Glycosylation in Axonal Guidance
Abstract
:1. Introduction
2. N-Linked and O-Linked Glycosylation in Axonal Guidance
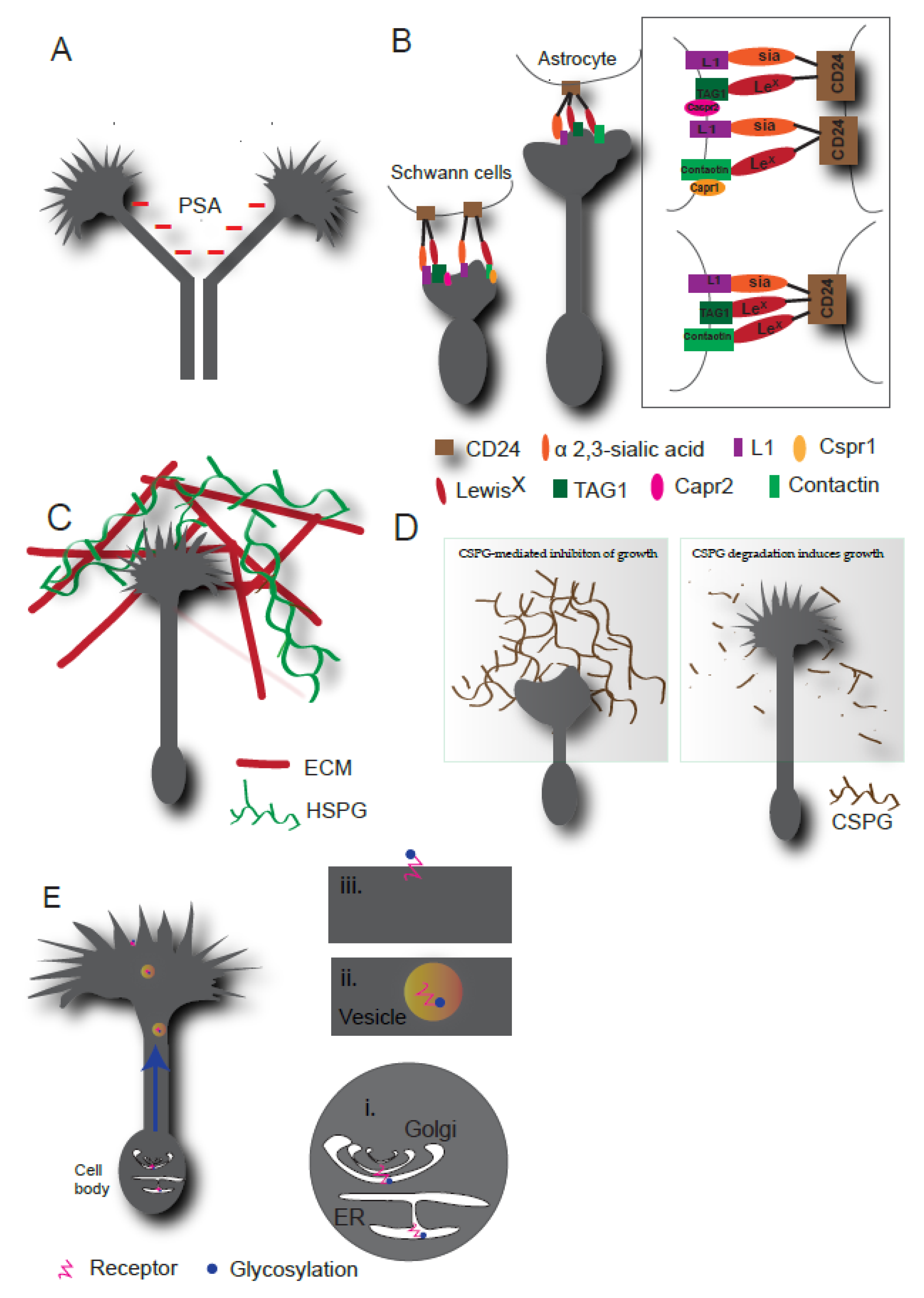
2.1. Glycosylation of Cell Adhesion Molecules in Axonal Guidance
2.2. Glycosylation of Dystroglycan in Axonal Guidance
2.3. Glycosylation of Endoglycan in Axonal Guidance
3. Glycosaminoglycans (GAGs) in Axonal Guidance
3.1. Hyaluronan (HA) in Axonal Guidance
3.2. Heparan Sulfate Proteoglycan (HSPGs) in Axonal Guidance
3.2.1. Regulation of Slit by HSPGs
3.2.2. Regulation of Netrin-1 by HSPGs
3.2.3. Regulation of Semaphorins by HSPGs
3.2.4. Regulation of Ephrins by HSPG
3.3. Chondroitin Sulfate Proteoglycan (CSPGs) in Axonal Guidance
4. Conclusions and Future Challenges
Author Contributions
Funding
Conflicts of Interest
References
- Comer, J.; Alvarez, S.; Butler, S.; Kaltschmidt, J. Commissural axon guidance in the developing spinal cord: From Cajal to the present day. Neural Dev. 2019, 14, 1–16. [Google Scholar]
- Stoeckli, E. Where does axon guidance lead us? F1000Research 2017, 6, 78. [Google Scholar] [CrossRef] [Green Version]
- Lowery, L.A.; Van Vactor, D. The trip of the tip: Understanding the growth cone machinery. Nat. Rev. Mol. cell Biol. 2009, 10, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Bellon, A.; Mann, F. Keeping up with advances in axon guidance. Curr. Opin. Neurobiol. 2018, 53, 183–191. [Google Scholar] [CrossRef] [PubMed]
- McCormick, L.E.; Gupton, S.L. Mechanistic advances in axon pathfinding. Curr. Opin. Cell Biol. 2020, 63, 11–19. [Google Scholar] [CrossRef]
- Boyer, N.P.; Gupton, S.L. Revisiting Netrin-1: One who guides (axons). Front. Cell. Neurosci. 2018, 12, 221. [Google Scholar] [CrossRef]
- Stoeckli, E.T. Understanding axon guidance: Are we nearly there yet? Development 2018, 145, dev151415. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Makihara, S.; Yam, P.T.; Teo, S.; Renier, N.; Balekoglu, N.; Moreno-Bravo, J.A.; Olsen, O.; Chédotal, A.; Charron, F. Long-range guidance of spinal commissural axons by netrin1 and sonic hedgehog from midline floor plate cells. Neuron 2019, 101, 635–647.e634. [Google Scholar] [CrossRef] [Green Version]
- Varadarajan, S.G.; Kong, J.H.; Phan, K.D.; Kao, T.-J.; Panaitof, S.C.; Cardin, J.; Eltzschig, H.; Kania, A.; Novitch, B.G.; Butler, S.J. Netrin1 produced by neural progenitors, not floor plate cells, is required for axon guidance in the spinal cord. Neuron 2017, 94, 790–799.e793. [Google Scholar] [CrossRef] [Green Version]
- Araújo, S.J.; Tear, G. Axon guidance mechanisms and molecules: Lessons from invertebrates. Nat. Rev. Neurosci. 2003, 4, 910–922. [Google Scholar] [CrossRef]
- Vysokov, N.V.; Silva, J.-P.; Lelianova, V.G.; Suckling, J.; Cassidy, J.; Blackburn, J.K.; Yankova, N.; Djamgoz, M.B.; Kozlov, S.V.; Tonevitsky, A.G. Proteolytically released Lasso/teneurin-2 induces axonal attraction by interacting with latrophilin-1 on axonal growth cones. Elife 2018, 7, e37935. [Google Scholar] [CrossRef]
