Implications of Extended Inhibitory Neuron Development
Abstract
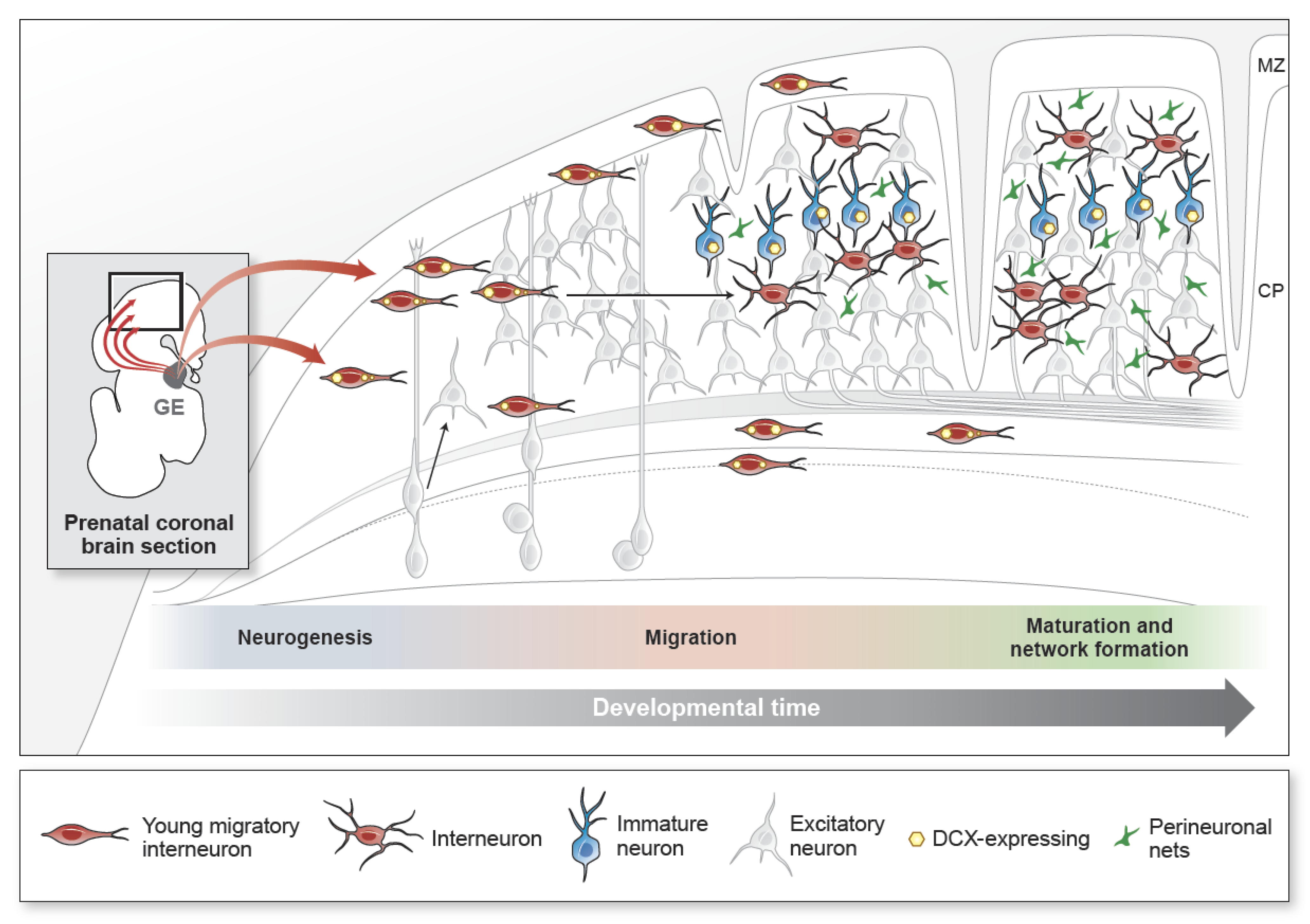
1. Introduction
2. Extended Production of Cortical GABAergic Interneuron
2.1. Neurogenesis of Cortical GABAergic Interneuron Extends Until the End of Gestation
2.2. Epigenetic Regulation during Neurogenesis
2.3. Vulnerability of Embryonic Neurogenesis to Environmental Risk Factors
3. Prolonged Migration of Coritcal GABAergic Interneurons
3.1. Early Postnatal Migration of GABAergic Interneuron into the Forebrain
3.2. Diverse Migratory Behaviors of GABAergic Interneurons
3.3. Regional Cortical Vulnerability and GABAergic Interneuron Migration
4. Establishment of the GABAergic Interneuron Network
4.1. Circuit Maturation of GABAergic Interneurons for Refinement of Cortical Functions
4.2. Extracellular Matrix Regulation of the Inhibitory Circuit Plasticity
4.3. Imbalanced Neural Networks Due to Interneuron Abnormality
5. Maintaining a Population of Immature Neurons in the Adult Brain
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, Z.J.; Di Cristo, G.; Ango, F. Development of GABA innervation in the cerebral and cerebellar cortices. Nat. Rev. Neurosci. 2007, 8, 673–686. [Google Scholar] [CrossRef]
- Kepecs, A.; Fishell, G. Interneuron cell types are fit to function. Nature 2014, 505, 318–326. [Google Scholar] [CrossRef]
- Buzsaki, G.; Draguhn, A. Neuronal oscillations in cortical networks. Science 2004, 304, 1926–1929. [Google Scholar] [CrossRef]
- Moore, C.I.; Carlen, M.; Knoblich, U.; Cardin, J.A. Neocortical interneurons: From diversity, strength. Cell 2010, 142, 189–193. [Google Scholar] [CrossRef]
- Satterstrom, F.K.; Kosmicki, J.A.; Wang, J.; Breen, M.S.; De Rubeis, S.; An, J.Y.; Peng, M.; Collins, R.; Grove, J.; Klei, L.; et al. Large-Scale Exome Sequencing Study Implicates Both Developmental and Functional Changes in the Neurobiology of Autism. Cell 2020, 180, 568–584.e523. [Google Scholar] [CrossRef]
- Kaar, S.J.; Angelescu, I.; Marques, T.R.; Howes, O.D. Pre-frontal parvalbumin interneurons in schizophrenia: A meta-analysis of post-mortem studies. J. Neural Transm. 2019, 126, 1637–1651. [Google Scholar] [CrossRef]
- Pfisterer, U.; Petukhov, V.; Demharter, S.; Meichsner, J.; Thompson, J.J.; Batiuk, M.Y.; Asenjo-Martinez, A.; Vasistha, N.A.; Thakur, A.; Mikkelsen, J.; et al. Identification of epilepsy-associated neuronal subtypes and gene expression underlying epileptogenesis. Nat. Commun. 2020, 11, 5038. [Google Scholar] [CrossRef]
- Hansen, D.V.; Lui, J.H.; Flandin, P.; Yoshikawa, K.; Rubenstein, J.L.; Alvarez-Buylla, A.; Kriegstein, A.R. Non-epithelial stem cells and cortical interneuron production in the human ganglionic eminences. Nat. Neurosci. 2013, 16, 1576–1587. [Google Scholar] [CrossRef]
- Ma, T.; Wang, C.; Wang, L.; Zhou, X.; Tian, M.; Zhang, Q.; Zhang, Y.; Li, J.; Liu, Z.; Cai, Y.; et al. Subcortical origins of human and monkey neocortical interneurons. Nat. Neurosci. 2013, 16, 1588–1597. [Google Scholar] [CrossRef]
- Luo, C.; Keown, C.L.; Kurihara, L.; Zhou, J.; He, Y.; Li, J.; Castanon, R.; Lucero, J.; Nery, J.R.; Sandoval, J.P.; et al. Single-cell methylomes identify neuronal subtypes and regulatory elements in mammalian cortex. Science 2017, 357, 600–604. [Google Scholar] [CrossRef]
- Lim, L.; Mi, D.; Llorca, A.; Marin, O. Development and Functional Diversification of Cortical Interneurons. Neuron 2018, 100, 294–313. [Google Scholar] [CrossRef]
- Nagashima, F.; Suzuki, I.K.; Shitamukai, A.; Sakaguchi, H.; Iwashita, M.; Kobayashi, T.; Tone, S.; Toida, K.; Vanderhaeghen, P.; Kosodo, Y. Novel and robust transplantation reveals the acquisition of polarized processes by cortical cells derived from mouse and human pluripotent stem cells. Stem Cells Dev. 2014, 23, 2129–2142. [Google Scholar] [CrossRef]
- Xu, G.; Broadbelt, K.G.; Haynes, R.L.; Folkerth, R.D.; Borenstein, N.S.; Belliveau, R.A.; Trachtenberg, F.L.; Volpe, J.J.; Kinney, H.C. Late development of the GABAergic system in the human cerebral cortex and white matter. J. Neuropathol. Exp. Neurol. 2011, 70, 841–858. [Google Scholar] [CrossRef]
- Hladnik, A.; Dzaja, D.; Darmopil, S.; Jovanov-Milosevic, N.; Petanjek, Z. Spatio-temporal extension in site of origin for cortical calretinin neurons in primates. Front. Neuroanat. 2014, 8, 50. [Google Scholar] [CrossRef]
- Dzaja, D.; Hladnik, A.; Bicanic, I.; Bakovic, M.; Petanjek, Z. Neocortical calretinin neurons in primates: Increase in proportion and microcircuitry structure. Front. Neuroanat. 2014, 8, 103. [Google Scholar] [CrossRef]
- Hansen, D.V.; Lui, J.H.; Parker, P.R.; Kriegstein, A.R. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature 2010, 464, 554–561. [Google Scholar] [CrossRef]
- Rakic, P. Radial unit hypothesis of neocortical expansion. Novartis Found. Symp. 2000, 228, 30–42. [Google Scholar] [CrossRef]
