A Prepubertal Mice Model to Study the Growth Pattern of Early Ovarian Follicles
Abstract
:1. Introduction
2. Results
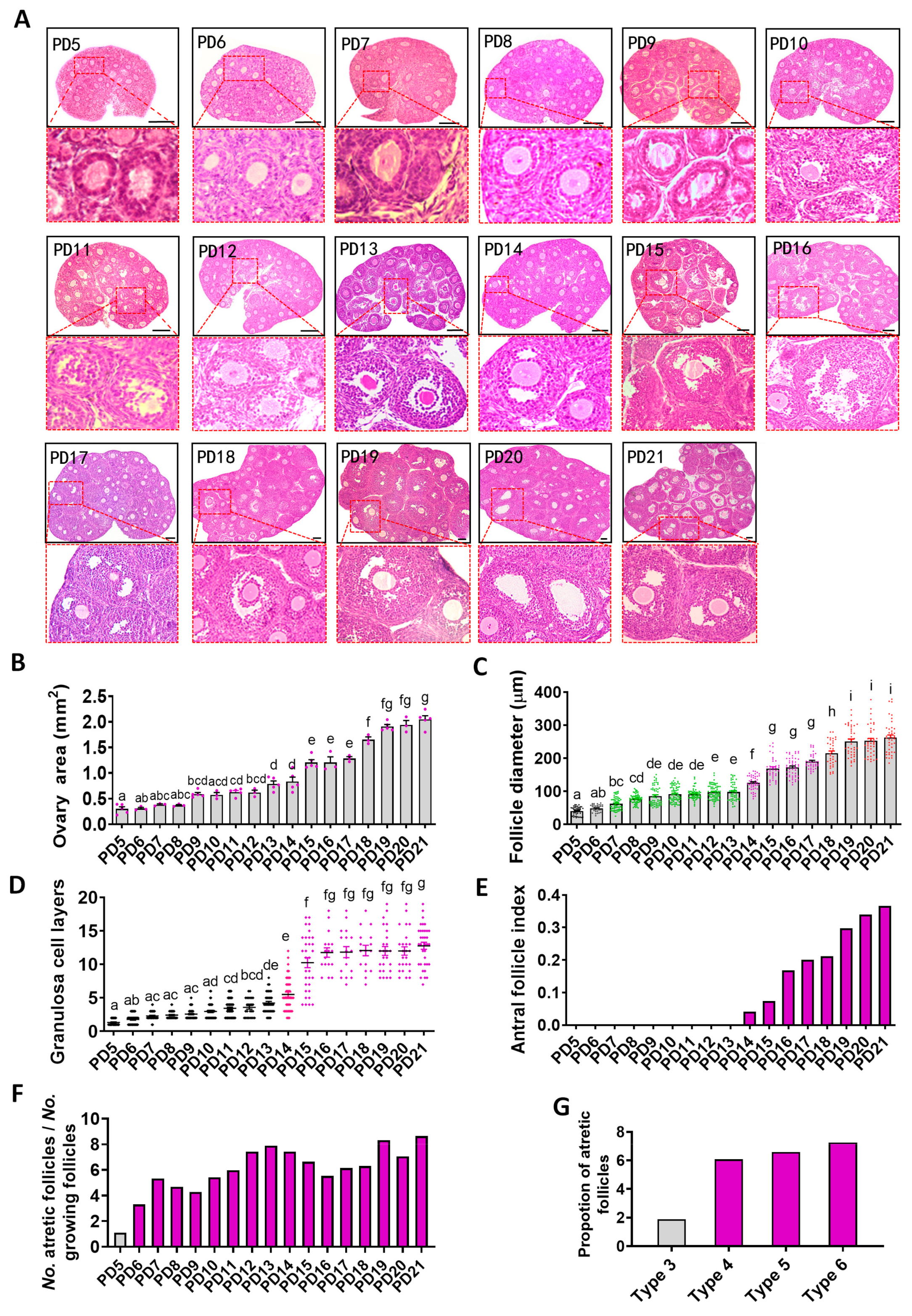
2.1. Histological Analysis of the Dynamics of Early Folliculogenesis
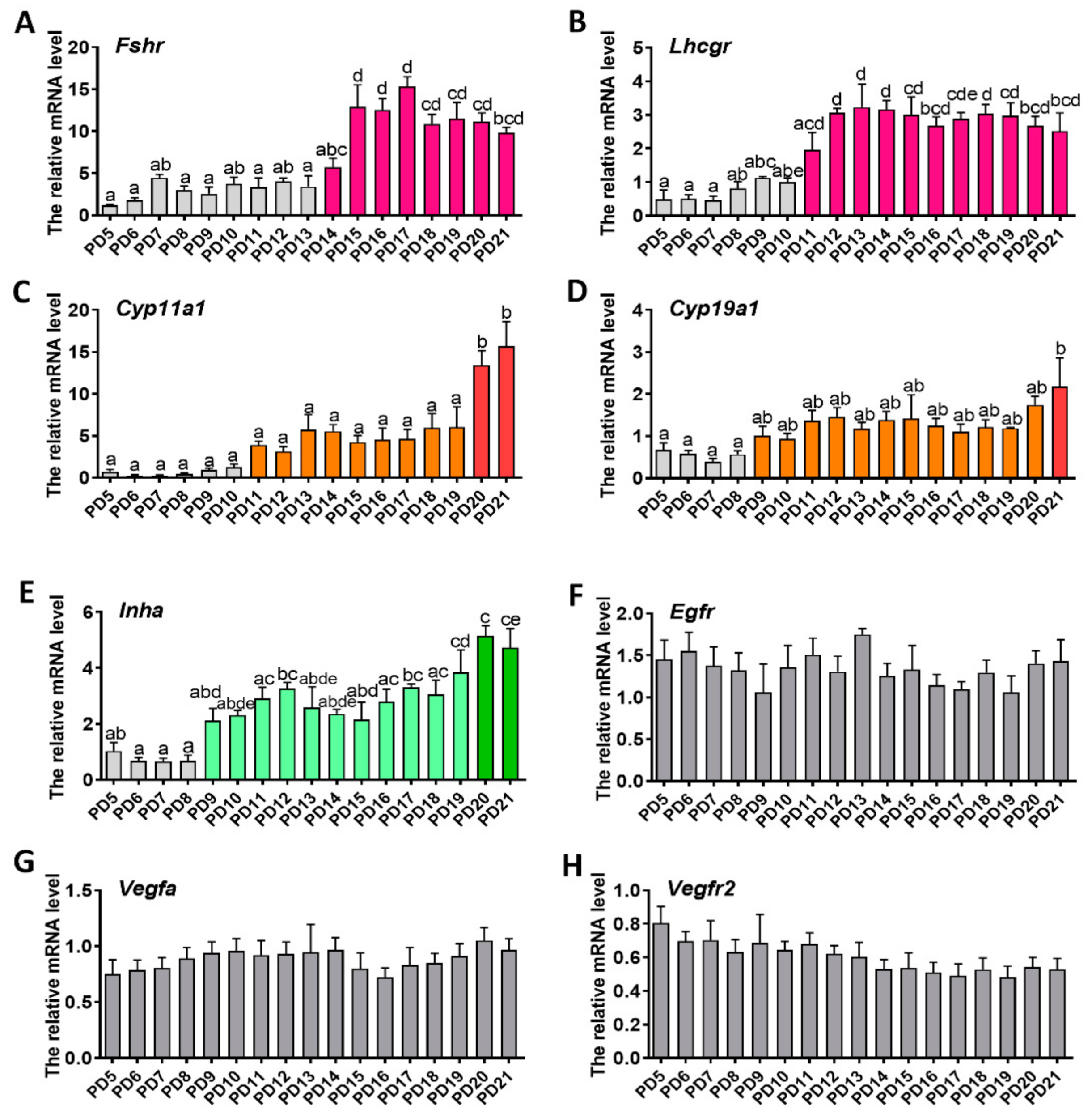
2.2. Expression Patterns of Genes Related to Folliculogenesis
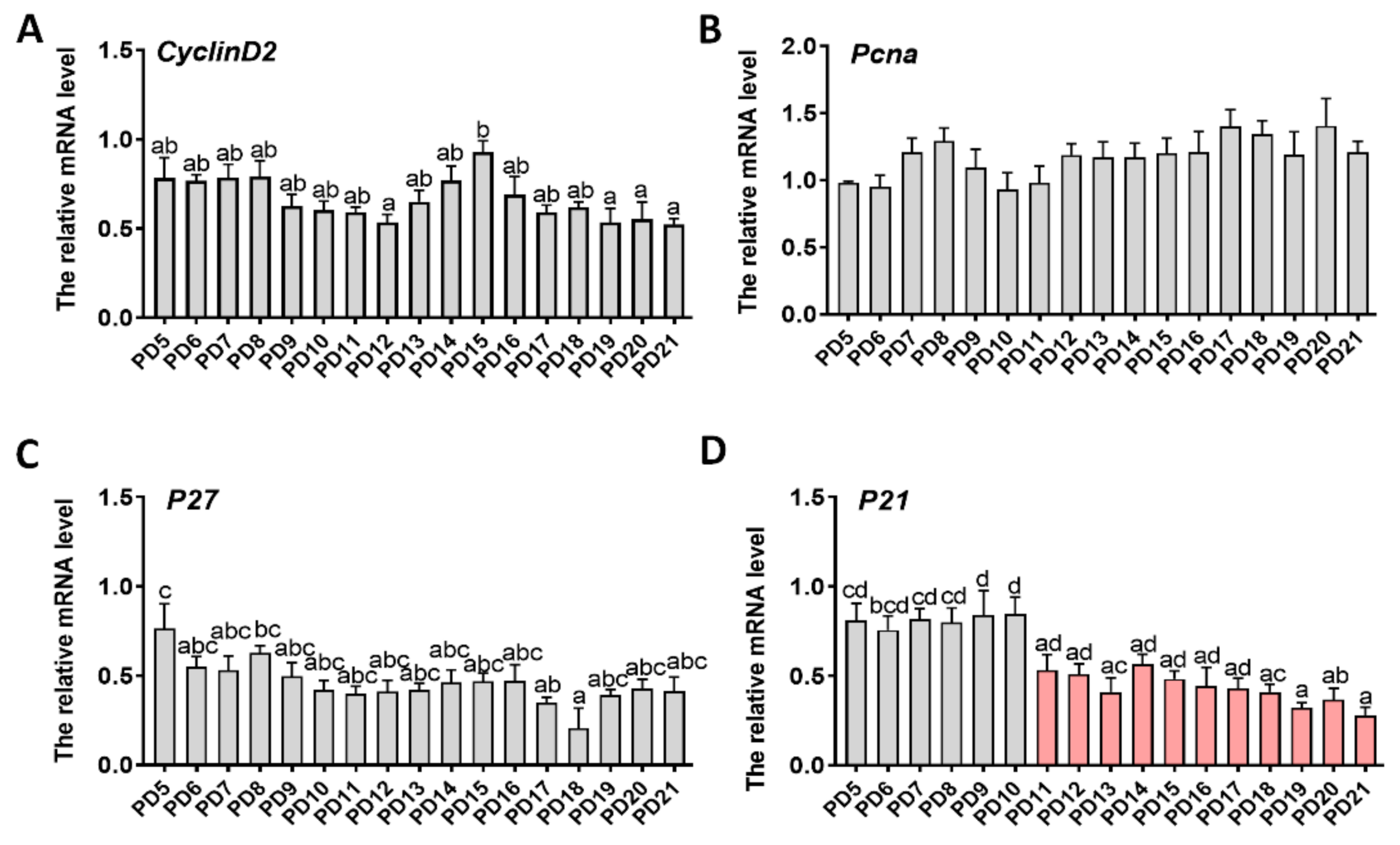
2.3. Expression Patterns of Genes Related to Proliferation
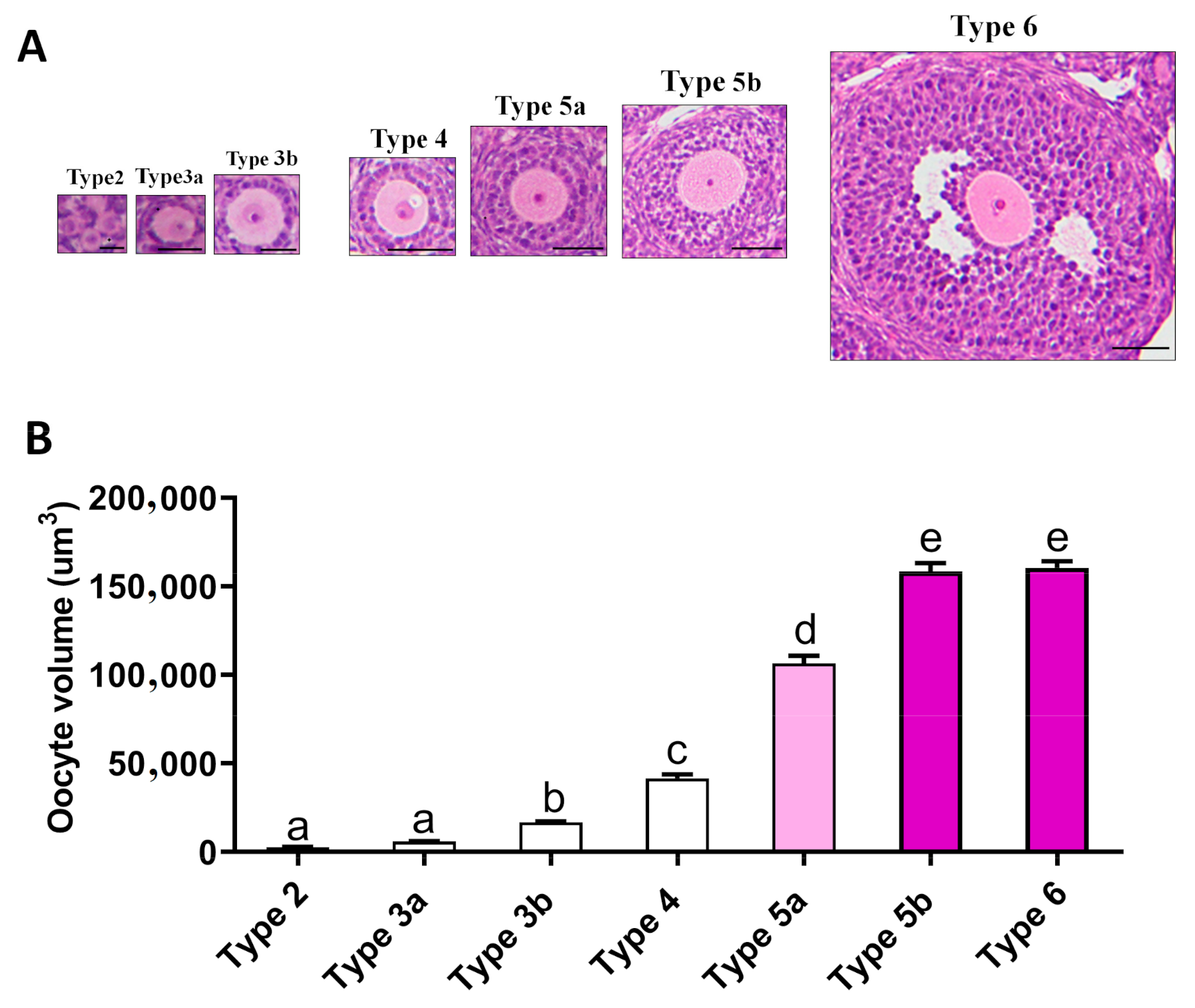
2.4. Relationship between Oogenesis and Early Folliculogenesis
2.5. Identification of the Day-Age of Follicles Entering the Gonadotropin-Dependent Phase
2.6. Identification of the Day-Age of Puberty Initiation
3. Discussion
4. Materials and Methods
4.1. Mice Handing
4.2. Histomorphological Analysis
4.3. Gene Expression Assay with Real-Time qPCR
4.4. Superovulation
4.5. Puberty Initiation Determination
4.6. FSH, LH, and Estradiol-17β Level Analyses by Radioimmunoassay
4.7. Statistics Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiong, J.; Wu, M.; Zhang, Q.; Zhang, C.; Xiong, G.; Ma, L.; Lu, Z.; Wang, S. Proteomic analysis of mouse ovaries during the prepubertal stages. Exp. Cell Res. 2019, 377, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, A.J.; Kawamura, K.; Cheng, Y.; Fauser, B.C. Intraovarian control of early folliculogenesis. Endocr. Rev. 2015, 36, 1–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, S.Q.; Zhu, M.; Wang, J.W.; Li, L.; He, H.; Hu, B.; Hu, J.W.; Xia, L. Histomorphology and gene expression profiles during early ovarian folliculogenesis in duck and goose. Poult. Sci. 2021, 100, 1098–1108. [Google Scholar] [CrossRef]
- Pedersen, T.; Peters, H. Proposal for a classification of oocytes and follicles in the mouse ovary. J. Reprod. Fertil. 1968, 17, 555–557. [Google Scholar] [CrossRef] [PubMed]