- Guy, A.T.; Nagatsuka, Y.; Ooashi, N.; Inoue, M.; Nakata, A.; Greimel, P.; Inoue, A.; Nabetani, T.; Murayama, A.; Ohta, K. Glycerophospholipid regulation of modality-specific sensory axon guidance in the spinal cord. Science 2015, 349, 974–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, X.; Qiu, Y.; Gao, Y.; Wan, D.; Zhu, H. A subtle network mediating axon guidance: Intrinsic dynamic structure of growth cone, attractive and repulsive molecular cues, and the intermediate role of signaling pathways. Neural Plast. 2019. [Google Scholar] [CrossRef] [PubMed]
- Chédotal, A. Roles of axon guidance molecules in neuronal wiring in the developing spinal cord. Nat. Rev. Neurosci. 2019, 20, 380–396. [Google Scholar] [CrossRef] [PubMed]
- Seiradake, E.; Jones, E.Y.; Klein, R. Structural perspectives on axon guidance. Annu. Rev. cell Dev. Biol. 2016, 32, 577–608. [Google Scholar] [CrossRef]
- He, W.; Wei, L.; Zou, Q. Research progress in protein posttranslational modification site prediction. Brief. Funct. Genom. 2019, 18, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Higuero, A.M.; Díez-Revuelta, N.; Abad-Rodríguez, J. The sugar code in neuronal physiology. Histochem. Cell Biol. 2017, 147, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Reily, C.; Stewart, T.J.; Renfrow, M.B.; Novak, J. Glycosylation in health and disease. Nat. Rev. Nephrol. 2019, 15, 346–366. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.; Wang, X.-J.; Xue, Y.; Liu, M.-Q.; Zeng, W.-F.; Zhang, Y.; Zhang, L.; Gao, X.; Yan, G.-Q.; Yao, J. In-Depth mapping of the mouse brain N-Glycoproteome reveals widespread N-Glycosylation of diverse brain proteins. Oncotarget 2016, 7, 38796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohtsubo, K.; Marth, J.D. Glycosylation in cellular mechanisms of health and disease. Cell 2006, 126, 855–867. [Google Scholar] [CrossRef] [Green Version]
- Stanley, P. What have we learned from glycosyltransferase knockouts in mice? J. Mol. Biol. 2016, 428, 3166–3182. [Google Scholar] [CrossRef] [Green Version]
- Scott, H.; Panin, V.M. N-glycosylation in regulation of the nervous system. In Glycobiology of the Nervous System; Springer: Berlin, Germany, 2014; pp. 367–394. [Google Scholar]
- Masu, M. Proteoglycans and axon guidance: A new relationship between old partners. J. Neurochem. 2016, 139, 58–75. [Google Scholar] [CrossRef]
- Sytnyk, V.; Leshchyns’ka, I.; Schachner, M. Neural glycomics: The sweet side of nervous system functions. Cell. Mol. Life Sci. 2020, 78, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Huang, Y.; Cho, B.G.; Zhong, J.; Gautam, S.; Peng, W.; Williamson, S.D.; Banazadeh, A.; Torres-Ulloa, K.Y.; Mechref, Y. Advances in mass spectrometry-based glycomics. Electrophoresis 2018, 39, 3063–3081. [Google Scholar] [CrossRef]
