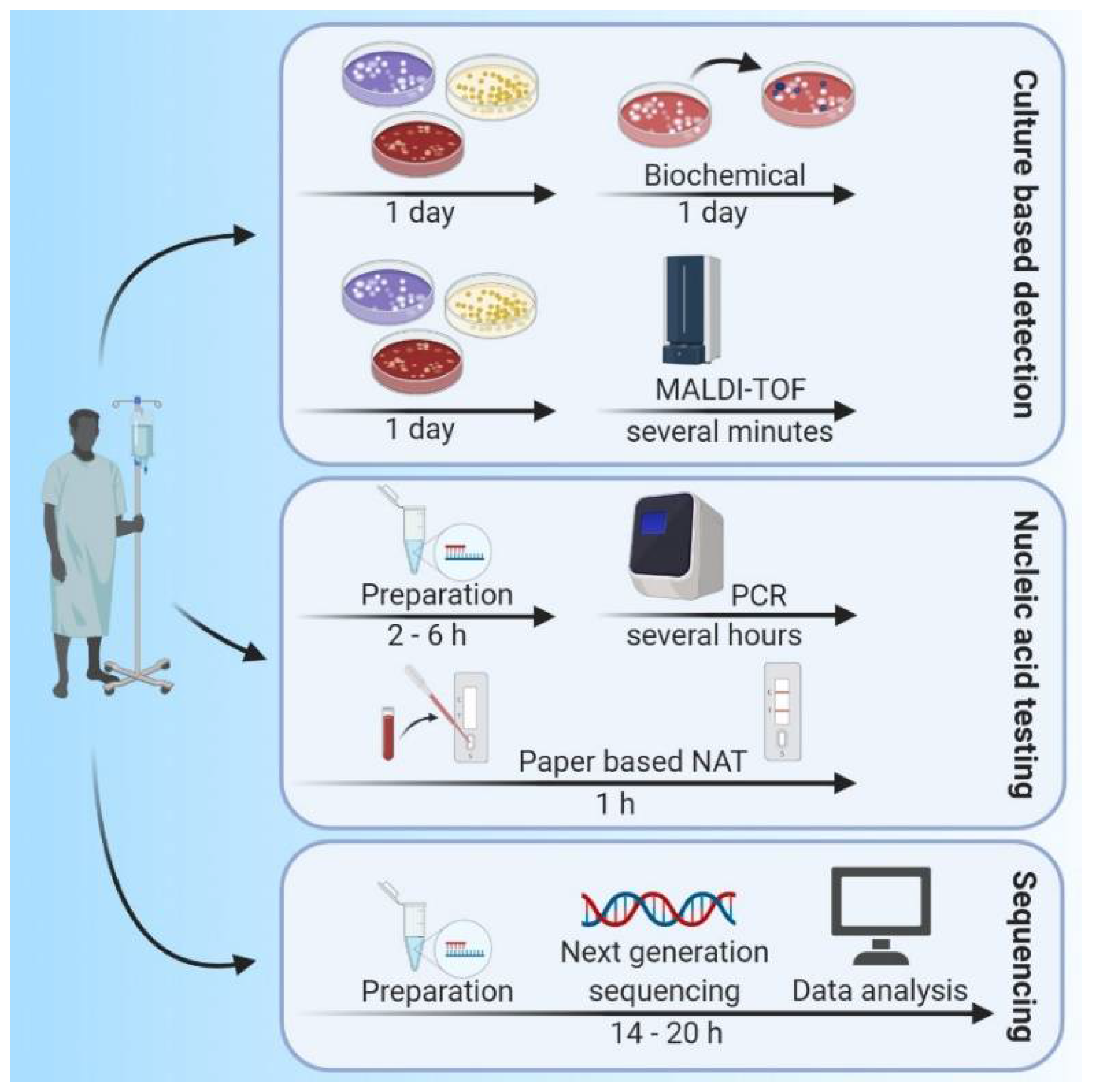
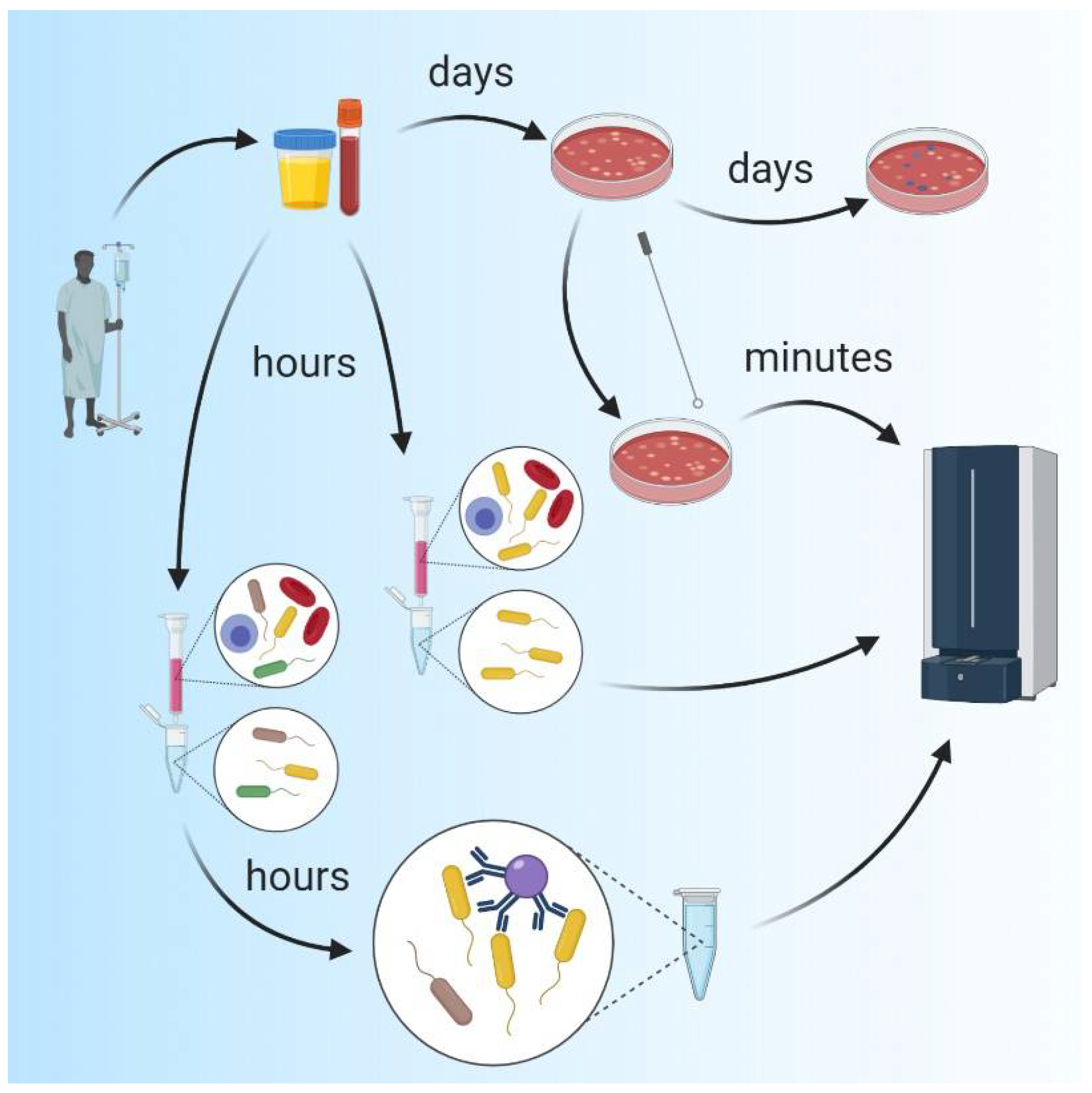
2. Methods for Identifying Infectious Agents
2.1. Traditional Microbiological Methods
2.2. Molecular Methods, Polymerase Chain Reaction (PCR) and DNA Sequencing
2.3. Mass Spectrometry (MS)
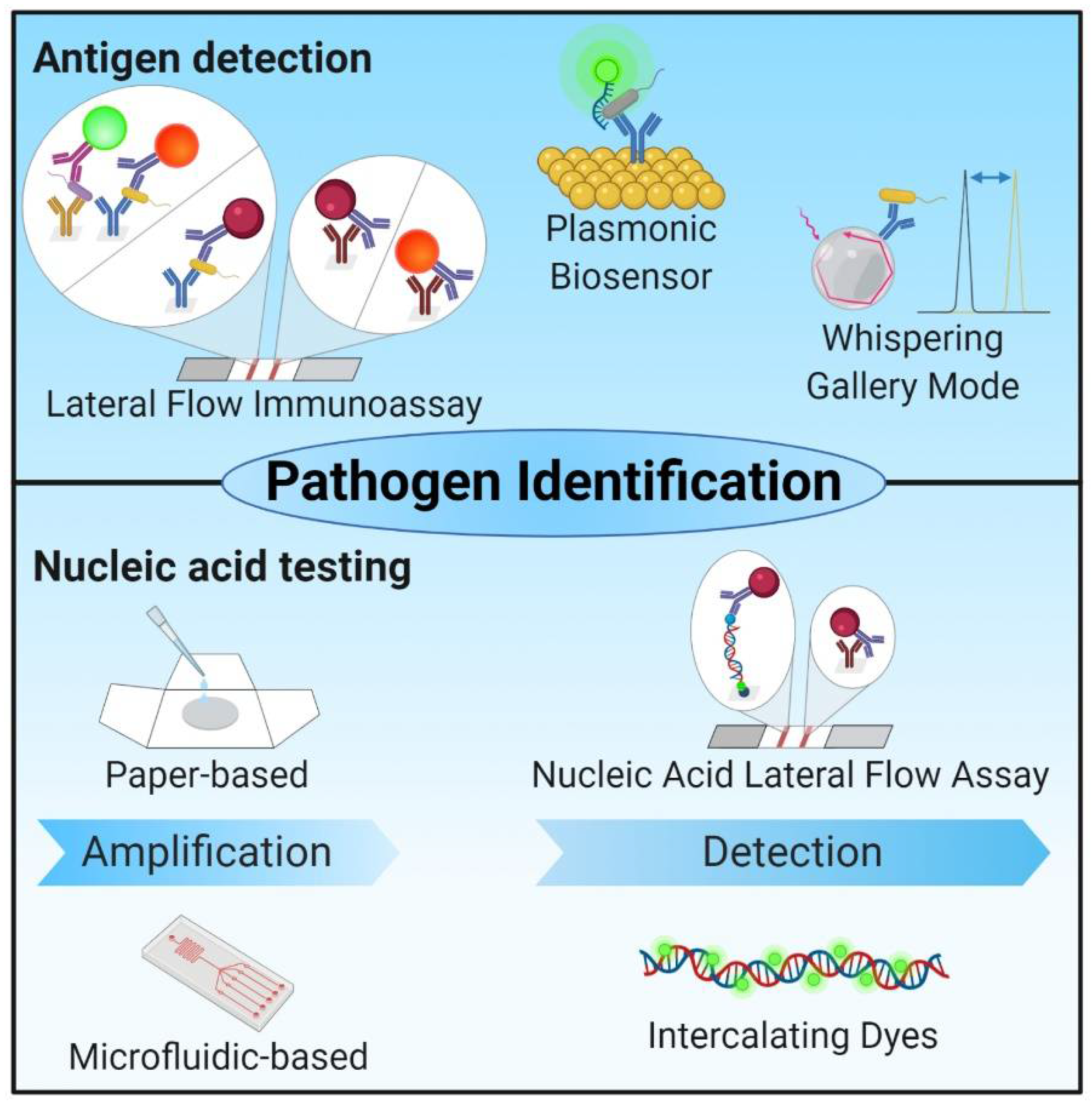
2.4. Biosensors
2.5. Technologies with Potential for POC Diagnosis of Bacterial Infections
| Method | Pathogen Identification (ID) | Time | RD | AST | Advantages and Disadvantages | POC | Ref |
|---|---|---|---|---|---|---|---|
| Cell culture | Growth based; all culturable bacteria | 24–72 h cultivation + 18–24 h for biochemical ID | - | √ | + Cost-effective + Good specificity − Long turnaround times − Lacking sensitivity − Prone to errors in workflow − Difficulties with fastidious organisms − Unculturable organisms not detectable | - | [5,6,13,14,16,21,27,133,134,135] |
| PCR-analysis and real-time PCR | Sequence dependent amplification of bacterial genes > pathogen-specific | One to several hours | √ | - | + No cultivation + Good performance − Expensive − A priori knowledge on suspected pathogens necessary − Turnaround time − High-end instrumentation | - | [8,13,16,17,19,21,27,136,137,138,139,140,141,142] |
| Next-generation sequencing | Simultaneous sequencing of billions of nucleic acid fragments contained in heterogenous samples > identification on subspecies or strain level based on SNPs | 14–20 h | √ | - | + Primer independent + Identification without a priori knowledge or suspicion + Faster adaption to new resistance mechanisms − Complex workflow with experimental pitfalls and biases − High overall error rate − Differentiation between colonization and infection critical | (√) | [5,22,23,24,27,28,29,31,32,33,136,143,144,145,146] |
| MALDI-TOF; Direct sample testing | Generated mass spectrum of molecular sample composition compared to spectral database containing spectra from pure colonies (pre-cultivation); Cell enrichment followed by specific isolation | 2–50 h | (√) | (√) | + Automatable + Low costs per test + Fast analysis − Pre-cultivation necessary − Several resistance mechanisms not detectable − Identification of subspecies limited − Polymicrobial analysis difficult + No pre-cultivation − A priori knowledge necessary | - | [15,38,39,41,43,44,45,46,47,48,49,50,51,52,53,54,55,147,148,149,150,151,152] |
| HPLC-MS | Separation of proteolytic digests of cell extracts via HPLC and identification of unique peptide markers | ~4 h | - | - | − Transferability to routine lab remains limited | - | [38,56,57,58] |
| Biosensors | Recognition of pathogen presence or their metabolic activity via biological recognition elements in intimate contact to transducers and detection systems | √ | - | + (Semi-) quantitative measurement + No or few additional reagents, pre-enrichment or processing steps | (√) | [60,61,62,63] | |
| Mass transduction (e.g., QCM, SAW) | Detection of mass changes on the sensor (e.g., piezoelectric crystals) | variable | (-) | (-) | + Results comparable to ELISA, PCR + Target selectivity better than SPR − Affordability (QCM) − Long incubation times (SAW) − High packing costs (SAW) | - | |
| Electrochemical transduction (e.g., EIS) | Variable (e.g., screen-printed electrodes with antibiotic-seeded hydrogel or bacterial growth in electrode containing micro-wells in presence of antibiotics) | 1–3 h | √ | √ | + Unaffected of samples optical properties + Low-power instrumentation − Limited in sensitivity and specificity than optical-based sensors | (√) | [60,68,98,153,154] |
| Optical transduction (e.g., SPR) | Variable (e.g., digital time-lapse microscopy, SPR) | variable | √ | √ | + High sensitivities and specificities + Sensors small and cost effective + Fast real-time detection − Label-based detection requires additional steps − Label-free detection often not easily accessible − Interference of non-specific binding − Trouble analyzing turbid samples − Interference in complex matrices | (√) | [60,68,77,78,98,155] |
| Whispering gallery mode (optical) | Label-free detection via capturing of pathogens and pathogen compounds with biological recognition molecules | ~15–30 min | √ | - | + Label-free and real-time + Detection of single molecules and atoms + No prior purification or amplification + Low manufacturing costs + Small test volume − Sensor stability and specificity | (√) | [85,86,89,90,91,92,93] |
| Lateral Flow Assays | detection via capturing of pathogens and pathogen compounds with biological recognition molecules, detection with colorimetric and optical detection molecules | Several minutes | √ | - | + Broad range of biological samples + Results confirmed by naked eye − Low accuracy − Limited sensitivities − Cross-reactivity in multiplexing − Interpretation of weakly positive tests difficult | √ | [99,100,102,103,104,109,110,111,112,156,157,158,159] |
| Low-cost paper-based NAT | Containing all three key steps of NAT for pathogen detection | 45 min–120 min | √ | - | + Higher sensitivities and specificities than immunoassays + Capability for multiplex detection | (√) | [19,114,115,116] |
| Micro-fluidic systems | Variable (e.g., NAT-based micro-fluidic systems, chip-based isothermal nano calorimetry, micro-fluidic channels with gold-micro-electrodes, nanoliter-sized-micro-chamber and micro-array based micro-fluidic) | 15 min–3 h | (√) | (√) | + faster and better LOD by simple adaption to micro-fluidic format − sensitive to air bubbles − Sample preparation necessary (RPA) | (√) | [106,160,161,162,163] |
| Biochemical tests (e.g., CarbaNP, BYG Carba test) | No pathogen identification | Several minutes | √ | - | − Applied amount of bacteria critical − Limitations in sensitivity for some lactamases | - | [164,165,166,167] |
3. Resistance Profiling and Tests for Antimicrobial Susceptibility
3.1. Culture-Based Methods
3.2. Molecular Detection, Genetic Methods, RNA Markers, and Sequencing-Based Methods
3.3. MALDI-TOF MS-Based
3.4. Innovative and Rapid Testing Systems: Efforts toward POC Testing of Antimicrobial Resistance
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| POC | Point-of-Care |
| ASSURED | Affordable, Sensitive, Specific, User-friendly, Rapid and Robust, Equipment-free, and Deliverable to end-users |
| WHO | World Health Organization |
| PCR | Polymerase Chain Reaction |
| RT-PCR | Reverse Transcriptase Polymerase Chain Reaction |
| LAMP | Loop-mediated isothermal amplification |
| NASBA | Nucleic acid sequence-based amplification |
| TMA | Transcription-mediated amplification |
| NAT | Nucleic acid testing |
| NGS | Next-generation sequencing |
| mNGS | Metagenomic Next-generation sequencing |
| SNPs | Single nucleotide polymorphisms |
| CSF | Cerebrospinal fluid |
