Interaction between Galectin-3 and Integrins Mediates Cell-Matrix Adhesion in Endothelial Cells and Mesenchymal Stem Cells
Abstract
:1. Introduction
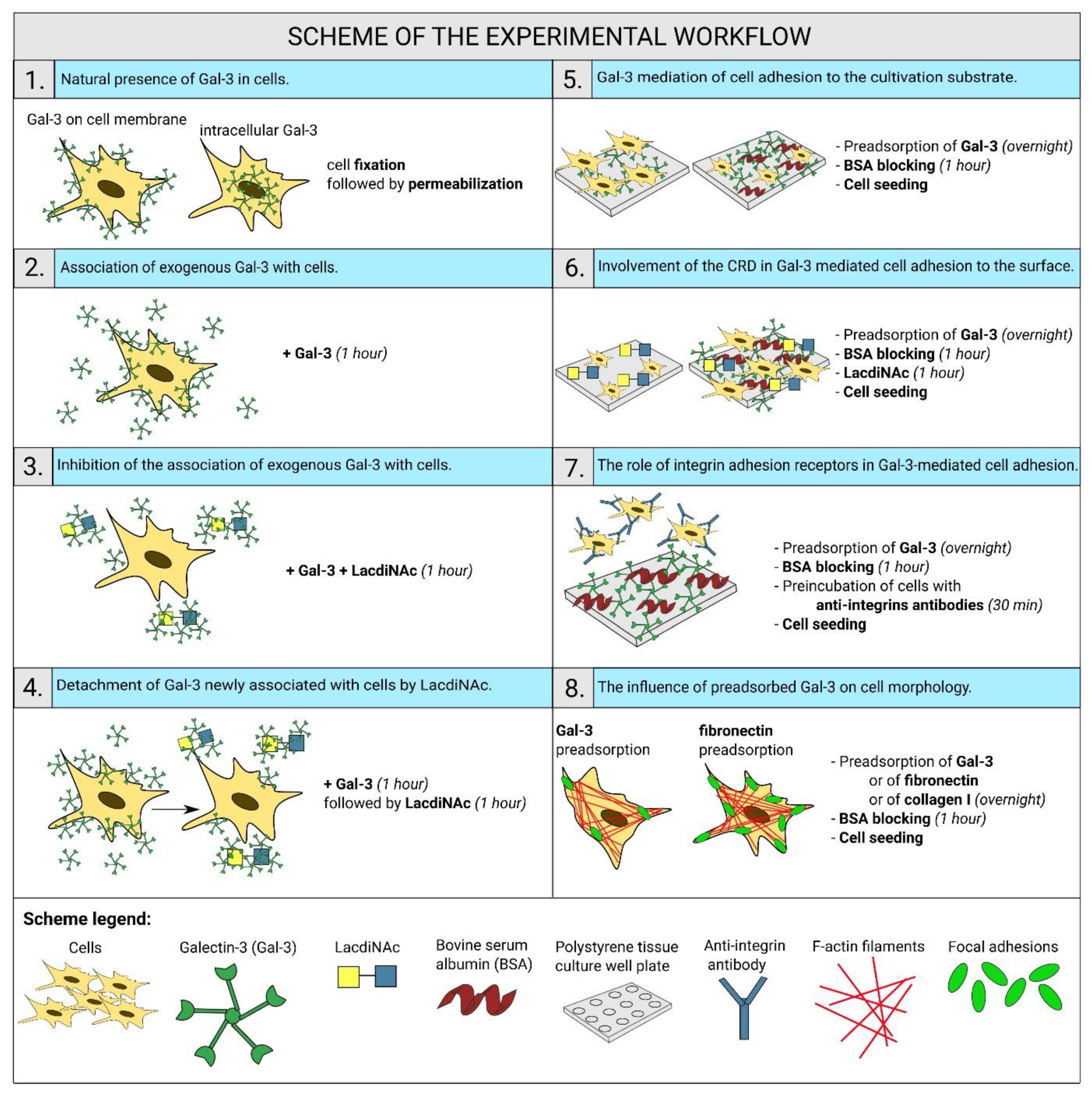
2. Results
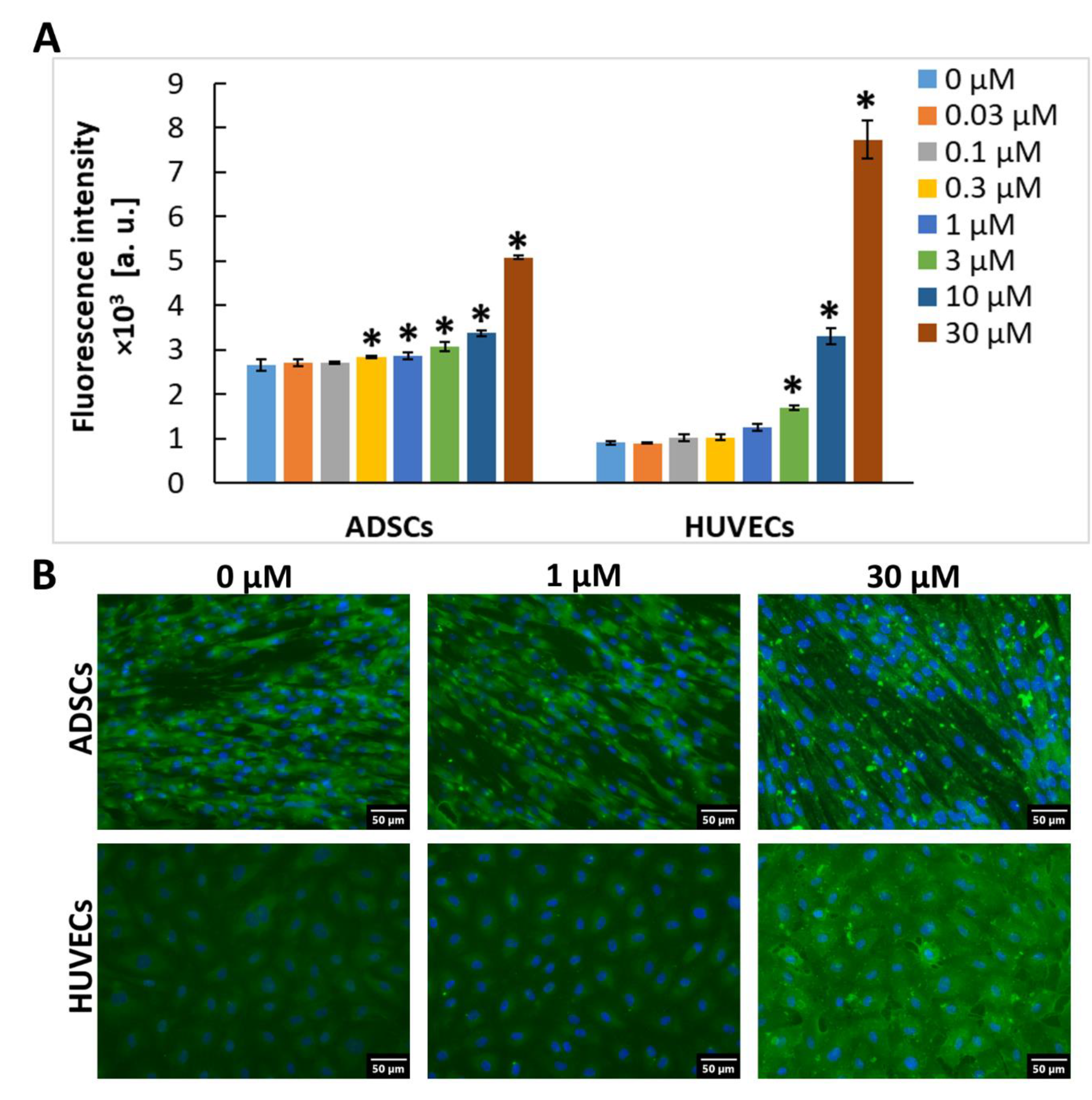
2.1. Gal-3 Is Naturally Present in ADSCs and HUVECs
2.2. Exogenous Gal-3 Associates with ADSCs and HUVECs
2.3. The Association of Gal-3 with Cells Is Reduced by LacdiNAc
2.4. LacdiNAc Is Capable to Detach Gal-3 Newly Associated with the Cell Membrane
2.5. Gal-3 Mediates Cell Adhesion to the Cultivation Substrate
2.6. Gal-3-Mediated Cell Adhesion to the Surface Does Not Involve the CRD-Domain
2.7. Integrins Are Cell Receptors for the Substrate-Bound Galectin
2.8. Cell Morphology on the Surface Preadsorbed with Gal-3
3. Discussion
4. Materials and Methods
4.1. Preparation of Human Recombinant Gal-3
4.2. Preparation and Characterization of LacdiNAc Ligand for Gal-3
4.3. Cell Models
4.4. Natural Presence of Gal-3 in ADSCs and HUVECs
4.5. Association of Free Gal-3 with the Cell Surface
4.6. Cell Adhesion to Gal-3 Immobilized on Cultivation Substrate
4.7. The Role of Integrin Adhesion Receptors in Gal-3-Mediated Cell Adhesion
4.8. Comparison of Gal-3 with Other Adhesion Ligands and Cell Morphology
4.9. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADSC | Adipose tissue-derived stem cells |
| BSA | Bovine serum albumin |
| CI | Cell index |
| CRD | Carbohydrate recognition domain |
| EBM-2 | Endothelial cell basal medium-2 |
| ECM | Extracellular matrix |
| EGF | Epidermal growth factor |
| EGM-2 | Endothelial cell growth medium-2 |
| EPC | Endothelial progenitor cell |
| FBS | Fetal bovine serum |
| FGF-2 | Fibroblast growth factor-2 |
| Gal-3 | Galectin-3 |
| GRGDSP | Gly-Arg-Gly-Asp-Ser-Pro integrin-blocking RGD-based peptide |
| HIV | Human immunodeficiency virus |
| HPMA | N-(2-Hydroxypropyl) methacrylamide |
| HUVEC | Human umbilical vein endothelial cell |
| IGF-1 | Insulin-like growth factor-1 |
| LacdiNAc | 2-Acetamido-2-deoxy-β-d-galactopyranosyl-(1→4)-2-acetamido-2-deoxy-d-glucopyranose |
| LacNAc | β-d-Galactopyranosyl-(1→4)-2-acetamido-2-deoxy-d-glucopyranose |
| PBS | Phosphate-buffered saline |
| RGD | Arg-Gly-Asp binding motif |
| RT | Room temperature |
References
- Hughes, R.C. Galectins as modulators of cell adhesion. Biochimie 2001, 83, 667–676. [Google Scholar] [CrossRef]
- Cummings, R.D.; Liu, F.T. Galectins. In Essentials of Glycobiology, 2nd ed.; Varki, A., Cummings, R.D., Esko, J.D., Freeze, H.H., Stanley, P., Bertozzi, C.R., Hart, G.W., Etzler, M.E., Eds.; Cold Spring Harbor: New York, NY, USA, 2009; ISBN 9780879697709. [Google Scholar]
- Xin, M.; Dong, X.W.; Guo, X.L. Role of the interaction between galectin-3 and cell adhesion molecules in cancer metastasis. Biomed. Pharmacother. 2015, 69, 179–185. [Google Scholar] [CrossRef]
