More Than Just Simple Interaction between STIM and Orai Proteins: CRAC Channel Function Enabled by a Network of Interactions with Regulatory Proteins
Abstract
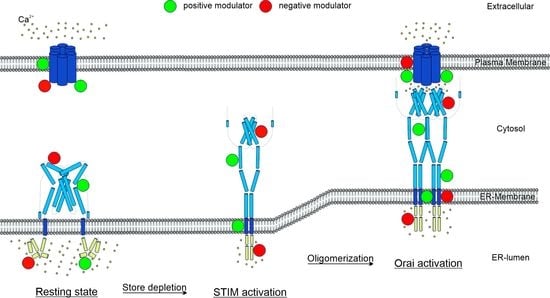
1. Introduction
2. Regulators of CRAC Channel Function
2.1. Protein Trafficking and Dynamics
2.2. ER Sculpturing Proteins, ER-PM Tethers, and PM Microdomains
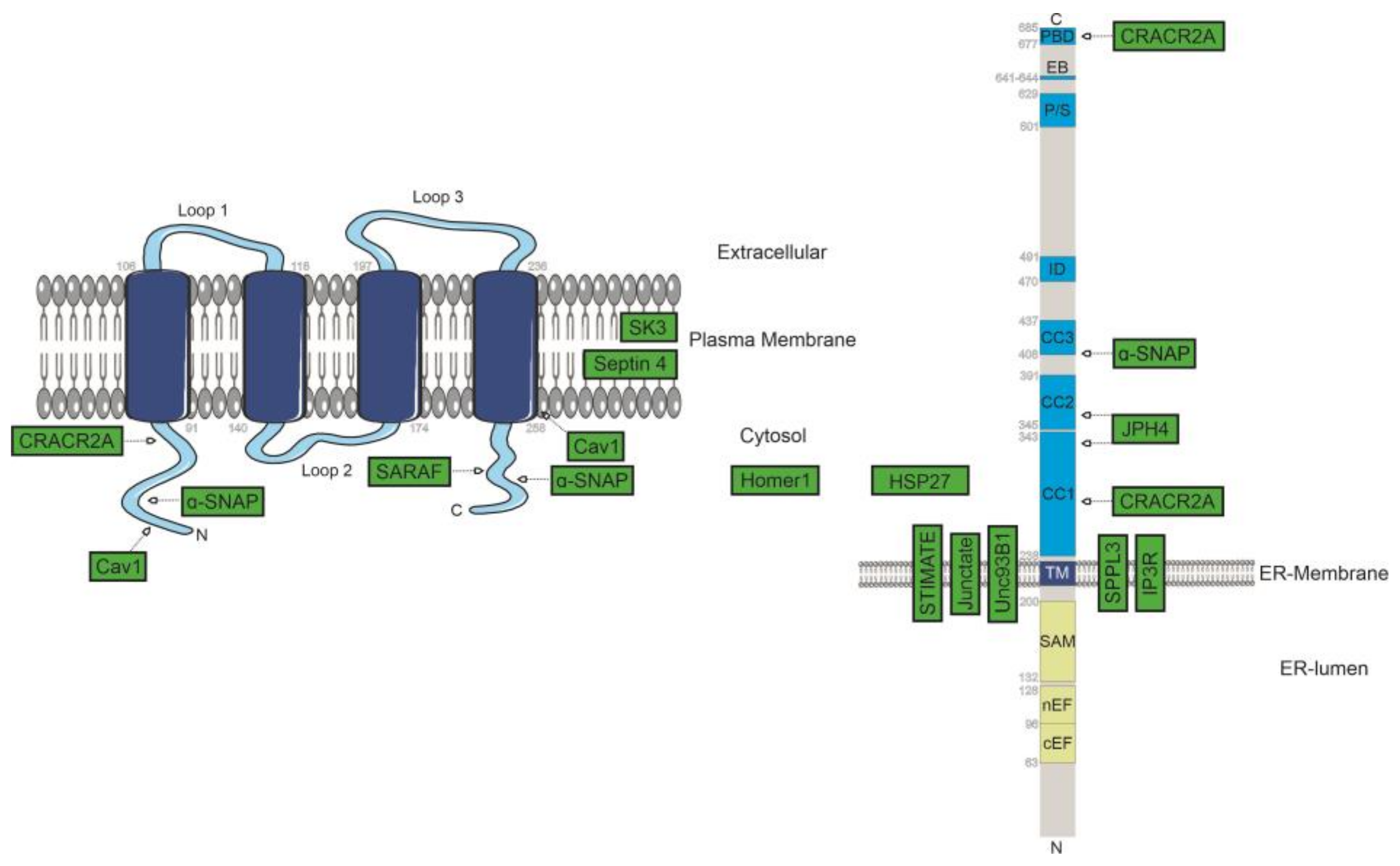
2.3. Positive Modulators of SOCE
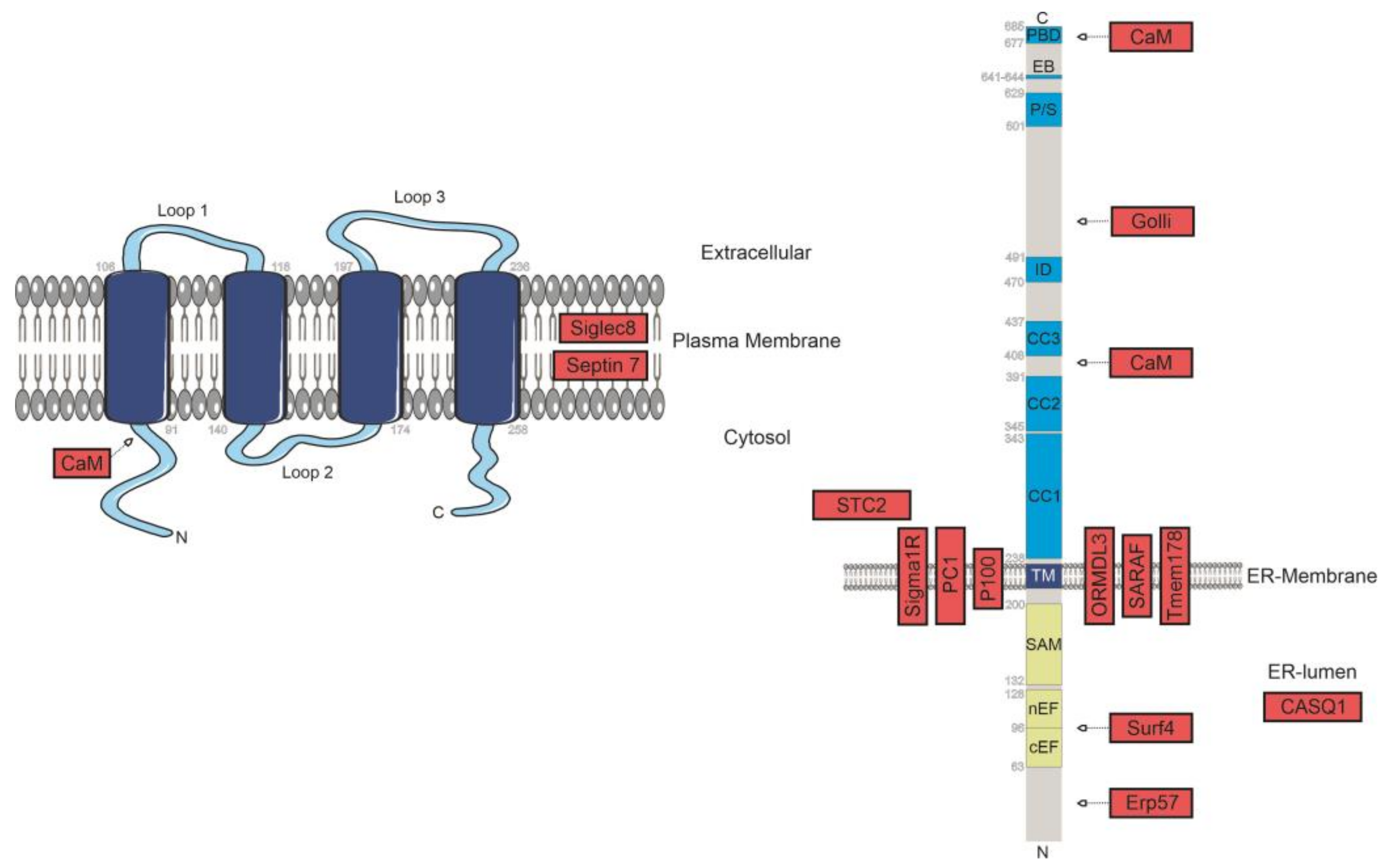
2.4. Negative Modulators of SOCE
2.5. Further Interaction Partners of STIM1/Orai1
3. Conclusions/Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carafoli, E.; Krebs, J. Why Calcium? How Calcium Became the Best Communicator. J. Biol. Chem. 2016, 291, 20849–20857. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S. Calcium Signaling: From Basic to Bedside. Adv. Exp. Med. Biol. 2020, 1131, 1–6. [Google Scholar] [PubMed]
- Lopez, J.J.; Jardin, I.; Albarrán, L.; Sanchez-Collado, J.; Cantonero, C.; Salido, G.M.; Smani, T.; Rosado, J.A. Molecular Basis and Regulation of Store-Operated Calcium Entry. In Taurine 6; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2020; Volume 1131, pp. 445–469. [Google Scholar]
- Fahrner, M.; Grabmayr, H.; Romanin, C. Mechanism of STIM activation. Curr. Opin. Physiol. 2020, 17, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Prakriya, M.; Lewis, R.S. Store-operated calcium channels. Physiol. Rev. 2015, 95, 1383–1436. [Google Scholar] [CrossRef]
- Lewis, R.S. Store-operated calcium channels: From function to structure and back again. Cold Spring Harb. Perspect. Biol. 2020, 12, a035055. [Google Scholar] [CrossRef]
- Liou, J.; Kim, M.L.; Heo, W.D.; Jones, J.T.; Myers, J.W.; Ferrell, J.E., Jr.; Meyer, T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr. Biol. 2005, 15, 1235–1241. [Google Scholar] [CrossRef]
- Stathopulos, P.B.; Zheng, L.; Li, G.-Y.; Plevin, M.J.; Ikura, M. Structural and Mechanistic Insights into STIM1-Mediated Initiation of Store-Operated Calcium Entry. Cell 2008, 135, 110–122. [Google Scholar] [CrossRef]
- Schober, R.; Bonhenry, D.; Lunz, V.; Zhu, J.; Tiffner, A.; Frischauf, I.; Fahrner, M.; Zhang, M.; Waldherr, L.; Schmidt, T.; et al. Sequential activation of STIM1 links Ca2+ with luminal domain unfolding. Sci. Signal. 2019, 12, eaax3194. [Google Scholar] [CrossRef]
- Ercan, E.; Momburg, F.; Engel, U.; Temmerman, K.; Nickel, W.; Seedorf, M. A Conserved, Lipid-Mediated Sorting Mechanism of Yeast Ist2 and Mammalian STIM Proteins to the Peripheral ER. Traffic 2009, 10, 1802–1818. [Google Scholar] [CrossRef]
- Muik, M.; Fahrner, M.; Schindl, R.; Stathopulos, P.; Frischauf, I.; Derler, I.; Plenk, P.; Lackner, B.; Groschner, K.; Ikura, M.; et al. STIM1 couples to ORAI1 via an intramolecular transition into an extended conformation. EMBO J. 2011, 30, 1678–1689. [Google Scholar] [CrossRef]
- Muik, M.; Fahrner, M.; Derler, I.; Schindl, R.; Bergsmann, J.; Frischauf, I.; Groschner, K.; Romanin, C. A Cytosolic Homomerization and a Modulatory Domain within STIM1 C Terminus Determine Coupling to ORAI1 Channels. J. Biol. Chem. 2009, 284, 8421–8426. [Google Scholar] [CrossRef] [PubMed]
- Frischauf, I.; Muik, M.; Derler, I.; Bergsmann, J.; Fahrner, M.; Schindl, R.; Groschner, K.; Romanin, C. Molecular determinants of the coupling between STIM1 and Orai channels: Differential activation of Orai1-3 channels by a STIM1 coiled-coil mutant. J. Biol. Chem. 2009, 284, 21696–21706. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lu, J.; Xu, P.; Xie, X.; Chen, L.; Xu, T. Mapping the Interacting Domains of STIM1 and Orai1 in Ca2+Release-activated Ca2+Channel Activation. J. Biol. Chem. 2007, 282, 29448–29456. [Google Scholar] [CrossRef] [PubMed]
- Park, C.Y.; Hoover, P.J.; Mullins, F.M.; Bachhawat, P.; Covington, E.D.; Raunser, S.; Walz, T.; Garcia, K.C.; Dolmetsch, R.E.; Lewis, R.S. STIM1 Clusters and Activates CRAC Channels via Direct Binding of a Cytosolic Domain to Orai1. Cell 2009, 136, 876–890. [Google Scholar] [CrossRef]
- Liu, X.; Wu, G.; Yu, Y.; Chen, X.; Ji, R.; Lu, J.; Li, X.; Zhang, X.; Yang, X.; Shen, Y. Molecular understanding of calcium permeation through the open Orai channel. PLoS Biol. 2019, 17, e3000096. [Google Scholar] [CrossRef]
- Hou, X.; Burstein, S.R.; Long, S.B. Structures reveal opening of the store-operated calcium channel Orai. eLife 2018, 7, e36758. [Google Scholar] [CrossRef]
- Hou, X.; Pedi, L.; Diver, M.M.; Long, S.B. Crystal structure of the calcium release-activated calcium channel Orai. Science 2012, 338, 1308–1313. [Google Scholar] [CrossRef]
- Lopez, J.J.; Albarran, L.; Gómez, L.J.; Smani, T.; Salido, G.M.; Rosado, J.A. Molecular modulators of store-operated calcium entry. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 2016, 1863, 2037–2043. [Google Scholar] [CrossRef]
- Sampieri, A.; Zepeda, A.; Asanov, A.; Vaca, L. Visualizing the store-operated channel complex assembly in real time: Identification of SERCA2 as a new member. Cell Calcium 2009, 45, 439–446. [Google Scholar] [CrossRef]
- Srikanth, S.; Jung, H.-J.; Kim, K.-D.; Souda, P.; Whitelegge, J.; Gwack, Y. A novel EF-hand protein, CRACR2A, is a cytosolic Ca2+ sensor that stabilizes CRAC channels in T cells. Nat. Cell Biol. 2010, 12, 436–446. [Google Scholar] [CrossRef]
- Kappel, S.; Borgström, A.; Stokłosa, P.; Dörr, K.; Peinelt, C. Store-operated calcium entry in disease: Beyond STIM/Orai expression levels. Semin. Cell Dev. Biol. 2019, 94, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Hodeify, R.; Nandakumar, M.; Own, M.; Courjaret, R.; Graumann, J.; Hubrack, S.Z.; Machaca, K. The CCT chaperonin is a novel regulator of Ca2+ signaling through modulation of Orai1 trafficking. Sci. Adv. 2018, 4, eaau1935. [Google Scholar] [CrossRef] [PubMed]
