Butyrate and Propionate Restore the Cytokine and House Dust Mite Compromised Barrier Function of Human Bronchial Airway Epithelial Cells
Abstract
1. Introduction
2. Results
2.1. Assessment of Cell Damage
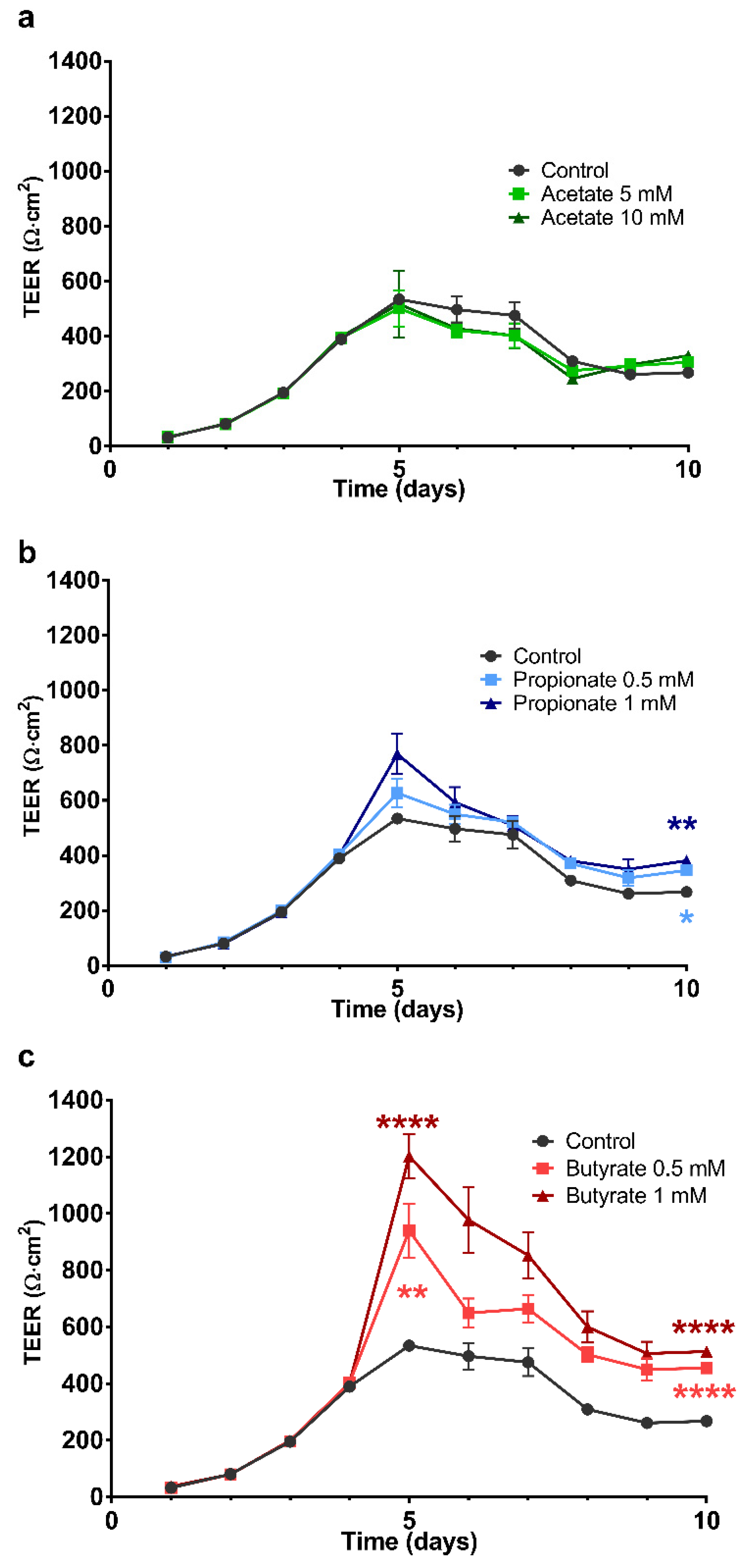
2.2. The Effect of SCFA on the Development of the Barrier Integrity of 16HBE Cells
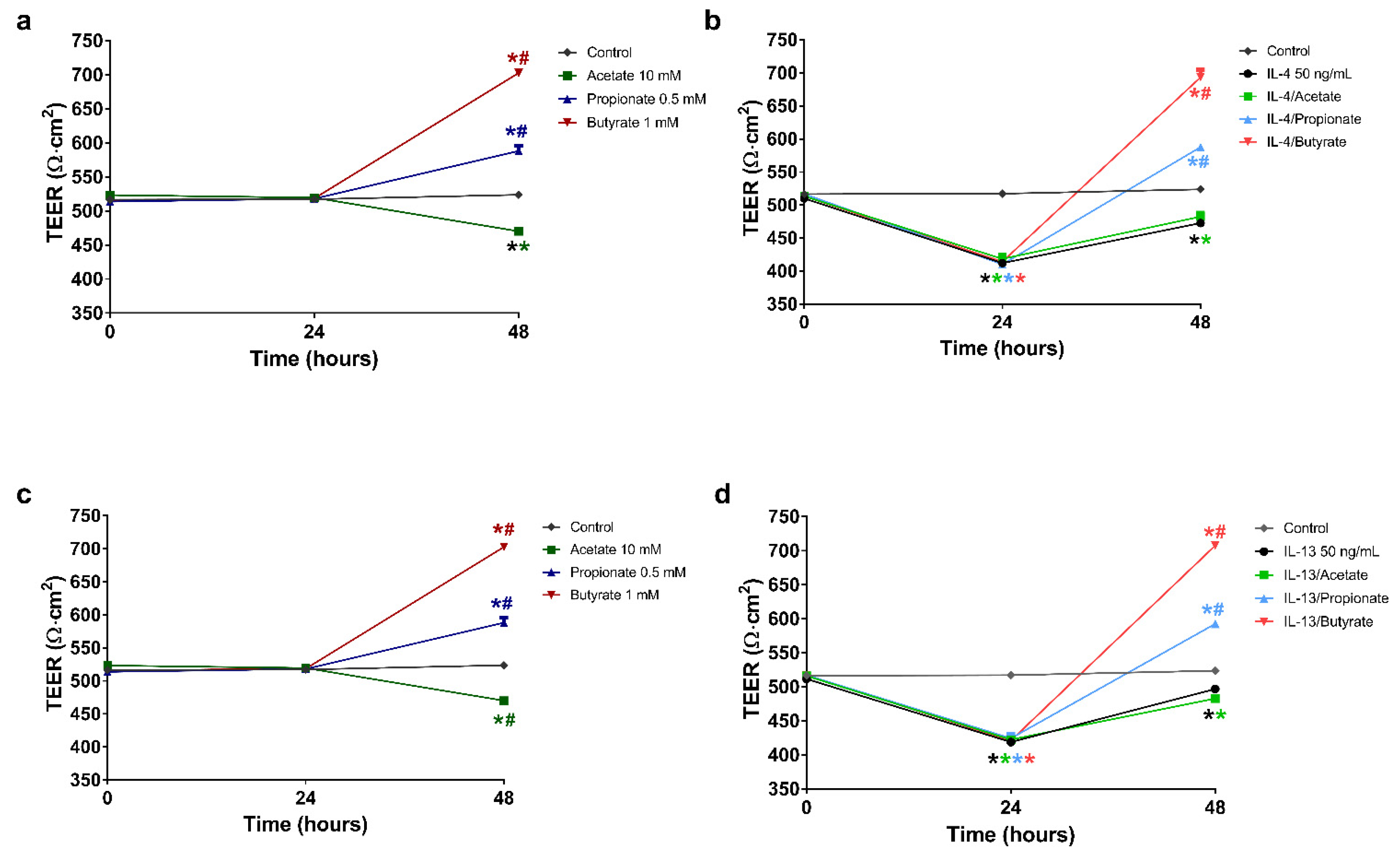
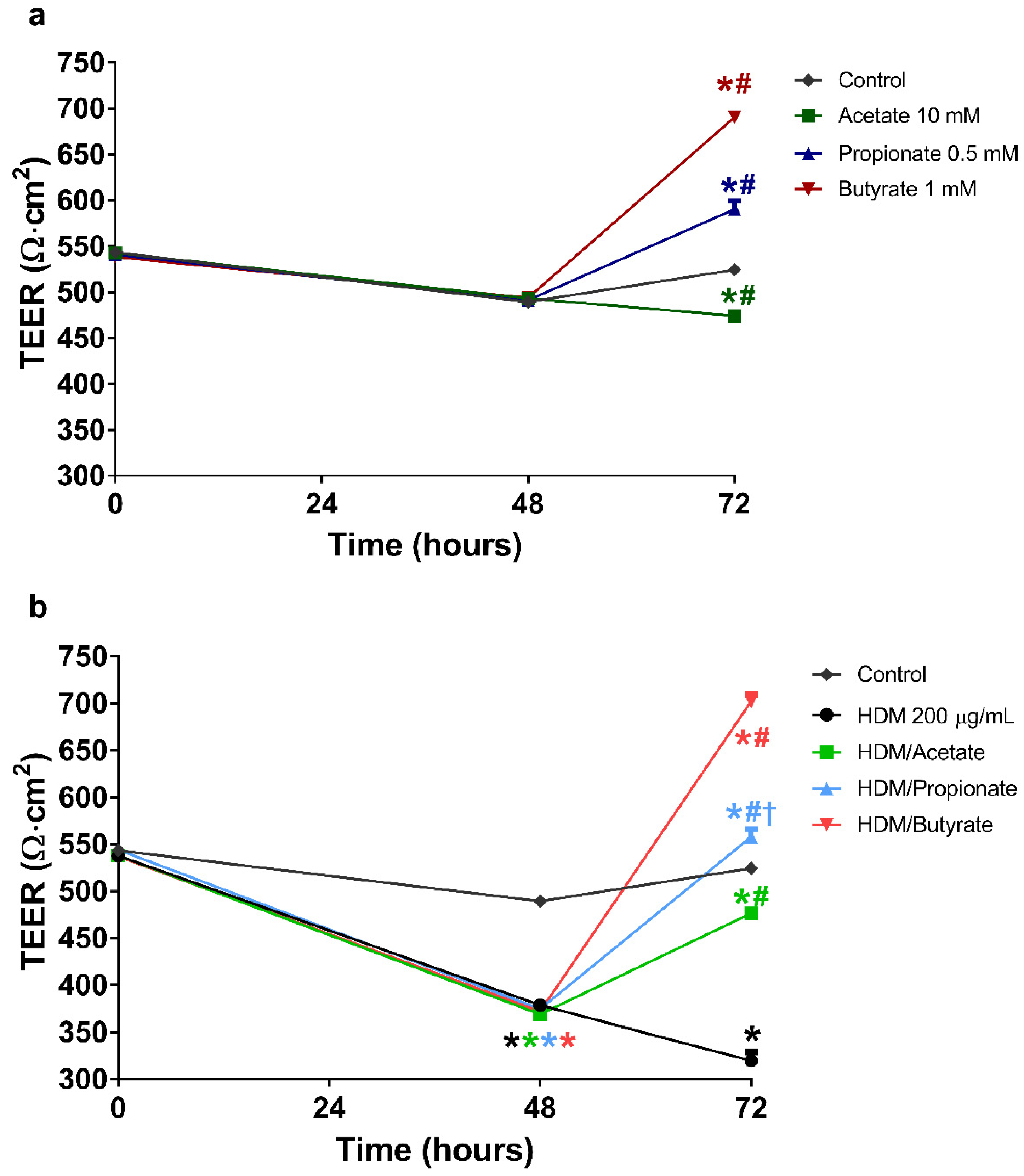