- Ye, Z.; Marth, J.D. N-glycan branching requirement in neuronal and postnatal viability. Glycobiology 2004, 14, 547–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Ha, S.; Kim, M.; Kim, S.-W.; Yun, J.; Ozcan, S.; Hwang, H.; Ji, I.J.; Yin, D.; Webster, M.J. Spatial and temporal diversity of glycome expression in mammalian brain. Proc. Natl. Acad. Sci. USA 2020, 117, 28743–28753. [Google Scholar] [CrossRef]
- Wang, A.C.; Jensen, E.H.; Rexach, J.E.; Vinters, H.V.; Hsieh-Wilson, L.C. Loss of O-GlcNAc glycosylation in forebrain excitatory neurons induces neurodegeneration. Proc. Natl. Acad. Sci. USA 2016, 113, 15120–15125. [Google Scholar] [CrossRef] [Green Version]
- Ono, K.; Tomasiewicz, H.; Magnuson, T.; Rutishauser, U. N-CAM mutation inhibits tangential neuronal migration and is phenocopied by enzymatic removal of polysialic acid. Neuron 1994, 13, 595–609. [Google Scholar] [CrossRef]
- Weinhold, B.; Seidenfaden, R.; Röckle, I.; Mühlenhoff, M.; Schertzinger, F.; Conzelmann, S.; Marth, J.D.; Gerardy-Schahn, R.; Hildebrandt, H. Genetic ablation of polysialic acid causes severe neurodevelopmental defects rescued by deletion of the neural cell adhesion molecule. J. Biol. Chem. 2005, 280, 42971–42977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hildebrandt, H.; Mühlenhoff, M.; Oltmann-Norden, I.; Röckle, I.; Burkhardt, H.; Weinhold, B.; Gerardy-Schahn, R. Imbalance of neural cell adhesion molecule and polysialyltransferase alleles causes defective brain connectivity. Brain 2009, 132, 2831–2838. [Google Scholar] [CrossRef] [Green Version]
- Monnier, P.P.; Beck, S.G.; Bolz, J.; Henke-Fahle, S. The polysialic acid moiety of the neural cell adhesion molecule is involved in intraretinal guidance of retinal ganglion cell axons. Dev. Biol. 2001, 229, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Rutishauser, U.; Watanabe, M.; Silver, J.; Troy, F.A.; Vimr, E.R. Specific alteration of NCAM-mediated cell adhesion by an endoneuraminidase. J. Cell Biol. 1985, 101, 1842–1849. [Google Scholar] [CrossRef] [Green Version]
- Koyama, R.; Ikegaya, Y. The molecular and cellular mechanisms of axon guidance in mossy fiber sprouting. Front. Neurol. 2018, 9, 382. [Google Scholar] [CrossRef]
- Angata, K.; Huckaby, V.; Ranscht, B.; Terskikh, A.; Marth, J.D.; Fukuda, M. Polysialic acid-directed migration and differentiation of neural precursors are essential for mouse brain development. Mol. Cell. Biol. 2007, 27, 6659–6668. [Google Scholar] [CrossRef] [Green Version]
- Rutishauser, U.; Landmesser, L. Polysialic acid in the vertebrate nervous system: A promoter of plasticity in cell-cell interactions. Trends Neurosci. 1996, 19, 422–427. [Google Scholar] [CrossRef]
- Kanato, Y.; Kitajima, K.; Sato, C. Direct binding of polysialic acid to a brain-derived neurotrophic factor depends on the degree of polymerization. Glycobiology 2008, 18, 1044–1053. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.-L.; Wu, G.-Z.; Dawe, G.S.; Zeng, L.; Cui, S.-S.; Loers, G.; Tilling, T.; Sun, L.; Schachner, M.; Xiao, Z.-C. Cell surface sialylation and fucosylation are regulated by L1 via phospholipase Cγ and cooperate to modulate neurite outgrowth, cell survival and migration. PLoS ONE 2008, 3, e3841. [Google Scholar] [CrossRef] [Green Version]