| IBMA | Isolate-based mixture assessment |
| HPLC | High-Performance Liquid Chromatography |
| QCM | Quartz crystal microbalance |
| SAW | Surface acoustic wave |
| ELISA | Enzyme-linked immunosorbent assay |
| SPR | Surface plasmon resonance |
| EIS | Electrochemical impedance spectroscopy |
| WGM | Whispering Gallery Mode |
| TIR | Total Internal Reflection |
| LFIA | Lateral Flow Immunoassay |
| RPA | Isothermal recombinase polymerase amplification |
| EHEC | Enterohemorrhagic E. coli |
| HRP | Horseradish peroxidase |
| AST | Antibiotic susceptibility testing |
| MIC | Minimum inhibitory concentration |
| ESBL | Extended-spectrum β-lactamase |
| CRE | Carbapenem-resistant Enterobacteriaceae |
| VRE | Vancomycin-resistant enterococci |
References
- Srivastava, S.; Singh, P.K.; Vatsalya, V.; Karch, R.C. Developments in the diagnostic techniques of infectious diseases: Rural and urban prospective. Adv. Infect. Dis. 2018, 8, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Malani, P.N. Contemporary challenges to human health infectious disease theme issue. JAMA J. Am. Med. Assoc. 2014, 312, 1407–1408. [Google Scholar] [CrossRef] [PubMed]
- Caliendo, A.M.; Gilbert, D.N.; Ginocchio, C.C.; Hanson, K.E.; May, L.; Quinn, T.C.; Tenover, F.C.; Alland, D.; Blaschke, A.J.; Bonomo, R.A.; et al. Better tests, better care: Improved diagnostics for infectious diseases. Clin. Infect. Dis. 2013, 57, S139–S170. [Google Scholar] [CrossRef] [PubMed]
- Hunter, P. Novel diagnostic technologies for clinical and frontline use. EMBO Rep. 2017, 18, 881–884. [Google Scholar] [CrossRef] [PubMed]
- Tenover, F.C. The role for rapid molecular diagnostic tests for infectious diseases in precision medicine. Expert Rev. Precis. Med. Drug Dev. 2018, 3. [Google Scholar] [CrossRef]
- Miao, Q.; Ma, Y.; Wang, Q.; Pan, J.; Zhang, Y.; Jin, W.; Yao, Y.; Su, Y.; Huang, Y.; Wang, M.; et al. Microbiological diagnostic performance of metagenomic next-generation sequencing when applied to clinical practice. Clin. Infect. Dis. 2018, 67, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Powers, J. Commentary: How to encourage more diagnostics for infectious diseases. BMJ 2016, 4744, 1–2. [Google Scholar] [CrossRef]
- Yoshida, A.; Iguchi, S.; Kikuchi, K. Translational applications of diagnostics of infectious diseases using infectomics approaches in clinical settings. J. Bacteriol. Mycol. 2016, 3. [Google Scholar] [CrossRef]
- Price, C.P. Clinical review Point of care testing. BMJ 2001, 322, 1285–1288. [Google Scholar] [CrossRef]
- Sun, A.C.; Hall, D.A. Point-of-Care Smartphone-based Electrochemical Biosensing. Electroanalysis 2019, 2–16. [Google Scholar] [CrossRef]
- Dave, V.P.; Anh, T.; Pernestig, N.A.; Tilevik, D.; Kant, K.; Nguyen, T.; Wolff, A.; Duong, D. MicroRNA amplification and detection technologies: Opportunities and challenges for point of care diagnostics. Lab. Investig. 2019, 452–469. [Google Scholar] [CrossRef] [PubMed]
- Peeling, R.W.; Mabey, D. Point-of-care tests for diagnosing infections in the developing world. Clin. Microbiol. Infect. 2010, 16, 1062–1069. [Google Scholar] [CrossRef]
- Tsalik, E.L.; Bonomo, R.A.; Fowler, V.G. New molecular diagnostic approaches to bacterial infections and antibacterial resistance. Annu. Rev. Med. 2018, 379–394. [Google Scholar] [CrossRef]
- Özenci, V.; Patel, R.; Ullberg, M.; Strålin, K. Demise of polymerase chain reaction/electrospray ionization-mass spectrometry as an infectious diseases diagnostic tool. Clin. Infect. Dis. 2018, 66, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Patel, R. MALDI-TOF MS for the diagnosis of infectious diseases. Clin. Chem. 2015, 61, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Lecuit, M.; Eloit, M. The potential of whole genome NGS for infectious disease diagnosis. Expert Rev. Mol. Diagn. 2015, 15, 1517–1519. [Google Scholar] [CrossRef] [PubMed]
- Niemz, A.; Ferguson, T.M.; Boyle, D.S. Point-of-care nucleic acid testing for infectious diseases. Trends Biotechnol. 2011, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Young, B.A.; Hanson, K.E.; Gomez, C.A.; Hanson, K.E. Molecular diagnostic advances in transplant infectious diseases. Curr. Infect. Dis. Rep. 2019, 21, 52. [Google Scholar] [CrossRef]
- Choi, J.R.; Yong, K.W.; Tang, R.; Gong, Y.; Wen, T.; Li, F. Advances and challenges of fully integrated paper-based point-of-care nucleic acid testing. Trends Anal. Chem. 2017. [Google Scholar] [CrossRef]
- Nilsson, A.C.; Björkman, P.; Persson, K. Polymerase chain reaction is superior to serology for the diagnosis of acute Mycoplasma pneumoniae infection and reveals a high rate of persistent infection. BMC Microbiol. 2008, 8, 93. [Google Scholar] [CrossRef]
- Yang, S.; Rothman, R.E. PCR-based diagnostics for infectious diseases: Uses, limitations, and future applications in acute-care settings. Lancet Infect. Dis. 2004, 4, 337–348. [Google Scholar] [CrossRef]
- Boers, S.A.; Jansen, R.; Hays, J.P. Understanding and overcoming the pitfalls and biases of next-generation sequencing (NGS) methods for use in the routine clinical microbiological diagnostic laboratory. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1059–1070. [Google Scholar] [CrossRef] [PubMed]
- Khodakov, D.; Wang, C.; Zhang, D.Y. Diagnostics based on nucleic acid sequence variant profiling: PCR, hybridization, and NGS approaches. Adv. Drug Deliv. Rev. 2016, 105, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, B.; Sichtig, H.; Geyer, C.; Ledeboer, N.; Weinstock, G.M. Making the leap from research laboratory to clinic: Challenges and opportunities for next-generation sequencing in infectious disease diagnostics. mBio 2015, 6, 1–10. [Google Scholar] [CrossRef]
- Mulcahy-O’Grady, H.; Workentine, M.L. The challenge and potential of metagenomics in the clinic. Front. Immunol. 2016, 7, 1–8. [Google Scholar] [CrossRef]
- Maljkovic Berry, I.; Melendrez, M.C.; Bishop-Lilly, K.A.; Rutvisuttinunt, W.; Pollett, S.; Talundzic, E.; Morton, L.; Jarman, R.G. Next Generation Sequencing and Bioinformatics Methodologies for Infectious Disease Research and Public Health: Approaches, Applications, and Considerations for Development of Laboratory Capacity. J. Infect. Dis. 2019, 221. [Google Scholar] [CrossRef]
- Maugeri, G.; Lychko, I.; Sobral, R.; Roque, A.C.A. Identification and Antibiotic-Susceptibility Profiling of Infectious Bacterial Agents: A Review of Current and Future Trends. Biotechnol. J. 2019, 1700750. [Google Scholar] [CrossRef]
- Afshinnekoo, E.; Chou, C.; Alexander, N.; Ahsanuddin, S.; Schuetz, A.N.; Mason, C.E. Precision Metagenomics: Rapid Metagenomic Analyses for Infectious Disease Diagnostics and Public Health Surveillance. J. Biomol. Tech. 2017, 28, 40–45. [Google Scholar] [CrossRef]
