An Eco-Friendly Approach to the Control of Pathogenic Microbes and Anopheles stephensi Malarial Vector Using Magnesium Oxide Nanoparticles (Mg-NPs) Fabricated by Penicillium chrysogenum
Abstract
:1. Introduction
2. Results and Discussion
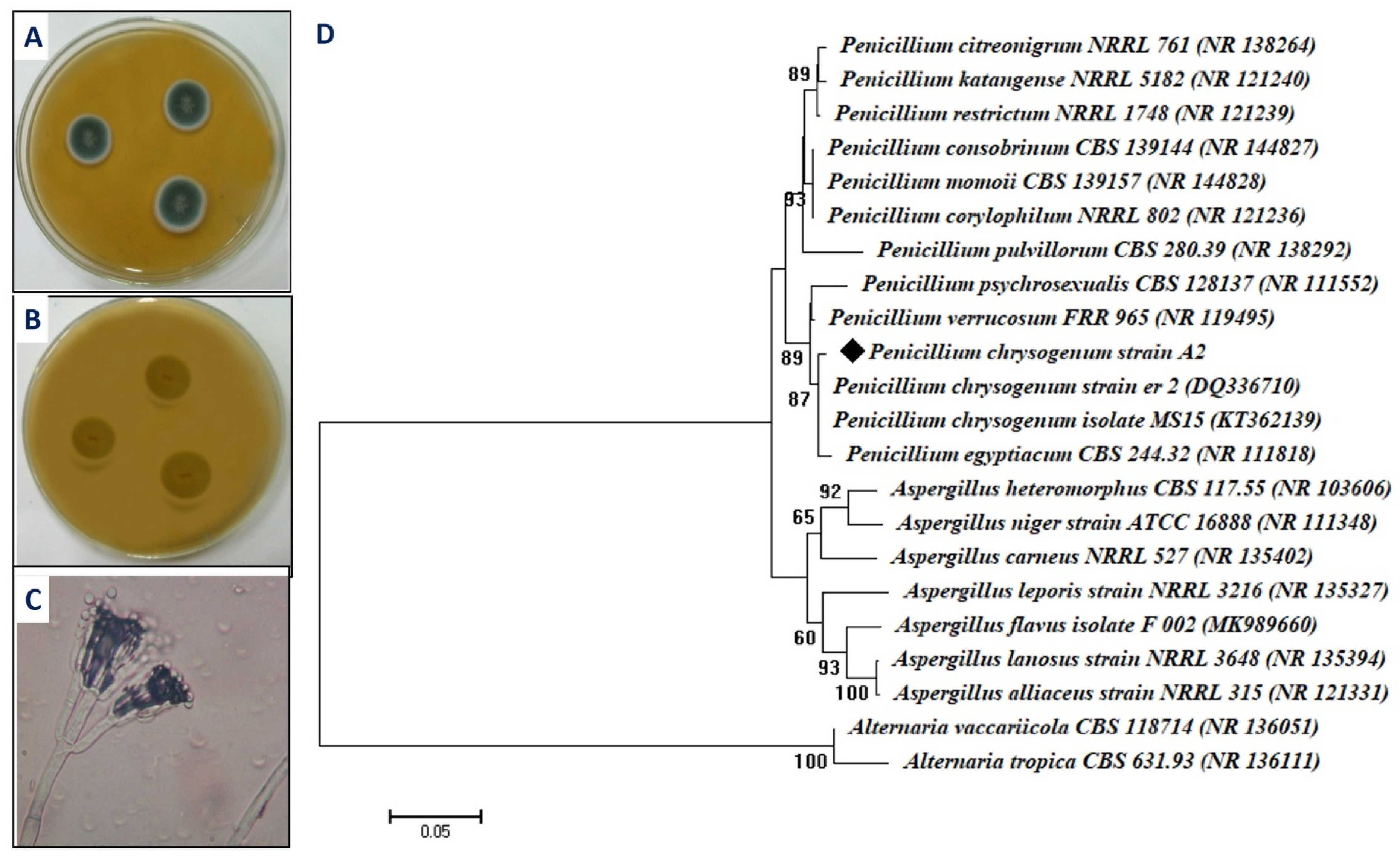
2.1. Isolation and Identification of the Fungal Isolate
2.2. Green Synthesis of MgO-NPs
2.3. Characterizations of Biosynthesized MgO-NPs
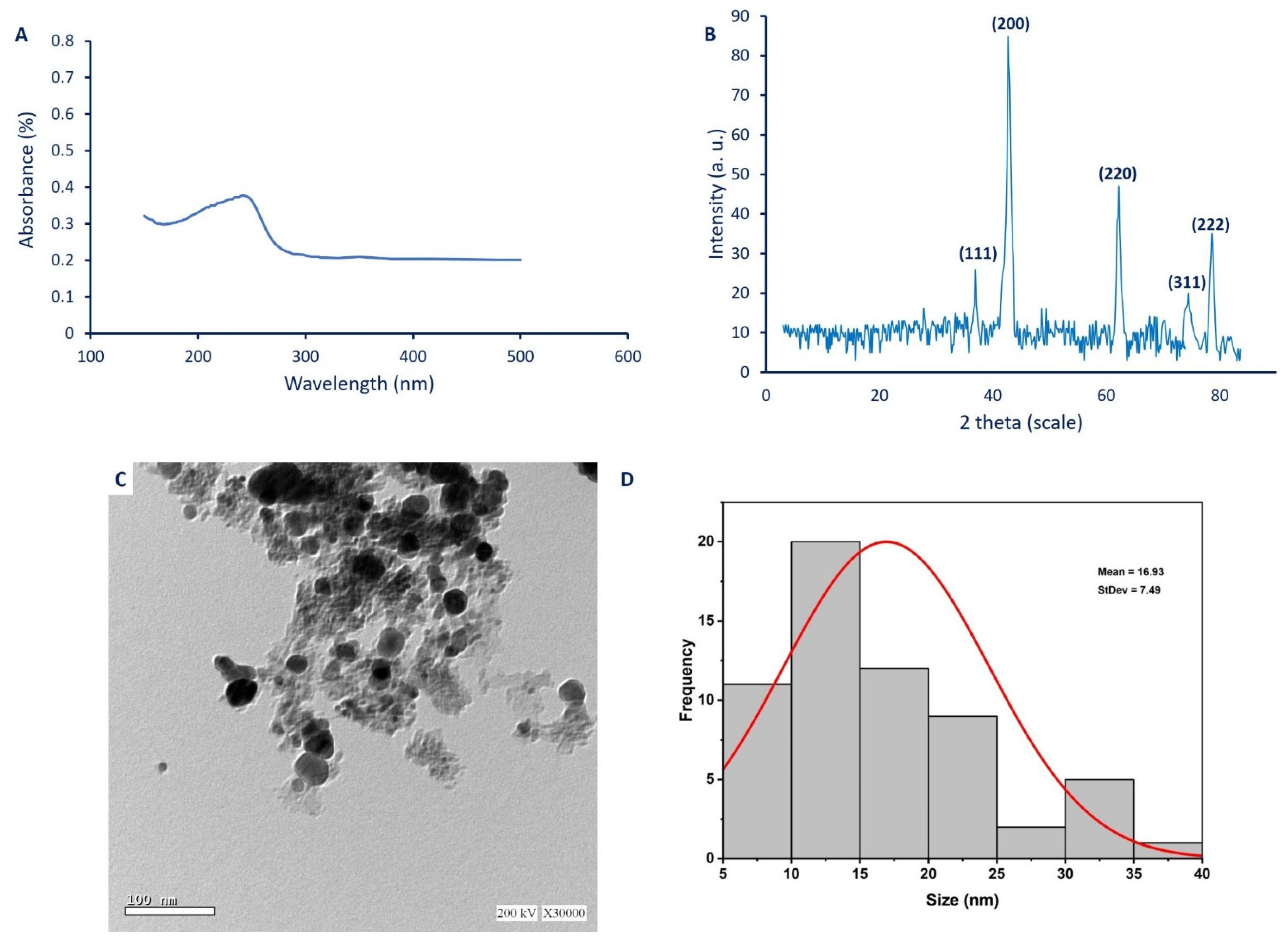
2.3.1. UV-Vis Spectroscopy Analysis
2.3.2. X-Ray Diffraction (XRD)
2.3.3. Transmission Electron Microscopy (TEM)
2.3.4. Dynamic Light Scattering (DLS) Analysis
2.3.5. Energy Dispersive X-ray (EDX) Analysis
2.3.6. Fourier Transform Infrared (FT-IR) Spectroscopy
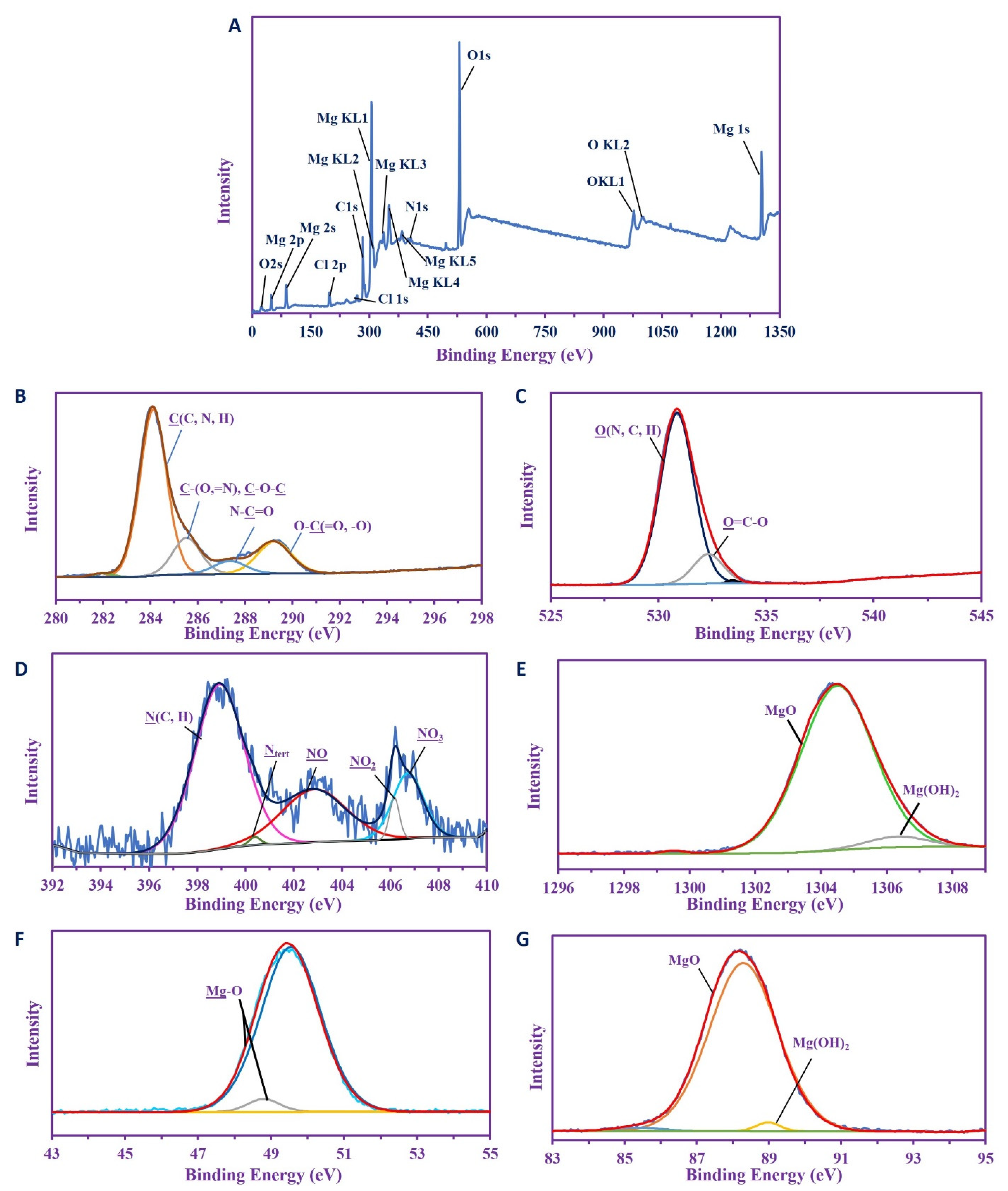
2.3.7. X-ray Photoelectron Spectroscopy (XPS) Analysis
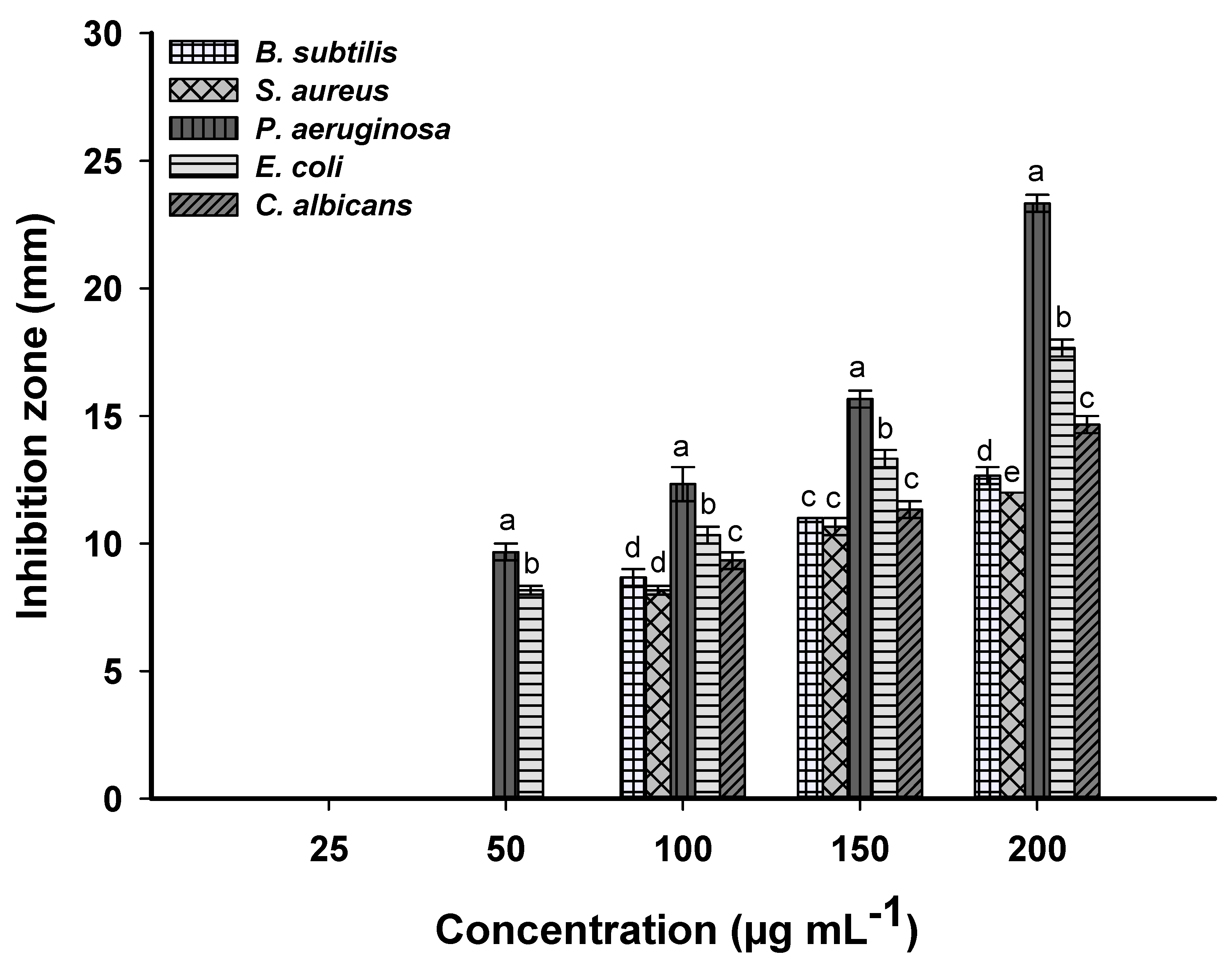
2.4. Antimicrobial Activity
2.5. Larvicidal/Pupicidal Bioassay
2.6. Repellent Activity
3. Materials and Methods
3.1. Chemicals Used
3.2. Isolation and Identification of the Fungal Strain
3.3. Green Synthesis of MgO-NPs
3.4. Characterization of Biosynthesized MgO-NPs
3.4.1. UV-Vis Spectroscopy
3.4.2. X-ray Diffraction (XRD)
3.4.3. Transmission Electron Microscopy (TEM)
3.4.4. Dynamic Light Scattering (DLS)
3.4.5. Energy Dispersive X-ray (EDX) Analysis
3.4.6. Fourier Transform Infrared (FT-IR)
3.4.7. X-Ray Photoelectron Spectroscopy (XPS)
3.5. Antimicrobial Activity
3.6. Mosquitocidal Assay
3.6.1. Rearing of Anopheles stephensi
3.6.2. Larvicidal/Pupicidal Assay
3.6.3. Repellent Activity
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rani, P.; Kaur, G.; Rao, K.V.; Singh, J.; Rawat, M. Impact of Green Synthesized Metal Oxide Nanoparticles on Seed Germination and Seedling Growth of Vigna radiata (Mung Bean) and Cajanus cajan (Red Gram). J. Inorg. Organomet. Polym. Mater. 2020, 30, 4053–4062. [Google Scholar] [CrossRef]
- Fouda, A.; Abdel-Maksoud, G.; Abdel-Rahman, M.A.; Salem, S.S.; Hassan, S.E.-D.; El-Sadany, M.A.-H. Eco-friendly approach utilizing green synthesized nanoparticles for paper conservation against microbes involved in biodeterioration of archaeological manuscript. Int. Biodeterior. Biodegrad. 2019, 142, 160–169. [Google Scholar] [CrossRef]
- Pham, D.T.N.; Khan, F.; Phan, T.T.V.; Park, S.-K.; Manivasagan, P.; Oh, J.; Kim, Y.-M. Biofilm inhibition, modulation of virulence and motility properties by FeOOH nanoparticle in Pseudomonas aeruginosa. Braz. J. Microbiol. 2019, 50, 791–805. [Google Scholar] [CrossRef] [PubMed]
- Raliya, R.; Tarafdar, J.; Choudhary, K.K.; Mal, P.; Raturi, A.; Gautam, R.; Singh, S. Synthesis of MgO Nanoparticles Using Aspergillus Tubingensis TFR-3. J. Bionanosci. 2014, 8. [Google Scholar] [CrossRef]
- Salem, S.S.; Fouda, A. Green Synthesis of Metallic Nanoparticles and Their Prospective Biotechnological Applications: An Overview. Biol. Trace Elem. Res. 2021, 199, 344–370. [Google Scholar] [CrossRef]
- Fatimah, I. Biosynthesis and characterization of ZnO nanoparticles using rice bran extract as low-cost templating agent. J. Eng. Sci. Technol. 2018, 13, 409–420. [Google Scholar]
- Eid, A.M.; Fouda, A.; Abdel-Rahman, M.A.; Salem, S.S.; Elsaied, A.; Oelmüller, R.; Hijri, M.; Bhowmik, A.; Elkelish, A.; Hassan, S.E.-D. Harnessing Bacterial Endophytes for Promotion of Plant Growth and Biotechnological Applications: An Overview. Plants 2021, 10, 935. [Google Scholar] [CrossRef]
- El-Belely, E.F.; Farag, M.M.S.; Said, H.A.; Amin, A.S.; Azab, E.; Gobouri, A.A.; Fouda, A. Green Synthesis of Zinc Oxide Nanoparticles (ZnO-NPs) Using Arthrospira platensis (Class: Cyanophyceae) and Evaluation of their Biomedical Activities. Nanomaterials 2021, 11, 95. [Google Scholar] [CrossRef] [PubMed]