2.3. Butyrate and Propionate Restored the Barrier in IL-4 Compromised 16HBE Cells
2.4. Butyrate and Propionate Restored the Barrier in IL-13 Compromised 16HBE Cells
2.5. Butyrate and Propionate Restored the Barrier in HDM Compromised 16HBE Cells
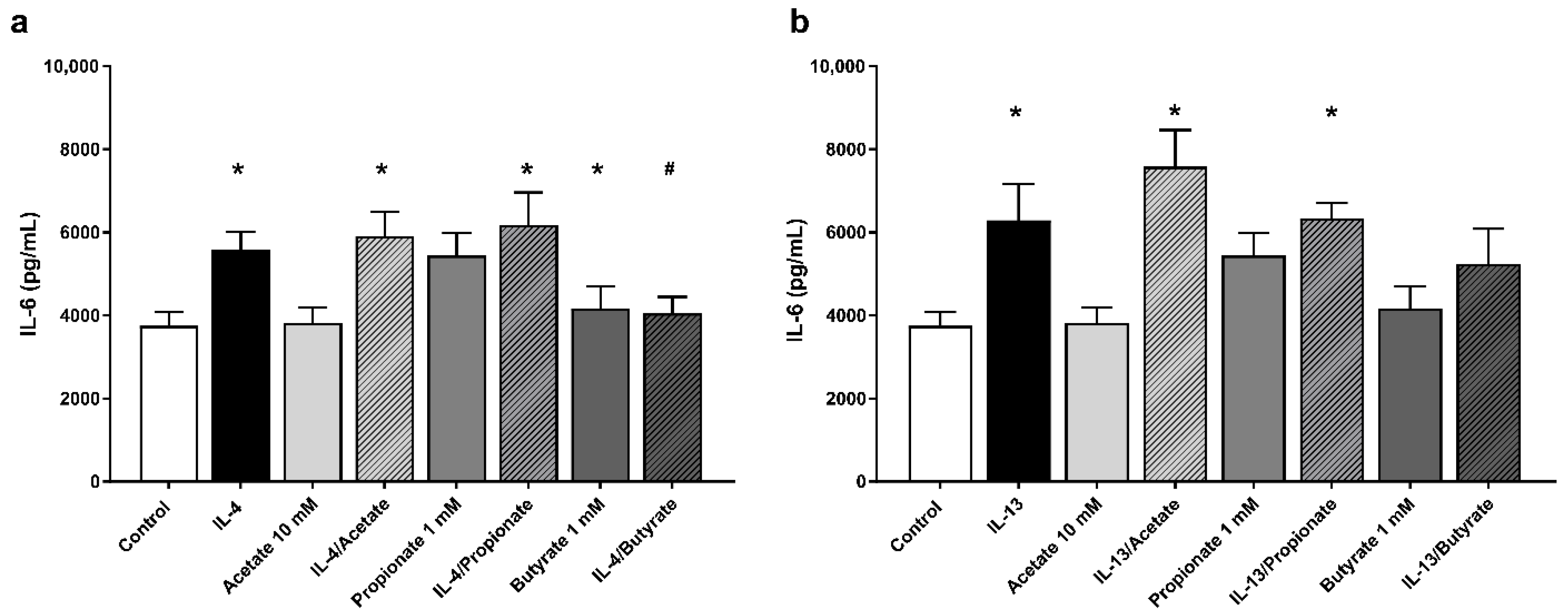
2.6. Butyrate Inhibited IL-6 Production by IL-4 Stimulated 16HBE Cells
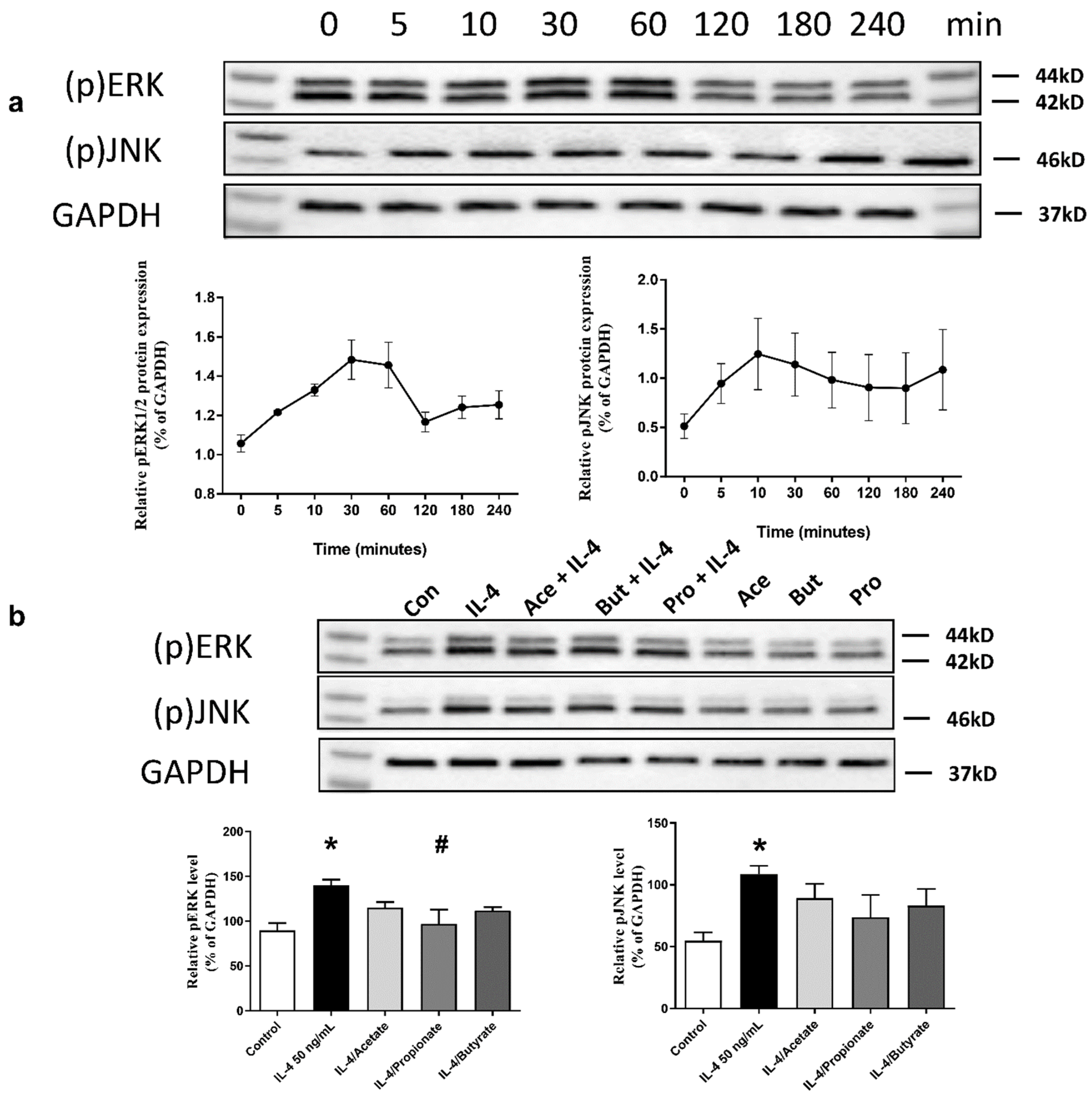