- Gertz, C.C.; Lui, J.H.; LaMonica, B.E.; Wang, X.; Kriegstein, A.R. Diverse behaviors of outer radial glia in developing ferret and human cortex. J. Neurosci. 2014, 34, 2559–2570. [Google Scholar] [CrossRef]
- Nowakowski, T.J.; Pollen, A.A.; Sandoval-Espinosa, C.; Kriegstein, A.R. Transformation of the Radial Glia Scaffold Demarcates Two Stages of Human Cerebral Cortex Development. Neuron 2016, 91, 1219–1227. [Google Scholar] [CrossRef]
- Rakic, P. Specification of cerebral cortical areas. Science 1988, 241, 170–176. [Google Scholar] [CrossRef]
- Parnavelas, J.G.; Alifragis, P.; Nadarajah, B. The origin and migration of cortical neurons. Prog. Brain Res. 2002, 136, 73–80. [Google Scholar] [CrossRef]
- Puelles, L.; Kuwana, E.; Puelles, E.; Bulfone, A.; Shimamura, K.; Keleher, J.; Smiga, S.; Rubenstein, J.L. Pallial and subpallial derivatives in the embryonic chick and mouse telencephalon, traced by the expression of the genes Dlx-2, Emx-1, Nkx-2.1, Pax-6, and Tbr-1. J. Comp. Neurol. 2000, 424, 409–438. [Google Scholar] [CrossRef]
- Fogarty, M.; Grist, M.; Gelman, D.; Marin, O.; Pachnis, V.; Kessaris, N. Spatial genetic patterning of the embryonic neuroepithelium generates GABAergic interneuron diversity in the adult cortex. J. Neurosci. 2007, 27, 10935–10946. [Google Scholar] [CrossRef]
- Xu, Q.; Cobos, I.; De La Cruz, E.; Rubenstein, J.L.; Anderson, S.A. Origins of cortical interneuron subtypes. J. Neurosci. 2004, 24, 2612–2622. [Google Scholar] [CrossRef]
- Kanatani, S.; Yozu, M.; Tabata, H.; Nakajima, K. COUP-TFII is preferentially expressed in the caudal ganglionic eminence and is involved in the caudal migratory stream. J. Neurosci. 2008, 28, 13582–13591. [Google Scholar] [CrossRef]
- Nery, S.; Fishell, G.; Corbin, J.G. The caudal ganglionic eminence is a source of distinct cortical and subcortical cell populations. Nat. Neurosci. 2002, 5, 1279–1287. [Google Scholar] [CrossRef]
- Miyoshi, G.; Young, A.; Petros, T.; Karayannis, T.; McKenzie Chang, M.; Lavado, A.; Iwano, T.; Nakajima, M.; Taniguchi, H.; Huang, Z.J.; et al. Prox1 Regulates the Subtype-Specific Development of Caudal Ganglionic Eminence-Derived GABAergic Cortical Interneurons. J. Neurosci. 2015, 35, 12869–12889. [Google Scholar] [CrossRef]
- Raju, C.S.; Spatazza, J.; Stanco, A.; Larimer, P.; Sorrells, S.F.; Kelley, K.W.; Nicholas, C.R.; Paredes, M.F.; Lui, J.H.; Hasenstaub, A.R.; et al. Secretagogin is Expressed by Developing Neocortical GABAergic Neurons in Humans but not Mice and Increases Neurite Arbor Size and Complexity. Cereb. Cortex 2018, 28, 1946–1958. [Google Scholar] [CrossRef]
- Lavdas, A.A.; Grigoriou, M.; Pachnis, V.; Parnavelas, J.G. The medial ganglionic eminence gives rise to a population of early neurons in the developing cerebral cortex. J. Neurosci. 1999, 19, 7881–7888. [Google Scholar] [CrossRef]
- Petanjek, Z.; Dujmovic, A.; Kostovic, I.; Esclapez, M. Distinct origin of GABA-ergic neurons in forebrain of man, nonhuman primates and lower mammals. Coll. Antropol. 2008, 32 (Suppl. S1), 9–17. [Google Scholar]
- Rakic, P. Neurogenesis in adult primate neocortex: An evaluation of the evidence. Nat. Rev. Neurosci. 2002, 3, 65–71. [Google Scholar] [CrossRef] [PubMed]
- van den Ameele, J.; Tiberi, L.; Vanderhaeghen, P.; Espuny-Camacho, I. Thinking out of the dish: What to learn about cortical development using pluripotent stem cells. Trends Neurosci. 2014, 37, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Tam, M.; Anderson, S.A. Fate mapping Nkx2.1-lineage cells in the mouse telencephalon. J. Comp. Neurol. 2008, 506, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Laclef, C.; Metin, C. Conserved rules in embryonic development of cortical interneurons. Semin. Cell Dev. Biol. 2018, 76, 86–100. [Google Scholar] [CrossRef]
- Anthony, T.E.; Klein, C.; Fishell, G.; Heintz, N. Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron 2004, 41, 881–890. [Google Scholar] [CrossRef]
- Arshad, A.; Vose, L.R.; Vinukonda, G.; Hu, F.; Yoshikawa, K.; Csiszar, A.; Brumberg, J.C.; Ballabh, P. Extended Production of Cortical Interneurons into the Third Trimester of Human Gestation. Cereb. Cortex 2016, 26, 2242–2256. [Google Scholar] [CrossRef]
- Malik, S.; Vinukonda, G.; Vose, L.R.; Diamond, D.; Bhimavarapu, B.B.; Hu, F.; Zia, M.T.; Hevner, R.; Zecevic, N.; Ballabh, P. Neurogenesis continues in the third trimester of pregnancy and is suppressed by premature birth. J. Neurosci. 2013, 33, 411–423. [Google Scholar] [CrossRef]
- Tibrewal, M.; Cheng, B.; Dohare, P.; Hu, F.; Mehdizadeh, R.; Wang, P.; Zheng, D.; Ungvari, Z.; Ballabh, P. Disruption of Interneuron Neurogenesis in Premature Newborns and Reversal with Estrogen Treatment. J. Neurosci. 2018, 38, 1100–1113. [Google Scholar] [CrossRef]
- Krienen, F.M.; Goldman, M.; Zhang, Q.; del Rosario, R.C.H.; Florio, M.; Machold, R.; Saunders, A.; Levandowski, K.; Zaniewski, H.; Schuman, B.; et al. Innovations present in the primate interneuron repertoire. Nature 2020, 586, 262–269. [Google Scholar] [CrossRef]