- Lashin, I.; Fouda, A.; Gobouri, A.A.; Azab, E.; Mohammedsaleh, Z.M.; Makharita, R.R. Antimicrobial and In Vitro Cytotoxic Efficacy of Biogenic Silver Nanoparticles (Ag-NPs) Fabricated by Callus Extract of Solanum incanum L. Biomolecules 2021, 11, 341. [Google Scholar] [CrossRef] [PubMed]
- Velgosova, O.; Veselovský, L. Synthesis of Ag nanoparticle using R. officinalis, U. dioica and V. vitis-idaea extracts. Mater. Lett. 2019, 248, 150–152. [Google Scholar] [CrossRef]
- Velgosova, O.; Dolinská, S.; Mražíková, A.; Briančin, J. Effect of P. kessleri extracts treatment on AgNPs synthesis. Inorg. Nano-Met. Chem. 2020, 50, 842–852. [Google Scholar] [CrossRef]
- Clarance, P.; Luvankar, B.; Sales, J.; Khusro, A.; Agastian, P.; Tack, J.C.; Al Khulaifi, M.M.; Al-Shwaiman, H.A.; Elgorban, A.M.; Syed, A.; et al. Green synthesis and characterization of gold nanoparticles using endophytic fungi Fusarium solani and its in-vitro anticancer and biomedical applications. Saudi J. Biol. Sci. 2020, 27, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.E.L.D.; Salem, S.S.; Fouda, A.; Awad, M.A.; El-Gamal, M.S.; Abdo, A.M. New approach for antimicrobial activity and bio-control of various pathogens by biosynthesized copper nanoparticles using endophytic actinomycetes. J. Radiat. Res. Appl. Sci. 2018, 11, 262–270. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, A.A.; Fouda, A.; Abdel-Rahman, M.A.; Hassan, S.E.-D.; El-Gamal, M.S.; Salem, S.S.; Shaheen, T.I. Fungal strain impacts the shape, bioactivity and multifunctional properties of green synthesized zinc oxide nanoparticles. Biocatal. Agric. Biotechnol. 2019, 19, 101103. [Google Scholar] [CrossRef]
- Aref, M.S.; Salem, S.S. Bio-callus synthesis of silver nanoparticles, characterization, and antibacterial activities via Cinnamomum camphora callus culture. Biocatal. Agric. Biotechnol. 2020, 27, 101689. [Google Scholar] [CrossRef]
- Fouda, A.; Hassan, S.E.-D.; Saied, E.; Azab, M.S. An eco-friendly approach to textile and tannery wastewater treatment using maghemite nanoparticles (γ-Fe2O3-NPs) fabricated by Penicillium expansum strain (K-w). J. Environ. Chem. Eng. 2021, 9, 104693. [Google Scholar] [CrossRef]
- Velgosova, O.; Mudra, E.; Vojtko, M. Preparing, Characterization and Anti-Biofilm Activity of Polymer Fibers Doped by Green Synthesized AgNPs. Polymers 2021, 13, 605. [Google Scholar] [CrossRef]
- Spagnoletti, F.N.; Spedalieri, C.; Kronberg, F.; Giacometti, R. Extracellular biosynthesis of bactericidal Ag/AgCl nanoparticles for crop protection using the fungus Macrophomina phaseolina. J. Environ. Manag. 2019, 231, 457–466. [Google Scholar] [CrossRef]
- Fouda, A.; Abdel-Maksoud, G.; Saad, H.A.; Gobouri, A.A.; Mohammedsaleh, Z.M.; El-Sadany, M.A. The efficacy of silver nitrate (AgNO3) as a coating agentto protect paper against high deteriorating microbes. Catalysts 2021, 11, 310. [Google Scholar] [CrossRef]
- Khalil, A.M.; Hassan, S.E.; Alsharif, S.M.; Eid, A.M.; Ewais, E.E.; Azab, E.; Gobouri, A.A.; Elkelish, A.; Fouda, A. Isolation and Characterization of Fungal Endophytes Isolated from Medicinal Plant Ephedra pachyclada as Plant Growth-Promoting. Biomolecules 2021, 11, 140. [Google Scholar] [CrossRef]
- Fouda, A.H.; Hassan, S.E.-D.; Eid, A.M.; Ewais, E.E.-D. Biotechnological applications of fungal endophytes associated with medicinal plant Asclepias sinaica (Bioss.). Ann. Agric. Sci. 2015, 60, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Fouda, A.; Salem, S.S.; Wassel, A.R.; Hamza, M.F.; Shaheen, T.I. Optimization of green biosynthesized visible light active CuO/ZnO nano-photocatalysts for the degradation of organic methylene blue dye. Heliyon 2020, 6, e04896. [Google Scholar] [CrossRef]
- Tso, C.P.; Zhung, C.M.; Shih, Y.H.; Tseng, Y.M.; Wu, S.C.; Doong, R.A. Stability of metal oxide nanoparticles in aqueous solutions. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2010, 61, 127–133. [Google Scholar] [CrossRef] [Green Version]