2.7. SCFA Inhibited IL-4-Induced Activation of ERK1/2 and JNK Signalling Pathways
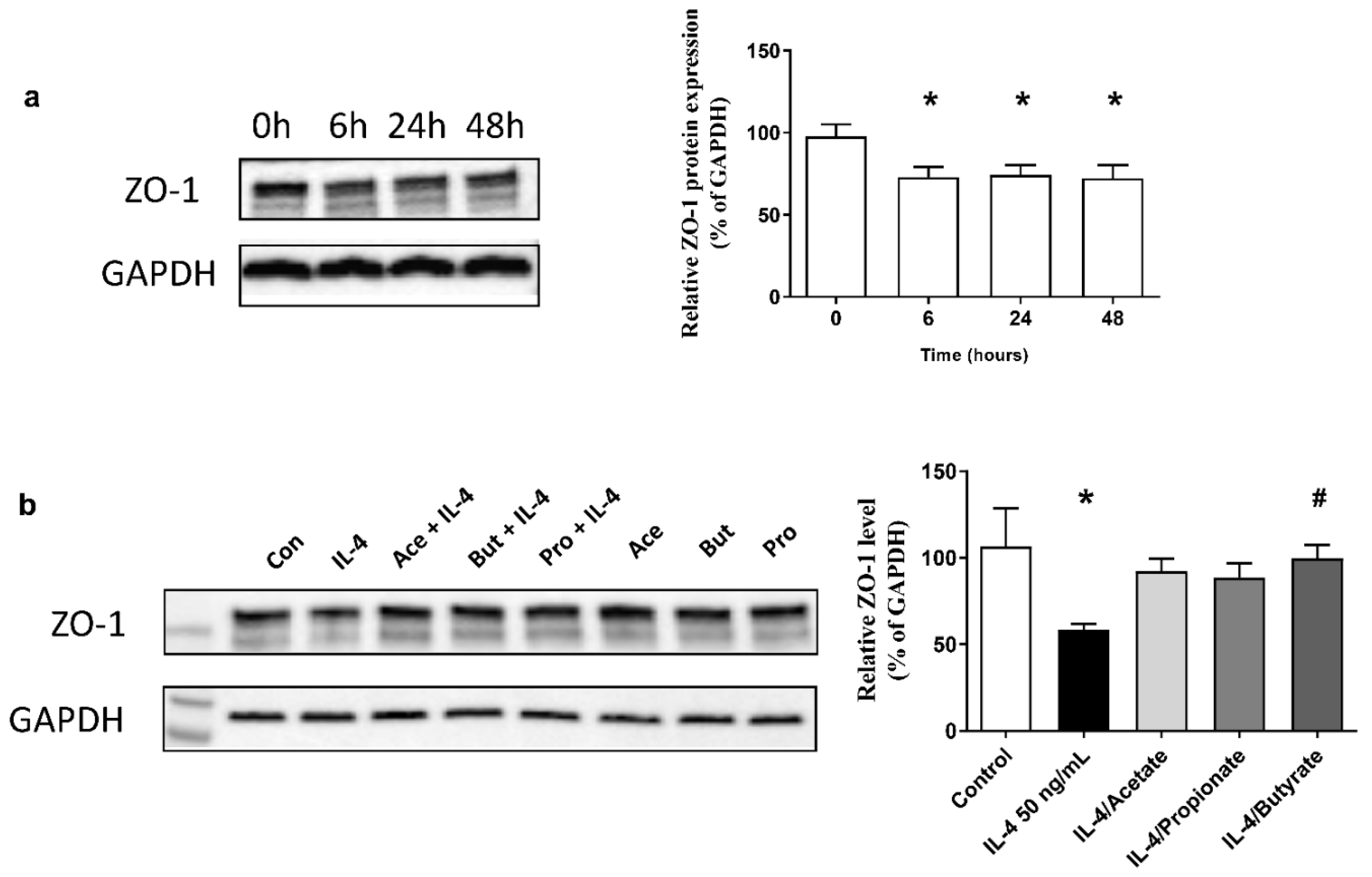
2.8. SCFA Increased ZO-1 Expression in IL-4-Stimulated 16HBE
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cells and Culture Conditions
4.3. Trans-Epithelial Electrical Resistance (TEER)
4.4. The Effect of SCFA on the Development of the Barrier Integrity of 16HBE Cells
4.5. The Restorative Effects of SCFA after Barrier Disruption
4.6. Measurement of IL-6 and IL-8 Cytokine Levels
4.7. Western Blot
4.8. Cytotoxicity
4.9. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| DMEM | Dulbercco’s Modified Eagle Medium |
| ECL | enhanced chemiluminescence |
| ERK1/2 | extracellular signal-regulated protein kinases 1/2 |
| FBS | fetal bovine serum |
| HDAC | histone deacetylases |
| HDM | house dust mite |
| JNK | c-Jun N-terminal kinases |
| LDH | lactate dehydrogenase |
| MAPK | mitogen-activated protein kinases |
| MEM | minimal essential medium |
| SCFA | Short-chain fatty acids |
| TEER | trans-epithelial electrical resistance |
| TJs | tight junctions |
| ZO-1 | zonula occludens-1 |
References and Note
- Global Initiative for Asthma Global Strategy for Asthma Management and prevention. 2018.