- Barman, S.A.; Li, X.Y.; Haigh, S.; Kondrikov, D.; Mahboubi, K.; Bordan, Z.; Stepp, D.W.; Zhou, J.L.; Wang, Y.S.; Weintraub, D.S.; et al. Galectin-3 is expressed in vascular smooth muscle cells and promotes pulmonary hypertension through changes in proliferation, apoptosis, and fibrosis. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2019, 316, L784–L797. [Google Scholar] [CrossRef]
- Brinchmann, M.F.; Patel, D.M.; Iversen, M.H. The role of galectins as modulators of metabolism and inflammation. Mediat. Inflamm. 2018, 2018, 9186940. [Google Scholar] [CrossRef] [Green Version]
- Zick, Y.; Eisenstein, M.; Goren, R.A.; Hadari, Y.R.; Levy, Y.; Ronen, D. Role of galectin-8 as a modulator of cell adhesion and cell growth. Glycoconj. J. 2002, 19, 517–526. [Google Scholar] [CrossRef]
- Bojarová, P.; Křen, V. Sugared biomaterial binding lectins: Achievements and perspectives. Biomat. Sci. 2016, 4, 1142–1160. [Google Scholar] [CrossRef] [PubMed]
- Johannes, L.; Jacob, R.; Leffler, H. Galectins at a glance. J. Cell Sci. 2018, 131, jcs208884. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.T.; Wan, L. Galectin-3 and inflammation. Glycobiol. Insights 2016, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Fashanu, O.E.; Heckbert, S.R.; Aguilar, D.; Jensen, P.N.; Ballantyne, C.M.; Basu, S.; Hoogeveen, R.C.; deFilippi, C.; Cushman, M.; Folsom, A.R. Galectin-3 and venous thromboembolism incidence: The atherosclerosis risk in communities (ARIC) study. Res. Pract. Thromb. Haemost. 2017, 1, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Bojarová, P.; Tavares, M.R.; Laaf, D.; Bumba, L.; Petrásková, L.; Konefal, R.; Bláhová, M.; Pelantová, H.; Elling, L.; Etrych, T.; et al. Biocompatible glyconanomaterials based on HPMA-copolymer for specific targeting of galectin-3. J. Nanobiotechnol. 2018, 16, 73. [Google Scholar] [CrossRef] [PubMed]
- Laaf, D.; Bojarová, P.; Pelantová, H.; Křen, V.; Elling, L. Tailored multivalent neo-glycoproteins: Synthesis, evaluation, and application of a library of galectin-3-binding glycan ligands. Bioconjug. Chem. 2017, 28, 2832–2840. [Google Scholar] [CrossRef] [PubMed]
- Laaf, D.; Bojarová, P.; Elling, L.; Křen, V. Galectin-carbohydrate interactions in biomedicine and biotechnology. Trends Biotechnol. 2019, 37, 402–415. [Google Scholar] [CrossRef]
- Panjwani, N. Role of galectins in re-epithelialization of wounds. Ann. Transl. Med. 2014, 2, 89. [Google Scholar] [CrossRef]
- Iacobini, C.; Menini, S.; Ricci, C.; Scipioni, A.; Sansoni, V.; Cordone, S.; Taurino, M.; Serino, M.; Marano, G.; Federici, M.; et al. Accelerated lipid-induced atherogenesis in galectin-3-deficient mice: Role of lipoxidation via receptor-mediated mechanisms. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 831–836. [Google Scholar] [CrossRef] [Green Version]