- Kleene, R.; Yang, H.; Kutsche, M.; Schachner, M. The neural recognition molecule L1 is a sialic acid-binding lectin for CD24, which induces promotion and inhibition of neurite outgrowth. J. Biol. Chem. 2001, 276, 21656–21663. [Google Scholar] [CrossRef] [Green Version]
- Lieberoth, A.; Splittstoesser, F.; Katagihallimath, N.; Jakovcevski, I.; Loers, G.; Ranscht, B.; Karagogeos, D.; Schachner, M.; Kleene, R. Lewisx and α2, 3-sialyl glycans and their receptors TAG-1, Contactin, and L1 mediate CD24-dependent neurite outgrowth. J. Neurosci. 2009, 29, 6677–6690. [Google Scholar] [CrossRef] [Green Version]
- Frei, J.A.; Stoeckli, E.T. SynCAMs–From axon guidance to neurodevelopmental disorders. Mol. Cell. Neurosci. 2017, 81, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Galuska, S.P.; Rollenhagen, M.; Kaup, M.; Eggers, K.; Oltmann-Norden, I.; Schiff, M.; Hartmann, M.; Weinhold, B.; Hildebrandt, H.; Geyer, R. Synaptic cell adhesion molecule SynCAM 1 is a target for polysialylation in postnatal mouse brain. Proc. Natl. Acad. Sci. USA 2010, 107, 10250–10255. [Google Scholar] [CrossRef] [Green Version]
- Agarwala, K.L.; Ganesh, S.; Amano, K.; Suzuki, T.; Yamakawa, K. DSCAM, a highly conserved gene in mammals, expressed in differentiating mouse brain. Biochem. Biophys. Res. Commun. 2001, 281, 697–705. [Google Scholar] [CrossRef]
- Li, S.-A.; Cheng, L.; Yu, Y.; Wang, J.-h.; Chen, Q. Structural basis of Dscam1 homodimerization: Insights into context constraint for protein recognition. Sci. Adv. 2016, 2, e1501118. [Google Scholar] [CrossRef] [Green Version]
- Medina-Cano, D.; Ucuncu, E.; Nguyen, L.S.; Nicouleau, M.; Lipecka, J.; Bizot, J.-C.; Thiel, C.; Foulquier, F.; Lefort, N.; Faivre-Sarrailh, C. High N-glycan multiplicity is critical for neuronal adhesion and sensitizes the developing cerebellum to N-glycosylation defect. Elife 2018, 7, e38309. [Google Scholar] [CrossRef]
- Henion, T.R.; Faden, A.A.; Knott, T.K.; Schwarting, G.A. β3GnT2 maintains adenylyl cyclase-3 signaling and axon guidance molecule expression in the olfactory epithelium. J. Neurosci. 2011, 31, 6576–6586. [Google Scholar] [CrossRef]
- Lindenmaier, L.B.; Parmentier, N.; Guo, C.; Tissir, F.; Wright, K.M. Dystroglycan is a scaffold for extracellular axon guidance decisions. Elife 2019, 8, e42143. [Google Scholar] [CrossRef]
- Moore, C.J.; Winder, S.J. Dystroglycan versatility in cell adhesion: A tale of multiple motifs. Cell Commun. Signal. 2010, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Clements, R.; Turk, R.; Campbell, K.P.; Wright, K.M. Dystroglycan maintains inner limiting membrane integrity to coordinate retinal development. J. Neurosci. 2017, 37, 8559–8574. [Google Scholar] [CrossRef] [Green Version]