- Dekker, J.P. Metagenomics for clinical infectious disease diagnostics steps closer to reality. J. Clin. Microbiol. 2018, 56, 1–7. [Google Scholar] [CrossRef]
- Castillo, D.J.; Rifkin, R.F.; Cowan, D.A.; Potgieter, M. The healthy human blood microbiome: Fact or fiction? Front. Cell. Infect. Microbiol. 2019, 9, 148. [Google Scholar] [CrossRef]
- Simner, P.J.; Miller, S.; Carroll, K.C. Understanding the Promises and Hurdles of Metagenomic Next-Generation Sequencing as a Diagnostic Tool for Infectious Diseases. Clin. Infect. Dis. 2018, 66, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Van Der Eijk, A.A.; Tintu, A.N.; Hays, J.P. Pre-implementation guidelines for infectious disease point-of-care testing in medical institutions. Future Microbiol. 2017, 12, 51–58. [Google Scholar] [CrossRef]
- Bhattacharyya, R.P.; Walker, M.; Boykin, R.; Son, S.S.; Liu, J.; Hachey, A.C.; Ma, P.; Wu, L.; Choi, K.; Cummins, K.C.; et al. Rapid identification and phylogenetic classification of diverse bacterial pathogens in a multiplexed hybridization assay targeting ribosomal RNA. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Taxt, A.M.; Avershina, E.; Frye, S.A.; Naseer, U.; Ahmad, R. Rapid identification of pathogens, antibiotic resistance genes and plasmids in blood cultures by nanopore sequencing. Sci. Rep. 2020, 10, 7622. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.L.; Watson, M.; Minot, S.S.; Rivera, M.C.; Franklin, R.B. MinIONTM nanopore sequencing of environmental metagenomes: A synthetic approach. Gigascience 2017, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kilianski, A.; Haas, J.L.; Corriveau, E.J.; Liem, A.T.; Willis, K.L.; Kadavy, D.R.; Rosenzweig, C.N.; Minot, S.S. Bacterial and viral identification and differentiation by amplicon sequencing on the MinION nanopore sequencer. Gigascience 2015, 4, 12. [Google Scholar] [CrossRef]
- Sinha, M.; Jupe, J.; Mack, H.; Coleman, T.P.; Lawrence, S.M.; Fraley, I. Towards Detection Directly From Whole Blood: Current and Emerging Technologies for Rapid Diagnosis of Microbial Infections Without. Clin. Microbiol. Rev. 2018, 31, 1–26. [Google Scholar]
- Roux-Dalvai, F.; Gotti, C.; Leclercq, M.; Hélie, M.C.; Boissinot, M.; Arrey, T.N.; Dauly, C.; Fournier, F.; Kelly, I.; Marcoux, J.; et al. Fast and accurate bacterial species identification in urine specimens using LC-MS/MS mass spectrometry and machine learning. Mol. Cell. Proteomics 2019, 18, 2492–2505. [Google Scholar] [CrossRef]
- Ho, Y.P.; Muralidhar Reddy, P. Identification of pathogens by mass spectrometry. Clin. Chem. 2010, 56, 525–536. [Google Scholar] [CrossRef]
- Afonso, C.; Fenselau, C. Use of Bioactive Glass Slides for Matrix-Assisted Laser Desorption/Ionization Analysis: Application to Microorganisms. Anal. Chem. 2003, 75, 694–697. [Google Scholar] [CrossRef]
- Gu, H.; Ho, P.L.; Tsang, K.W.T.; Wang, L.; Xu, B. Using Biofunctional Magnetic Nanoparticles to Capture Vancomycin-Resistant Enterococci and Other Gram-Positive Bacteria at Ultralow Concentration. J. Am. Chem. Soc. 2003, 125, 15702–15703. [Google Scholar] [CrossRef]
- Madonna, A.J.; Van Cuyk, S.; Voorhees, K.J. Detection of Escherichia coli using immunomagnetic separation and bacteriophage amplification coupled with matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2003, 17, 257–263. [Google Scholar] [CrossRef]
- DeMarco, M.L.; Burnham, C.-A.D. Diafiltration MALDI-TOF Mass Spectrometry Method for Culture-Independent Detection and Identification of Pathogens Directly From Urine Specimens. Am. J. Clin. Pathol. 2014, 141, 204–212. [Google Scholar] [CrossRef]
- Sandrin, T.R.; Demirev, P.A. Characterization of microbial mixtures by mass spectrometry. Mass Spectrom. Rev. 2018, 37, 321–349. [Google Scholar] [CrossRef]
- Altun, O.; Botero-Kleiven, S.; Carlsson, S.; Ullberg, M.; Özenci, V. Rapid identification of bacteria from positive blood culture bottles by MALDI-TOF MS following short-term incubation on solid media. J. Med. Microbiol. 2015, 64, 1346–1352. [Google Scholar] [CrossRef]
- Vlek, A.L.M.; Bonten, M.J.M.; Boel, C.H.E. Direct matrix-assisted laser desorption ionization time-of-flight mass spectrometry improves appropriateness of antibiotic treatment of bacteremia. PLoS ONE 2012, 7, 32589. [Google Scholar] [CrossRef]
- Armstrong, D.W.; Schulte, G.; Schneiderheinze, J.M.; Westenberg, D.J. Separating microbes in the manner of molecules. 1. Capillary electrokinetic approaches. Anal. Chem. 1999, 71, 5465–5469. [Google Scholar] [CrossRef]
- Lantz, A.W.; Bao, Y.; Armstrong, D.W. Single-cell detection: Test of microbial contamination using capillary electrophoresis. Anal. Chem. 2007, 79, 1720–1724. [Google Scholar] [CrossRef]
- Horká, M.; Karásek, P.; Růžička, F.; Dvořáčková, M.; Sittová, M.; Roth, M. Separation of methicillin-resistant from methicillin-susceptible staphylococcus aureus by electrophoretic methods in fused silica capillaries etched with supercritical water. Anal. Chem. 2014, 86, 9701–9708. [Google Scholar] [CrossRef]
- Horká, M.; Karásek, P.; Šalplachta, J.; Růžička, F.; Vykydalová, M.; Kubesová, A.; Dráb, V.; Roth, M.; Šlais, K. Capillary isoelectric focusing of probiotic bacteria from cow’s milk in tapered fused silica capillary with off-line matrix-assisted laser desorption/ionization time-of-flight mass spectrometry identification. Anal. Chim. Acta 2013, 788, 193–199. [Google Scholar] [CrossRef]
- Reschiglian, P.; Zattoni, A.; Cinque, L.; Roda, B.; Dal Piaz, F.; Roda, A.; Moon, M.H.; Min, B.R. Hollow-Fiber Flow Field-Flow Fractionation for Whole Bacteria Analysis by Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry. Anal. Chem. 2004, 76, 2103–2111. [Google Scholar] [CrossRef]
- Lee, H.; Williams, S.K.R.; Wahl, K.L.; Valentine, N.B. Analysis of whole bacterial cells by flow field-flow fractionation and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Anal. Chem. 2003, 75, 2746–2752. [Google Scholar] [CrossRef]
- Madonna, A.J.; Basile, F.; Furlong, E.; Voorhees, K.J. Detection of bacteria from biological mixtures using immunomagnetic separation combined with matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2001, 15, 1068–1074. [Google Scholar] [CrossRef]
- Mandrell, R.E.; Harden, L.A.; Bates, A.; Miller, W.G.; Haddon, W.F.; Fagerquist, C.K. Speciation of Campylobacter coli, C. jejuni, C. helveticus, C. lari, C. sputorum, and C. upsaliensis by matrix-assisted laser desorption ionization-time of flight mass spectrometry. Appl. Environ. Microbiol. 2005, 71, 6292–6307. [Google Scholar] [CrossRef]
- Li, S.; Guo, Z.; Wu, H.F.; Liu, Y.; Yang, Z.; Woo, C.H. Rapid analysis of Gram-positive bacteria in water via membrane filtration coupled with nanoprobe-based MALDI-MS. Anal. Bioanal. Chem. 2010, 397, 2465–2476. [Google Scholar] [CrossRef] [PubMed]