- Kumar, T.R.; Wang, Y.; Lu, N.; Matzuk, M.M. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat. Genet. 1997, 15, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Dong, Y.; Matzuk, M.M.; Kumar, T.R. Targeted disruption of luteinizing hormone beta-subunit leads to hypogonadism, defects in gonadal steroidogenesis, and infertility. Proc. Natl. Acad. Sci. USA 2004, 101, 17294–17299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aerts, J.M.; Bols, P.E. Ovarian follicular dynamics. A review with emphasis on the bovine species. Part II: Antral development, exogenous influence and future prospects. Reprod. Domest. Anim. 2010, 45, 180–187. [Google Scholar] [CrossRef]
- Hutt, K.J.; McLaughlin, E.A.; Holland, M.K. Primordial follicle activation and follicular development in the juvenile rabbit ovary. Cell Tissue Res. 2006, 326, 809–822. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, H.; Gorre, N.; Risal, S.; Shen, Y.; Liu, K. Two classes of ovarian primordial follicles exhibit distinct developmental dynamics and physiological functions. Hum. Mol. Genet. 2014, 23, 920–928. [Google Scholar] [CrossRef]
- Knight, P.G.; Glister, C. TGF-beta superfamily members and ovarian follicle development. Reproduction 2006, 132, 191–206. [Google Scholar] [CrossRef] [Green Version]
- McGee, E.A.; Perlas, E.; LaPolt, P.S.; Tsafriri, A.; Hsueh, A.J. Follicle-Stimulating hormone enhances the development of preantral follicles in juvenile rats. Biol. Reprod. 1997, 57, 990–998. [Google Scholar] [CrossRef] [Green Version]
- Xi, G.; Wang, W.; Fazlani, S.A.; Yao, F.; Yang, M.; Hao, J.; An, L.; Tian, J. C-Type natriuretic peptide enhances mouse preantral follicle growth. Reproduction 2019, 157, 445–455. [Google Scholar] [CrossRef]