- Wright, K.M.; Lyon, K.A.; Leung, H.; Leahy, D.J.; Ma, L.; Ginty, D.D. Dystroglycan organizes axon guidance cue localization and axonal pathfinding. Neuron 2012, 76, 931–944. [Google Scholar] [CrossRef] [Green Version]
- Godfrey, C.; Foley, A.R.; Clement, E.; Muntoni, F. Dystroglycanopathies: Coming into focus. Curr. Opin. Genet. Dev. 2011, 21, 278–285. [Google Scholar] [CrossRef]
- Baeriswyl, T.; Dumoulin, A.; Schaettin, M.; Tsapara, G.; Niederkofler, V.; Helbling, D.; Avilés, E.; Frei, J.A.; Wilson, N.H.; Gesemann, M. Endoglycan plays a role in axon guidance by modulating cell adhesion. Elife 2021, 10, e64767. [Google Scholar] [CrossRef]
- Miyata, S.; Kitagawa, H. Formation and remodeling of the brain extracellular matrix in neural plasticity: Roles of chondroitin sulfate and hyaluronan. Biochim. et Biophys. Acta (BBA)-Gen. Subj. 2017, 1861, 2420–2434. [Google Scholar] [CrossRef]
- Lin, L.; Wang, J.; Chan, C.K.; Chan, S.O. Effects of exogenous hyaluronan on midline crossing and axon divergence in the optic chiasm of mouse embryos. Eur. J. Neurosci. 2007, 26, 1–11. [Google Scholar] [CrossRef]
- Balashova, A.; Pershin, V.; Zaborskaya, O.; Tkachenko, N.; Mironov, A.; Guryev, E.; Kurbatov, L.; Gainullin, M.; Mukhina, I. Enzymatic digestion of hyaluronan-based brain extracellular matrix in vivo can induce seizures in neonatal mice. Front. Neurosci. 2019, 13, 1033. [Google Scholar] [CrossRef] [Green Version]
- Haupt, C.; Huber, A.B. How axons see their way–axonal guidance in the visual system. Front. Biosci 2008, 13, 3136–3149. [Google Scholar] [CrossRef] [Green Version]
- Förster, E.; Zhao, S.; Frotscher, M. Hyaluronan-associated adhesive cues control fiber segregation in the hippocampus. Development 2001, 128, 3029–3039. [Google Scholar] [CrossRef]
- Bülow, H.E.; Hobert, O. Differential sulfations and epimerization define heparan sulfate specificity in nervous system development. Neuron 2004, 41, 723–736. [Google Scholar] [CrossRef] [Green Version]
- Smart, A.D.; Course, M.M.; Rawson, J.; Selleck, S.; Van Vactor, D.; Johnson, K.G. Heparan sulfate proteoglycan specificity during axon pathway formation in the Drosophila embryo. Dev. Neurobiol. 2011, 71, 608–618. [Google Scholar] [CrossRef] [Green Version]
- Inatani, M.; Irie, F.; Plump, A.S.; Tessier-Lavigne, M.; Yamaguchi, Y. Mammalian brain morphogenesis and midline axon guidance require heparan sulfate. Science 2003, 302, 1044–1046. [Google Scholar] [CrossRef]
- Esko, J.D.; Lindahl, U. Molecular diversity of heparan sulfate. J. Clin. Investig. 2001, 108, 169–173. [Google Scholar] [CrossRef]
- Esko, J.D.; Selleck, S.B. Order out of chaos: Assembly of ligand binding sites in heparan sulfate. Annu. Rev. Biochem. 2002, 71, 435–471. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.G.; Ghose, A.; Epstein, E.; Lincecum, J.; O’Connor, M.B.; Van Vactor, D. Axonal heparan sulfate proteoglycans regulate the distribution and efficiency of the repellent slit during midline axon guidance. Curr. Biol. 2004, 14, 499–504. [Google Scholar] [CrossRef] [Green Version]