- Ivask, A.; Titma, T.; Visnapuu, M.; Vija, H.; Kakinen, A.; Sihtmae, M.; Pokhrel, S.; Madler, L.; Heinlaan, M.; Kisand, V.; et al. Toxicity of Metal Oxide Nanoparticles to Three Mammalian Cell Types In Vitro. Curr. Top. Med. Chem. 2015, 15, 1914–1929. [Google Scholar] [CrossRef]
- Mirzaei, H.; Davoodnia, A. Microwave Assisted Sol-Gel Synthesis of MgO Nanoparticles and Their Catalytic Activity in the Synthesis of Hantzsch 1,4-Dihydropyridines. Chin. J. Catal. 2012, 33, 1502–1507. [Google Scholar] [CrossRef]
- Fouda, A.; Hassan, S.E.-D.; Saied, E.; Hamza, M.F. Photocatalytic degradation of real textile and tannery effluent using biosynthesized magnesium oxide nanoparticles (MgO-NPs), heavy metal adsorption, phytotoxicity, and antimicrobial activity. J. Environ. Chem. Eng. 2021, 9, 105346. [Google Scholar] [CrossRef]
- Hassan, S.E.-D.; Fouda, A.; Saied, E.; Farag, M.M.S.; Eid, A.M.; Barghoth, M.G.; Awad, M.A.; Hamza, M.F.; Awad, M.F. Rhizopus Oryzae-mediated green synthesis of magnesium oxide nanoparticles (MgO-NPs): A promising tool for antimicrobial, mosquitocidal action, and tanning effluent treatment. J. Fungi 2021, 7, 372. [Google Scholar] [CrossRef]
- Di, D.R.; He, Z.Z.; Sun, Z.Q.; Liu, J. A new nano-cryosurgical modality for tumor treatment using biodegradable MgO nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 1233–1241. [Google Scholar] [CrossRef]
- Khan, A.; Shabbier, D.; Ahmad, P.; Khandaker, M.; Faruque, M.R.; Din, I.U. Biosynthesis and antibacterial activity of MgO-NPs produced from Camellia-sinensis leaves extract. Mater. Res. Express 2020, 8. [Google Scholar] [CrossRef]
- Cai, L.; Chen, J.; Liu, Z.; Wang, H.; Yang, H.; Ding, W. Magnesium Oxide Nanoparticles: Effective Agricultural Antibacterial Agent Against Ralstonia solanacearum. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Ammulu, M.A.; Vinay Viswanath, K.; Giduturi, A.K.; Vemuri, P.K.; Mangamuri, U.; Poda, S. Phytoassisted synthesis of magnesium oxide nanoparticles from Pterocarpus marsupium rox.b heartwood extract and its biomedical applications. J. Genet. Eng. Biotechnol. 2021, 19, 21. [Google Scholar] [CrossRef]
- Moubasher, A.H.; Moustafa, A.F. A survey of Egyptian soil fungi with special reference to Aspergillus, Penicillium and Penicillium-related genera. Trans. Br. Mycol. Soc. 1970, 54, 35–44. [Google Scholar] [CrossRef]
- Housseiny, M.M.; Gomaa, E.Z. Enhancement of Antimicrobial and Antitumor Activities of Zinc Nanoparticles Biosynthesized by Penicillium chrysogenum AUMC 10608 Using Gamma Radiation. Egypt. J. Bot. 2019, 59, 319–337. [Google Scholar] [CrossRef]
- Barabadi, H.; Kobarfard, F.; Vahidi, H. Biosynthesis and Characterization of Biogenic Tellurium Nanoparticles by Using Penicillium chrysogenum PTCC 5031: A Novel Approach in Gold Biotechnology. Iran. J. Pharm. Res. IJPR 2018, 17, 87–97. [Google Scholar]
- Grijseels, S.; Nielsen, J.C.; Nielsen, J.; Larsen, T.O.; Frisvad, J.C.; Nielsen, K.F.; Frandsen, R.J.N.; Workman, M. Physiological characterization of secondary metabolite producing Penicillium cell factories. Fungal Biol. Biotechnol. 2017, 4, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes, F.C.; Tichota, D.M.; Sauter, I.P.; Meira, S.M.M.; Segalin, J.; Rott, M.B.; Rios, A.O.; Brandelli, A. Active metabolites produced by Penicillium chrysogenum IFL1 growing on agro-industrial residues. Ann. Microbiol. 2013, 63, 771–778. [Google Scholar] [CrossRef]
- Khanra, K.; Panja, S.; Choudhuri, I.; Bhattacharyya, N. Evaluation of Antibacterial Activity and Cytotoxicity of Green Synthesized Silver Nanoparticles Using Scoparia dulcis. Nano Biomed. Eng. 2015, 7, 128–133. [Google Scholar] [CrossRef] [Green Version]