- Hodeify, R.; Selvaraj, S.; Wen, J.; Arredouani, A.; Hubrack, S.Z.; Dib, M.; Al-Thani, S.N.; McGraw, T.; Machaca, K. A STIM1-dependent ’trafficking trap’ mechanism regulates Orai1 plasma membrane residence and Ca²? influx levels. J. Cell Sci. 2015, 128, 3143–3154. [Google Scholar] [CrossRef] [PubMed]
- Woodard, G.E.; Salido, G.M.; Rosado, J.A. Enhanced exocytotic-like insertion of Orai1 into the plasma membrane upon intracellular Ca2+ store depletion. Am. J. Physiol. Physiol. 2008, 294, C1323–C1331. [Google Scholar] [CrossRef] [PubMed]
- Skach, W.R. Defects in processing and trafficking of the cystic fibrosis transmembrane conductance regulator. Kidney Int. 2000, 57, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Perrin, M.J.; Subbiah, R.N.; Vandenberg, J.I.; Hill, A.P. Human ether-a-go-go related gene (hERG) K+ channels: Function and dysfunction. Prog. Biophys. Mol. Biol. 2008, 98, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.B.; Lockwich, T.P.; Bandyopadhyay, B.C.; Liu, X.; Bollimuntha, S.; Brazer, S.-C.; Combs, C.; Das, S.; Leenders, A.; Sheng, Z.-H.; et al. VAMP2-Dependent Exocytosis Regulates Plasma Membrane Insertion of TRPC3 Channels and Contributes to Agonist-Stimulated Ca2+ Influx. Mol. Cell 2004, 15, 635–646. [Google Scholar] [CrossRef]
- Lockwich, T.P.; Liu, X.; Singh, B.B.; Jadlowiec, J.; Weiland, S.; Ambudkar, I.S. Assembly of Trp1 in a signaling complex associated with caveolin-scaffolding lipid raft domains. J. Biol. Chem. 2000, 275, 11934–11942. [Google Scholar] [CrossRef]
- Yu, F.; Sun, L.; Machaca, K. Constitutive recycling of the store-operated Ca2+ channel Orai1 and its internalization during meiosis. J. Cell Biol. 2010, 191, 523–535. [Google Scholar] [CrossRef]
- Yeh, Y.-C.; Lin, Y.-P.; Kramer, H.; Parekh, A.B. Single-nucleotide polymorphisms in Orai1 associated with atopic dermatitis inhibit protein turnover, decrease calcium entry and disrupt calcium-dependent gene expression. Hum. Mol. Genet. 2019, 29, 1808–1823. [Google Scholar] [CrossRef]
- Cross, B.M.; Hack, A.; Reinhardt, T.A.; Rao, R. SPCA2 Regulates Orai1 Trafficking and Store Independent Ca2+ Entry in a Model of Lactation. PLoS ONE 2013, 8, e67348. [Google Scholar] [CrossRef] [PubMed]
- Smaardijk, S.; Chen, J.; Wuytack, F.; Vangheluwe, P. SPCA2 couples Ca2+ influx via Orai1 to Ca2+ uptake into the Golgi/secretory pathway. Tissue Cell 2017, 49, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Peretti, M.; Badaoui, M.; Girault, A.; Van Gulick, L.; Mabille, M.-P.; Tebbakha, R.; Sevestre, H.; Morjani, H.; Ouadid-Ahidouch, H. Original association of ion transporters mediates the ECM-induced breast cancer cell survival: Kv10.1-Orai1-SPCA2 partnership. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Grice, D.M.; Faddy, H.M.; Nguyen, N.; Leitch, S.; Wang, Y.; Muend, S.; Kenny, P.A.; Sukumar, S.; Roberts-Thomson, S.J.; et al. Store-Independent Activation of Orai1 by SPCA2 in Mammary Tumors. Cell 2010, 143, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Fenech, M.A.; Carter, M.M.; Stathopulos, P.B.; Pin, C.L. The pancreas-specific form of secretory pathway calcium ATPase 2 regulates multiple pathways involved in calcium homeostasis. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 2020, 1867, 118567. [Google Scholar] [CrossRef]
- Zeng, B.; Chen, G.-L.; Garcia-Vaz, E.; Bhandari, S.; Daskoulidou, N.; Berglund, L.M.; Jiang, H.; Hallett, T.; Zhou, L.-P.; Huang, L.; et al. ORAI channels are critical for receptor-mediated endocytosis of albumin. Nat. Commun. 2017, 8, 1920. [Google Scholar] [CrossRef]
- Grieve, A.G.; Yeh, Y.-C.; Zarcone, L.; Breuning, J.; Johnson, N.; Stříšovský, K.; Brown, M.H.; Parekh, A.B.; Freeman, M. Conformational surveillance of Orai1 by a rhomboid intramembrane protease prevents inappropriate CRAC channel activation. bioRxiv 2020. [Google Scholar] [CrossRef]
- Saitoh, N.; Oritani, K.; Saito, K.; Yokota, T.; Ichii, M.; Sudo, T.; Fujita, N.; Nakajima, K.; Okada, M.; Kanakura, Y. Identification of functional domains and novel binding partners of STIM proteins. J. Cell. Biochem. 2011, 112, 147–156. [Google Scholar] [CrossRef]
- Pozo-Guisado, E.; Casas-Rua, V.; Tomas-Martin, P.; Lopez-Guerrero, A.M.; Alvarez-Barrientos, A.; Martin-Romero, F.J. Phosphorylation of STIM1 at ERK1/2 target sites regulates interaction with the microtubule plus-end binding protein EB1. J. Cell Sci. 2013, 126, 3170–3180. [Google Scholar] [CrossRef]
- Asanov, A.; Sherry, R.; Sampieri, A.; Vaca, L. A relay mechanism between EB1 and APC facilitate STIM1 puncta assembly at endoplasmic reticulum–plasma membrane junctions. Cell Calcium 2013, 54, 246–256. [Google Scholar] [CrossRef]
- Munemitsu, S.; Souza, B.; Müller, O.; Albert, I.; Rubinfeld, B.; Polakis, P. The APC gene product associates with microtubules in vivo and promotes their assembly in vitro. Cancer Res. 1994, 54, 3676–3681. [Google Scholar] [PubMed]
- Lee, J.-E.; Jeon, I.-S.; Han, N.-E.; Song, H.-J.; Kim, E.-G.; Choi, J.-W.; Song, K.-D.; Lee, H.-K.; Choi, J.-K. Ubiquilin 1 interacts with Orai1 to regulate calcium mobilization. Mol. Cells 2013, 35, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Jozsef, L.; Tashiro, K.; Kuo, A.; Park, E.J.; Skoura, A.; Albinsson, S.; Rivera-Molina, F.; Harrison, K.D.; Iwakiri, Y.; Toomre, D.; et al. Reticulon 4 Is Necessary for Endoplasmic Reticulum Tubulation, STIM1-Orai1 Coupling, and Store-operated Calcium Entry. J. Biol. Chem. 2014, 289, 9380–9395. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.; Brotto, M.; Weisleder, N.; Chu, Y.; Lin, P.-H.; Zhao, X.; Thornton, A.; Komazaki, S.; Takeshima, H.; Ma, J.; et al. Uncoupling Store-Operated Ca2+ Entry and Altered Ca2+ Release from Sarcoplasmic Reticulum through Silencing of Junctophilin Genes. Biophys. J. 2006, 90, 4418–4427. [Google Scholar] [CrossRef]