- Manavalan, M.A.; Jayasinghe, V.R.; Grewal, R.; Bhat, K.M. The glycosylation pathway is required for the secretion of Slit and for the maintenance of the Slit receptor Robo on axons. Sci. Signal. 2017, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Pasquale, V.; Pavone, L.M. Heparan sulfate proteoglycans: The sweet side of development turns sour in mucopolysaccharidoses. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2019, 1865, 165539. [Google Scholar] [CrossRef] [PubMed]
- Ypsilanti, A.R.; Zagar, Y.; Chédotal, A. Moving away from the midline: New developments for Slit and Robo. Development 2010, 137, 1939–1952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piper, M.; Anderson, R.; Dwivedy, A.; Weinl, C.; Van Horck, F.; Leung, K.M.; Cogill, E.; Holt, C. Signaling mechanisms underlying Slit2-induced collapse of Xenopus retinal growth cones. Neuron 2006, 49, 215–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pratt, T.; Conway, C.D.; Tian, N.M.-L.; Price, D.J.; Mason, J.O. Heparan sulphation patterns generated by specific heparan sulfotransferase enzymes direct distinct aspects of retinal axon guidance at the optic chiasm. J. Neurosci. 2006, 26, 6911–6923. [Google Scholar] [CrossRef] [Green Version]
- Yung, A.R.; Nishitani, A.M.; Goodrich, L.V. Phenotypic analysis of mice completely lacking netrin 1. Development 2015, 142, 3686–3691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glasgow, S.D.; Ruthazer, E.S.; Kennedy, T.E. Guiding synaptic plasticity: Novel roles for netrin-1 in synaptic plasticity and memory formation in the adult brain. J. Physiol. 2021, 599, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Dun, X.-P.; Parkinson, D.B. Role of netrin-1 signaling in nerve regeneration. Int. J. Mol. Sci. 2017, 18, 491. [Google Scholar] [CrossRef] [Green Version]
- Serafini, T.; Colamarino, S.A.; Leonardo, E.D.; Wang, H.; Beddington, R.; Skarnes, W.C.; Tessier-Lavigne, M. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell 1996, 87, 1001–1014. [Google Scholar] [CrossRef] [Green Version]
- Finci, L.I.; Krüger, N.; Sun, X.; Zhang, J.; Chegkazi, M.; Wu, Y.; Schenk, G.; Mertens, H.D.; Svergun, D.I.; Zhang, Y. The crystal structure of netrin-1 in complex with DCC reveals the bifunctionality of netrin-1 as a guidance cue. Neuron 2014, 83, 839–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumoto, Y.; Irie, F.; Inatani, M.; Tessier-Lavigne, M.; Yamaguchi, Y. Netrin-1/DCC signaling in commissural axon guidance requires cell-autonomous expression of heparan sulfate. J. Neurosci. 2007, 27, 4342–4350. [Google Scholar] [CrossRef] [PubMed]
- Blanchette, C.R.; Perrat, P.N.; Thackeray, A.; Bénard, C.Y. Glypican is a modulator of netrin-mediated axon guidance. PLoS Biol. 2015, 13, e1002183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alto, L.T.; Terman, J.R. Semaphorins and their signaling mechanisms. Method Mol. Bio. 2017, 1493, 1–25. [Google Scholar]