- Murphy, V.E.; Smith, R.; Giles, W.B.; Clifton, V.L. Endocrine regulation of human fetal growth: The role of the mother, placenta, and fetus. Endocr. Rev. 2006, 27, 141–169. [Google Scholar] [CrossRef]
- Salinas, R.D.; Connolly, D.R.; Song, H. Invited Review: Epigenetics in neurodevelopment. Neuropathol. Appl. Neurobiol. 2020, 46, 6–27. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Ye, J.; Weber, C.; Sun, W.; Zhang, H.; Zhou, Y.; Cai, C.; Qian, G.; Capel, B. The histone demethylase KDM6B regulates temperature-dependent sex determination in a turtle species. Science 2018, 360, 645–648. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Crews, D. Molecular mechanisms of temperature-dependent sex determination in the context of ecological developmental biology. Mol. Cell. Endocrinol. 2012, 354, 103–110. [Google Scholar] [CrossRef]
- Toepfer, P.; O’Donnell, K.J.; Entringer, S.; Garg, E.; Heim, C.M.; Lin, D.T.S.; MacIsaac, J.L.; Kobor, M.S.; Meaney, M.J.; Provencal, N.; et al. Dynamic DNA methylation changes in the maternal oxytocin gene locus (OXT) during pregnancy predict postpartum maternal intrusiveness. Psychoneuroendocrinology 2019, 103, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Maire, C.L.; Bilenky, M.; Carles, A.; Heravi-Moussavi, A.; Hong, C.; Tam, A.; Kamoh, B.; Cho, S.; Cheung, D.; et al. Epigenomic programming in early fetal brain development. Epigenomics 2020, 12, 1053–1070. [Google Scholar] [CrossRef] [PubMed]
- Spiers, H.; Hannon, E.; Schalkwyk, L.C.; Smith, R.; Wong, C.C.; O’Donovan, M.C.; Bray, N.J.; Mill, J. Methylomic trajectories across human fetal brain development. Genome Res. 2015, 25, 338–352. [Google Scholar] [CrossRef]
- Sharif, J.; Muto, M.; Takebayashi, S.; Suetake, I.; Iwamatsu, A.; Endo, T.A.; Shinga, J.; Mizutani-Koseki, Y.; Toyoda, T.; Okamura, K.; et al. The SRA protein Np95 mediates epigenetic inheritance by recruiting Dnmt1 to methylated DNA. Nature 2007, 450, 908–912. [Google Scholar] [CrossRef]
- Watanabe, D.; Uchiyama, K.; Hanaoka, K. Transition of mouse de novo methyltransferases expression from Dnmt3b to Dnmt3a during neural progenitor cell development. Neuroscience 2006, 142, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Chang, H.; Li, E.; Fan, G. Dynamic expression of de novo DNA methyltransferases Dnmt3a and Dnmt3b in the central nervous system. J. Neurosci. Res. 2005, 79, 734–746. [Google Scholar] [CrossRef]
- Wu, H.; Coskun, V.; Tao, J.; Xie, W.; Ge, W.; Yoshikawa, K.; Li, E.; Zhang, Y.; Sun, Y.E. Dnmt3a-dependent nonpromoter DNA methylation facilitates transcription of neurogenic genes. Science 2010, 329, 444–448. [Google Scholar] [CrossRef]
- Kadriu, B.; Guidotti, A.; Chen, Y.; Grayson, D.R. DNA methyltransferases1 (DNMT1) and 3a (DNMT3a) colocalize with GAD67-positive neurons in the GAD67-GFP mouse brain. J. Comp. Neurol. 2012, 520, 1951–1964. [Google Scholar] [CrossRef] [PubMed]
- Ruzicka, W.B.; Zhubi, A.; Veldic, M.; Grayson, D.R.; Costa, E.; Guidotti, A. Selective epigenetic alteration of layer I GABAergic neurons isolated from prefrontal cortex of schizophrenia patients using laser-assisted microdissection. Mol. Psychiatry 2007, 12, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Alzayady, K.; Stewart, R.; Ye, P.; Yang, S.; Li, W.; Shi, Y. Histone demethylase LSD1 regulates neural stem cell proliferation. Mol. Cell. Biol. 2010, 30, 1997–2005. [Google Scholar] [CrossRef]
- Hirano, K.; Namihira, M. New insight into LSD1 function in human cortical neurogenesis. Neurogenesis 2016, 3, e1249195. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Lancaster, M.A.; Castanon, R.; Nery, J.R.; Knoblich, J.A.; Ecker, J.R. Cerebral Organoids Recapitulate Epigenomic Signatures of the Human Fetal Brain. Cell Rep. 2016, 17, 3369–3384. [Google Scholar] [CrossRef] [PubMed]
- Salmaso, N.; Jablonska, B.; Scafidi, J.; Vaccarino, F.M.; Gallo, V. Neurobiology of premature brain injury. Nat. Neurosci. 2014, 17, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Miranda, R.C. MicroRNAs and Fetal Brain Development: Implications for Ethanol Teratology during the Second Trimester Period of Neurogenesis. Front. Genet. 2012, 3, 77. [Google Scholar] [CrossRef]
- Panchision, D.M. The role of oxygen in regulating neural stem cells in development and disease. J. Cell Physiol. 2009, 220, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Rosen, N.J.; Yoshida, C.K.; Croen, L.A. Infection in the first 2 years of life and autism spectrum disorders. Pediatrics 2007, 119, e61–e69. [Google Scholar] [CrossRef]
- Stolp, H.B.; Fleiss, B.; Arai, Y.; Supramaniam, V.; Vontell, R.; Birtles, S.; Yates, A.G.; Baburamani, A.A.; Thornton, C.; Rutherford, M.; et al. Interneuron Development Is Disrupted in Preterm Brains With Diffuse White Matter Injury: Observations in Mouse and Human. Front. Physiol. 2019, 10, 955. [Google Scholar] [CrossRef]