- Woo, J.S.; Cho, C.-H.; Lee, K.J.; Kim, D.H.; Ma, J.; Lee, E.H. Hypertrophy in Skeletal Myotubes Induced by Junctophilin-2 Mutant, Y141H, Involves an Increase in Store-operated Ca2+Entry via Orai1. J. Biol. Chem. 2012, 287, 14336–14348. [Google Scholar] [CrossRef] [PubMed]
- Jardin, I.; Albarran, L.; Bermejo, N.; Salido, G.M.; Rosado, J.A. Homers regulate calcium entry and aggregation in human platelets: A role for Homers in the association between STIM1 and Orai1. Biochem. J. 2012, 445, 29–38. [Google Scholar] [CrossRef]
- Shambharkar, P.B.; Bittinger, M.; Latario, B.; Xiong, Z.; Bandyopadhyay, S.; Davis, V.; Lin, V.; Yang, Y.; Valdez, R.; Labow, M.A. TMEM203 Is a Novel Regulator of Intracellular Calcium Homeostasis and Is Required for Spermatogenesis. PLoS ONE 2015, 10, e0127480. [Google Scholar] [CrossRef]
- Smyth, J.T.; Petranka, J.G.; Boyles, R.R.; DeHaven, W.I.; Fukushima, M.; Johnson, K.L.; Williams, J.G.; Putney, J.W., Jr. Phosphorylation of STIM1 underlies suppression of store-operated calcium entry during mitosis. Nat. Cell Biol. 2009, 11, 1465–1472. [Google Scholar] [CrossRef]
- Ritchie, M.F.; Samakai, E.; Soboloff, J. STIM1 is required for attenuation of PMCA-mediated Ca2+clearance during T-cell activation. EMBO J. 2012, 31, 1123–1133. [Google Scholar] [CrossRef]
- Park, C.Y.; Shcheglovitov, A.; Dolmetsch, R.E. The CRAC Channel Activator STIM1 Binds and Inhibits L-Type Voltage-Gated Calcium Channels. Science 2010, 330, 101–105. [Google Scholar] [CrossRef]
- Krapivinsky, G.; Stotz, S.C.; Manasian, Y.; Clapham, D.E.; Krapivinsky, L. POST, partner of stromal interaction molecule 1 (STIM1), targets STIM1 to multiple transporters. Proc. Natl. Acad. Sci. USA 2011, 108, 19234–19239. [Google Scholar] [CrossRef] [PubMed]
- Kar, P.; Samanta, K.; Kramer, H.; Morris, O.; Bakowski, D.; Parekh, A.B. Dynamic Assembly of a Membrane Signaling Complex Enables Selective Activation of NFAT by Orai1. Curr. Biol. 2014, 24, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, D.; Everett, K.L.; Halls, M.L.; Pacheco, J.; Skroblin, P.; Vaca, L.; Klussmann, E.; Cooper, D.M.F. Direct Binding Between Orai1 and AC8 Mediates Dynamic Interplay Between Ca2+ and cAMP Signaling. Sci. Signal. 2012, 5, ra29. [Google Scholar] [CrossRef] [PubMed]
- Smaardijk, S.; Chen, J.; Kerselaers, S.; Voets, T.; Eggermont, J.; Vangheluwe, P. Store-independent coupling between the Secretory Pathway Ca2+ transport ATPase SPCA1 and Orai1 in Golgi stress and Hailey-Hailey disease. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 2018, 1865, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Sun, L.; Machaca, K. Orai1 internalization and STIM1 clustering inhibition modulate SOCE inactivation during meiosis. Proc. Natl. Acad. Sci. USA 2009, 106, 17401–17406. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.T.; Senior, P.V.; Van Stekelenburg, L.; Layton, J.E.; Smith, P.J.; Dziadek, M.A. Stromal interaction molecule 1 (STIM1), a transmembrane protein with growth suppressor activity, contains an extracellular SAM domain modified by N-linked glycosylation. Biochim. Biophys. Acta Protein Struct. Mol. Enzym. 2002, 1596, 131–137. [Google Scholar] [CrossRef]
- Keil, J.M.; Shen, Z.; Briggs, S.P.; Patrick, G.N. Regulation of STIM1 and SOCE by the Ubiquitin-Proteasome System (UPS). PLoS ONE 2010, 5, e13465. [Google Scholar] [CrossRef]
- Honnappa, S.; Gouveia, S.M.; Weisbrich, A.; Damberger, F.F.; Bhavesh, N.S.; Jawhari, H.; Grigoriev, I.; Van Rijssel, F.J.; Buey, R.M.; Lawera, A.; et al. An EB1-Binding Motif Acts as a Microtubule Tip Localization Signal. Cell 2009, 138, 366–376. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Wei, P.-L.; Chen, W.-Y.; Chang, W.-C.; Chang, Y.-J. Silencing Heat Shock Protein 27 Inhibits the Progression and Metastasis of Colorectal Cancer (CRC) by Maintaining the Stability of Stromal Interaction Molecule 1 (STIM1) Proteins. Cells 2018, 7, 262. [Google Scholar] [CrossRef]
- Moon, H.; Min, C.; Kim, G.; Kim, D.; Kim, K.; Lee, S.-A.; Moon, B.; Yang, S.; Lee, J.; Yang, S.-J.; et al. Crbn modulates calcium influx by regulating Orai1 during efferocytosis. Nat. Commun. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Cao, X.; Choi, S.; Maléth, J.J.; Park, S.; Ahuja, M.; Muallem, S. The ER/PM microdomain, PI(4,5)P₂ and the regulation of STIM1-Orai1 channel function. Cell Calcium 2015, 58, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Zaman, M.F.; Nenadic, A.; Radojičić, A.; Rosado, A.; Beh, C.T. Sticking With It: ER-PM Membrane Contact Sites as a Coordinating Nexus for Regulating Lipids and Proteins at the Cell Cortex. Front. Cell Dev. Biol. 2020, 8, 675. [Google Scholar] [CrossRef] [PubMed]
- Pla-Martín, D.; Calpena, E.; Lupo, V.; Márquez, C.; Rivas, E.; Sivera, R.; Sevilla, T.; Palau, F.; Espinós, C. Junctophilin-1 is a modifier gene of GDAP1-related Charcot–Marie–Tooth disease. Human Mol. Genet. 2014, 24, 213–229. [Google Scholar]
- Woo, J.S.; Srikanth, S.; Nishi, M.; Ping, P.; Takeshima, H.; Gwack, Y. Junctophilin-4, a component of the endoplasmic reticulum–plasma membrane junctions, regulates Ca2+ dynamics in T cells. Proc. Natl. Acad. Sci. USA 2016, 113, 2762–2767. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, S.; Jew, M.; Kim, K.-D.; Yee, M.-K.; Abramson, J.; Gwack, Y. Junctate is a Ca2+-sensing structural component of Orai1 and stromal interaction molecule 1 (STIM1). Proc. Natl. Acad. Sci. USA 2012, 109, 8682–8687. [Google Scholar] [CrossRef]
- Guido, D.; Demaurex, N.; Nunes-Hasler, P. Junctate boosts phagocytosis by recruiting endoplasmic reticulum Ca2+ stores near phagosomes. J. Cell Sci. 2015, 128, 4074–4082. [Google Scholar] [CrossRef]
- Prakash, Y.; Thompson, M.A.; Vaa, B.; Matabdin, I.; Peterson, T.E.; He, T.; Pabelick, C.M. Caveolins and intracellular calcium regulation in human airway smooth muscle. Am. J. Physiol. Cell. Mol. Physiol. 2007, 293, L1118–L1126. [Google Scholar] [CrossRef]