- De Wit, J.; De Winter, F.; Klooster, J.; Verhaagen, J. Semaphorin 3A displays a punctate distribution on the surface of neuronal cells and interacts with proteoglycans in the extracellular matrix. Mol. Cell. Neurosci. 2005, 29, 40–55. [Google Scholar] [CrossRef]
- Klein, R. Eph/ephrin signaling in morphogenesis, neural development and plasticity. Curr. Opin. Cell Biol. 2004, 16, 580–589. [Google Scholar] [CrossRef]
- Kolodkin, A.L.; Tessier-Lavigne, M. Mechanisms and molecules of neuronal wiring: A primer. Cold Spring Harb. Perspect. Biol. 2011, 3, a001727. [Google Scholar] [CrossRef] [Green Version]
- Irie, F.; Okuno, M.; Matsumoto, K.; Pasquale, E.B.; Yamaguchi, Y. Heparan sulfate regulates ephrin-A3/EphA receptor signaling. Proc. Natl. Acad. Sci. USA 2008, 105, 12307–12312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mire, E.; Hocine, M.; Bazellières, E.; Jungas, T.; Davy, A.; Chauvet, S.; Mann, F. Developmental upregulation of Ephrin-B1 silences Sema3C/Neuropilin-1 signaling during post-crossing navigation of corpus callosum axons. Curr. Biol. 2018, 28, 1768–1782.e1764. [Google Scholar] [CrossRef] [Green Version]
- Chung, K.; Taylor, J.; Shum, D.; Chan, S. Axon routing at the optic chiasm after enzymatic removal of chondroitin sulfate in mouse embryos. Development 2000, 127, 2673–2683. [Google Scholar] [CrossRef]
- Bernhardt, R.R.; Schachner, M. Chondroitin sulfates affect the formation of the segmental motor nerves in zebrafish embryos. Dev. Biol. 2000, 221, 206–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walz, A.; McFarlane, S.; Brickman, Y.G.; Nurcombe, V.; Bartlett, P.F.; Holt, C.E. Essential role of heparan sulfates in axon navigation and targeting in the developing visual system. Development 1997, 124, 2421–2430. [Google Scholar] [CrossRef]
- Ichijo, H.; Kawabata, I. Roles of the telencephalic cells and their chondroitin sulfate proteoglycans in delimiting an anterior border of the retinal pathway. J. Neurosci. 2001, 21, 9304–9314. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.; Tilve, S.; Huang, Z.; Zhou, L.; Geller, H.M.; Yu, P. Effect of chondroitin sulfate proteoglycans on neuronal cell adhesion, spreading and neurite growth in culture. Neural Regen. Res. 2018, 13, 289. [Google Scholar]
- Tan, C.L.; Kwok, J.C.; Patani, R.; Chandran, S.; Fawcett, J.W. Integrin activation promotes axon growth on inhibitory chondroitin sulfate proteoglycans by enhancing integrin signaling. J. Neurosci. 2011, 31, 6289–6295. [Google Scholar] [CrossRef]
- Vo, T.; Carulli, D.; Ehlert, E.M.; Kwok, J.C.; Dick, G.; Mecollari, V.; Moloney, E.B.; Neufeld, G.; de Winter, F.; Fawcett, J.W. The chemorepulsive axon guidance protein semaphorin3A is a constituent of perineuronal nets in the adult rodent brain. Mol. Cell. Neurosci. 2013, 56, 186–200. [Google Scholar] [CrossRef] [PubMed]