- Yang, C.; Liu, Q.; Chen, Y.; Wang, X.; Ran, Z.; Fang, F.; Xiong, J.; Liu, G.; Li, X.; Yang, L.; et al. Melatonin delays ovarian aging in mice by slowing down the exhaustion of ovarian reserve. Commun. Biol. 2021, 4, 534. [Google Scholar] [CrossRef]
- Hsueh, A.J.; Kawamura, K. Hippo signaling disruption and ovarian follicle activation in infertile patients. Fertil. Steril. 2020, 114, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.A.; Beckett, E.L.; Roman, S.D.; McLaughlin, E.A.; Sutherland, J.M. Advances in human primordial follicle activation and premature ovarian insufficiency. Reproduction 2020, 159, R15–R29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.N.; Chang, E.M. Primordial follicle activation as new treatment for primary ovarian insufficiency. Clin. Exp. Reprod. Med. 2019, 46, 43–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlsson, I.B.; Scott, J.E.; Visser, J.A.; Ritvos, O.; Themmen, A.P.N.; Hovatta, O. Anti-Müllerian hormone inhibits initiation of growth of human primordial ovarian follicles in vitro. Hum. Reprod. 2006, 21, 2223–2227. [Google Scholar] [CrossRef] [Green Version]
- Pankhurst, M.W. A putative role for anti-Müllerian hormone (AMH) in optimising ovarian reserve expenditure. J. Endocrinol. 2017, 233, R1–R13. [Google Scholar] [CrossRef] [PubMed]
- Abel, M.H.; Wootton, A.N.; Wilkins, V.; Huhtaniemi, I.; Knight, P.G.; Charlton, H.M. The effect of a null mutation in the follicle-stimulating hormone receptor gene on mouse reproduction. Endocrinology 2000, 141, 1795–1803. [Google Scholar] [CrossRef]
- Fortune, J.E. Ovarian follicular growth and development in mammals. Biol. Reprod. 1994, 50, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Pepling, M.E. From primordial germ cell to primordial follicle: Mammalian female germ cell development. Genesis 2006, 44, 622–632. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H. Research advances in regulating mechanisms of mammalian ovarian folliculogenesis. Sheng Li Xue Bao 2020, 72, 63–74. [Google Scholar]