- Nangia-Makker, P.; Honjo, Y.; Sarvis, R.; Akahani, S.; Hogan, V.; Pienta, K.J.; Raz, A. Galectin-3 induces endothelial cell morphogenesis and angiogenesis. Am. J. Pathol. 2000, 156, 899–909. [Google Scholar] [CrossRef] [Green Version]
- Laaf, D.; Bojarová, P.; Mikulová, B.; Pelantová, H.; Křen, V.; Elling, L. Two-step enzymatic synthesis of ß-d-N-acetylgalactosamine-(1→4)-d-N-acetylglucosamine (LacdiNAc) chitooligomers for deciphering galectin binding behavior. Adv. Synth. Catal. 2017, 359, 2101–2108. [Google Scholar] [CrossRef] [Green Version]
- Tavares, M.R.; Bláhová, M.; Sedláková, L.; Elling, L.; Pelantová, H.; Konefal, R.; Etrych, T.; Křen, V.; Bojarová, P.; Chytil, P. High-affinity N-(2-hydroxypropyl) methacrylamide copolymers with tailored N-acetyllactosamine presentation discriminate between galectins. Biomacromolecules 2020, 21, 641–652. [Google Scholar] [CrossRef]
- Bratteby, K.; Torkelsson, E.; L’Estrade, E.T.; Peterson, K.; Shalgunov, V.; Xiong, M.F.; Leffler, H.; Zetterberg, F.R.; Olsson, T.G.; Gillings, N.; et al. In Vivo veritas: F-18-radiolabeled glycomimetics allow insights into the pharmacological fate of galectin-3 inhibitors. J. Med. Chem. 2020, 63, 747–755. [Google Scholar] [CrossRef]
- Vašíček, T.; Spiwok, V.; Červený, J.; Petrásková, L.; Bumba, L.; Vrbata, D.; Pelantová, H.; Křen, V.; Bojarová, P. Regioselective 3-O-substitution of unprotected thiodigalactosides: Direct route to galectin inhibitors. Chem. Eur. J. 2020, 26, 9620–9631. [Google Scholar] [CrossRef] [PubMed]
- Bumba, L.; Laaf, D.; Spiwok, V.; Elling, L.; Křen, V.; Bojarová, P. Poly-N-acetyllactosamine neo-glycoproteins as nanomolar ligands of human galectin-3: Binding kinetics and modeling. Int. J. Mol. Sci. 2018, 19, 372. [Google Scholar] [CrossRef] [Green Version]
- Furuhata, S.; Ando, K.; Oki, M.; Aoki, K.; Ohnishi, S.; Aoyagi, K.; Sasaki, H.; Sakamoto, H.; Yoshida, T.; Ohnami, S. Gene expression profiles of endothelial progenitor cells by oligonucleotide microarray analysis. Mol. Cell. Biochem. 2007, 298, 125–138. [Google Scholar] [CrossRef]
- Chen, W.T.; Zhang, F.; Zhao, X.Q.; Yu, B.; Wang, B.W. Galectin-3 and TRIM16 coregulate osteogenic differentiation of human bone marrow-derived mesenchymal stem cells at least partly via enhancing autophagy. Bone 2020, 131. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Xu, X.; Wang, L.H.; Liu, G.J.; Li, Y.Q.; Wu, X.B.; Jing, Y.G.; Li, H.Y.; Wang, G.H. Senescent mesenchymal stem cells promote colorectal cancer cells growth via galectin-3 expression. Cell. Biosci. 2015, 5, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Šimonová, A.; Kupper, C.E.; Böcker, S.; Müller, A.; Hofbauerová, K.; Pelantová, H.; Elling, L.; Křen, V.; Bojarová, P. Chemo-enzymatic synthesis of LacdiNAc dimers of varying length as novel galectin ligands. J. Mol. Catal. B Enzym. 2014, 101, 47–55. [Google Scholar] [CrossRef]