- Kantor, D.B.; Chivatakarn, O.; Peer, K.L.; Oster, S.F.; Inatani, M.; Hansen, M.J.; Flanagan, J.G.; Yamaguchi, Y.; Sretavan, D.W.; Giger, R.J. Semaphorin 5A is a bifunctional axon guidance cue regulated by heparan and chondroitin sulfate proteoglycans. Neuron 2004, 44, 961–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Sajid, M.S.; Trakhtenberg, E.F. The extent of extra-axonal tissue damage determines the levels of CSPG upregulation and the success of experimental axon regeneration in the CNS. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rauvala, H.; Paveliev, M.; Kuja-Panula, J.; Kulesskaya, N. Inhibition and enhancement of neural regeneration by chondroitin sulfate proteoglycans. Neural Regen. Res. 2017, 12, 687. [Google Scholar] [CrossRef]
- Li, F.; Li, C.; Revote, J.; Zhang, Y.; Webb, G.I.; Li, J.; Song, J.; Lithgow, T. GlycoMine struct: A new bioinformatics tool for highly accurate mapping of the human N-linked and O-linked glycoproteomes by incorporating structural features. Sci. Rep. 2016, 6, 1–16. [Google Scholar]
- Grandin, M.; Meier, M.; Delcros, J.G.; Nikodemus, D.; Reuten, R.; Patel, T.R.; Goldschneider, D.; Orriss, G.; Krahn, N.; Boussouar, A. Structural decoding of the Netrin-1/UNC5 interaction and its therapeutical implications in cancers. Cancer Cell 2016, 29, 173–185. [Google Scholar] [CrossRef] [Green Version]
- Roy, R.; Cao, Y.; Kaltner, H.; Kottari, N.; Shiao, T.C.; Belkhadem, K.; André, S.; Manning, J.C.; Murphy, P.V.; Gabius, H.-J. Teaming up synthetic chemistry and histochemistry for activity screening in galectin-directed inhibitor design. Histochem. Cell Biol. 2017, 147, 285–301. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Chen, W.; Smeekens, J.M.; Wu, R. An enrichment method based on synergistic and reversible covalent interactions for large-scale analysis of glycoproteins. Nat. Commun. 2018, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Osimanjiang, W.; Roballo, K.C.S.; Houck, B.D.; Ito, M.; Antonopoulos, A.; Dell, A.; Haslam, S.M.; Bushman, J.S. Analysis of N-and O-Linked Glycosylation: Differential Glycosylation after Rat Spinal Cord Injury. J. Neurotrauma 2020, 37, 1954–1962. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mutalik, S.P.; Gupton, S.L. Glycosylation in Axonal Guidance. Int. J. Mol. Sci. 2021, 22, 5143. https://doi.org/10.3390/ijms22105143
Mutalik SP, Gupton SL. Glycosylation in Axonal Guidance. International Journal of Molecular Sciences. 2021; 22(10):5143. https://doi.org/10.3390/ijms22105143
Chicago/Turabian StyleMutalik, Sampada P., and Stephanie L. Gupton. 2021. "Glycosylation in Axonal Guidance" International Journal of Molecular Sciences 22, no. 10: 5143. https://doi.org/10.3390/ijms22105143
APA StyleMutalik, S. P., & Gupton, S. L. (2021). Glycosylation in Axonal Guidance. International Journal of Molecular Sciences, 22(10), 5143. https://doi.org/10.3390/ijms22105143