- Lo, A.A.L.; Hu, A.; Ho, Y.P. Identification of microbial mixtures by LC-selective proteotypic-peptide analysis (SPA). J. Mass Spectrom. 2006, 41, 1049–1060. [Google Scholar] [CrossRef]
- Chenau, J.; Fenaille, F.; Ezan, E.; Morel, N.; Lamourette, P.; Goossens, P.L.; Becher, F. Sensitive detection of bacillus anthracis spores by immunocapture and liquid chromatography-tandem mass spectrometry. Anal. Chem. 2011, 83, 8675–8682. [Google Scholar] [CrossRef]
- Chenau, J.; Fenaille, F.; Simon, S.; Filali, S.; Volland, H.; Junot, C.; Carniel, E.; Becher, F. Detection of Yersinia pestis in environmental and food samples by intact cell immunocapture and liquid chromatography-tandem mass spectrometry. Anal. Chem. 2014, 86, 6144–6152. [Google Scholar] [CrossRef]
- Lasch, P.; Schneider, A.; Blumenscheit, C.; Doellinger, J. Identification of Microorganisms by Liquid Chromatography-Mass Spectrometry (LC-MS 1 ) and in Silico Peptide Mass Libraries. Mol. Cell. Proteomics 2020, 19, 2125–2138. [Google Scholar] [CrossRef]
- Alahi, M.E.E.; Mukhopadhyay, S.C. Detection methodologies for pathogen and toxins: A review. Sensors 2017, 17, 1885. [Google Scholar] [CrossRef]
- Liu, R.; Ye, X.; Cui, T. Recent Progress of Biomarker Detection Sensors. Research 2020, 2020, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Davis, F.; Altintas, Z. General Introduction to Biosensors and Recognition Receptors. In Biosensors and Nanotechnology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 1–15. [Google Scholar]
- Ivnitski, D.; Abdel-Hamid, I.; Atanasov, P.; Wilkins, E. Biosensors for detection of pathogenic bacteria. Biosens. Bioelectron. 1999, 14, 599–624. [Google Scholar] [CrossRef]
- Cesewski, E.; Johnson, B.N. Electrochemical biosensors for pathogen detection. Biosens. Bioelectron. 2020, 159, 112214. [Google Scholar] [CrossRef] [PubMed]
- Velusamy, V.; Arshak, K.; Korostynska, O.; Oliwa, K.; Adley, C. An overview of foodborne pathogen detection: In the perspective of biosensors. Biotechnol. Adv. 2010, 28, 232–254. [Google Scholar] [CrossRef]
- Lim, H.J.; Saha, T.; Tey, B.T.; Tan, W.S.; Ooi, C.W. Quartz crystal microbalance-based biosensors as rapid diagnostic devices for infectious diseases. Biosens. Bioelectron. 2020, 168, 112513. [Google Scholar] [CrossRef]
- Rocha-Gaso, M.-I.; March-Iborra, C.; Montoya-Baides, Á.; Arnau-Vives, A. Surface Generated Acoustic Wave Biosensors for the Detection of Pathogens: A Review. Sensors 2009, 9, 5740–5769. [Google Scholar] [CrossRef]
- Lazcka, O.; del Campo, F.J.; Muñoz, F.X. Pathogen detection: A perspective of traditional methods and biosensors. Biosens. Bioelectron. 2007, 22, 1205–1217. [Google Scholar] [CrossRef]
- Aizawa, M. Principles and applications of electrochemical and optical biosensors. Anal. Chim. Acta 1991, 250, 249–256. [Google Scholar] [CrossRef]
- Gau, J.J.; Lan, E.H.; Dunn, B.; Ho, C.M.; Woo, J.C.S. A MEMS based amperometric detector for E. coli bacteria using self-assembled monolayers. Biosens. Bioelectron. 2001, 16, 745–755. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, P.; Gong, J.; Fang, L.; Deng, J.; Liang, W.; Zheng, J. Amperometric immunosensor for the detection of Escherichia coli O157:H7 in food specimens. Anal. Biochem. 2012, 421, 227–233. [Google Scholar] [CrossRef]
- Neufeld, T.; Schwartz-Mittelmann, A.; Biran, D.; Ron, E.Z.; Rishpon, J. Combined phage typing and amperometric detection of released enzymatic activity for the specific identification and quantification of bacteria. Anal. Chem. 2003, 75, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Radke, S.M.; Alocilja, E.C. Design and fabrication of a microimpedance biosensor for bacterial detection. IEEE Sens. J. 2004, 4, 434–440. [Google Scholar] [CrossRef]
- Chowdhury, A.D.; De, A.; Chaudhuri, C.R.; Bandyopadhyay, K.; Sen, P. Label free polyaniline based impedimetric biosensor for detection of E. coli O157:H7 Bacteria. Sens. Actuators B Chem. 2012, 171–172, 916–923. [Google Scholar] [CrossRef]
- Mannoor, M.S.; Zhang, S.; Link, A.J.; McAlpine, M.C. Electrical detection of pathogenic bacteria via immobilized antimicrobial peptides. Proc. Natl. Acad. Sci. USA 2010, 107, 19207–19212. [Google Scholar] [CrossRef]
- Henihan, G.; Schulze, H.; Corrigan, D.K.; Giraud, G.; Terry, J.G.; Hardie, A.; Campbell, C.J.; Walton, A.J.; Crain, J.; Pethig, R.; et al. Label- and amplification-free electrochemical detection of bacterial ribosomal RNA. Biosens. Bioelectron. 2016, 81, 487–494. [Google Scholar] [CrossRef]
- Pirzada, M.; Altintas, Z. Recent Progress in Optical Sensors for Biomedical Diagnostics. Micromachines 2020, 11, 356. [Google Scholar] [CrossRef]
- Anderson, M.; O’Brien, E.; Grayek, E.; Hermansen, J.; Hunt, H. The Detection of Helicobacter hepaticus Using Whispering-Gallery Mode Microcavity Optical Sensors. Biosensors 2015, 5, 562–576. [Google Scholar] [CrossRef]
- Tokel, O.; Inci, F.; Demirci, U. Advances in plasmonic technologies for point of care applications. Chem. Rev. 2014, 114, 5728–5752. [Google Scholar] [CrossRef]
- Taylor, A.D.; Ladd, J.; Homola, J.; Jiang, S. Surface Plasmon Resonance (SPR) Sensors for the Detection of Bacterial Pathogens. In Principles of Bacterial Detection: Biosensors, Recognition Receptors and Microsystems; Springer: New York, NY, USA, 2008; pp. 83–108. [Google Scholar]
- Baccar, H.; Mejri, M.B.; Hafaiedh, I.; Ktari, T.; Aouni, M.; Abdelghani, A. Surface plasmon resonance immunosensor for bacteria detection. Talanta 2010, 82, 810–814. [Google Scholar] [CrossRef]
- Boulade, M.; Morlay, A.; Piat, F.; Roupioz, Y.; Livache, T.; Charette, P.G.; Canva, M.; Leroy, L. Early detection of bacteria using SPR imaging and event counting: Experiments with: Listeria monocytogenes and Listeria innocua. RSC Adv. 2019, 9, 15554–15560. [Google Scholar] [CrossRef]
- Puiu, M.; Bala, C. SPR and SPR imaging: Recent trends in developing nanodevices for detection and real-time monitoringof biomolecular events. Sensors 2016, 16, 870. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.M.; Lee, S.Y. Optical Biosensors for the Detection of Pathogenic Microorganisms. Trends Biotechnol. 2016, 34, 7–25. [Google Scholar] [CrossRef] [PubMed]
- Foreman, M.R.; Swaim, J.D.; Vollmer, F. Whispering gallery mode sensors: Erratum. Adv. Opt. Photonics 2015, 7, 632. [Google Scholar] [CrossRef]
- Bischler, R.; Olszyna, M.; Himmelhaus, M.; Dähne, L. Development of a fully automated in-vitro diagnostics system based on low-Q whispering gallery modes in fluorescent microparticles. Eur. Phys. J. Spec. Top. 2014, 223, 2041–2055. [Google Scholar] [CrossRef]