- Hutchings, H.; Ortega, N.; Plouet, J. Extracellular matrix-bound vascular endothelial growth factor promotes endothelial cell adhesion, migration, and survival through integrin ligation. FASEB J. 2003, 17, 1520–1522. [Google Scholar] [CrossRef]
- Lee, M.H.; Brass, D.A.; Morris, R.; Composto, R.J.; Ducheyne, P. The effect of non-specific interactions on cellular adhesion using model surfaces. Biomaterials 2005, 26, 1721–1730. [Google Scholar] [CrossRef] [PubMed]
- da Silva, R.G.; Tavora, B.; Robinson, S.D.; Reynolds, L.E.; Szekeres, C.; Lamar, J.; Batista, S.; Kostourou, V.; Germain, M.A.; Reynolds, A.R.; et al. Endothelial alpha3beta1-integrin represses pathological angiogenesis and sustains endothelial-VEGF. Am. J. Pathol. 2010, 177, 1534–1548. [Google Scholar] [CrossRef]
- Hynes, R.O. Integrins: Bidirectional, allosteric signaling machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef] [Green Version]
- Goessler, U.R.; Bugert, P.; Bieback, K.; Stern-Straeter, J.; Bran, G.; Hormann, K.; Riedel, F. Integrin expression in stem cells from bone marrow and adipose tissue during chondrogenic differentiation. Int. J. Mol. Med. 2008, 21, 271–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reed, N.I.; Jo, H.; Chen, C.; Tsujino, K.; Arnold, T.D.; DeGrado, W.F.; Sheppard, D. The αvβ1 integrin plays a critical in vivo role in tissue fibrosis. Sci. Transl. Med. 2015, 7, 288ra79. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, A.L.; Barrett, J.W.; Slack, R.J. Pharmacological characterisation of a tool αvβ1 integrin small molecule RGD-mimetic inhibitor. Eur. J. Pharmacol. 2019, 842, 239–247. [Google Scholar] [CrossRef]
- Ahrens, I.; Domeij, H.; Topcic, D.; Haviv, I.; Merivirta, R.M.; Agrotis, A.; Leitner, E.; Jowett, J.B.; Bode, C.; Lappas, M.; et al. Successful in vitro expansion and differentiation of cord blood derived CD34+ cells into early endothelial progenitor cells reveals highly differential gene expression. PLoS ONE 2011, 6, e23210. [Google Scholar] [CrossRef] [Green Version]
- Fukumori, T.; Takenaka, Y.; Yoshii, T.; Kim, H.R.C.; Hogan, V.; Inohara, H.; Kagawa, S.; Raz, A. CD29 and CD7 mediate galectin-3-induced type II T-cell apoptosis. Cancer Res. 2003, 63, 8302–8311. [Google Scholar] [PubMed]
- Suzuki, O.; Abe, M. Cell surface N-glycosylation and sialylation regulate galectin-3-induced apoptosis in human diffuse large B cell lymphoma. Oncol. Rep. 2008, 19, 743–748. [Google Scholar] [CrossRef] [Green Version]
- Tadokoro, T.; Ikekita, M.; Toda, T.; Ito, H.; Sato, T.; Nakatani, R.; Hamaguchi, Y.; Furukawa, K. Involvement of galectin-3 with vascular cell adhesion molecule-1 in growth regulation of mouse BALB/3T3 cells. J. Biol. Chem. 2009, 284, 35556–35563. [Google Scholar] [CrossRef] [Green Version]
- Zhuo, Y.; Chammas, R.; Bellis, S.L. Sialylation of β1 Integrins blocks cell adhesion to galectin-3 and protects cells against galectin-3-induced apoptosis. J. Biol. Chem. 2008, 283, 22177–22185. [Google Scholar] [CrossRef] [PubMed]