- Peters, H. The development of the mouse ovary from birth to maturity. Acta Endocrinol. 1969, 62, 98–116. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, H.; Liu, K. The two classes of primordial follicles in the mouse ovary: Their development, physiological functions and implications for future research. Mol. Hum. Reprod. 2014, 20, 286–292. [Google Scholar] [CrossRef] [Green Version]
- Byskov, A.G. Cell kinetic studies of follicular atresia in the mouse ovary. J. Reprod. Fertil. 1974, 37, 277–285. [Google Scholar] [CrossRef] [Green Version]
- Hirshfield, A.N.; Midgley, A.R., Jr. Morphometric analysis of follicular development in the rat. Biol. Reprod. 1978, 19, 597–605. [Google Scholar] [CrossRef] [Green Version]
- Hirshfield, A.N. Size-frequency analysis of atresia in cycling rats. Biol. Reprod. 1988, 38, 1181–1188. [Google Scholar] [CrossRef] [Green Version]
- Chun, S.Y.; Eisenhauer, K.M.; Minami, S.; Billig, H.; Perlas, E.; Hsueh, A.J. Hormonal regulation of apoptosis in early antral follicles: Follicle-Stimulating hormone as a major survival factor. Endocrinology 1996, 137, 1447–1456. [Google Scholar] [CrossRef] [Green Version]
- Menon, K.M.; Menon, B. Structure, function and regulation of gonadotropin receptors—A perspective. Mol. Cell Endocrinol. 2012, 356, 88–97. [Google Scholar] [CrossRef] [Green Version]
- Palermo, R. Differential actions of FSH and LH during folliculogenesis. Reprod. Biomed. Online 2007, 15, 326–337. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Yang, C.; Liu, Q.; Ran, Z.; Li, X.; He, C. A mouse model reveals the events and underlying regulatory signals during the gonadotrophin-dependent phase of follicle development. Mol. Hum. Reprod. 2020, 26, 920–937. [Google Scholar] [CrossRef] [PubMed]