- François, A.; Krishnamoorthy, S.; Himmelhaus, M. Novel detection scheme for optical biosensing using whispering gallery modes in clusters of dielectric particles. Single Mol. Spectrosc. Imaging 2008, 6862, 68620Q. [Google Scholar] [CrossRef]
- Schweiger, G.; Horn, M. Effect of changes in size and index of refraction on the resonance wavelength of microspheres. J. Opt. Soc. Am. B 2006, 23, 212. [Google Scholar] [CrossRef]
- Vollmer, F.; Braun, D.; Libchaber, A.; Khoshsima, M.; Teraoka, I.; Arnold, S. Protein detection by optical shift of a resonant microcavity. Appl. Phys. Lett. 2002, 80, 4057–4059. [Google Scholar] [CrossRef]
- Vollmer, F.; Arnold, S.; Braun, D.; Teraoka, I.; Libchaber, A. Multiplexed DNA quantification by spectroscopic shift of two microsphere cavities. Biophys. J. 2003, 85, 1974–1979. [Google Scholar] [CrossRef]
- Kim, E.; Baaske, M.D.; Vollmer, F. Towards next-generation label-free biosensors: Recent advances in whispering gallery mode sensors. Lab Chip 2017, 17, 1190–1205. [Google Scholar] [CrossRef]
- Jiang, X.; Qavi, A.J.; Huang, S.H.; Yang, L. Whispering-Gallery Sensors. Matter 2020, 3, 371–392. [Google Scholar] [CrossRef]
- Vollmer, F.; Arnold, S.; Keng, D. Single virus detection from the reactive shift of a whispering-gallery mode. Proc. Natl. Acad. Sci. USA 2008, 105, 20701–20704. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Özdemir, Ş.K.; Zhu, J.; Kim, W.; Yang, L. Detecting single viruses and nanoparticles using whispering gallery microlasers. Nat. Nanotechnol. 2011, 6, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Dantham, V.R.; Holler, S.; Kolchenko, V.; Wan, Z.; Arnold, S. Taking whispering gallery-mode single virus detection and sizing to the limit. Appl. Phys. Lett. 2012, 101. [Google Scholar] [CrossRef]
- Ghali, H.; Chibli, H.; Nadeau, J.L.; Bianucci, P.; Peter, Y.A. Real-time detection of Staphylococcus aureus using Whispering Gallery Mode optical microdisks. Biosensors 2016, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Jiang, X.; Zhao, G.; Yang, L. Phone-sized whispering-gallery microresonator sensing system. Opt. Express 2016, 24, 25905. [Google Scholar] [CrossRef]
- Singh, R.; Mukherjee, M.D.; Sumana, G.; Gupta, R.K.; Sood, S.; Malhotra, B.D. Biosensors for pathogen detection: A smart approach towards clinical diagnosis. Sens. Actuators B Chem. 2014, 197, 385–404. [Google Scholar] [CrossRef]
- Borse, V.; Srivastava, R. Fluorescence lateral flow immunoassay based point-of-care nanodiagnostics for orthopedic implant-associated infection. Sens. Actuators B. Chem. 2018. [Google Scholar] [CrossRef]
- Kim, H.; Chung, D.-R.; Kang, M. A new point-of-care test for diagnosis of infectious diseases based on multiplex lateral flow immunoassay. Analyst 2019. [Google Scholar] [CrossRef]
- Gong, Y.; Zheng, Y.; Jin, B.; You, M.; Wang, J.; Li, X.; Lin, M.; Xu, F.; Li, F. Talanta A portable and universal upconversion nanoparticle-based lateral flow assay platform for point-of-care testing. Talanta 2019, 201, 126–133. [Google Scholar] [CrossRef]
- Drancourt, M.; Michel-Lepage, A.; Boyer, S.; Raoult, D. The Point-of-Care Laboratory in Clinical Microbiology. Clin. Microbiol. Rev. 2016, 29, 429–447. [Google Scholar] [CrossRef]
- Dincer, C.; Bruch, R.; Kling, A.; Dittrich, S.; Urban, G.A. Multiplexed Point-of-Care Testing − xPOCT. Trends Biotechnol. 2017, 35, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Rashid, H.; Shafi, S.; Haworth, E.; El Bashir, H.; Ali, K.A.; Memish, Z.A.; Booy, R. Value of rapid testing for influenza among Hajj pilgrims. Travel Med. Infect. Dis. 2007, 5, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Minopoli, A.; Ventura, B.D.; Lenyk, B.; Offenhäusser, A.; Mayer, D.; Velotta, R.; Gentile, F.; Tanner, J.A. Ultrasensitive antibody-aptamer plasmonic biosensor for malaria biomarker detection in whole blood. Nat. Commun. 2020, 1–10. [Google Scholar] [CrossRef]
- Nagatani, N.; Yamanaka, K.; Ushijima, H.; Koketsu, R.; Sasaki, T.; Ikuta, K.; Saito, M.; Miyahara, T.; Tamiya, E. Detection of influenza virus using a lateral flow immunoassay for amplified DNA by a microfluidic RT-PCR chip. Analyst 2012, 137, 3422–3426. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Lee, S.; Choo, J. Application of a SERS-based Lateral Flow Immunoassay Strip for Rapid and Sensitive Detection of Staphylococcal Enterotoxin B. Nanoscale 2016, 8, 11418–11425. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Wang, J.; Tang, Z.; Pounds, J.G.; Lin, Y. Rapid and Sensitive Detection of Protein Biomarker Using a Portable Fluorescence Biosensor Based on Quantum Dots and a Lateral Flow Test Strip. Anal. Chem. 2010, 82, 7008–7014. [Google Scholar] [CrossRef]
- Mohd Hanafiah, K.; Arifin, N.; Bustami, Y.; Noordin, R.; Garcia, M.; Anderson, D. Development of Multiplexed Infectious Disease Lateral Flow Assays: Challenges and Opportunities. Diagnostics 2017, 7, 51. [Google Scholar] [CrossRef]
- Rajendran, V.K.; Bakthavathsalam, P.; Jaffar Ali, B.M. Smartphone based bacterial detection using biofunctionalized fluorescent nanoparticles. Microchim. Acta 2014, 181, 1815–1821. [Google Scholar] [CrossRef]
- Lee, S.; Kim, G.; Moon, J. Development of a Smartphone-Based Reading System for Lateral Flow Immunoassay. J. Nanosci. Nanotechnol. 2014, 14, 8453–8457. [Google Scholar] [CrossRef]
- Ruppert, C.; Phogat, N.; Laufer, S.; Kohl, M.; Deigner, H.-P. A smartphone readout system for gold nanoparticle-based lateral flow assays: Application to monitoring of digoxigenin. Microchim. Acta 2019, 186, 119. [Google Scholar] [CrossRef]
- Clerc, O.; Greub, G. Routine use of point-of-care tests: Usefulness and application in clinical microbiology. Eur. Soc. Clin. Infect. Dis. 2010, 16, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Peeling, R.W.; Holmes, K.K.; Mabey, D.; Ronald, A. Rapid tests for sexually transmitted infections (STIs): The way forward. Sex. Transm. Infect. 2006, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Maffert, P.; Reverchon, S.; Nasser, W.; Rozand, C.; Abaibou, H. New nucleic acid testing devices to diagnose infectious diseases in resource-limited settings. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 1717–1731. [Google Scholar] [CrossRef]
- Chen, H.; Liu, K.; Li, Z.; Wang, P. Point of care testing for infectious diseases. Clin. Chim. Acta 2019, 493, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Wang, G.A.; Li, F. Shaping up field-deployable nucleic acid testing using microfluidic paper-based analytical devices. Anal. Bioanal. Chem. 2019, 411, 4401–4414. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, S.W.; Kang, J.Y.; Ahn, C.H. A polymer lab-on-a-chip for reverse transcription (RT)-PCR based point-of-care clinical diagnostics. Lab Chip 2008, 8, 2121–2127. [Google Scholar] [CrossRef] [PubMed]