- Zhu, H.; Weisleder, N.; Wu, P.; Cai, C.; Chen, J.-W. Caveolae/caveolin-1 are important modulators of store-operated calcium entry in Hs578/T breast cancer cells. J. Pharmacol. Sci. 2008, 106, 287–294. [Google Scholar] [CrossRef]
- Yeh, Y.-C.; Parekh, A.B. Distinct Structural Domains of Caveolin-1 Independently Regulate Ca2+Release-Activated Ca2+Channels and Ca2+Microdomain-Dependent Gene Expression. Mol. Cell. Biol. 2015, 35, 1341–1349. [Google Scholar] [CrossRef]
- Bóhorquez-Hernández, A.; Gratton, E.; Pacheco, J.; Asanov, A.; Vaca, L. Cholesterol modulates the cellular localization of Orai1 channels and its disposition among membrane domains. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 1481–1490. [Google Scholar] [CrossRef]
- Jiao, H.-X.; Mu, Y.-P.; Gui, L.-X.; Yan, F.-R.; Lin, D.-C.; Sham, J.S.K.; Lin, M.-J. Increase in caveolae and caveolin-1 expression modulates agonist-induced contraction and store- and receptor-operated Ca2+ entry in pulmonary arteries of pulmonary hypertensive rats. Vasc. Pharmacol. 2016, 84, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Pani, B.; Singh, B.B. Lipid rafts/caveolae as microdomains of calcium signaling. Cell Calcium 2009, 45, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Sathish, V.; Abcejo, A.J.; Thompson, M.A.; Sieck, G.C.; Prakash, Y.S.; Pabelick, C. Caveolin-1 regulation of store-operated Ca2+influx in human airway smooth muscle. Eur. Respir. J. 2012, 40, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Quintana, A.; Findlay, G.M.; Mettlen, M.; Baust, B.; Jain, M.R.; Nilsson, R.; Rao, A.; Hogan, P.G. An siRNA screen for NFAT activation identifies septins as coordinators of store-operated Ca2+ entry. Nat. Cell Biol. 2013, 499, 238–242. [Google Scholar] [CrossRef]
- Deb, B.K.; Pathak, T.; Hasan, G. Store-independent modulation of Ca2+ entry through Orai by Septin 7. Nat. Commun. 2016, 7, 11751. [Google Scholar] [CrossRef] [PubMed]
- Deb, B.K.; Hasan, G. Regulation of Store-Operated Ca2+ Entry by Septins. Front. Cell Dev. Biol. 2016, 4, 142. [Google Scholar] [CrossRef]
- Katz, Z.B.; Zhang, C.; Quintana, A.; Lillemeier, B.F.; Hogan, P.G. Septins organize endoplasmic reticulum-plasma membrane junctions for STIM1-ORAI1 calcium signalling. Sci. Rep. 2019, 9, 10839. [Google Scholar] [CrossRef]
- Deb, B.K.; Chakraborty, P.; Gopurappilly, R.; Hasan, G. SEPT7 regulates Ca2+ entry through Orai channels in human neural progenitor cells and neurons. Cell Calcium 2020, 90, 102252. [Google Scholar] [CrossRef]
- Miao, Y.; Miner, C.; Zhang, L.; Hanson, P.I.; Dani, A.; Vig, M. An essential and NSF independent role for α-SNAP in store-operated calcium entry. eLife 2013, 2, e00802. [Google Scholar] [CrossRef]
- Li, P.; Miao, Y.; Dani, A.; Vig, M. α-SNAP regulates dynamic, on-site assembly and calcium selectivity of Orai1 channels. Mol. Biol. Cell 2016, 27, 2542–2553. [Google Scholar] [CrossRef]
- Miao, Y.; Bhushan, J.; Dani, A.; Vig, M. Na+ influx via Orai1 inhibits intracellular ATP-induced mTORC2 signaling to disrupt CD4 T cell gene expression and differentiation. eLife 2017, 6, 6. [Google Scholar] [CrossRef]
- Bauer, M.C.; O’Connell, D.; Cahill, L.J.; Linse, S. Calmodulin Binding to the Polybasic C-Termini of STIM Proteins Involved in Store-Operated Calcium Entry†. Biochemistry 2008, 47, 6089–6091. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.S.; Srikanth, S.; Kim, K.D.; Elsaesser, H.; Lu, J.; Pellegrini, M.; Brooks, D.G.; Sun, Z.; Gwack, Y. CRACR2A-Mediated TCR Signaling Promotes Local Effector Th1 and Th17 Responses. J. Immunol. 2018, 201, 1174–1185. [Google Scholar] [CrossRef]
- Srikanth, S.; Woo, J.S.; Gwack, Y. A large Rab GTPase family in a small GTPase world. Small GTPases 2017, 8, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Quintana, A.; Rajanikanth, V.; Farber-Katz, S.; Gudlur, A.; Zhang, C.; Jing, J.; Zhou, Y.; Rao, A.; Hogan, P.G. TMEM110 regulates the maintenance and remodeling of mammalian ER–plasma membrane junctions competent for STIM–ORAI signaling. Proc. Natl. Acad. Sci. USA 2015, 112, E7083–E7092. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.; He, L.; Sun, A.; Quintana, A.; Ding, Y.; Ma, G.; Tan, P.; Liang, X.; Zheng, X.; Chen, L.; et al. Proteomic mapping of ER–PM junctions identifies STIMATE as a regulator of Ca2+ influx. Nat. Cell Biol. 2015, 17, 1339–1347. [Google Scholar] [CrossRef]
- Hooper, R.; Soboloff, J. STIMATE reveals a STIM1 transitional state. Nat. Cell Biol. 2015, 17, 1232–1234. [Google Scholar] [CrossRef][Green Version]
- Worley, P.F.; Zeng, W.; Huang, G.; Kim, J.Y.; Shin, D.M.; Kim, M.S.; Yuan, J.P.; Kiselyov, K.; Muallem, S. Homer proteins in Ca2+ signaling by excitable and non-excitable cells. Cell Calcium 2007, 42, 363–371. [Google Scholar] [CrossRef]
- Chen, T.; Zhu, J.; Zhang, C.; Huo, K.; Fei, Z.; Jiang, X.-F. Protective Effects of SKF-96365, a Non-Specific Inhibitor of SOCE, against MPP+-Induced Cytotoxicity in PC12 Cells: Potential Role of Homer1. PLoS ONE 2013, 8, e55601. [Google Scholar] [CrossRef]
- Tu, J.C.; Xiao, B.; Yuan, J.P.; A Lanahan, A.; Leoffert, K.; Li, M.; Linden, D.J.; Worley, P.F. Homer Binds a Novel Proline-Rich Motif and Links Group 1 Metabotropic Glutamate Receptors with IP3 Receptors. Neuron 1998, 21, 717–726. [Google Scholar] [CrossRef]