- Essien, E.R.; Atasie, V.N.; Okeafor, A.O.; Nwude, D.O. Biogenic synthesis of magnesium oxide nanoparticles using Manihot esculenta (Crantz) leaf extract. Int. Nano Lett. 2020, 10, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Fedlheim, D.L.; Foss, C.A. Metal Nanoparticles: Synthesis, Characterization, and Applications; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Jeevanandam, J.; Chan, Y.S.; Danquah, M.K. Biosynthesis and characterization of MgO nanoparticles from plant extracts via induced molecular nucleation. New J. Chem. 2017, 41, 2800–2814. [Google Scholar] [CrossRef]
- Abdallah, Y.; Ogunyemi, S.O.; Abdelazez, A.; Zhang, M.; Hong, X.; Ibrahim, E.; Hossain, A.; Fouad, H.; Li, B.; Chen, J. The Green Synthesis of MgO Nano-Flowers Using Rosmarinus officinalis L. (Rosemary) and the Antibacterial Activities against Xanthomonas oryzae pv. oryzae. BioMed Res. Int. 2019, 2019, 5620989. [Google Scholar] [CrossRef] [Green Version]
- Sivaselvam, S.; Premasudha, P.; Viswanathan, C.; Ponpandian, N. Enhanced removal of emerging pharmaceutical contaminant ciprofloxacin and pathogen inactivation using morphologically tuned MgO nanostructures. J. Environ. Chem. Eng. 2020, 8, 104256. [Google Scholar] [CrossRef]
- Lekota, M.W.; Dimpe, K.M.; Nomngongo, P.N. MgO-ZnO/carbon nanofiber nanocomposite as an adsorbent for ultrasound-assisted dispersive solid-phase microextraction of carbamazepine from wastewater prior to high-performance liquid chromatographic detection. J. Anal. Sci. Technol. 2019, 10, 25. [Google Scholar] [CrossRef]
- Dhaouadi, H.; Chaabane, H.; Touati, F. Mg(OH)2 Nanorods Synthesized by A Facile Hydrothermal Method in the Presence of CTAB. Nano-Micro Lett. 2011, 3, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Fouda, A.; Hassan, S.E.-D.; Abdel-Rahman, M.A.; Farag, M.M.S.; Shehal-deen, A.; Mohamed, A.A.; Alsharif, S.M.; Saied, E.; Moghanim, S.A.; Azab, M.S. Catalytic degradation of wastewater from the textile and tannery industries by green synthesized hematite (α-Fe2O3) and magnesium oxide (MgO) nanoparticles. Curr. Res. Biotechnol. 2021, 3, 29–41. [Google Scholar] [CrossRef]
- Moorthy, S.K.; Ashok, C.H.; Rao, K.V.; Viswanathan, C. Synthesis and Characterization of Mgo Nanoparticles by Neem Leaves through Green Method. Mater. Today: Proc. 2015, 2, 4360–4368. [Google Scholar] [CrossRef]
- Eppler, A.; Zhu, J.; Anderson, E.; Somorjai, G. Model catalysts fabricated by electron beam lithography: AFM and TPD surface studies and hydrogenation/dehydrogenation of cyclohexene + H2 on a Pt nanoparticle array supported by silica. Top. Catal. 2000, 13, 33–41. [Google Scholar] [CrossRef]
- Bindhu, M.R.; Umadevi, M.; Kavin Micheal, M.; Arasu, M.V.; Abdullah Al-Dhabi, N. Structural, morphological and optical properties of MgO nanoparticles for antibacterial applications. Mater. Lett. 2016, 166, 19–22. [Google Scholar] [CrossRef]
- Huang, L.; Li, D.; Lin, Y.; Evans, D.G.; Duan, X. Influence of nano-MgO particle size on bactericidal action against Bacillus subtilis var. niger. Chin. Sci. Bull. 2005, 50, 514–519. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Soliwoda, K.; Kadziola, K.; Tkacz-Szczesna, B.; Celichowski, G.; Cichomski, M.; Szmaja, W.; Grobelny, J. Detection Limits of DLS and UV-Vis Spectroscopy in Characterization of Polydisperse Nanoparticles Colloids. J. Nanomater. 2013, 2013, 313081. [Google Scholar] [CrossRef] [Green Version]
- Fouda, A.; Hassan, S.E.; Abdo, A.M.; El-Gamal, M.S. Antimicrobial, Antioxidant and Larvicidal Activities of Spherical Silver Nanoparticles Synthesized by Endophytic Streptomyces spp. Biol. Trace Elem. Res. 2020, 195, 707–724. [Google Scholar] [CrossRef] [PubMed]