- Neuzil, P.; Pipper, J.; Hsieh, T.M. Disposable real-time microPCR device: Lab-on-a-chip at a low cost. Mol. Biosyst. 2006, 2, 292–298. [Google Scholar] [CrossRef]
- Houssin, T.; Cramer, J.; Grojsman, R.; Bellahsene, L.; Colas, G.; Moulet, H.; Minnella, W.; Pannetier, C.; Leberre, M.; Plecis, A.; et al. Ultrafast, sensitive and large-volume on-chip real-time PCR for the molecular diagnosis of bacterial and viral infections. Lab Chip 2016, 16, 1401–1411. [Google Scholar] [CrossRef]
- Han, N.; Shin, J.H.; Han, K.H. An on-chip RT-PCR microfluidic device, that integrates mRNA extraction, cDNA synthesis, and gene amplification. RSC Adv. 2014, 4, 9160–9165. [Google Scholar] [CrossRef]
- Lutz, S.; Weber, P.; Focke, M.; Faltin, B.; Hoffmann, J.; Müller, C.; Mark, D.; Roth, G.; Munday, P.; Armes, N.; et al. Microfluidic lab-on-a-foil for nucleic acid analysis based on isothermal recombinase polymerase amplification (RPA). Lab Chip 2010, 10, 887–893. [Google Scholar] [CrossRef]
- Jauset-Rubio, M.; Svobodová, M.; Mairal, T.; McNeil, C.; Keegan, N.; Saeed, A.; Abbas, M.N.; El-Shahawi, M.S.; Bashammakh, A.S.; Alyoubi, A.O.; et al. Ultrasensitive, rapid and inexpensive detection of DNA using paper based lateral flow assay. Sci. Rep. 2016, 6, 37732. [Google Scholar] [CrossRef] [PubMed]
- Gulliksen, A.; Solli, L.; Karlsen, F.; Rogne, H.; Hovig, E.; Nordstrøm, T.; Sirevåg, R. Real-Time Nucleic Acid Sequence-Based Amplification in Nanoliter Volumes. Anal. Chem. 2004, 76, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Dimov, I.K.; Garcia-Cordero, J.L.; O’Grady, J.; Poulsen, C.R.; Viguier, C.; Kent, L.; Daly, P.; Lincoln, B.; Maher, M.; O’Kennedy, R.; et al. Integrated microfluidic tmRNA purification and real-time NASBA device for molecular diagnostics. Lab Chip 2008, 8, 2071–2078. [Google Scholar] [CrossRef] [PubMed]
- Lam, L.; Sakakihara, S.; Ishizuka, K.; Takeuchi, S.; Arata, H.F.; Fujita, H.; Noji, H. Loop-mediated isothermal amplification of a single DNA molecule in polyacrylamide gel-based microchamber. Biomed. Microdevices 2008, 10, 539–546. [Google Scholar] [CrossRef]
- Hataoka, Y.; Zhang, L.; Mori, Y.; Tomita, N.; Notomi, T.; Baba, Y. Analysis of specific gene by integration of isothermal amplification and electrophoresis on poly(methyl methacrylate) microchips. Anal. Chem. 2004, 76, 3689–3693. [Google Scholar] [CrossRef]
- Chen, Z.; Mauk, M.G.; Wang, J.; Abrams, W.R.; Corstjens, P.L.A.M.; Niedbala, R.S.; Malamud, D.; Bau, H.H. A Microfluidic System for Saliva-Based Detection of Infectious Diseases. Ann. N. Y. Acad. Sci. 2007, 1098, 429–436. [Google Scholar] [CrossRef]
- Shatzkes, K.; Teferedegne, B.; Murata, H. A simple, inexpensive method for preparing cell lysates suitable for downstream reverse transcription quantitative PCR. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef]
- Li, H.Y.; Jia, W.N.; Li, X.Y.; Zhang, L.; Liu, C.; Wu, J. Advances in Detection of Infectious Agents by Aptamer-based Technologies. Emerg. Microbes Infect. 2020, 1–38. [Google Scholar] [CrossRef]
- Mahmoud, M.; Ruppert, C.; Rentschler, S.; Laufer, S.; Deigner, H.-P. Combining aptamers and antibodies: Lateral flow quantification for thrombin and interleukin-6 with smartphone readout. Sens. Actuators B Chem. 2020, 129246. [Google Scholar] [CrossRef]
- Wiersinga, W.J.; Leopold, S.J.; Cranendonk, D.R.; van der Poll, T. Host innate immune responses to sepsis. Virulence 2014, 5, 36–44. [Google Scholar] [CrossRef]
- Jorgensen, J.H.; Ferraro, M.J. Antimicrobial Susceptibility Testing: A Review of General Principles and Contemporary Practices. Clin. Infect. Dis. 2009, 49, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- van Belkum, A.; Burnham, C.A.D.; Rossen, J.W.A.; Mallard, F.; Rochas, O.; Dunne, W.M. Innovative and rapid antimicrobial susceptibility testing systems. Nat. Rev. Microbiol. 2020, 18, 299–311. [Google Scholar] [CrossRef] [PubMed]
- van Belkum, A.; Bachmann, T.T.; Lüdke, G.; Lisby, J.G.; Kahlmeter, G.; Mohess, A.; Becker, K.; Hays, J.P.; Woodford, N.; Mitsakakis, K.; et al. Developmental roadmap for antimicrobial susceptibility testing systems. Nat. Rev. Microbiol. 2019, 17, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, X.; Zhao, W. Emerging Microtechnologies and Automated Systems for Rapid Bacterial Identification and Antibiotic Susceptibility Testing. SLAS Technol. Transl. Life Sci. Innov. 2017, 22, 585–608. [Google Scholar] [CrossRef] [PubMed]
- Abram, T.J.; Cherukury, H.; Ou, C.Y.; Vu, T.; Toledano, M.; Li, Y.; Grunwald, J.T.; Toosky, M.N.; Tifrea, D.F.; Slepenkin, A.; et al. Rapid bacterial detection and antibiotic susceptibility testing in whole blood using one-step, high throughput blood digital PCR. Lab Chip 2020, 20, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Pholwat, S.; Stroup, S.; Foongladda, S.; Houpt, E. Digital PCR to Detect and Quantify Heteroresistance in Drug Resistant Mycobacterium tuberculosis. PLoS ONE 2013, 8, e57238. [Google Scholar] [CrossRef] [PubMed]
- Bradford, P.A. Extended-Spectrum β-Lactamases in the 21st Century: Characterization, Epidemiology, and Detection of This Important Resistance Threat. Clin. Microbiol. Rev. 2001, 14, 933–951. [Google Scholar] [CrossRef]
- Fluit, A.C.; Visser, M.R.; Schmitz, F.J. Molecular detection of antimicrobial resistance. Clin. Microbiol. Rev. 2001, 14, 836–871. [Google Scholar] [CrossRef]
- Van Belkum, A.; Dunne, W.M. Next-generation antimicrobial susceptibility testing. J. Clin. Microbiol. 2013, 51, 2018–2024. [Google Scholar] [CrossRef]
- Sundsfjord, A.; Simonsen, G.S.; Haldorsen, B.C.; Haaheim, H.; Hjelmevoll, S.O.; Littauer, P.; Dahl, K.H. Genetic methods for detection of antimicrobial resistance. Apmis 2004, 112, 815–837. [Google Scholar] [CrossRef]
- Pereckaite, L.; Tatarunas, V.; Giedraitiene, A. Current antimicrobial susceptibility testing for beta-lactamase-producing Enterobacteriaceae in clinical settings. J. Microbiol. Methods 2018, 152, 154–164. [Google Scholar] [CrossRef] [PubMed]
- McDermott, P.F.; Tyson, G.H.; Kabera, C.; Chen, Y.; Li, C.; Folster, J.P.; Ayers, S.L.; Lam, C.; Tate, H.P.; Zhao, S. Whole-genome sequencing for detecting antimicrobial resistance in nontyphoidal Salmonella. Antimicrob. Agents Chemother. 2016, 60, 5515–5520. [Google Scholar] [CrossRef] [PubMed]
- Rakeman, J.L.; Id, A.L.H.; Id, N.W.; Sa, L.; Id, S.R.H.; Id, Y.H.G. Evaluation of parameters affecting performance and reliability of machine learning-based antibiotic susceptibility testing from whole genome sequencing data. PLoS Comput. Biol. 2019, 15, e1007349. [Google Scholar] [CrossRef]
- Souza, A.W.D. Sequencing-based methods and resources to study antimicrobial resistance. Nat. Rev. Genet. 2019, 20. [Google Scholar] [CrossRef]