- Casarini, L.; Santi, D.; Simoni, M.; Potì, F. ‘Spare’ Luteinizing Hormone Receptors: Facts and Fiction. Trends Endocrinol. Metab. 2018, 29, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Asiabi, P.; Leonel, E.; Marbaix, E.; Dolmans, M.; Amorim, C. Immunodetection and quantification of enzymatic markers in theca cells: The early process of ovarian steroidogenesis. Biol. Reprod. 2020, 102, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Gilep, A.A.; Sushko, T.A.; Usanov, S.A. At the crossroads of steroid hormone biosynthesis: The role, substrate specificity and evolutionary development of CYP17. Biochim. Biophys. Acta 2011, 1814, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Ball, B.A.; Davolli, G.M.; Esteller-Vico, A.; Fleming, B.O.; Wynn, M.; Conley, A.J. Inhibin–A and Inhibin-B in stallions: Seasonal changes and changes after down-regulation of the hypothalamic-pituitary-gonadal axis. Theriogenology 2019, 123, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Sicinski, P.; Donaher, J.L.; Geng, Y.; Parker, S.B.; Gardner, H.; Park, M.Y.; Eppig, J.J. Cyclin D2 is an FSH-responsive gene involved in gonadal cell proliferation and oncogenesis. Nature 1996, 384, 470. [Google Scholar] [CrossRef]
- Coqueret, O. New roles for p21 and p27 cell–cycle inhibitors: A function for each cell compartment. Trends Cell Biol. 2003, 13, 65–70. [Google Scholar] [CrossRef]
- Watson, A.J. Regulation of blastocyst formation. Front. Biosci. 2001, 6, 708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frum, T.; Ralston, A. Cell signaling and transcription factors regulating cell fate during formation of the mouse blastocyst. Trends Genet. 2015, 31, 402–410. [Google Scholar] [CrossRef] [Green Version]