- Salem, S.S.; El-Belely, E.F.; Niedbała, G.; Alnoman, M.M.; Hassan, S.E.-D.; Eid, A.M.; Shaheen, T.I.; Elkelish, A.; Fouda, A. Bactericidal and In-Vitro Cytotoxic Efficacy of Silver Nanoparticles (Ag-NPs) Fabricated by Endophytic Actinomycetes and Their Use as Coating for the Textile Fabrics. Nanomaterials 2020, 10, 2082. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Jyoti, K.; Patnaik, A.; Singh, A.; Chauhan, R.; Chandel, S.S. Biosynthesis, characterization and antibacterial activity of silver nanoparticles using an endophytic fungal supernatant of Raphanus sativus. J. Genet. Eng. Biotechnol. 2017, 15, 31–39. [Google Scholar] [CrossRef]
- Eid, A.M.; Fouda, A.; Niedbała, G.; Hassan, S.E.; Salem, S.S.; Abdo, A.M.; Hetta, H.F.; Shaheen, T.I. Endophytic Streptomyces laurentii Mediated Green Synthesis of Ag-NPs with Antibacterial and Anticancer Properties for Developing Functional Textile Fabric Properties. Antibiotics 2020, 9, 641. [Google Scholar] [CrossRef] [PubMed]
- Dobrucka, R. Synthesis of MgO Nanoparticles Using Artemisia abrotanum Herba Extract and Their Antioxidant and Photocatalytic Properties. Iran. J. Sci. Technol. Trans. Sci. 2018, 42, 547–555. [Google Scholar] [CrossRef] [Green Version]
- Alsharif, S.M.; Salem, S.S.; Abdel-Rahman, M.A.; Fouda, A.; Eid, A.M.; El-Din Hassan, S.; Awad, M.A.; Mohamed, A.A. Multifunctional properties of spherical silver nanoparticles fabricated by different microbial taxa. Heliyon 2020, 6, e03943. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, T.I.; Fouda, A.; Salem, S.S. Integration of Cotton Fabrics with Biosynthesized CuO Nanoparticles for Bactericidal Activity in the Terms of Their Cytotoxicity Assessment. Ind. Eng. Chem. Res. 2021, 60, 1553–1563. [Google Scholar] [CrossRef]
- Ramanujam, K.; Sundrarajan, M. Antibacterial effects of biosynthesized MgO nanoparticles using ethanolic fruit extract of Emblica officinalis. J. Photochem. Photobiol. B Biol. 2014, 141, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Hamza, M. Uranium recovery from concentrated chloride solution produced from direct acid leaching of calcareous shale, Allouga ore materials, southwestern Sinai, Egypt. J. Radioanal. Nucl. Chem. 2018, 315. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of infrared spectra, a practical approach. Encycl. Anal. Chem. Appl. Theory Instrum. 2006. [Google Scholar] [CrossRef]
- Hamza, M.F.; Salih, K.A.M.; Abdel-Rahman, A.A.H.; Zayed, Y.E.; Wei, Y.; Liang, J.; Guibal, E. Sulfonic-functionalized algal/PEI beads for scandium, cerium and holmium sorption from aqueous solutions (synthetic and industrial samples). Chem. Eng. J. 2021, 403, 126399. [Google Scholar] [CrossRef]
- Hamza, M.F.; Wei, Y.; Mira, H.I.; Abdel-Rahman, A.A.H.; Guibal, E. Synthesis and adsorption characteristics of grafted hydrazinyl amine magnetite-chitosan for Ni(II) and Pb(II) recovery. Chem. Eng. J. 2019, 362, 310–324. [Google Scholar] [CrossRef] [Green Version]
- Karthik, K.; Dhanuskodi, S.; Gobinath, C.; Prabukumar, S.; Sivaramakrishnan, S. Fabrication of MgO nanostructures and its efficient photocatalytic, antibacterial and anticancer performance. J. Photochem. Photobiol. B Biol. 2019, 190, 8–20. [Google Scholar] [CrossRef]
- Hamza, M.F.; Wei, Y.; Benettayeb, A.; Wang, X.; Guibal, E. Efficient removal of uranium, cadmium and mercury from aqueous solutions using grafted hydrazide-micro-magnetite chitosan derivative. J. Mater. Sci. 2020, 55, 4193–4212. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Prabhu, R.; Muruganantham, K.; Shanmuganathan, R.; Natarajan, S. Anticancer, antimicrobial and photocatalytic activities of green synthesized magnesium oxide nanoparticles (MgONPs) using aqueous extract of Sargassum wightii. J. Photochem. Photobiol. B Biol. 2019, 190, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Salih, K.A.M.; Lu, S.; Hamza, M.F.; Fujita, T.; Vincent, T.; Guibal, E. Amidoxime Functionalization of Algal/Polyethyleneimine Beads for the Sorption of Sr(II) from Aqueous Solutions. Molecules 2019, 24, 3893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salih, K.A.M.; Hamza, M.F.; Mira, H.; Wei, Y.; Gao, F.; Atta, A.M.; Fujita, T.; Guibal, E. Nd(III) and Gd(III) sorption on mesoporous amine-functionalized polymer/SiO2 composite. Molecules 2021, 26, 1049. [Google Scholar] [CrossRef]