- Brown, R.P.A.; Aplin, R.T.; Schofield, C.J. Inhibition of TEM-2 β-lactamase from Escherichia coli by clavulanic acid: Observation of intermediates by electrospray ionization mass spectrometry. Biochemistry 1996, 35, 12421–12432. [Google Scholar] [CrossRef]
- Vrioni, G.; Tsiamis, C.; Oikonomidis, G.; Theodoridou, K.; Kapsimali, V.; Tsakris, A. MALDI-TOF mass spectrometry technology for detecting biomarkers of antimicrobial resistance: Current achievements and future perspectives. Ann. Transl. Med. 2018, 6, 240. [Google Scholar] [CrossRef]
- Hrabák, J.; Walková, R.; Študentová, V.; Chudáčková, E.; Bergerová, T. Carbapenemase activity detection by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 2011, 49, 3222–3227. [Google Scholar] [CrossRef]
- Sparbier, K.; Schubert, S.; Weller, U.; Boogen, C.; Kostrzewa, M. Matrix-assisted laser desorption ionization-time of flight mass spectrometry-based functional assay for rapid detection of resistance against β-lactam antibiotics. J. Clin. Microbiol. 2012, 50, 927–937. [Google Scholar] [CrossRef]
- Sparbier, K.; Lange, C.; Jung, J.; Wieser, A.; Schubert, S.; Kostrzewa, M. Maldi biotyper-based rapid resistance detection by stable-isotope labeling. J. Clin. Microbiol. 2013, 51, 3741–3748. [Google Scholar] [CrossRef]
- Lange, C.; Schubert, S.; Jung, J.; Kostrzewa, M.; Sparbier, K. Quantitative matrix-assisted laser desorption ionization-time of flight mass spectrometry for rapid resistance detection. J. Clin. Microbiol. 2014, 52, 4155–4162. [Google Scholar] [CrossRef]
- Besant, J.D.; Sargent, E.H.; Kelley, S.O. Rapid electrochemical phenotypic profiling of antibiotic-resistant bacteria. Lab Chip 2015, 15, 2799–2807. [Google Scholar] [CrossRef] [PubMed]
- Hannah, S.; Dobrea, A.; Lasserre, P.; Blair, E.O.; Alcorn, D.; Hoskisson, P.A.; Corrigan, D.K. Development of a Rapid, Antimicrobial Susceptibility Test for E. coli Based on Low-Cost, Screen-Printed Electrodes. Biosensors 2020, 10, 153. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, H.P.; Sue, D. Rapid antimicrobial susceptibility testing and β-lactam-induced cell morphology changes of Gram-negative biological threat pathogens by optical screening. BMC Microbiol. 2018, 18, 1–15. [Google Scholar] [CrossRef]
- Volland, H.; Dortet, L.; Bernabeu, S.; Boutal, H.; Haenni, M.; Madec, J.Y.; Robin, F.; Beyrouthy, R.; Naas, T.; Simon, S. Development and multicentric validation of a lateral flow immunoassay for rapid detection of mcr-1-producing Enterobacteriaceae. J. Clin. Microbiol. 2019, 57, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rösner, S.; Kamalanabhaiah, S.; Küsters, U.; Kolbert, M.; Pfennigwerth, N.; Mack, D. Evaluation of a novel immunochromatographic lateral flow assay for rapid detection of OXA-48, NDM, KPC and VIM carbapenemases in multidrug-resistant Enterobacteriaceae. J. Med. Microbiol. 2019, 68, 379–381. [Google Scholar] [CrossRef]
- Tada, T.; Sekiguchi, J.I.; Watanabe, S.; Kuwahara-Arai, K.; Mizutani, N.; Yanagisawa, I.; Hishinuma, T.; Zan, K.N.; Mya, S.; Tin, H.H.; et al. Assessment of a newly developed immunochromatographic assay for NDM-type metallo-β-lactamase producing Gram-negative pathogens in Myanmar. BMC Infect. Dis. 2019, 19, 1–5. [Google Scholar] [CrossRef]
- Pasteran, F.; Denorme, L.; Ote, I.; Gomez, S.; De Belder, D.; Glupczynski, Y.; Bogaerts, P.; Ghiglione, B.; Power, P.; Mertens, P.; et al. Rapid identification of OXA-48 and OXA-163 subfamilies in carbapenem-resistant gram-negative bacilli with a novel immunochromatographic lateral flow assay. J. Clin. Microbiol. 2016, 54, 2832–2836. [Google Scholar] [CrossRef]
- Nemr, C.R.; Smith, S.J.; Liu, W.; Mepham, A.H.; Mohamadi, R.M.; Labib, M.; Kelley, S.O. Nanoparticle-Mediated Capture and Electrochemical Detection of Methicillin-Resistant Staphylococcus aureus. Anal. Chem. 2019, 91, 2847–2853. [Google Scholar] [CrossRef]
- Liu, Y.; Lehnert, T.; Gijs, M.A.M. Fast antimicrobial susceptibility testing on: Escherichia coli by metabolic heat nanocalorimetry. Lab Chip 2020, 20, 3144–3157. [Google Scholar] [CrossRef]
- Jeon, H.; Khan, Z.A.; Barakat, E.; Park, S. Label-free electrochemical microfluidic chip for the antimicrobial susceptibility testing. Antibiotics 2020, 9, 348. [Google Scholar] [CrossRef]
- Azizi, M.; Zaferani, M.; Dogan, B.; Zhang, S.; Simpson, K.W.; Abbaspourrad, A. Nanoliter-Sized Microchamber/Microarray Microfluidic Platform for Antibiotic Susceptibility Testing. Anal. Chem. 2018, 90, 14137–14144. [Google Scholar] [CrossRef] [PubMed]
- Maurer, F.P.; Castelberg, C.; Quiblier, C.; Bloemberg, G.V.; Hombach, M. Evaluation of carbapenemase screening and confirmation tests with Enterobacteriaceae and development of a practical diagnostic algorithm. J. Clin. Microbiol. 2015, 53, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Österblad, M.; Hakanen, A.J.; Jalavaa, J. Evaluation of the Carba NP test for carbapenemase detection. Antimicrob. Agents Chemother. 2014, 58, 7553–7556. [Google Scholar] [CrossRef] [PubMed]
- Tijet, N.; Boyd, D.; Patel, S.N.; Mulvey, M.R.; Melano, R.G. Evaluation of the carba NP test for rapid detection of carbapenemase- producing enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2013, 57, 4578–4580. [Google Scholar] [CrossRef]
- Bogaerts, P.; Yunus, S.; Massart, M.; Huang, T.D.; Glupczynski, Y. Evaluation of the BYG carba test, a new electrochemical assay for rapid laboratory detection of carbapenemase-producing enterobacteriaceae. J. Clin. Microbiol. 2016, 54, 349–358. [Google Scholar] [CrossRef][Green Version]
- Kim, S.; Masum, F.; Jeon, J.S. Recent Developments of Chip-based Phenotypic Antibiotic Susceptibility Testing. Biochip J. 2019, 13, 43–52. [Google Scholar] [CrossRef]
- Hoffman, S.B. Mechanisms of Antibiotic Resistance. Compend. Contin. Educ. Pract. Vet. 2001, 23, 464–472. [Google Scholar] [CrossRef]
- Wright, G.D. Molecular mechanisms of antibiotic resistance. Chem. Commun. 2011, 47, 4055–4061. [Google Scholar] [CrossRef]
- Blair, J.M.A.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J.V. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef]
- Lambert, P.A. Mechanisms of antibiotic resistance in Pseudomonas aeruginosa. J. R. Soc. Med. Suppl. 2002, 95, 22–26. [Google Scholar]
- Webber, M.A.; Piddock, L.J.V. The importance of efflux pumps in bacterial antibiotic resistance. J. Antimicrob. Chemother. 2003, 51, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Clark, C.G.; Langner, S.; Boyd, D.A.; Bharat, A.; McCorrister, S.J.; McArthur, A.G.; Graham, M.R.; Westmacott, G.R.; Van Domselaar, G. Detection of Antimicrobial Resistance Using Proteomics and the Comprehensive Antibiotic Resistance Database: A Case Study. Proteomics Clin. Appl. 2020, 14, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Strommenger, B.; Kettlitz, C.; Werner, G.; Witte, W. Multiplex PCR assay for simultaneous detection of nine clinically relevant antibiotic resistance genes in Staphylococcus aureus. J. Clin. Microbiol. 2003, 41, 4089–4094. [Google Scholar] [CrossRef] [PubMed]