- Lacaille, H.; Vacher, C.M.; Bakalar, D.; O’Reilly, J.J.; Salzbank, J.; Penn, A.A. Impaired Interneuron Development in a Novel Model of Neonatal Brain Injury. eNeuro 2019, 6. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.; Li, Q.; Dechant, A.; Cohen, M.L. Neonatal loss of gamma-aminobutyric acid pathway expression after human perinatal brain injury. J. Neurosurg. 2006, 104, 396–408. [Google Scholar] [CrossRef]
- Paterno, R.; Casalia, M.; Baraban, S.C. Interneuron deficits in neurodevelopmental disorders: Implications for disease pathology and interneuron-based therapies. Eur. J. Paediatr. Neurol. 2020, 24, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, L.; Calcagnotto, M.E.; Paredes, M.F. Cortical Malformations: Lessons in Human Brain Development. Front. Cell Neurosci. 2019, 13, 576. [Google Scholar] [CrossRef] [PubMed]
- Weaver, I.C.; Diorio, J.; Seckl, J.R.; Szyf, M.; Meaney, M.J. Early environmental regulation of hippocampal glucocorticoid receptor gene expression: Characterization of intracellular mediators and potential genomic target sites. Ann. N. Y. Acad. Sci. 2004, 1024, 182–212. [Google Scholar] [CrossRef]
- Oberlander, T.F.; Weinberg, J.; Papsdorf, M.; Grunau, R.; Misri, S.; Devlin, A.M. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics 2008, 3, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Veldic, M.; Caruncho, H.J.; Liu, W.S.; Davis, J.; Satta, R.; Grayson, D.R.; Guidotti, A.; Costa, E. DNA-methyltransferase 1 mRNA is selectively overexpressed in telencephalic GABAergic interneurons of schizophrenia brains. Proc. Natl. Acad. Sci. USA 2004, 101, 348–353. [Google Scholar] [CrossRef]
- Veldic, M.; Guidotti, A.; Maloku, E.; Davis, J.M.; Costa, E. In psychosis, cortical interneurons overexpress DNA-methyltransferase 1. Proc. Natl. Acad. Sci. USA 2005, 102, 2152–2157. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Z.; Zhi, X.; Du, Y.; Lin, Z.; Wu, J. Identification of De Novo DNMT3A Mutations That Cause West Syndrome by Using Whole-Exome Sequencing. Mol. Neurobiol. 2018, 55, 2483–2493. [Google Scholar] [CrossRef]
- Kim, H.G.; Rosenfeld, J.A.; Scott, D.A.; Benedicte, G.; Labonne, J.D.; Brown, J.; McGuire, M.; Mahida, S.; Naidu, S.; Gutierrez, J.; et al. Disruption of PHF21A causes syndromic intellectual disability with craniofacial anomalies, epilepsy, hypotonia, and neurobehavioral problems including autism. Mol. Autism 2019, 10, 35. [Google Scholar] [CrossRef]
- Fang, Y.; Liao, G.; Yu, B. LSD1/KDM1A inhibitors in clinical trials: Advances and prospects. J. Hematol. Oncol. 2019, 12, 129. [Google Scholar] [CrossRef]
- Xu, Z.; Li, H.; Jin, P. Epigenetics-Based Therapeutics for Neurodegenerative Disorders. Curr. Transl. Geriatr. Exp. Gerontol. Rep. 2012, 1, 229–236. [Google Scholar] [CrossRef]
- Iraola-Guzman, S.; Estivill, X.; Rabionet, R. DNA methylation in neurodegenerative disorders: A missing link between genome and environment? Clin. Genet. 2011, 80, 1–14. [Google Scholar] [CrossRef]
- Buchsbaum, I.Y.; Cappello, S. Neuronal migration in the CNS during development and disease: Insights from in vivo and in vitro models. Development 2019, 146. [Google Scholar] [CrossRef] [PubMed]
- Friedl, P.; Wolf, K. Plasticity of cell migration: A multiscale tuning model. J. Cell Biol. 2010, 188, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Ayala, R.; Shu, T.; Tsai, L.H. Trekking across the brain: The journey of neuronal migration. Cell 2007, 128, 29–43. [Google Scholar] [CrossRef]
- Faux, C.; Rakic, S.; Andrews, W.; Britto, J.M. Neurons on the move: Migration and lamination of cortical interneurons. Neurosignals 2012, 20, 168–189. [Google Scholar] [CrossRef] [PubMed]
- Inta, D.; Alfonso, J.; von Engelhardt, J.; Kreuzberg, M.M.; Meyer, A.H.; van Hooft, J.A.; Monyer, H. Neurogenesis and widespread forebrain migration of distinct GABAergic neurons from the postnatal subventricular zone. Proc. Natl. Acad. Sci. USA 2008, 105, 20994–20999. [Google Scholar] [CrossRef]
- Ellis, J.K.; Sorrells, S.F.; Mikhailova, S.; Chavali, M.; Chang, S.; Sabeur, K.; McQuillen, P.; Rowitch, D.H. Ferret brain possesses young interneuron collections equivalent to human postnatal migratory streams. J. Comp. Neurol. 2019, 527, 2843–2859. [Google Scholar] [CrossRef]
- Paredes, M.F.; James, D.; Gil-Perotin, S.; Kim, H.; Cotter, J.A.; Ng, C.; Sandoval, K.; Rowitch, D.H.; Xu, D.; McQuillen, P.S.; et al. Extensive migration of young neurons into the infant human frontal lobe. Science 2016, 354. [Google Scholar] [CrossRef]
- Sanai, N.; Nguyen, T.; Ihrie, R.A.; Mirzadeh, Z.; Tsai, H.H.; Wong, M.; Gupta, N.; Berger, M.S.; Huang, E.; Garcia-Verdugo, J.M.; et al. Corridors of migrating neurons in the human brain and their decline during infancy. Nature 2011, 478, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Grangeray Vilmint, A.; Lelievre, V. The medial migratory stream: A new turn in postnatal neurogenesis! Cell. Adh. Migr. 2012, 6, 454–456. [Google Scholar] [CrossRef][Green Version]