- Hamza, M.F.; Lu, S.; Salih, K.A.M.; Mira, H.; Dhmees, A.S.; Fujita, T.; Wei, Y.; Vincent, T.; Guibal, E. As(V) sorption from aqueous solutions using quaternized algal/polyethyleneimine composite beads. Sci. Total Environ. 2020, 719, 137396. [Google Scholar] [CrossRef]
- Wei, Y.; Rakhatkyzy, M.; Salih, K.A.M.; Wang, K.; Hamza, M.F.; Guibal, E. Controlled bi-functionalization of silica microbeads through grafting of amidoxime/methacrylic acid for Sr(II) enhanced sorption. Chem. Eng. J. 2020, 402, 125220. [Google Scholar] [CrossRef]
- Lu, S.; Chen, L.; Hamza, M.F.; He, C.; Wang, X.; Wei, Y.; Guibal, E. Amidoxime functionalization of a poly(acrylonitrile)/silica composite for the sorption of Ga(III)—Application to the treatment of Bayer liquor. Chem. Eng. J. 2019, 368, 459–473. [Google Scholar] [CrossRef]
- Hamza, M.F.; Hamad, N.A.; Hamad, D.M.; Khalafalla, M.S.; Abdel-Rahman, A.A.-H.; Zeid, I.F.; Wei, Y.; Hessien, M.M.; Fouda, A.; Salem, W.M. Synthesis of Eco-Friendly Biopolymer, Alginate-Chitosan Composite to Adsorb the Heavy Metals, Cd(II) and Pb(II) from Contaminated Effluents. Materials 2021, 14, 2189. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Gao, B.; Chen, J.; Yang, L. Engineered Biochar Reclaiming Phosphate from Aqueous Solutions: Mechanisms and Potential Application as a Slow-Release Fertilizer. Environ. Sci. Technol. 2013, 47, 8700–8708. [Google Scholar] [CrossRef]
- Le Febvrier, A.; Jensen, J.; Eklund, P. Wet-cleaning of MgO: Modification of surface chemistry and effects on thin film growth investigated by x-ray photoelectron spectroscopy and time-of-flight secondary ion mass spectroscopy. J. Vac. Sci. Technol. A Vac. Surf. Film. 2017, 35, 021407. [Google Scholar] [CrossRef] [Green Version]
- Dang, L.; Nai, X.; Dong, Y.; Li, W. Functional group effect on flame retardancy, thermal, and mechanical properties of organophosphorus-based magnesium oxysulfate whiskers as a flame retardant in polypropylene. RSC Adv. 2017, 7, 21655–21665. [Google Scholar] [CrossRef] [Green Version]
- Jin, T.; He, Y. Antibacterial activities of magnesium oxide (MgO) nanoparticles against foodborne pathogens. J. Nanopart. Res. 2011, 13, 6877–6885. [Google Scholar] [CrossRef]
- Shaheen, T.I.; Fouda, A. Green approach for one-pot synthesis of silver nanorod using cellulose nanocrystal and their cytotoxicity and antibacterial assessment. Int. J. Biol. Macromol. 2018, 106, 784–792. [Google Scholar] [CrossRef]
- Badawy, A.A.; Abdelfattah, N.A.H.; Salem, S.S.; Awad, M.F.; Fouda, A. Efficacy Assessment of Biosynthesized Copper Oxide Nanoparticles (CuO-NPs) on Stored Grain Insects and Their Impacts on Morphological and Physiological Traits of Wheat (Triticum aestivum L.) Plant. Biology 2021, 10, 233. [Google Scholar] [CrossRef]
- Umaralikhan, L.; Jaffar, M. Green Synthesis of MgO Nanoparticles and it Antibacterial Activity. Iran. J. Sci. Technol. Trans. A Sci. 2016, 42. [Google Scholar] [CrossRef]
- Hamza, M.F.; Fouda, A.; Elwakeel, K.Z.; Wei, Y.; Guibal, E.; Hamad, N.A. Phosphorylation of Guar Gum/Magnetite/Chitosan Nanocomposites for Uranium (VI) Sorption and Antibacterial Applications. Molecules 2021, 26, 1290. [Google Scholar] [CrossRef]
- Nguyen, N.-Y.T.; Grelling, N.; Wetteland, C.L.; Rosario, R.; Liu, H. Antimicrobial Activities and Mechanisms of Magnesium Oxide Nanoparticles (nMgO) against Pathogenic Bacteria, Yeasts, and Biofilms. Sci. Rep. 2018, 8, 16260. [Google Scholar] [CrossRef] [Green Version]
- Holm, A.; Vikström, E. Quorum sensing communication between bacteria and human cells: Signals, targets, and functions. Front. Plant Sci. 2014, 5, 309. [Google Scholar] [CrossRef]