- Gordon, N.C.; Price, J.R.; Cole, K.; Everitt, R.; Morgan, M.; Finney, J.; Kearns, A.M.; Pichon, B.; Young, B.; Wilson, D.J.; et al. Prediction of staphylococcus aureus antimicrobial resistance by whole-genome sequencing. J. Clin. Microbiol. 2014, 52, 1182–1191. [Google Scholar] [CrossRef] [PubMed]
- Sakai, J.; Tarumoto, N.; Kodana, M.; Ashikawa, S.; Imai, K.; Kawamura, T.; Ikebuchi, K.; Murakami, T.; Mitsutake, K.; Maeda, T.; et al. An identification protocol for ESBL-producing gramnegative bacteria bloodstream infections using a MinION nanopore sequencer. J. Med. Microbiol. 2019, 68, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, F.; Zhu, C.; Xiu, L.; Li, Y.; Li, L.; Liu, B.; Li, Y.; Zeng, Y.; Guo, B.; et al. Determining antimicrobial resistance profiles and identifying novel mutations of Neisseria gonorrhoeae genomes obtained by multiplexed MinION sequencing. Sci. China Life Sci. 2020, 63, 1063–1070. [Google Scholar] [CrossRef]
- Idelevich, E.A.; Becker, K. How to accelerate antimicrobial susceptibility testing. Clin. Microbiol. Infect. 2019, 25, 1347–1355. [Google Scholar] [CrossRef]
- Huang, J.M.Y.; Henihan, G.; Macdonald, D.; Michalowski, A.; Templeton, K.; Gibb, A.P.; Schulze, H.; Bachmann, T.T. Rapid Electrochemical Detection of New Delhi Metallo-beta-lactamase Genes To Enable Point-of-Care Testing of Carbapenem-Resistant Enterobacteriaceae. Anal. Chem. 2015, 87, 7738–7745. [Google Scholar] [CrossRef]
- Xu, L.; Liang, W.; Wen, Y.; Wang, L.; Yang, X.; Ren, S.; Jia, N.; Zuo, X.; Liu, G. An ultrasensitive electrochemical biosensor for the detection of mecA gene in methicillin-resistant Staphylococcus aureus. Biosens. Bioelectron. 2018, 99, 424–430. [Google Scholar] [CrossRef]
- Liu, M.; Xiang, H.; Hua, E.; Wang, L.; Jing, X.; Cao, X.; Sheng, S.; Xie, G. Ultrasensitive Electrochemical Biosensor for the Detection of the mecA Gene Sequence in Methicillin Resistant Strains of Staphylococcus aureus Employing Gold Nanoparticles. Anal. Lett. 2014, 47, 579–591. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, Z.; Li, Y.; Xie, G. Amplified electrochemical detection of mecA gene in methicillin-resistant Staphylococcus aureus based on target recycling amplification and isothermal strand-displacement polymerization reaction. Sens. Actuators B Chem. 2015, 221, 148–154. [Google Scholar] [CrossRef]
- Nordmann, P.; Poirel, L.; Dortet, L. Rapid detection of carbapenemase-producing Enterobacteriaceae. Emerg. Infect. Dis. 2012, 18, 1503–1507. [Google Scholar] [CrossRef]
- Dortet, L.; Poirel, L.; Nordmann, P. Rapid detection of carbapenemase-producing Pseudomonas spp. J. Clin. Microbiol. 2012, 50, 3773–3776. [Google Scholar] [CrossRef]
- Dortet, L.; Poirel, L.; Nordmann, P. Rapid identification of carbapenemase types in Enterobacteriaceae and Pseudomonas spp. by using a biochemical test. Antimicrob. Agents Chemother. 2012, 56, 6437–6440. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Nordmann, P. Rapidec carba NP test for rapid detection of carbapenemase producers. J. Clin. Microbiol. 2015, 53, 3003–3008. [Google Scholar] [CrossRef] [PubMed]
- Hennebique, A.; Bidart, M.; Jarraud, S.; Beraud, L.; Schwebel, C. Digital PCR for Detection and Resistance in Legionella pneumophila. Am. Soc. Microbiol. 2017, 61, 1–12. [Google Scholar]
- Sun, L.; Talarico, S.; Yao, L.; He, L.; Self, S.; You, Y.; Zhang, H.; Zhang, Y.; Guo, Y.; Liu, G.; et al. Droplet digital PCR-based detection of clarithromycin resistance in Helicobacter pylori isolates reveals frequent heteroresistance. J. Clin. Microbiol. 2018, 56, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.; McBeth, C.; Kalashnikov, M.; Boardman, A.K.; Sharon, A.; Sauer-Budge, A.F. Microfluidic advances in phenotypic antibiotic susceptibility testing. Biomed. Microdevices 2016, 18, 103. [Google Scholar] [CrossRef]
- Dai, J.; Hamon, M.; Jambovane, S. Microfluidics for antibiotic susceptibility and toxicity testing. Bioengineering 2016, 3, 25. [Google Scholar] [CrossRef]
- Messacar, K.; Parker, S.K.; Todd, J.K.; Dominguez, S.R. Implementation of Rapid Molecular Infectious Disease Diagnostics: The Role of Diagnostic and Antimicrobial. J. Clin. Microbiol. 2017, 55, 715–723. [Google Scholar] [CrossRef]
- Holcomb, Z.E.; Tsalik, E.L.; Woods, C.W.; McClain, M.T. Host-Based Peripheral Blood Gene Expression Analysus for Diagnosis of Infectious Diseases. J. Clin. Microbiol. 2017, 55, 360–368. [Google Scholar] [CrossRef]
- Belushkin, A.; Yesilkoy, F.; González-López, J.J.; Ruiz-Rodríguez, J.C.; Ferrer, R.; Fàbrega, A.; Altug, H. Rapid and Digital Detection of Inflammatory Biomarkers Enabled by a Novel Portable Nanoplasmonic Imager. Small 2020, 16, 1906108. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Cheng, C. Minireview Diagnostics for Infectious Disease Minireview. Cell Chem. Biol. 2016, 23, 1056–1066. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, R.P.; Thakku, S.G.; Hung, D.T. Harnessing CRISPR Effectors for Infectious Disease Diagnostics. ACS Infect. Dis. 2018, 4, 1278–1282. [Google Scholar] [CrossRef] [PubMed]
- Chertow, D.S. Next-generation diagnostics with CRISPR. Science 2018, 360, 381–382. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, S.; Ali, Z.; Jiang, X.; Abdolvand, R.; Ünlü, M.S.; Da Silva, H.P.; Baca, J.T.; Kim, B.; Scott, S.; Sajid, M.I.; et al. Developments in transduction, connectivity and AI/machine learning for point-of-care testing. Sensors 2019, 19, 1917. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rentschler, S.; Kaiser, L.; Deigner, H.-P. Emerging Options for the Diagnosis of Bacterial Infections and the Characterization of Antimicrobial Resistance. Int. J. Mol. Sci. 2021, 22, 456. https://doi.org/10.3390/ijms22010456
Rentschler S, Kaiser L, Deigner H-P. Emerging Options for the Diagnosis of Bacterial Infections and the Characterization of Antimicrobial Resistance. International Journal of Molecular Sciences. 2021; 22(1):456. https://doi.org/10.3390/ijms22010456
Chicago/Turabian StyleRentschler, Simone, Lars Kaiser, and Hans-Peter Deigner. 2021. "Emerging Options for the Diagnosis of Bacterial Infections and the Characterization of Antimicrobial Resistance" International Journal of Molecular Sciences 22, no. 1: 456. https://doi.org/10.3390/ijms22010456
APA StyleRentschler, S., Kaiser, L., & Deigner, H.-P. (2021). Emerging Options for the Diagnosis of Bacterial Infections and the Characterization of Antimicrobial Resistance. International Journal of Molecular Sciences, 22(1), 456. https://doi.org/10.3390/ijms22010456