- Shalygin, A.; Ryazantseva, M.A.; Glushankova, L.; Mozhayeva, G.N.; Bezprozvanny, I.; Kaznacheyeva, E. Homer regulation of native plasma membrane calcium channels in A431 cells. Cell Calcium 2010, 48, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Shalygin, A.V.; Ryazantseva, M.A.; Glushankova, L.N.; Bezprozvanny, I.B.; Mozhayeva, G.N.; Kaznacheyeva, E.V. Delay in I(min) channel activation induced by dissociation of Homer proteins in A431 cells. Dokl. Biol. Sci. 2011, 438, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Rodriguez, M.; Williams, A.G.; Yuan, J.P. Homer binds to Orai1 and TRPC channels in the neointima and regulates vascular smooth muscle cell migration and proliferation. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Aubart, F.C.; Sassi, Y.; Coulombe, A.; Mougenot, N.; Vrignaud, C.; Leprince, P.; Lechat, P.; Lompre, A.-M.; Hulot, J.-S. RNA Interference Targeting STIM1 Suppresses Vascular Smooth Muscle Cell Proliferation and Neointima Formation in the Rat. Mol. Ther. 2009, 17, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Halligan, K.E.; Zhang, X.; Bisaillon, J.M.; Gonzalez-Cobos, J.C.; Motiani, R.K.; Hu, G.; Vincent, P.A.; Zhou, J.; Barroso, M.; et al. Orai1-Mediated I CRAC Is Essential for Neointima Formation After Vascular Injury. Circ. Res. 2011, 109, 534–542. [Google Scholar] [CrossRef]
- Li, X.; Chen, W.; Zhang, L.; Liu, W.-B.; Fei, Z. Inhibition of Store-Operated Calcium Entry Attenuates MPP+-Induced Oxidative Stress via Preservation of Mitochondrial Function in PC12 Cells: Involvement of Homer1a. PLoS ONE 2013, 8, e83638. [Google Scholar] [CrossRef] [PubMed]
- Mentrup, T.; Cabrera-Cabrera, F.; Fluhrer, R.; Schröder, B. Physiological functions of SPP/SPPL intramembrane proteases. Cell. Mol. Life Sci. 2020, 77, 2959–2979. [Google Scholar] [CrossRef]
- Makowski, S.L.; Wang, Z.; Pomerantz, J.L. A Protease-Independent Function for SPPL3 in NFAT Activation. Mol. Cell. Biol. 2014, 35, 451–467. [Google Scholar] [CrossRef]
- Lichtenthaler, S.F.; Lemberg, M.K.; Fluhrer, R. Proteolytic ectodomain shedding of membrane proteins in mammals—Hardware, concepts, and recent developments. EMBO J. 2018, 37, e99456. [Google Scholar] [CrossRef]
- Clarysse, L.; Guéguinou, M.; Potier-Cartereau, M.; Vandecasteele, G.; Bougnoux, P.; Chevalier, S.; Chantôme, A.; Vandier, C. cAMP-PKA inhibition of SK3 channel reduced both Ca2+ entry and cancer cell migration by regulation of SK3-Orai1 complex. Pflugers. Arch. 2014, 466, 1921–1932. [Google Scholar] [CrossRef]
- Guéguinou, M.; Harnois, T.; Crottes, D.; Uguen, A.; Deliot, N.; Gambade, A.; Chantôme, A.; Haelters, J.P.; Jaffrès, P.A.; Jourdan, M.L.; et al. SK3/TRPC1/Orai1 complex regulates SOCE-dependent colon cancer cell migration: A novel opportunity to modulate anti-EGFR mAb action by the alkyl-lipid Ohmline. Oncotarget 2016, 7, 36168–36184. [Google Scholar]
- Chen, M.; Li, J.; Jiang, F.; Fu, J.; Xia, X.; Du, J.; Hu, M.; Huang, J.; Shen, B. Orai1 forms a signal complex with BKCa channel in mesenteric artery smooth muscle cells. Physiol. Rep. 2016, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.; Schlichter, L.C. Selective Activation of KCa3.1 and CRAC Channels by P2Y2 Receptors Promotes Ca2+ Signaling, Store Refilling and Migration of Rat Microglial Cells. PLoS ONE 2013, 8, e62345. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Deng, X.; Mancarella, S.; Hendron, E.; Eguchi, S.; Soboloff, J.; Tang, X.D.; Gill, D.L. The Calcium Store Sensor, STIM1, Reciprocally Controls Orai and CaV1.2 Channels. Science 2010, 330, 105–109. [Google Scholar] [CrossRef]
- Lee, B.L.; Moon, J.E.; Shu, J.H.; Yuan, L.; Newman, Z.R.; Schekman, R.; Barton, G.M. UNC93B1 mediates differential trafficking of endosomal TLRs. eLife 2013, 2, e00291. [Google Scholar] [CrossRef]
- Kashuba, V.; Protopopov, A.I.; Kvasha, S.M.; Gizatullin, R.Z.; Wahlestedt, C.; Kisselev, L.L.; Klein, G.; Zabarovsky, E.R. hUNC93B1: A novel human gene representing a new gene family and encoding an unc-93-like protein. Gene 2002, 283, 209–217. [Google Scholar] [CrossRef]
- Pelka, K.; Bertheloot, D.; Reimer, E.; Phulphagar, K.; Schmidt, S.V.; Christ, A.; Stahl, R.; Watson, N.; Miyake, K.; Hacohen, N.; et al. The Chaperone UNC93B1 Regulates Toll-like Receptor Stability Independently of Endosomal TLR Transport. Immunity 2018, 48, 911–922.e7. [Google Scholar] [CrossRef]
- Maschalidi, S.; Nunes-Hasler, P.; Nascimento, C.R.; Sallent, I.; Lannoy, V.; Garfa-Traore, M.; Cagnard, N.; Sepulveda, F.E.; Vargas, P.; Lennon-Duménil, A.-M.; et al. UNC93B1 interacts with the calcium sensor STIM1 for efficient antigen cross-presentation in dendritic cells. Nat. Commun. 2017, 8, 1640. [Google Scholar] [CrossRef]
- Brinkmann, M.M.; Spooner, E.; Hoebe, K.; Beutler, B.; Ploegh, H.L.; Kim, Y.M. The interaction between the ER membrane protein UNC93B and TLR3, 7, and 9 is crucial for TLR signaling. J. Cell Biol. 2007, 177, 265–275. [Google Scholar] [CrossRef]
- Fujii, Y.; Shiota, M.; Ohkawa, Y.; Baba, A.; Wanibuchi, H.; Kinashi, T.; Kurosaki, T.; Baba, Y. Surf4 modulates STIM1-dependent calcium entry. Biochem. Biophys. Res. Commun. 2012, 422, 615–620. [Google Scholar] [CrossRef]
- Prins, D.; Groenendyk, J.; Touret, N.; Michalak, M. Modulation of STIM1 and capacitative Ca2+ entry by the endoplasmic reticulum luminal oxidoreductase ERp57. EMBO Rep. 2011, 12, 1182–1188. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yan, H.; Dai, W.; Jing, J.; Yang, Y.; Mahajan, S.; Zhou, Y.; Li, W.; Macaubas, C.; Mellins, E.D.; et al. Tmem178 negatively regulates store-operated calcium entry in myeloid cells via association with STIM1. J. Autoimmun. 2019, 101, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Decker, C.E.; Yang, Z.; Rimer, R.; Park-Min, K.-H.; Macaubas, C.; Mellins, E.D.; Novack, D.V.; Faccio, R. Tmem178 acts in a novel negative feedback loop targeting NFATc1 to regulate bone mass. Proc. Natl. Acad. Sci. USA 2015, 112, 15654–15659. [Google Scholar] [CrossRef] [PubMed]
- Santoso, N.G.; Cebotaru, L.; Guggino, W.B. Polycystin-1, 2, and STIM1 Interact with IP3R to Modulate ER Ca2+ Release through the PI3K/Akt Pathway. Cell. Physiol. Biochem. 2011, 27, 715–726. [Google Scholar] [CrossRef]