- Metin, C.; Vallee, R.B.; Rakic, P.; Bhide, P.G. Modes and mishaps of neuronal migration in the mammalian brain. J. Neurosci. 2008, 28, 11746–11752. [Google Scholar] [CrossRef]
- Flames, N.; Long, J.E.; Garratt, A.N.; Fischer, T.M.; Gassmann, M.; Birchmeier, C.; Lai, C.; Rubenstein, J.L.; Marin, O. Short- and long-range attraction of cortical GABAergic interneurons by neuregulin-1. Neuron 2004, 44, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z. Molecular regulation of neuronal migration during neocortical development. Mol. Cell. Neurosci. 2009, 42, 11–22. [Google Scholar] [CrossRef]
- Laurie, D.J.; Wisden, W.; Seeburg, P.H. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J. Neurosci. 1992, 12, 4151–4172. [Google Scholar] [CrossRef]
- Cuzon Carlson, V.C.; Yeh, H.H. GABAA receptor subunit profiles of tangentially migrating neurons derived from the medial ganglionic eminence. Cereb. Cortex 2011, 21, 1792–1802. [Google Scholar] [CrossRef] [PubMed]
- Cuzon, V.C.; Yeh, P.W.; Cheng, Q.; Yeh, H.H. Ambient GABA promotes cortical entry of tangentially migrating cells derived from the medial ganglionic eminence. Cereb. Cortex 2006, 16, 1377–1388. [Google Scholar] [CrossRef]
- Inada, H.; Watanabe, M.; Uchida, T.; Ishibashi, H.; Wake, H.; Nemoto, T.; Yanagawa, Y.; Fukuda, A.; Nabekura, J. GABA regulates the multidirectional tangential migration of GABAergic interneurons in living neonatal mice. PLoS ONE 2011, 6, e27048. [Google Scholar] [CrossRef]
- Martini, F.J.; Valdeolmillos, M. Actomyosin contraction at the cell rear drives nuclear translocation in migrating cortical interneurons. J. Neurosci. 2010, 30, 8660–8670. [Google Scholar] [CrossRef]
- Horigane, S.I.; Ozawa, Y.; Yamada, H.; Takemoto-Kimura, S. Calcium signalling: A key regulator of neuronal migration. J. Biochem. 2019, 165, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, J.; Penkert, H.; Duman, C.; Zuccotti, A.; Monyer, H. Downregulation of Sphingosine 1-Phosphate Receptor 1 Promotes the Switch from Tangential to Radial Migration in the OB. J. Neurosci. 2015, 35, 13659–13672. [Google Scholar] [CrossRef] [PubMed]
- Gengatharan, A.; Bammann, R.R.; Saghatelyan, A. The Role of Astrocytes in the Generation, Migration, and Integration of New Neurons in the Adult Olfactory Bulb. Front. Neurosci. 2016, 10, 149. [Google Scholar] [CrossRef]
- Bovetti, S.; Hsieh, Y.C.; Bovolin, P.; Perroteau, I.; Kazunori, T.; Puche, A.C. Blood vessels form a scaffold for neuroblast migration in the adult olfactory bulb. J. Neurosci. 2007, 27, 5976–5980. [Google Scholar] [CrossRef]
- Rezazadeh, A.; Bercovici, E.; Kiehl, T.R.; Chow, E.W.; Krings, T.; Bassett, A.S.; Andrade, D.M. Periventricular nodular heterotopia in 22q11.2 deletion and frontal lobe migration. Ann. Clin. Transl. Neurol. 2018, 5, 1314–1322. [Google Scholar] [CrossRef]
- Ozmen, M.; Yilmaz, Y.; Caliskan, M.; Minareci, O.; Aydinli, N. Clinical features of 21 patients with lissencephaly type I (agyria-pachygyria). Turk. J. Pediatr. 2000, 42, 210–214. [Google Scholar]
- Courchesne, E. Abnormal early brain development in autism. Mol. Psychiatry 2002, 7 (Suppl. S2), S21–S23. [Google Scholar] [CrossRef]
- Hazlett, H.C.; Poe, M.D.; Gerig, G.; Styner, M.; Chappell, C.; Smith, R.G.; Vachet, C.; Piven, J. Early brain overgrowth in autism associated with an increase in cortical surface area before age 2 years. Arch. Gen. Psychiatry 2011, 68, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Carper, R.A.; Moses, P.; Tigue, Z.D.; Courchesne, E. Cerebral lobes in autism: Early hyperplasia and abnormal age effects. Neuroimage 2002, 16, 1038–1051. [Google Scholar] [CrossRef]
- Ha, S.; Sohn, I.J.; Kim, N.; Sim, H.J.; Cheon, K.A. Characteristics of Brains in Autism Spectrum Disorder: Structure, Function and Connectivity across the Lifespan. Exp. Neurobiol. 2015, 24, 273–284. [Google Scholar] [CrossRef]
- Herrero, M.J.; Velmeshev, D.; Hernandez-Pineda, D.; Sethi, S.; Sorrells, S.; Banerjee, P.; Sullivan, C.; Gupta, A.R.; Kriegstein, A.R.; Corbin, J.G. Identification of amygdala-expressed genes associated with autism spectrum disorder. Mol. Autism 2020, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Sorrells, S.F.; Paredes, M.F.; Velmeshev, D.; Herranz-Perez, V.; Sandoval, K.; Mayer, S.; Chang, E.F.; Insausti, R.; Kriegstein, A.R.; Rubenstein, J.L.; et al. Immature excitatory neurons develop during adolescence in the human amygdala. Nat. Commun. 2019, 10, 2748. [Google Scholar] [CrossRef]
- Redcay, E. The superior temporal sulcus performs a common function for social and speech perception: Implications for the emergence of autism. Neurosci. Biobehav. Rev. 2008, 32, 123–142. [Google Scholar] [CrossRef]