- Lepur, A.; Carlsson, M.C.; Novak, R.; Dumic, J.; Nilsson, U.J.; Leffler, H. Galectin-3 endocytosis by carbohydrate independent and dependent pathways in different macrophage like cell types. Biochim. Biophys. Acta Gen. Subj. 2012, 1820, 804–818. [Google Scholar] [CrossRef]
- Gao, X.G.; Liu, D.; Fan, Y.Y.; Li, X.Z.; Xue, H.T.; Ma, Y.Y.; Zhou, Y.F.; Tai, G.H. The two endocytic pathways mediated by the carbohydrate recognition domain and regulated by the collagen-like domain of galectin-3 in vascular endothelial cells. PLoS ONE 2012, 7, e52430. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.G.; Zhi, Y.; Sun, L.; Peng, X.X.; Zhang, T.; Xue, H.T.; Tai, G.H.; Zhou, Y.F. The inhibitory effects of a rhamnogalacturonan I (RG-I) domain from ginseng pectin on galectin-3 and its structure-activity relationship. J. Biol. Chem. 2013, 288, 33953–33965. [Google Scholar] [CrossRef] [Green Version]
- John, C.M.; Leffler, H.; Kahl-Knutsson, B.; Svensson, I.; Jarvis, G.A. Truncated galectin-3 inhibits tumor growth and metastasis in orthotopic nude mouse model of human breast cancer. Clin. Cancer Res. 2003, 9, 2374–2383. [Google Scholar] [PubMed]
- Filipová, M.; Bojarová, P.; Tavares, M.R.; Bumba, L.; Elling, L.; Chytil, P.; Gunár, K.; Křen, V.; Etrych, T.; Janoušková, O. Glycopolymers for efficient inhibition of galectin-3: In Vitro proof of efficacy using suppression of T lymphocyte apoptosis and tumor cell migration. Biomacromolecules 2020, 21, 3122–3133. [Google Scholar] [CrossRef]
- MacKinnon, A.C.; Gibbons, M.A.; Farnworth, S.L.; Leffler, H.; Nilsson, U.J.; Delaine, T.; Simpson, A.J.; Forbes, S.J.; Hirani, N.; Gauldie, J.; et al. Regulation of transforming growth factor-β1-driven lung fibrosis by galectin-3. J. Respir. Crit. Care Med. 2012, 185, 537–546. [Google Scholar] [CrossRef] [Green Version]
- Ludwig, A.K.; Michalak, M.; Xiao, Q.; Gilles, U.; Medrano, F.J.; Ma, H.; FitzGerald, F.G.; Hasley, W.D.; Melendez-Davila, A.; Liu, M.; et al. Design-functionality relationships for adhesion/growth-regulatory galectins. Proc. Natl. Acad. Sci. USA 2019, 116, 2837–2842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thijssen, V.L.; HuIsmans, S.; Griffioen, A.W. The galectin profile of the endothelium—Altered expression and localization in activated and tumor endothelial cells. Am. J. Pathol. 2008, 172, 545–553. [Google Scholar] [CrossRef] [Green Version]
- Gieseke, F.; Bohringer, J.; Bussolari, R.; Dominici, M.; Handgretinger, R.; Muller, I. Human multipotent mesenchymal stromal cells use galectin-1 to inhibit immune effector cells. Blood 2010, 116, 3770–3779. [Google Scholar] [CrossRef]
- Gonen, T.; Donaldson, P.; Kistler, J. Galectin-3 is associated with the plasma membrane of lens fiber cells. Investig. Ophthal. Vis. Sci. 2000, 41, 199–203. [Google Scholar] [PubMed]