- Li, Y.; Santoso, N.G.; Yu, S.; Woodward, O.M.; Qian, F.; Guggino, W.B. Polycystin-1 Interacts with Inositol 1,4,5-Trisphosphate Receptor to Modulate Intracellular Ca2+Signaling with Implications for Polycystic Kidney Disease. J. Biol. Chem. 2009, 284, 36431–36441. [Google Scholar] [CrossRef]
- Woodward, O.M.; Li, Y.; Yu, S.; Greenwell, P.; Wodarczyk, C.; Boletta, A.; Guggino, W.B.; Qian, F. Identification of a Polycystin-1 Cleavage Product, P100, That Regulates Store Operated Ca2+ Entry through Interactions with STIM1. PLoS ONE 2010, 5, e12305. [Google Scholar] [CrossRef]
- Sampieri, A.; Santoyo, K.; Asanov, A.; Vaca, L. Association of the IP3R to STIM1 provides a reduced intraluminal calcium microenvironment, resulting in enhanced store-operated calcium entry. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Feng, J.-M.; Hu, Y.K.; Xie, L.-H.; Colwell, C.S.; Shao, X.M.; Sun, X.-P.; Chen, B.; Tang, H.; Campagnoni, A.T. Golli Protein Negatively Regulates Store Depletion-Induced Calcium Influx in T Cells. Immunity 2006, 24, 717–727. [Google Scholar] [CrossRef]
- Feng, J.-M.; O Fernandes, A.; Campagnoni, C.W.; Hu, Y.-H.; Campagnoni, A.T. The golli-myelin basic protein negatively regulates signal transduction in T lymphocytes. J. Neuroimmunol. 2004, 152, 57–66. [Google Scholar] [CrossRef]
- Campagnoni, A.T.; Pribyl, T.M.; Campagnoni, C.W.; Kampf, K.; Amur-Umarjee, S.; Landry, C.F.; Handley, V.W.; Newman, S.L.; Garbay, B.; Kitamura, K. Structure and developmental regulation of Golli-mbp, a 105-kilobase gene that encompasses the myelin basic protein gene and is expressed in cells in the oligodendrocyte lineage in the brain. J. Biol. Chem. 1993, 268, 4930–4938. [Google Scholar] [CrossRef]
- Feng, J.-M.; Givogri, I.M.; Bongarzone, E.R.; Campagnoni, C.; Jacobs, E.; Handley, V.W.; Schonmann, V.; Campagnoni, A.T. Thymocytes express the golli products of the myelin basic protein gene and levels of expression are stage dependent. J. Immunol. 2000, 165, 5443–5450. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.M.; Doherty, M.K.; Tepikin, A.V.; Burgoyne, R.D. Evidence for an interaction between Golli and STIM1 in store-operated calcium entry. Biochem. J. 2010, 430, 453–460. [Google Scholar] [CrossRef][Green Version]
- Palty, R.; Raveh, A.; Kaminsky, I.; Meller, R.; Reuveny, E. SARAF Inactivates the Store Operated Calcium Entry Machinery to Prevent Excess Calcium Refilling. Cell 2012, 149, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Albarran, L.; Regodón, S.; Salido, G.M.; López, J.J.; Rosado, J.A. Role of STIM1 in the surface expression of SARAF. Channels 2016, 11, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Albarran, L.; Lopez, J.J.; Ben Amor, N.; Martin-Cano, F.E.; Berna-Erro, A.; Smani, T.; Salido, G.M.; Rosado, J.A. Dynamic interaction of SARAF with STIM1 and Orai1 to modulate store-operated calcium entry. Sci. Rep. 2016, 6, 24452. [Google Scholar] [CrossRef] [PubMed]
- Jardin, I.; Albarran, L.; Salido, G.M.; López, J.J.; Sage, S.O.; Rosado, J.A. Fine-tuning of store-operated calcium entry by fast and slow Ca2+-dependent inactivation: Involvement of SARAF. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 2018, 1865, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Kimberlin, C.R.; Meshcheriakova, A.; Palty, R.; Raveh, A.; Karbat, I.; Reuveny, E.; Minor, D.L. SARAF Luminal Domain Structure Reveals a Novel Domain-Swapped β-Sandwich Fold Important for SOCE Modulation. J. Mol. Biol. 2019, 431, 2869–2883. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.; Ahuja, M.; Maléth, J.; Moreno, C.M.; Yuan, J.P.; Kim, M.S.; Muallem, S. The STIM1 CTID domain determines access of SARAF to SOAR to regulate Orai1 channel function. J. Cell Biol. 2013, 202, 71–79. [Google Scholar] [CrossRef]
- Parekh, A.B. Regulation of CRAC channels by Ca2+-dependent inactivation. Cell Calcium 2017, 63, 20–23. [Google Scholar] [CrossRef]
- Maléth, J.; Choi, S.; Muallem, S.; Ahuja, M. Translocation between PI(4,5)P2-poor and PI(4,5)P2-rich microdomains during store depletion determines STIM1 conformation and Orai1 gating. Nat. Commun. 2014, 5, 1–10. [Google Scholar] [CrossRef]
- Lopez, E.; Frischauf, I.; Jardin, I.; Derler, I.; Muik, M.; Cantonero, C.; Salido, G.M.; Smani, T.; Rosado, J.A.; Redondo, P.C. STIM1 phosphorylation at Y316 modulates its interaction with SARAF and the activation of SOCE and ICRAC. J. Cell Sci. 2019, 132. [Google Scholar] [CrossRef] [PubMed]
- Son, A.; Ahuja, M.; Schwartz, D.M.; Varga, A.; Swaim, W.; Kang, N.; Maléth, J.; Shin, D.M.; Muallem, S. Ca2+ Influx Channel Inhibitor SARAF Protects Mice From Acute Pancreatitis. Gastroenterology 2019, 157, 1660–1672. [Google Scholar] [CrossRef] [PubMed]
- Dai, F.; Zhang, Y.; Wang, Q.; Li, D.; Yang, Y.; Ma, S.; Yang, D. Overexpression of SARAF Ameliorates Pressure Overload–Induced Cardiac Hypertrophy Through Suppressing STIM1-Orai1 in Mice. Cell. Physiol. Biochem. 2018, 47, 817–826. [Google Scholar] [CrossRef] [PubMed]
- La Russa, D.; Frisina, M.; Secondo, A.; Bagetta, G.; Amantea, D. Modulation of Cerebral Store-operated Calcium Entry-regulatory Factor (SARAF) and Peripheral Orai1 Following Focal Cerebral Ischemia and Preconditioning in Mice. Neuroscience 2020, 441, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Albarran, L.; Lopez, J.J.; Jardin, I.; Sanchez-Collado, J.; Berna-Erro, A.; Smani, T.; Camello, P.J.; Salido, G.M.; Rosado, J.A. EFHB is a Novel Cytosolic Ca2+ Sensor That Modulates STIM1-SARAF Interaction. Cell. Physiol. Biochem. 2018, 51, 1164–1178. [Google Scholar] [CrossRef]
- Marshall, C.B.; Nishikawa, T.; Osawa, M.; Stathopulos, P.B.; Ikura, M. Calmodulin and STIM proteins: Two major calcium sensors in the cytoplasm and endoplasmic reticulum. Biochem. Biophys. Res. Commun. 2015, 460, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Linse, S.; Helmersson, A.; Forsén, S. Calcium binding to calmodulin and its globular domains. J. Biol. Chem. 1991, 266, 8050–8054. [Google Scholar]