- Adolphs, R. The neurobiology of social cognition. Curr. Opin. Neurobiol. 2001, 11, 231–239. [Google Scholar] [CrossRef]
- Kim, J.E.; Lyoo, I.K.; Estes, A.M.; Renshaw, P.F.; Shaw, D.W.; Friedman, S.D.; Kim, D.J.; Yoon, S.J.; Hwang, J.; Dager, S.R. Laterobasal amygdalar enlargement in 6- to 7-year-old children with autism spectrum disorder. Arch. Gen. Psychiatry 2010, 67, 1187–1197. [Google Scholar] [CrossRef]
- Bigler, E.D.; Tate, D.F.; Neeley, E.S.; Wolfson, L.J.; Miller, M.J.; Rice, S.A.; Cleavinger, H.; Anderson, C.; Coon, H.; Ozonoff, S.; et al. Temporal lobe, autism, and macrocephaly. AJNR Am. J. Neuroradiol. 2003, 24, 2066–2076. [Google Scholar] [PubMed]
- Kinney, H.C.; Haynes, R.L.; Xu, G.; Andiman, S.E.; Folkerth, R.D.; Sleeper, L.A.; Volpe, J.J. Neuron deficit in the white matter and subplate in periventricular leukomalacia. Ann. Neurol. 2012, 71, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Hensch, T.K. Critical period plasticity in local cortical circuits. Nat. Rev. Neurosci. 2005, 6, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Feldman, D.E.; Brecht, M. Map plasticity in somatosensory cortex. Science 2005, 310, 810–815. [Google Scholar] [CrossRef]
- Ferguson, B.R.; Gao, W.J. Development of thalamocortical connections between the mediodorsal thalamus and the prefrontal cortex and its implication in cognition. Front. Hum. Neurosci. 2014, 8, 1027. [Google Scholar] [CrossRef]
- Takesian, A.E.; Hensch, T.K. Balancing plasticity/stability across brain development. Prog. Brain Res. 2013, 207, 3–34. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.; Durand, S.; LeBlanc, J.J.; Hensch, T.K.; Chen, C.; Fagiolini, M. Visual acuity development and plasticity in the absence of sensory experience. J. Neurosci. 2013, 33, 17789–17796. [Google Scholar] [CrossRef] [PubMed]
- Barkat, T.R.; Polley, D.B.; Hensch, T.K. A critical period for auditory thalamocortical connectivity. Nat. Neurosci. 2011, 14, 1189–1194. [Google Scholar] [CrossRef]
- Miyashita-Lin, E.M.; Hevner, R.; Wassarman, K.M.; Martinez, S.; Rubenstein, J.L. Early neocortical regionalization in the absence of thalamic innervation. Science 1999, 285, 906–909. [Google Scholar] [CrossRef]
- Wang, L.; Fontanini, A.; Maffei, A. Experience-dependent switch in sign and mechanisms for plasticity in layer 4 of primary visual cortex. J. Neurosci. 2012, 32, 10562–10573. [Google Scholar] [CrossRef]
- Consorti, A.; Sansevero, G.; Torelli, C.; Berardi, N.; Sale, A. From Basic Visual Science to Neurodevelopmental Disorders: The Voyage of Environmental Enrichment-Like Stimulation. Neural Plast. 2019, 2019, 5653180. [Google Scholar] [CrossRef]
- Wang, B.S.; Sarnaik, R.; Cang, J. Critical period plasticity matches binocular orientation preference in the visual cortex. Neuron 2010, 65, 246–256. [Google Scholar] [CrossRef]
- Kalia, A.; Lesmes, L.A.; Dorr, M.; Gandhi, T.; Chatterjee, G.; Ganesh, S.; Bex, P.J.; Sinha, P. Development of pattern vision following early and extended blindness. Proc. Natl. Acad. Sci. USA 2014, 111, 2035–2039. [Google Scholar] [CrossRef] [PubMed]
- Levi, D.M. Perceptual learning in adults with amblyopia: A reevaluation of critical periods in human vision. Dev. Psychobiol. 2005, 46, 222–232. [Google Scholar] [CrossRef]
- Mataga, N.; Mizuguchi, Y.; Hensch, T.K. Experience-dependent pruning of dendritic spines in visual cortex by tissue plasminogen activator. Neuron 2004, 44, 1031–1041. [Google Scholar] [CrossRef]
- Fagiolini, M.; Hensch, T.K. Inhibitory threshold for critical-period activation in primary visual cortex. Nature 2000, 404, 183–186. [Google Scholar] [CrossRef]
- Southwell, D.G.; Froemke, R.C.; Alvarez-Buylla, A.; Stryker, M.P.; Gandhi, S.P. Cortical plasticity induced by inhibitory neuron transplantation. Science 2010, 327, 1145–1148. [Google Scholar] [CrossRef]
- Lodato, S.; Rouaux, C.; Quast, K.B.; Jantrachotechatchawan, C.; Studer, M.; Hensch, T.K.; Arlotta, P. Excitatory projection neuron subtypes control the distribution of local inhibitory interneurons in the cerebral cortex. Neuron 2011, 69, 763–779. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.J.; Kirkwood, A.; Pizzorusso, T.; Porciatti, V.; Morales, B.; Bear, M.F.; Maffei, L.; Tonegawa, S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell 1999, 98, 739–755. [Google Scholar] [CrossRef]
- Sugiyama, S.; Di Nardo, A.A.; Aizawa, S.; Matsuo, I.; Volovitch, M.; Prochiantz, A.; Hensch, T.K. Experience-dependent transfer of Otx2 homeoprotein into the visual cortex activates postnatal plasticity. Cell 2008, 134, 508–520. [Google Scholar] [CrossRef]
- Kreczko, A.; Goel, A.; Song, L.; Lee, H.K. Visual deprivation decreases somatic GAD65 puncta number on layer 2/3 pyramidal neurons in mouse visual cortex. Neural Plast. 2009, 2009, 415135. [Google Scholar] [CrossRef]