- Sawai, J.; Kojima, H.; Igarashi, H.; Hashimoto, A.; Shoji, S.; Takehara, A.; Sawaki, T.; Kokugan, T.; Shimizu, M. Escherichia coli damage by ceramic powder slurries. J. Chem. Eng. Jpn. J. Chem. Eng. Jpn. 1997, 30, 1034–1039. [Google Scholar] [CrossRef] [Green Version]
- Dhanasekaran, D.; Thangaraj, R. Evaluation of larvicidal activity of biogenic nanoparticles against filariasis causing Culex mosquito vector. Asian Pac. J. Trop. Dis. 2013, 3, 174–179. [Google Scholar] [CrossRef]
- Madhiyazhagan, P.; Murugan, K.; Kumar, A.N.; Nataraj, T.; Dinesh, D.; Panneerselvam, C.; Subramaniam, J.; Mahesh Kumar, P.; Suresh, U.; Roni, M.; et al. S argassum muticum-synthesized silver nanoparticles: An effective control tool against mosquito vectors and bacterial pathogens. Parasitol. Res. 2015, 114, 4305–4317. [Google Scholar] [CrossRef]
- Murugan, K.; Jaganathan, A.; Rajaganesh, R.; Suresh, U.; Madhavan, J.; Senthil-Nathan, S.; Rajasekar, A.; Higuchi, A.; Kumar, S.; Alarfaj, A.A.; et al. Poly(Styrene Sulfonate)/Poly(Allylamine Hydrochloride) Encapsulation of TiO2 Nanoparticles Boosts Their Toxic and Repellent Activity Against Zika Virus Mosquito Vectors. J. Clust. Sci. 2017, 29, 27–39. [Google Scholar] [CrossRef]
- Murugan, K.; Jaganathan, A.; Suresh, U.; Rajaganesh, R.; Jayasanthini, S.; Higuchi, A.; Kumar, S.; Benelli, G. Towards Bio-Encapsulation of Chitosan-Silver Nanocomplex? Impact on Malaria Mosquito Vectors, Human Breast Adenocarcinoma Cells (MCF-7) and Behavioral Traits of Non-target Fishes. J. Clust. Sci. 2017, 28, 529–550. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Manivannan, G.; Kim, S.J.; Jeyasubramanian, K.; Premanathan, M. Antibacterial activity of MgO nanoparticles based on lipid peroxidation by oxygen vacancy. J. Nanopart. Res. 2012, 14, 1063. [Google Scholar] [CrossRef]
- Ma, B.; Yu, N.; Han, Y.; Gao, M.; Wang, S.; Li, S.; Guo, L.; She, Z.; Zhao, Y.; Jin, C.; et al. Effect of magnesium oxide nanoparticles on microbial diversity and removal performance of sequencing batch reactor. J Environ. Manag. 2018, 222, 475–482. [Google Scholar] [CrossRef]
- Mangalampalli, B.; Dumala, N.; Grover, P. Allium cepa root tip assay in assessment of toxicity of magnesium oxide nanoparticles and microparticles. J. Environ. Sci. 2018, 66, 125–137. [Google Scholar] [CrossRef]
- Magdolenova, Z.; Collins, A.; Kumar, A.; Dhawan, A.; Stone, V.; Dusinska, M. Mechanisms of genotoxicity. A review of in vitro and in vivo studies with engineered nanoparticles. Nanotoxicology 2014, 8, 233–278. [Google Scholar] [CrossRef]
- Panneerselvam, C.; Murugan, K. Adulticidal, repellent, and ovicidal properties of indigenous plant extracts against the malarial vector, Anopheles stephensi (Diptera: Culicidae). Parasitol. Res. 2013, 112, 679–692. [Google Scholar] [CrossRef]
- Mullai, K.; Jebanesan, A.; Pushpanathan, T. Effect of bioactive fractions of Citrullus vulgaris Schrad. leaf extract against Anopheles stephensi and Aedes Aegypti. Parasitol. Res. 2008, 102, 951–955. [Google Scholar] [CrossRef]
- Fouda, A.; Khalil, A.; El-Sheikh, H.; Abdel-Rhaman, E.; Hashem, A. Biodegradation and detoxification of bisphenol-A by filamentous fungi screened from nature. J. Adv. Biol. Biotechnol. 2015, 123–132. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. Pcr Protoc. Guide Methods Appl. 1990, 18, 315–322. [Google Scholar]
- Soliman, A.M.; Abdel-Latif, W.; Shehata, I.H.; Fouda, A.; Abdo, A.M.; Ahmed, Y.M. Green Approach to Overcome the Resistance Pattern of Candida spp. Using Biosynthesized Silver Nanoparticles Fabricated by Penicillium chrysogenum F9. Biol. Trace Elem. Res. 2021, 199, 800–811. [Google Scholar] [CrossRef]