- Miller, M.C.; Ippel, H.; Suylen, D.; Klyosov, A.A.; Traber, P.G.; Hackeng, T.; Mayo, K.H. Binding of polysaccharides to human galectin-3 at a noncanonical site in its carbohydrate recognition domain. Glycobiology 2016, 26, 88–99. [Google Scholar] [CrossRef] [Green Version]
- Stegmayr, J.; Lepur, A.; Kahl-Knutson, B.; Aguilar-Moncayo, M.; Klyosov, A.A.; Field, R.A.; Oredsson, S.; Nilsson, U.J.; Leffler, H. Low or no inhibitory potency of the canonical galectin carbohydrate-binding site by pectins and galactomannans. J. Biol. Chem. 2016, 291, 13318–13334. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.Y.; Miller, M.C.; Xu, X.J.; Song, C.C.; Zhang, F.; Zheng, Y.; Zhou, Y.F.; Tai, G.H.; Mayo, K.H. NMR-based insight into galectin-3 binding to endothelial cell adhesion molecule CD146: Evidence for noncanonical interactions with the lectin’s CRD beta-sandwich F-face. Glycobiology 2019, 29, 608–618. [Google Scholar] [CrossRef]
- LeMarer, N.; Hughes, R.C. Effects of the carbohydrate-binding protein galectin-3 on the invasiveness of human breast carcinoma cells. Cell. Physiol. 1996, 168, 51–58. [Google Scholar] [CrossRef]
- Birdsall, B.; Feeney, J.; Burdett, I.D.J.; Bawumia, S.; Barboni, E.A.M.; Hughes, R.C. NMR solution studies of hamster galectin-3 and electron microscopic visualization of surface-adsorbed complexes: Evidence for interactions between the N- and C-terminal domains. Biochemistry 2001, 40, 4859–4866. [Google Scholar] [CrossRef]
- Benitez, P.L.; Mascharak, S.; Proctor, A.C.; Heilshorn, S.C. Use of protein-engineered fabrics to identify design rules for integrin ligand clustering in biomaterials. Integr. Biol. 2016, 8, 50–61. [Google Scholar] [CrossRef] [Green Version]
- Morandi, E.M.; Verstappen, R.; Zwierzina, M.E.; Geley, S.; Pierer, G.; Ploner, C. ITGAV and ITGA5 diversely regulate proliferation and adipogenic differentiation of human adipose derived stem cells. Sci. Rep. 2016, 6, 28889. [Google Scholar] [CrossRef] [Green Version]
- Fang, J.; Wei, Y.D.; Lv, C.R.; Peng, S.; Zhao, S.T.; Hua, J.L. CD61 promotes the differentiation of canine ADMSCs into PGC-like cells through modulation of TGF-beta signaling. Sci. Rep. 2017, 7, 43851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rico, P.; Rodrigo-Navarro, A.; de la Pena, M.; Moulisova, V.; Costell, M.; Salmeron-Sanchez, M. Simultaneous boron ion-channel/growth factor receptor activation for enhanced vascularization. Adv. Biosyst. 2019, 3, e1800220. [Google Scholar] [CrossRef]
- Foubert, P.; Varner, J.A. Integrins in tumor angiogenesis and lymphangiogenesis. Methods Mol. Biol. 2011, 757, 471–486. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, O.; Abe, M.; Hashimoto, Y. Sialylation and glycosylation modulate cell adhesion and invasion to extracellular matrix in human malignant lymphoma: Dependency on integrin and the Rho GTPase family. Int. J. Oncol. 2015, 47, 2091–2099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horton, M.A. The αvβ3 integrin “vitronectin receptor”. Int. J. Biochem. Cell B 1997, 29, 721–725. [Google Scholar] [CrossRef]