- Traxler, L.; Rathner, P.; Fahrner, M.; Stadlbauer, M.; Faschinger, F.; Charnavets, T.; Müller, N.; Romanin, C.; Hinterdorfer, P.; Gruber, H.J. Detailed Evidence for an Unparalleled Interaction Mode between Calmodulin and Orai Proteins. Angew. Chem. Int. Ed. 2017, 56, 15755–15759. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, X.; Mueller, G.A.; Sobhany, M.; Derose, E.F.; Zhang, Y.; London, R.E.; Birnbaumer, L. Crystal Structure of Calmodulin Binding Domain of Orai1 in Complex with Ca2+•Calmodulin Displays a Unique Binding Mode*. J. Biol. Chem. 2012, 287, 43030–43041. [Google Scholar] [CrossRef]
- Mullins, F.M.; Park, C.Y.; Dolmetsch, R.E.; Lewis, R.S. STIM1 and calmodulin interact with Orai1 to induce Ca2+-dependent inactivation of CRAC channels. Proc. Natl. Acad. Sci. USA 2009, 106, 15495–15500. [Google Scholar] [CrossRef]
- Mullins, F.M.; Yen, M.; Lewis, R.S. Orai1 pore residues control CRAC channel inactivation independently of calmodulin. J. Gen. Physiol. 2016, 147, 137–152. [Google Scholar] [CrossRef]
- Li, X.; Wu, G.; Yang, Y.; Fu, S.; Liu, X.; Kang, H.; Yang, X.; Su, X.-C.; Shen, Y. Calmodulin dissociates the STIM1-Orai1 complex and STIM1 oligomers. Nat. Commun. 2017, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, R.; Augustynek, B.S.; Ercan-Herbst, E.; Kandasamy, P.; Seedorf, M.; Peinelt, C.; Hediger, M.A. Ca2+/Calmodulin Binding to STIM1 Hydrophobic Residues Facilitates Slow Ca2+-Dependent Inactivation of the Orai1 Channel. Cell. Physiol. Biochem. 2020, 54, 252–270. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Li, S.; Zheng, Y.; Yan, X.; Chen, M.; Wang, H.; Putney, J.W.; Luo, D. Retrograde regulation of STIM1-Orai1 interaction and store-operated Ca2+ entry by calsequestrin. Sci. Rep. 2015, 5, 11349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, L.; Li, S.; Xue, J.; Luo, D. Calsequestrin-1 Regulates Store-Operated Ca2+ Entry by Inhibiting STIM1 Aggregation. Cell. Physiol. Biochem. 2016, 38, 2183–2193. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.W.; Pan, Z.; Kim, E.K.; Lee, J.M.; Bhat, M.B.; Parness, J.; Kim, D.H.; Ma, J. A Retrograde Signal from Calsequestrin for the Regulation of Store-operated Ca2+Entry in Skeletal Muscle. J. Biol. Chem. 2002, 278, 3286–3292. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhou, H.; Yu, X.; Chen, L.; Zhang, H.; Ren, S.; Wu, Y.; Luo, D. Potential regulatory role of calsequestrin in platelet Ca2+ homeostasis and its association with platelet hyperactivity in diabetes mellitus. J. Thromb. Haemost. 2011, 10, 116–124. [Google Scholar] [CrossRef]
- Györke, I.; Hester, N.; Jones, L.R.; Györke, S. The Role of Calsequestrin, Triadin, and Junctin in Conferring Cardiac Ryanodine Receptor Responsiveness to Luminal Calcium. Biophys. J. 2004, 86, 2121–2128. [Google Scholar] [CrossRef]
- Li, Y.; James, S.J.; Wyllie, D.; Wynne, C.; Czibula, A.; Bukhari, A.; Pye, K.; Mustafah, S.M.B.; Fajka-Boja, R.; Szabo, E.; et al. TMEM203 is a binding partner and regulator of STING-mediated inflammatory signaling in macrophages. Proc. Natl. Acad. Sci. USA 2019, 116, 16479–16488. [Google Scholar] [CrossRef]
- Srikanth, S.; Woo, J.S.; Wu, B.; El-Sherbiny, Y.M.; Leung, J.; Chupradit, K.; Rice, L.; Seo, G.J.; Calmettes, G.; Ramakrishna, C.; et al. The Ca2+ sensor STIM1 regulates the type I interferon response by retaining the signaling adaptor STING at the endoplasmic reticulum. Nat. Immunol. 2019, 20, 152–162. [Google Scholar] [CrossRef]
- Zeiger, W.; Ito, D.; Swetlik, C.; Oh-Hora, M.; Villereal, M.L.; Thinakaran, G. Stanniocalcin 2 Is a Negative Modulator of Store-Operated Calcium Entry. Mol. Cell. Biol. 2011, 31, 3710–3722. [Google Scholar] [CrossRef] [PubMed]
- López, E.; Gómez-Gordo, L.; Cantonero, C.; Bermejo, N.; Pérez-Gómez, J.; Granados, M.P.; Salido, G.M.; Dionisio, J.A.R.; Liberal, P.C.R. Stanniocalcin 2 Regulates Non-capacitative Ca2+ Entry and Aggregation in Mouse Platelets. Front. Physiol. 2018, 9, 266. [Google Scholar] [CrossRef] [PubMed]
- Srivats, S.; Balasuriya, D.; Pasche, M.; Vistal, G.; Edwardson, J.M.; Taylor, C.W.; Murrell-Lagnado, R.D. Sigma1 receptors inhibit store-operated Ca2+ entry by attenuating coupling of STIM1 to Orai1. J. Cell Biol. 2016, 213, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Su, T.-P. Sigma-1 Receptor Chaperones at the ER-Mitochondrion Interface Regulate Ca2+ Signaling and Cell Survival. Cell 2007, 131, 596–610. [Google Scholar] [CrossRef] [PubMed]
- Gasparre, G.; Abate, C.; Carlucci, R.; Berardi, F.; Cassano, G. The σ(1) receptor agonist (+)-pentazocine increases store-operated Ca(2+) entry in MCF7σ(1) and SK-N-SH cell lines. Pharmacol. Rep. 2017, 69, 542–545. [Google Scholar] [CrossRef]
- Brailoiu, G.C.; Deliu, E.; Console-Bram, L.M.; Soboloff, J.; Abood, M.E.; Unterwald, E.M.; Brailoiu, E. Cocaine inhibits store-operated Ca2+ entry in brain microvascular endothelial cells: Critical role for sigma-1 receptors. Biochem. J. 2016, 473, 1–5. [Google Scholar] [CrossRef]
- Dörr, K.; Kilch, T.; Kappel, S.; AlAnsary, D.; Schwär, G.; Niemeyer, B.A.; Peinelt, C. Cell type–specific glycosylation of Orai1 modulates store-operated Ca2+entry. Sci. Signal. 2016, 9, ra25. [Google Scholar] [CrossRef]
- Kiwamoto, T.; Kawasaki, N.; Paulson, J.C.; Bochner, B.S. Siglec-8 as a drugable target to treat eosinophil and mast cell-associated conditions. Pharmacol. Ther. 2012, 135, 327–336. [Google Scholar] [CrossRef]
- Carreras-Sureda, A.; Cantero-Recasens, G.; Rubio-Moscardo, F.; Kiefer, K.; Peinelt, C.; Niemeyer, B.A.; Valverde, M.A.; Vicente, R. ORMDL3 modulates store-operated calcium entry and lymphocyte activation. Hum. Mol. Genet. 2013, 22, 519–530. [Google Scholar] [CrossRef]