- Hensch, T.K.; Fagiolini, M.; Mataga, N.; Stryker, M.P.; Baekkeskov, S.; Kash, S.F. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science 1998, 282, 1504–1508. [Google Scholar] [CrossRef]
- Sarro, E.C.; Kotak, V.C.; Sanes, D.H.; Aoki, C. Hearing loss alters the subcellular distribution of presynaptic GAD and postsynaptic GABAA receptors in the auditory cortex. Cereb. Cortex 2008, 18, 2855–2867. [Google Scholar] [CrossRef]
- Reh, R.K.; Dias, B.G.; Nelson, C.A., 3rd; Kaufer, D.; Werker, J.F.; Kolb, B.; Levine, J.D.; Hensch, T.K. Critical period regulation across multiple timescales. Proc. Natl. Acad. Sci. USA 2020, 117, 23242–23251. [Google Scholar] [CrossRef]
- Kuhlman, S.J.; Lu, J.; Lazarus, M.S.; Huang, Z.J. Maturation of GABAergic inhibition promotes strengthening of temporally coherent inputs among convergent pathways. PLoS Comput. Biol. 2010, 6, e1000797. [Google Scholar] [CrossRef] [PubMed]
- Kalish, B.T.; Barkat, T.R.; Diel, E.E.; Zhang, E.J.; Greenberg, M.E.; Hensch, T.K. Single-nucleus RNA sequencing of mouse auditory cortex reveals critical period triggers and brakes. Proc. Natl. Acad. Sci. USA 2020, 117, 11744–11752. [Google Scholar] [CrossRef]
- Doischer, D.; Hosp, J.A.; Yanagawa, Y.; Obata, K.; Jonas, P.; Vida, I.; Bartos, M. Postnatal differentiation of basket cells from slow to fast signaling devices. J. Neurosci. 2008, 28, 12956–12968. [Google Scholar] [CrossRef] [PubMed]
- Kujala, J.; Jung, J.; Bouvard, S.; Lecaignard, F.; Lothe, A.; Bouet, R.; Ciumas, C.; Ryvlin, P.; Jerbi, K. Gamma oscillations in V1 are correlated with GABA(A) receptor density: A multi-modal MEG and Flumazenil-PET study. Sci. Rep. 2015, 5, 16347. [Google Scholar] [CrossRef]
- Vianney-Rodrigues, P.; Iancu, O.D.; Welsh, J.P. Gamma oscillations in the auditory cortex of awake rats. Eur. J. Neurosci. 2011, 33, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Kuki, T.; Fujihara, K.; Miwa, H.; Tamamaki, N.; Yanagawa, Y.; Mushiake, H. Contribution of parvalbumin and somatostatin-expressing GABAergic neurons to slow oscillations and the balance in beta-gamma oscillations across cortical layers. Front. Neural Circuits 2015, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhang, Y.; Li, X.; Zhao, X.; Ye, Q.; Lin, Y.; Tao, H.W.; Rasch, M.J.; Zhang, X. Distinct Inhibitory Circuits Orchestrate Cortical beta and gamma Band Oscillations. Neuron 2017, 96, 1403–1418.e1406. [Google Scholar] [CrossRef]
- Fries, P.; Reynolds, J.H.; Rorie, A.E.; Desimone, R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science 2001, 291, 1560–1563. [Google Scholar] [CrossRef]
- Cho, K.K.; Hoch, R.; Lee, A.T.; Patel, T.; Rubenstein, J.L.; Sohal, V.S. Gamma rhythms link prefrontal interneuron dysfunction with cognitive inflexibility in Dlx5/6(+/−) mice. Neuron 2015, 85, 1332–1343. [Google Scholar] [CrossRef]
- Conde, F.; Lund, J.S.; Lewis, D.A. The hierarchical development of monkey visual cortical regions as revealed by the maturation of parvalbumin-immunoreactive neurons. Dev. Brain Res. 1996, 96, 261–276. [Google Scholar] [CrossRef]
- Nelson, C.A., 3rd; Zeanah, C.H.; Fox, N.A. How Early Experience Shapes Human Development: The Case of Psychosocial Deprivation. Neural Plast. 2019, 2019, 1676285. [Google Scholar] [CrossRef]
- Fuster, J.M. Frontal lobe and cognitive development. J. Neurocytol. 2002, 31, 373–385. [Google Scholar] [CrossRef]
- Cho, R.Y.; Konecky, R.O.; Carter, C.S. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proc. Natl. Acad. Sci. USA 2006, 103, 19878–19883. [Google Scholar] [CrossRef]
- Minzenberg, M.J.; Firl, A.J.; Yoon, J.H.; Gomes, G.C.; Reinking, C.; Carter, C.S. Gamma oscillatory power is impaired during cognitive control independent of medication status in first-episode schizophrenia. Neuropsychopharmacology 2010, 35, 2590–2599. [Google Scholar] [CrossRef]
- Volk, D.W.; Lewis, D.A. Prenatal ontogeny as a susceptibility period for cortical GABA neuron disturbances in schizophrenia. Neuroscience 2013, 248, 154–164. [Google Scholar] [CrossRef]
- Berardi, N.; Pizzorusso, T.; Maffei, L. Extracellular matrix and visual cortical plasticity: Freeing the synapse. Neuron 2004, 44, 905–908. [Google Scholar] [CrossRef] [PubMed]
- Carceller, H.; Guirado, R.; Ripolles-Campos, E.; Teruel-Marti, V.; Nacher, J. Perineuronal Nets Regulate the Inhibitory Perisomatic Input onto Parvalbumin Interneurons and gamma Activity in the Prefrontal Cortex. J. Neurosci. 2020, 40, 5008–5018. [Google Scholar] [CrossRef] [PubMed]
- Pesarico, A.P.; Bueno-Fernandez, C.; Guirado, R.; Gomez-Climent, M.A.; Curto, Y.; Carceller, H.; Nacher, J. Chronic Stress Modulates Interneuronal Plasticity: Effects on PSA-NCAM and Perineuronal Nets in Cortical and Extracortical Regions. Front. Cell Neurosci. 2019, 13, 197. [Google Scholar] [CrossRef]