- Saravanan, C.; Liu, F.T.; Gipson, I.K.; Panjwani, N. Galectin-3 promotes lamellipodia formation in epithelial cells by interacting with complex N-glycans on alpha3beta1 integrin. J. Cell Sci. 2009, 122, 3684–3693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivkova, R.; Taborska, J.; Reparaz, A.; de Los Santos Pereira, A.; Kotelnikov, I.; Proks, V.; Kucka, J.; Svoboda, J.; Riedel, T.; Pop-Georgievski, O. Surface design of antifouling vascular constructs bearing biofunctional peptides for tissue regeneration applications. Int. J. Mol. Sci. 2020, 21, 6800. [Google Scholar] [CrossRef]
- Tang, D.; Chen, S.; Hou, D.; Gao, J.; Jiang, L.; Shi, J.; Liang, Q.; Kong, D.; Wang, S. Regulation of macrophage polarization and promotion of endothelialization by NO generating and PEG-YIGSR modified vascular graft. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 84, 1–11. [Google Scholar] [CrossRef]
- Devalliere, J.; Chen, Y.; Dooley, K.; Yarmush, M.L.; Uygun, B.E. Improving functional re-endothelialization of acellular liver scaffold using REDV cell-binding domain. Acta Biomater. 2018, 78, 151–164. [Google Scholar] [CrossRef]
- Estes, B.T.; Diekman, B.O.; Gimble, J.M.; Guilak, F. Isolation of adipose-derived stem cells and their induction to a chondrogenic phenotype. Nat. Protoc. 2010, 5, 1294–1311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bačáková, L.; Zárubová, J.; Trávníčková, M.; Musílková, J.; Pajorová, J.; Slepička, P.; Kasálková, N.S.; Švorčík, V.; Kolská, Z.; Motarjemi, H.; et al. Stem cells: Their source, potency and use in regenerative therapies with focus on adipose-derived stem cells—A review. Biotechnol. Adv. 2018, 36, 1111–1126. [Google Scholar] [CrossRef] [PubMed]
- Trávníčková, M.; Pajorová, J.; Zárubová, J.; Kročilová, N.; Molitor, M.; Bačáková, L. The influence of negative pressure and of the harvesting site on the characteristics of human adipose tissue-derived stromal cells from lipoaspirates. Stem Cells Int. 2020, 2020, 1016231. [Google Scholar] [CrossRef] [PubMed]
- Ratnikov, B.I.; Rozanov, D.V.; Postnova, T.I.; Baciu, P.G.; Zhang, H.; DiScipio, R.G.; Chestukhina, G.G.; Smith, J.W.; Deryugina, E.I.; Strongin, A.Y. An alternative processing of integrin alpha(v) subunit in tumor cells by membrane type-1 matrix metalloproteinase. J. Biol. Chem. 2002, 277, 7377–7385. [Google Scholar] [CrossRef] [Green Version]