- Hjelmqvist, L.; Tuson, M.; Marfany, G.; Herrero, E.; Balcells, S.; Gonzalez-Duarte, R. ORMDL proteins are a conserved new family of endoplasmic reticulum membrane proteins. Genome Biol. 2002, 3, research0027-1. [Google Scholar] [CrossRef]
- Catterall, W.A.; Perez-Reyes, E.; Snutch, T.P.; Striessnig, J. International Union of Pharmacology. XLVIII. Nomenclature and Structure-Function Relationships of Voltage-Gated Calcium Channels. Pharmacol. Rev. 2005, 57, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Caro, C.; Berrocal, M.; Lopez-Guerrero, A.M.; Alvarez-Barrientos, A.; Pozo-Guisado, E.; Gutierrez-Merino, C.; Mata, A.M.; Martin-Romero, F.J. STIM1 deficiency is linked to Alzheimer’s disease and triggers cell death in SH-SY5Y cells by upregulation of L-type voltage-operated Ca(2+) entry. J. Mol. Med. 2018, 96, 1061–1079. [Google Scholar] [CrossRef] [PubMed]
- Dionisio, N.; Smani, T.; Woodard, G.E.; Castellano, A.; Salido, G.M.; Rosado, J.A. Homer proteins mediate the interaction between STIM1 and Cav1.2 channels. Biochim. Biophys. Acta Bioenerg. 2015, 1853, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Kar, P.; Parekh, A.B. Distinct Spatial Ca2+ Signatures Selectively Activate Different NFAT Transcription Factor Isoforms. Mol. Cell 2015, 58, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.C.L.; Willoughby, D.; Ciruela, A.; Ayling, L.-J.; Pagano, M.; Wachten, S.; Tengholm, A.; Cooper, D.M.F. Capacitative Ca2+Entry via Orai1 and Stromal Interacting Molecule 1 (STIM1) Regulates Adenylyl Cyclase Type 8. Mol. Pharmacol. 2009, 75, 830–842. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Collado, J.; López, J.J.; Jardin, I.; Camello, P.J.; Falcon, D.; Regodon, S.; Salido, G.M.; Smani, T.; Rosado, J.A. Adenylyl Cyclase Type 8 Overexpression Impairs Phosphorylation-Dependent Orai1 Inactivation and Promotes Migration in MDA-MB-231 Breast Cancer Cells. Cancers 2019, 11, 1624. [Google Scholar] [CrossRef]
| Protein | Interaction-Site within STIM1/Orai | Function | References |
|---|---|---|---|
| CCT | Orai1157–167 | Promotes Orai1 endocytosis | Hodeify et al., 2018 [23] |
| SNAP-25 | not reported | Involved in the increase in cell surface expression of Orai1 upon activation | Woodard et al., 2008 [25] |
| SPCA2 | Orai1 | Plasma membrane expression of Orai1, STIM1-independent activation, regulation of SOCE (SPCA2C) | Cross et al., 2013 [32] Smaardijk et al., 2017 [33] Peretti et al., 2019 [34] Feng et al., 2020 [35,36] |
| Amnionless | Orai1 | Endocytosis of Orai1 in the proximal tubulus; critical for albumin re-uptake | Zeng et al., 2017 [37] |
| RHBDL2 | Orai1 | Surveillance of Orai1; prevents inappropriate activation and regulates STIM1-Orai1 stoichiometry | Grieve et al., 2020 [38] |
| Calnexin | STIM1 | Plasma membrane expression | Saitoh et al., 2011 [39] |
| EB1 | STIM1642–645 | Microtubule + end tracking, ER-remodelling, STIM1 motion upon rest | Pozo-Guisado et al., 2013 [40] Sampieri et al., 2009 [20] |
| APC | STIM1650–685 | STIM1 clustering at ER-PM junctions | Asanov et al., 2013 [41] Munemitsu et al., 1994 [42] |
| UBQLN1 | Orai1 | Promotes Orai1 ubiquitinylation, correlates with a downregulation in CRAC channel activity | Lee et al., 2013 [43] |
| RTN4 | not identified | ER tubulation | Jozsef et al., 2014 [44] |
| JPH1/2 | not identified | ER-PM junctions | Hirata et al., 2006 [45] Woo et al., 2012 [46] |
| Homer 1a | STIM1 | Stabilization of STIM1-Orai1 complex under conditions of rising intracellular Ca2+ levels | Jardin et al., 2012 [47] |
| TM203 | STIM1 | Regulates interferon signalling (STING-pathway) | Shambharkar, et al., 2015 [48] |
| CDK1 | STIM1 | Phosphorylation of STIM1, suppression of SOCE during mitosis | Smyth et al., 2009 [49] |
| PMCA | STIM1 | Suppresses PMCA in its activity upon CRAC channel activation, contributes to the establishment of Ca2+ microdomains | Ritchie et al., 2012 [50] |
| VGCC | STIM1 | Inhibits opening of CaV1.2 and leads to withdrawal of CaV1.2 from the plasma membrane | Park et al., 2010 [51] |
| POST | STIM1/Orai1 | negative modulator of energy-consuming transmembrane Ca2+ transport | Krapivinsky et al. (2011) [52] |
| AKAP79 | Orai1 | Formation of a signalling complex that sequesters downstream acting proteins close to Orai1-formed pores | Kar et al., 2014 [53] |
| ADCY8 | Orai1 N-terminus | Increases in ADCY8 activity upon SOCE at cellular subdomains | Willoughby et al., 2012 [54] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berlansky, S.; Humer, C.; Sallinger, M.; Frischauf, I. More Than Just Simple Interaction between STIM and Orai Proteins: CRAC Channel Function Enabled by a Network of Interactions with Regulatory Proteins. Int. J. Mol. Sci. 2021, 22, 471. https://doi.org/10.3390/ijms22010471
Berlansky S, Humer C, Sallinger M, Frischauf I. More Than Just Simple Interaction between STIM and Orai Proteins: CRAC Channel Function Enabled by a Network of Interactions with Regulatory Proteins. International Journal of Molecular Sciences. 2021; 22(1):471. https://doi.org/10.3390/ijms22010471
Chicago/Turabian StyleBerlansky, Sascha, Christina Humer, Matthias Sallinger, and Irene Frischauf. 2021. "More Than Just Simple Interaction between STIM and Orai Proteins: CRAC Channel Function Enabled by a Network of Interactions with Regulatory Proteins" International Journal of Molecular Sciences 22, no. 1: 471. https://doi.org/10.3390/ijms22010471
APA StyleBerlansky, S., Humer, C., Sallinger, M., & Frischauf, I. (2021). More Than Just Simple Interaction between STIM and Orai Proteins: CRAC Channel Function Enabled by a Network of Interactions with Regulatory Proteins. International Journal of Molecular Sciences, 22(1), 471. https://doi.org/10.3390/ijms22010471