- Fahy, J.V. Type 2 inflammation in asthma—Present in most, absent in many. Nat. Rev. Immunol. 2015, 15, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Hammad, H.; Lambrecht, B.N. Barrier Epithelial Cells and the Control of Type 2 Immunity. Immunity 2015, 43, 29–40. [Google Scholar] [CrossRef]
- Loxham, M.; Davies, D.E.; Blume, C. Epithelial function and dysfunction in asthma. Clin. Exp. Allergy 2014, 44, 1299–1313. [Google Scholar] [CrossRef] [PubMed]
- Georas, S.N.; Rezaee, F. Epithelial barrier function: At the frontline of asthma immunology and allergic airway inflammation. J. Allergy Clin. Immunol. 2014, 134, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, S.; Comstock, A.T.; Sajjan, U.S. Barrier function of airway tract epithelium. Tissue Barriers 2013, 1, e24997. [Google Scholar] [CrossRef]
- Saatian, B.; Rezaee, F.; Desando, S.; Emo, J.; Chapman, T.; Knowlden, S.; Georas, S.N. Interleukin-4 and interleukin-13 cause barrier dysfunction in human airway epithelial cells. Tissue Barriers 2013, 1, e24333. [Google Scholar] [CrossRef]
- Davies, D.E. Epithelial barrier function and immunity in asthma. Ann. Am. Thorac. Soc. 2014, 11 (Suppl. 5), S244–S251. [Google Scholar] [CrossRef]
- Colgan, S.P.; Resnick, M.B.; Parkos, C.A.; Delp-Archer, C.; McGuirk, D.; Bacarra, A.E.; Weller, P.F.; Madara, J.L. IL-4 directly modulates function of a model human intestinal epithelium. J. Immunol. 1994, 153, 2122–2129. [Google Scholar]
- Shin, K.; Fogg, V.C.; Margolis, B. Tight junctions and cell polarity. Annu. Rev. Cell Dev. Biol. 2006, 22, 207–235. [Google Scholar] [CrossRef]
- Matter, K.; Aijaz, S.; Tsapara, A.; Balda, M.S. Mammalian tight junctions in the regulation of epithelial differentiation and proliferation. Curr. Opin. Cell Biol. 2005, 17, 453–458. [Google Scholar] [CrossRef]
- Xiao, C.; Puddicombe, S.M.; Field, S.; Haywood, J.; Broughton-Head, V.; Puxeddu, I.; Haitchi, H.M.; Vernon-Wilson, E.; Sammut, D.; Bedke, N.; et al. Defective epithelial barrier function in asthma. J. Allergy Clin. Immunol. 2011, 128, 549–556.e12. [Google Scholar] [CrossRef]
- Gandhi, V.D.; Vliagoftis, H. Airway epithelium interactions with aeroallergens: Role of secreted cytokines and chemokines in innate immunity. Front. Immunol. 2015, 6, 147. [Google Scholar] [CrossRef] [PubMed]
- de Boer, W.I.; Sharma, H.S.; Baelemans, S.M.I.; Hoogsteden, H.C.; Lambrecht, B.N.; Braunstahl, G.J. Altered expression of epithelial junctional proteins in atopic asthma: Possible role in inflammation. Can. J. Physiol. Pharm. 2008, 86, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Mariscal, L.; Tapia, R.; Chamorro, D. Crosstalk of tight junction components with signaling pathways. Biochim. Biophys. Acta 2008, 1778, 729–756. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.H.; Englyst, H.N. Fermentation in the human large intestine and the available substrates. Am. J. Clin. Nutr. 1987, 45, 1243–1255. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.H.; Macfarlane, G.T. The control and consequences of bacterial fermentation in the human colon. J. Appl. Bacteriol. 1991, 70, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Richards, L.B.; Li, M.; van Esch, B.C.A.M.; Garssen, J.; Folkerts, G. The effects of short-chain fatty acids on the cardiovascular system. PharmaNutrition 2016, 4, 68–111. [Google Scholar] [CrossRef]
- Yan, H.; Ajuwon, K.M. Butyrate modifies intestinal barrier function in IPEC-J2 cells through a selective upregulation of tight junction proteins and activation of the Akt signaling pathway. PLoS ONE 2017, 12, e0179586. [Google Scholar] [CrossRef]
- Hooper, L.V.; Gordon, J.I. Commensal host-bacterial relationships in the gut. Science 2001, 292, 1115–1118. [Google Scholar] [CrossRef]
- Elamin, E.E.; Masclee, A.A.; Dekker, J.; Pieters, H.J.; Jonkers, D.M. Short-chain fatty acids activate AMP-activated protein kinase and ameliorate ethanol-induced intestinal barrier dysfunction in Caco-2 cell monolayers. J. Nutr. 2013, 143, 1872–1881. [Google Scholar] [CrossRef]