- Eppig, J.J.; Schroeder, A.C. Capacity of mouse oocytes from preantral follicles to undergo embryogenesis and development to live young after growth, maturation, and fertilization in vitro. Biol. Reprod. 1989, 41, 268–276. [Google Scholar] [CrossRef] [Green Version]
- Eppig, J.J. Oocyte control of ovarian follicular development and function in mammals. Reproduction 2001, 122, 829–838. [Google Scholar] [CrossRef]
- Aerts, J.M.; Bols, P.E. Ovarian follicular dynamics: A review with emphasis on the bovine species. Part I: Folliculogenesis and pre-antral follicle development. Reprod. Domest. Anim. 2010, 45, 171–179. [Google Scholar] [CrossRef]
- Bronson, F.H.; Desjardins, C. Circulating concentrations of FSH, LH, estradiol, and progesterone associated with acute, male-induced puberty in female mice. Endocrinology 1974, 94, 1658–1668. [Google Scholar] [CrossRef]
- Jayes, F.C.L.; Britt, J.H.; Esbenshade, K.L. Role of gonadotropin-releasing hormone pulse frequency in differential regulation of gonadotropins in the gilt. Biol. Reprod. 1997, 56, 1012–1019. [Google Scholar] [CrossRef] [Green Version]


| Genes | Primer Sequence (5’–3’) | Product Size (bp) | Tm (°C) |
|---|---|---|---|
| Actb | Forward: CCAGCCTTCCTTCTTGGGTAT | 93 | 60 |
| Reverse: AGGTCTTTACGGATGTCAACG | |||
| Lhcgr | Forward: CTGAGGAGATTTGGTTGCTGTA | 234 | 60 |
| Reverse: ATTTGGGTGGACTTTTTTGGGG | |||
| Cyp11α1 | Forward: GGGCAGTTTGGAGTCAGTTTAC | 186 | 60 |
| Reverse: TTTAGGACGATTCGGTCTTTCTT | |||
| Fshr | Forward: GCAGATGTGTTCTCCAACCTACC | 172 | 60 |
| Reverse: GGAGAGACTGGATCTTGTGAAAGG | |||
| Egfr | Forward: AAGGCACAAGTAACAGGCTCA | 114 | 60 |
| Reverse: CCAAGTTCCCAAGGACCACT | |||
| Cyp19α1 | Forward: GACACATCATGCTGGACACC | 179 | 60 |