- Fouda, A.; El-Din Hassan, S.; Salem, S.S.; Shaheen, T.I. In-Vitro cytotoxicity, antibacterial, and UV protection properties of the biosynthesized Zinc oxide nanoparticles for medical textile applications. Microb. Pathog. 2018, 125, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.E.; Fouda, A.; Radwan, A.A.; Salem, S.S.; Barghoth, M.G.; Awad, M.A.; Abdo, A.M.; El-Gamal, M.S. Endophytic actinomycetes Streptomyces spp mediated biosynthesis of copper oxide nanoparticles as a promising tool for biotechnological applications. J. Biol. Inorg. Chem. JBIC Publ. Soc. Biol. Inorg. Chem. 2019, 24, 377–393. [Google Scholar] [CrossRef]
- Gunathilaka, N.; Ranathunge, T.; Udayanga, L.; Abeyewickreme, W. Efficacy of Blood Sources and Artificial Blood Feeding Methods in Rearing of Aedes aegypti (Diptera: Culicidae) for Sterile Insect Technique and Incompatible Insect Technique Approaches in Sri Lanka. BioMed Res. Int. 2017, 2017, 3196924. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Target Instar | Mortality Percentages (%) ± SD * | LC50 | LC90 | ||||
|---|---|---|---|---|---|---|---|
| 5 ppm | 10 ppm | 15 ppm | 20 ppm | 25 ppm | |||
| I | 30.2 ± 1.1 | 48.6 ± 1.7 | 67.6 ± 2.5 | 79.2 ± 2.2 | 91.8 ± 2.4 | 12.4 | 22.3 |
| II | 29.6 ± 1.1 | 41.8 ± 1.1 | 64.2 ± 1.9 | 75.6 ± 1.1 | 88.2 ± 1.6 | 13.10 | 23.5 |
| III | 27.8 ± 1.3 | 41.4 ± 1.8 | 59.8 ± 2.9 | 72.4 ± 2.9 | 81.4 ± 0.9 | 13.93 | 25.09 |
| IV | 24.4 ± 1.9 | 35.6 ± 2.3 | 51.4 ± 1.5 | 67.6 ± 2.6 | 72.8 ± 2.6 | 15.5 | 27.99 |
| Pupa | 22.8 ± 1.9 | 34.8 ± 1.3 | 48.2 ± 1.5 | 61.8 ± 3.5 | 69.2 ± 2.8 | 16.5 | 29.8 |
| Concentrations of MgO-NPs (mg cm−2) | Repellent Percentages (%) ± SD | |||||||
|---|---|---|---|---|---|---|---|---|
| 15 min | 30 min | 60 min | 90 min | 120 min | 150 min | 180 min | 210 min | |
| 1 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 69.3 ± 1.6 | 57.2 ± 1.1 | 43.1 ± 1.6 |
| 2.5 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 72.4 ± 1.5 | 64.2 ± 1.6 | 49.4 ± 1.8 |
| 5 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 76.6 ± 1.2 | 67.6 ± 1.4 |
| 7.5 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 76.6 ± 1.6 | 70.0 ± 1.6 | 50.6 ± 1.4 |
| 10 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 80.9 ± 1.9 | 74.9 ± 1.3 | 59.6 ± 1.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fouda, A.; Awad, M.A.; Eid, A.M.; Saied, E.; Barghoth, M.G.; Hamza, M.F.; Awad, M.F.; Abdelbary, S.; Hassan, S.E.-D. An Eco-Friendly Approach to the Control of Pathogenic Microbes and Anopheles stephensi Malarial Vector Using Magnesium Oxide Nanoparticles (Mg-NPs) Fabricated by Penicillium chrysogenum. Int. J. Mol. Sci. 2021, 22, 5096. https://doi.org/10.3390/ijms22105096
Fouda A, Awad MA, Eid AM, Saied E, Barghoth MG, Hamza MF, Awad MF, Abdelbary S, Hassan SE-D. An Eco-Friendly Approach to the Control of Pathogenic Microbes and Anopheles stephensi Malarial Vector Using Magnesium Oxide Nanoparticles (Mg-NPs) Fabricated by Penicillium chrysogenum. International Journal of Molecular Sciences. 2021; 22(10):5096. https://doi.org/10.3390/ijms22105096
Chicago/Turabian StyleFouda, Amr, Mohamed A. Awad, Ahmed M. Eid, Ebrahim Saied, Mohammed G. Barghoth, Mohammed F. Hamza, Mohamed F. Awad, Salah Abdelbary, and Saad El-Din Hassan. 2021. "An Eco-Friendly Approach to the Control of Pathogenic Microbes and Anopheles stephensi Malarial Vector Using Magnesium Oxide Nanoparticles (Mg-NPs) Fabricated by Penicillium chrysogenum" International Journal of Molecular Sciences 22, no. 10: 5096. https://doi.org/10.3390/ijms22105096
APA StyleFouda, A., Awad, M. A., Eid, A. M., Saied, E., Barghoth, M. G., Hamza, M. F., Awad, M. F., Abdelbary, S., & Hassan, S. E.-D. (2021). An Eco-Friendly Approach to the Control of Pathogenic Microbes and Anopheles stephensi Malarial Vector Using Magnesium Oxide Nanoparticles (Mg-NPs) Fabricated by Penicillium chrysogenum. International Journal of Molecular Sciences, 22(10), 5096. https://doi.org/10.3390/ijms22105096