- Luke, M.P.; Brown, R.E.; Clarke, D.B. Polysialylated—Neural cell adhesion molecule (PSA-NCAM) promotes recovery of vision after the critical period. Mol. Cell. Neurosci. 2020, 107, 103527. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, J.L.; Merzenich, M.M. Model of autism: Increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003, 2, 255–267. [Google Scholar] [CrossRef]
- Gao, R.; Penzes, P. Common mechanisms of excitatory and inhibitory imbalance in schizophrenia and autism spectrum disorders. Curr. Mol. Med. 2015, 15, 146–167. [Google Scholar] [CrossRef]
- Rojas, D.C.; Wilson, L.B. Gamma-band abnormalities as markers of autism spectrum disorders. Biomark. Med. 2014, 8, 353–368. [Google Scholar] [CrossRef]
- An, K.M.; Ikeda, T.; Yoshimura, Y.; Hasegawa, C.; Saito, D.N.; Kumazaki, H.; Hirosawa, T.; Minabe, Y.; Kikuchi, M. Altered Gamma Oscillations during Motor Control in Children with Autism Spectrum Disorder. J. Neurosci. 2018, 38, 7878–7886. [Google Scholar] [CrossRef]
- Stoner, R.; Chow, M.L.; Boyle, M.P.; Sunkin, S.M.; Mouton, P.R.; Roy, S.; Wynshaw-Boris, A.; Colamarino, S.A.; Lein, E.S.; Courchesne, E. Patches of disorganization in the neocortex of children with autism. N. Engl. J. Med. 2014, 370, 1209–1219. [Google Scholar] [CrossRef]
- Harada, M.; Taki, M.M.; Nose, A.; Kubo, H.; Mori, K.; Nishitani, H.; Matsuda, T. Non-invasive evaluation of the GABAergic/glutamatergic system in autistic patients observed by MEGA-editing proton MR spectroscopy using a clinical 3 tesla instrument. J. Autism Dev. Disord. 2011, 41, 447–454. [Google Scholar] [CrossRef]
- La Rosa, C.; Cavallo, F.; Pecora, A.; Chincarini, M.; Ala, U.; Faulkes, C.G.; Nacher, J.; Cozzi, B.; Sherwood, C.C.; Amrein, I.; et al. Phylogenetic variation in cortical layer II immature neuron reservoir of mammals. eLife 2020, 9. [Google Scholar] [CrossRef]
- Palazzo, O.; La Rosa, C.; Piumatti, M.; Bonfanti, L. Do large brains of long-living mammals prefer non-newly generated, immature neurons? Neural Regen. Res. 2018, 13, 633–634. [Google Scholar] [CrossRef]
- La Rosa, C.; Parolisi, R.; Bonfanti, L. Brain Structural Plasticity: From Adult Neurogenesis to Immature Neurons. Front. Neurosci. 2020, 14, 75. [Google Scholar] [CrossRef] [PubMed]
- Piumatti, M.; Palazzo, O.; La Rosa, C.; Crociara, P.; Parolisi, R.; Luzzati, F.; Levy, F.; Bonfanti, L. Non-Newly Generated, “Immature” Neurons in the Sheep Brain Are Not Restricted to Cerebral Cortex. J. Neurosci. 2018, 38, 826–842. [Google Scholar] [CrossRef] [PubMed]
- Varea, E.; Belles, M.; Vidueira, S.; Blasco-Ibanez, J.M.; Crespo, C.; Pastor, A.M.; Nacher, J. PSA-NCAM is Expressed in Immature, but not Recently Generated, Neurons in the Adult Cat Cerebral Cortex Layer II. Front. Neurosci. 2011, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xie, M.X.; Li, J.M.; Hu, X.; Patrylo, P.R.; Luo, X.G.; Cai, Y.; Li, Z.; Yan, X.X. Prenatal genesis of layer II doublecortin expressing neurons in neonatal and young adult guinea pig cerebral cortex. Front. Neuroanat. 2015, 9, 109. [Google Scholar] [CrossRef][Green Version]
- Cai, Y.; Xiong, K.; Chu, Y.; Luo, D.W.; Luo, X.G.; Yuan, X.Y.; Struble, R.G.; Clough, R.W.; Spencer, D.D.; Williamson, A.; et al. Doublecortin expression in adult cat and primate cerebral cortex relates to immature neurons that develop into GABAergic subgroups. Exp. Neurol. 2009, 216, 342–356. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.W.; Matarin, M.; Reeves, C.; McEvoy, A.W.; Miserocchi, A.; Thompson, P.; Sisodiya, S.M.; Thom, M. Doublecortin-expressing cell types in temporal lobe epilepsy. Acta. Neuropathol. Commun. 2018, 6, 60. [Google Scholar] [CrossRef] [PubMed]
- Marti-Mengual, U.; Varea, E.; Crespo, C.; Blasco-Ibanez, J.M.; Nacher, J. Cells expressing markers of immature neurons in the amygdala of adult humans. Eur. J. Neurosci. 2013, 37, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Luzzati, F.; Bonfanti, L.; Fasolo, A.; Peretto, P. DCX and PSA-NCAM expression identifies a population of neurons preferentially distributed in associative areas of different pallial derivatives and vertebrate species. Cereb. Cortex 2009, 19, 1028–1041. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-Y.; Paredes, M.F. Implications of Extended Inhibitory Neuron Development. Int. J. Mol. Sci. 2021, 22, 5113. https://doi.org/10.3390/ijms22105113
Kim J-Y, Paredes MF. Implications of Extended Inhibitory Neuron Development. International Journal of Molecular Sciences. 2021; 22(10):5113. https://doi.org/10.3390/ijms22105113
Chicago/Turabian StyleKim, Jae-Yeon, and Mercedes F. Paredes. 2021. "Implications of Extended Inhibitory Neuron Development" International Journal of Molecular Sciences 22, no. 10: 5113. https://doi.org/10.3390/ijms22105113
APA StyleKim, J.-Y., & Paredes, M. F. (2021). Implications of Extended Inhibitory Neuron Development. International Journal of Molecular Sciences, 22(10), 5113. https://doi.org/10.3390/ijms22105113