| Reverse: CAAGTCCTTGACGGATCGTT | |||
| Vegfα | Forward: GAGAAGACAGGGTGGTGGAAG | 193 | 60 |
| Reverse: GAAGGGAAGATGAGGAAGGGT | |||
| Vegfr2 | Forward: CCTGCCTACCTCACCTGTTTC | 205 | 60 |
| Reverse: CCACTGTCTGTCTGGCTGTC | |||
| Inhα | Forward: CTTTCCCTCTGCTGACCCA | 184 | 60 |
| Reverse: AAAGCCGCAGGAGACCAA | |||
| CyclinD2 | Forward: GCTATGGAGCTGCTGTGCT | 263 | 60 |
| Reverse: CCAAGAAACGGTCCAGGTAA | |||
| PCNA | Forward: ACCTGCAGAGCATGGACTCG | 83 | 60 |
| Reverse: GCAGCGGTATGTGTCGAAGC | |||
| P21 | Forward: CCTGGTGATGTCCGACCTG | 130 | 60 |
| Reverse: CCATGAGCGCATCGCAATC | |||
| P27 | Forward: TCAAACGTGAGAGTGTCTAACG | 238 | 60 |
| Reverse: CCGGGCCGAAGAGATTTCTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Liu, Q.; Liu, R.; Yang, C.; Wang, X.; Ran, Z.; Zhou, S.; Li, X.; He, C. A Prepubertal Mice Model to Study the Growth Pattern of Early Ovarian Follicles. Int. J. Mol. Sci. 2021, 22, 5130. https://doi.org/10.3390/ijms22105130
Chen Y, Liu Q, Liu R, Yang C, Wang X, Ran Z, Zhou S, Li X, He C. A Prepubertal Mice Model to Study the Growth Pattern of Early Ovarian Follicles. International Journal of Molecular Sciences. 2021; 22(10):5130. https://doi.org/10.3390/ijms22105130
Chicago/Turabian StyleChen, Yingjun, Qinghua Liu, Ruiyan Liu, Chan Yang, Xiaodong Wang, Zaohong Ran, Shanshan Zhou, Xiang Li, and Changjiu He. 2021. "A Prepubertal Mice Model to Study the Growth Pattern of Early Ovarian Follicles" International Journal of Molecular Sciences 22, no. 10: 5130. https://doi.org/10.3390/ijms22105130
APA StyleChen, Y., Liu, Q., Liu, R., Yang, C., Wang, X., Ran, Z., Zhou, S., Li, X., & He, C. (2021). A Prepubertal Mice Model to Study the Growth Pattern of Early Ovarian Follicles. International Journal of Molecular Sciences, 22(10), 5130. https://doi.org/10.3390/ijms22105130