- Kamitani, H.; Ikawa, H.; Hsi, L.C.; Watanabe, T.; DuBois, R.N.; Eling, T.E. Regulation of 12-lipoxygenase in rat intestinal epithelial cells during differentiation and apoptosis induced by sodium butyrate. Arch. Biochem. Biophys. 1999, 368, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Macia, L.; Tan, J.; Vieira, A.T.; Leach, K.; Stanley, D.; Luong, S.; Maruya, M.; Ian McKenzie, C.; Hijikata, A.; Wong, C.; et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat. Commun. 2015, 6, 6734. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.B.; Wang, P.Y.; Wang, X.; Wan, Y.L.; Liu, Y.C. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein Claudin-1 transcription. Dig. Dis. Sci. 2012, 57, 3126–3135. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Lu, L.; Huang, L.; Wen, Q.; Xie, J.; Jin, W.; Li, H.; Jiang, L. The effect of neonatal maternal separation on short-chain fatty acids and airway inflammation in adult asthma mice. Allergol. Immunopathol. (Madr.) 2019, 47, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Cait, A.; Hughes, M.R.; Antignano, F.; Cait, J.; Dimitriu, P.A.; Maas, K.R.; Reynolds, L.A.; Hacker, L.; Mohr, J.; Finlay, B.B.; et al. Microbiome-driven allergic lung inflammation is ameliorated by short-chain fatty acids. Mucosal Immunol. 2018, 11, 785–795. [Google Scholar] [CrossRef]
- Roscioli, E.; Hamon, R.; Lester, S.E.; Jersmann, H.P.A.; Reynolds, P.N.; Hodge, S. Airway epithelial cells exposed to wildfire smoke extract exhibit dysregulated autophagy and barrier dysfunction consistent with COPD. Respir. Res. 2018, 19, 234. [Google Scholar] [CrossRef] [PubMed]
- Gurram, R.K.; Zhu, J. Orchestration between ILC2s and Th2 cells in shaping type 2 immune responses. Cell Mol. Immunol. 2019, 16, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Borger, P.; Koëter, G.H.; Timmerman, J.A.; Vellenga, E.; Tomee, J.F.; Kauffman, H.F. Proteases from Aspergillus fumigatus induce interleukin (IL)-6 and IL-8 production in airway epithelial cell lines by transcriptional mechanisms. J. Infect. Dis. 1999, 180, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, H.F.; Tomee, J.F.; van de Riet, M.A.; Timmerman, A.J.; Borger, P. Protease-dependent activation of epithelial cells by fungal allergens leads to morphologic changes and cytokine production. J. Allergy Clin. Immunol. 2000, 105, 1185–1193. [Google Scholar] [CrossRef]
- King, C.; Brennan, S.; Thompson, P.J.; Stewart, G.A. Dust mite proteolytic allergens induce cytokine release from cultured airway epithelium. J. Immunol. Baltim. Md. 1950 1998, 161, 3645–3651. [Google Scholar]
- Tomee, J.F.; van Weissenbruch, R.; de Monchy, J.G.; Kauffman, H.F. Interactions between inhalant allergen extracts and airway epithelial cells: Effect on cytokine production and cell detachment. J. Allergy Clin. Immunol. 1998, 102, 75–85. [Google Scholar] [CrossRef]
- Chin, A.C.; Parkos, C.A. Pathobiology of neutrophil transepithelial migration: Implications in mediating epithelial injury. Annu. Rev. Pathol. 2007, 2, 111–143. [Google Scholar] [CrossRef]
- Jevnikar, Z.; Östling, J.; Ax, E.; Calvén, J.; Thörn, K.; Israelsson, E.; Öberg, L.; Singhania, A.; Lau, L.C.K.; Wilson, S.J.; et al. Epithelial IL-6 trans-signaling defines a new asthma phenotype with increased airway inflammation. J. Allergy Clin. Immunol. 2019, 143, 577–590. [Google Scholar] [CrossRef] [PubMed]
- Bengalli, R.; Longhin, E.; Marchetti, S.; Proverbio, M.C.; Battaglia, C.; Camatini, M. The role of IL-6 released from pulmonary epithelial cells in diesel UFP-induced endothelial activation. Environ. Pollut. Barking Essex 1987 2017, 231, 1314–1321. [Google Scholar] [CrossRef] [PubMed]
- Biancone, L.; Monteleone, I.; Del Vecchio Blanco, G.; Vavassori, P.; Pallone, F. Resident bacterial flora and immune system. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2002, 34 (Suppl. 2), S37–S43. [Google Scholar] [CrossRef]
- Floch, M.H.; Hong-Curtiss, J. Probiotics and Functional Foods in Gastrointestinal Disorders. Curr. Treat Options Gastroenterol. 2002, 5, 311–321. [Google Scholar] [CrossRef]
- Post, S.; Nawijn, M.C.; Hackett, T.L.; Baranowska, M.; Gras, R.; van Oosterhout, A.J.M.; Heijink, I.H. The composition of house dust mite is critical for mucosal barrier dysfunction and allergic sensitisation. Thorax 2012, 67, 488–495. [Google Scholar] [CrossRef]
- Rutting, S.; Xenaki, D.; Malouf, M.; Horvat, J.C.; Wood, L.G.; Hansbro, P.M.; Oliver, B.G. Short-chain fatty acids increase TNFα-induced inflammation in primary human lung mesenchymal cells through the activation of p38 MAPK. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2018, 316, L157–L174. [Google Scholar] [CrossRef]
- Rutting, S.; Zakarya, R.; Bozier, J.; Xenaki, D.; Horvat, J.C.; Wood, L.G.; Hansbro, P.M.; Oliver, B.G. Dietary Fatty Acids Amplify Inflammatory Responses to Infection through p38 MAPK Signaling. Am. J. Respir. Cell Mol. Biol. 2019, 60, 554–568. [Google Scholar] [CrossRef]
- Petecchia, L.; Sabatini, F.; Usai, C.; Caci, E.; Varesio, L.; Rossi, G.A. Cytokines induce tight junction disassembly in airway cells via an EGFR-dependent MAPK/ERK1/2-pathway. Lab. Investig. 2012, 92, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Kang, S.G.; Park, J.H.; Yanagisawa, M.; Kim, C.H. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology 2013, 145, 396–406.e1-10. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, S.; Wang, J.; Luo, C.; Zhao, S.; Zheng, N. Modulation of Intestinal Epithelial Permeability in Differentiated Caco-2 Cells Exposed to Aflatoxin M1 and Ochratoxin A Individually or Collectively. Toxins 2017, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.; Brocklebank, D.; Ram, F. Inhaler devices for the treatment of asthma and chronic obstructive airways disease (COPD). Qual. Saf. Health Care 2002, 11, 376–382. [Google Scholar] [CrossRef]
- Mash, B.R.; Bheekie, A.; Jones, P. Inhaled versus oral steroids for adults with chronic asthma. Cochrane Database Syst. Rev. 2001. [Google Scholar] [CrossRef]
- Labiris, N.R.; Dolovich, M.B. Pulmonary drug delivery. Part I: Physiological factors affecting therapeutic effectiveness of aerosolized medications. Br. J. Clin. Pharm. 2003, 56, 588–599. [Google Scholar] [CrossRef]
- Cozens, A.L.; Yezzi, M.J.; Yamaya, M.; Steiger, D.; Wagner, J.A.; Garber, S.S.; Chin, L.; Simon, E.M.; Cutting, G.R.; Gardner, P.; et al. A transformed human epithelial cell line that retains tight junctions post crisis. In Vitro Cell. Dev. Biol. 1992, 28a, 735–744. [Google Scholar] [CrossRef]
- Mortaz, E.; Henricks, P.A.; Kraneveld, A.D.; Givi, M.E.; Garssen, J.; Folkerts, G. Cigarette smoke induces the release of CXCL-8 from human bronchial epithelial cells via TLRs and induction of the inflammasome. Biochim. Biophys. Acta 2011, 1812, 1104–1110. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Richards, L.B.; Li, M.; Folkerts, G.; Henricks, P.A.J.; Garssen, J.; van Esch, B.C.A.M. Butyrate and Propionate Restore the Cytokine and House Dust Mite Compromised Barrier Function of Human Bronchial Airway Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 65. https://doi.org/10.3390/ijms22010065
Richards LB, Li M, Folkerts G, Henricks PAJ, Garssen J, van Esch BCAM. Butyrate and Propionate Restore the Cytokine and House Dust Mite Compromised Barrier Function of Human Bronchial Airway Epithelial Cells. International Journal of Molecular Sciences. 2021; 22(1):65. https://doi.org/10.3390/ijms22010065
Chicago/Turabian StyleRichards, Levi B., Meng Li, Gert Folkerts, Paul A.J. Henricks, Johan Garssen, and Betty C.A.M. van Esch. 2021. "Butyrate and Propionate Restore the Cytokine and House Dust Mite Compromised Barrier Function of Human Bronchial Airway Epithelial Cells" International Journal of Molecular Sciences 22, no. 1: 65. https://doi.org/10.3390/ijms22010065
APA StyleRichards, L. B., Li, M., Folkerts, G., Henricks, P. A. J., Garssen, J., & van Esch, B. C. A. M. (2021). Butyrate and Propionate Restore the Cytokine and House Dust Mite Compromised Barrier Function of Human Bronchial Airway Epithelial Cells. International Journal of Molecular Sciences, 22(1), 65. https://doi.org/10.3390/ijms22010065