- Benoit, Y.D.; Lussier, C.; Ducharme, P.A.; Sivret, S.; Schnapp, L.M.; Basora, N.; Beaulieu, J.F. Integrin alpha 8 beta 1 regulates adhesion, migration and proliferation of human intestinal crypt cells via a predominant RhoA/ROCK-dependent mechanism. Biol. Cell 2009, 101, 695–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wayner, E.A.; Carter, W.G. Identification of multiple cell-adhesion receptors for collagen and fibronectin in human fibrosarcoma cells possessing unique alpha-subunits and common beta-subunits. J. Cell Biol. 1987, 105, 1873–1884. [Google Scholar] [CrossRef]
- Wilkins, J.A.; Li, A.L.; Ni, H.Y.; Stupack, D.G.; Shen, C.X. Control of beta(1) integrin function—Localization of stimulatory epitopes. J. Biol. Chem. 1996, 271, 3046–3051. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.T.B.; Ward, J.P.T.; Hirst, S.J. Beta 1-integrins mediate enhancement of airway smooth muscle proliferation by collagen and fibronectin. Am. J. Respir. Crit. Care 2005, 171, 217–223. [Google Scholar] [CrossRef]
- Maeshima, Y.; Yerramalla, U.L.; Dhanabal, M.; Holthaus, K.A.; Barbashov, S.; Kharbanda, S.; Reimer, C.; Manfredi, M.; Dickerson, W.M.; Kalluri, R. Extracellular matrix-derived peptide binds to alpha(v)beta(3) integrin and inhibits angiogenesis. J. Biol. Chem. 2001, 276, 31959–31968. [Google Scholar] [CrossRef] [Green Version]
- Irigoyen, M.; Pajares, M.J.; Agorreta, J.; Ponz-Sarvise, M.; Salvo, E.; Lozano, M.D.; Pio, R.; Gil-Bazo, I.; Rouzaut, A. TGFBI expression is associated with a better response to chemotherapy in NSCLC. Mol. Cancer 2010, 9, 130. [Google Scholar] [CrossRef] [Green Version]
- Cheresh, D.A.; Spiro, R.C. Biosynthetic and functional-properties of an Arg-Gly-Asp-directed receptor involved in human-melanoma cell attachment to vitronectin, fibrinogen, and vonwillebrand-factor. J. Biol. Chem. 1987, 262, 17703–17711. [Google Scholar] [CrossRef] [PubMed]
- Mould, A.P.; Garratt, A.N.; Puzon-McLaughlin, W.; Takada, Y.; Humphries, M.J. Regulation of integrin function: Evidence that bivalent-cation-induced conformational changes lead to the unmasking of ligand-binding sites within integrin alpha 5 beta 1. Biochem. J. 1998, 331, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Imoto, E.; Kakuta, S.; Hori, M.; Yagami, K.; Nagumo, M. Adhesion of a chondrocytic cell line (USAC) to fibronectin and its regulation by proteoglycan. J. Oral Pathol. Med. 2002, 31, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Hangan, D.; Uniyal, S.; Morris, V.L.; MacDonald, I.C.; vonBallestrem, C.; Chau, T.; Schmidt, E.E.; Chambers, A.F.; Groom, A.C.; Chan, B.M.C. Integrin VLA-2 (alpha(2)beta(1)) function in postextravasation movement of human rhabdomyosarcoma RD cells in the liver. Cancer Res. 1996, 56, 3142–3149. [Google Scholar] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sedlář, A.; Trávníčková, M.; Bojarová, P.; Vlachová, M.; Slámová, K.; Křen, V.; Bačáková, L. Interaction between Galectin-3 and Integrins Mediates Cell-Matrix Adhesion in Endothelial Cells and Mesenchymal Stem Cells. Int. J. Mol. Sci. 2021, 22, 5144. https://doi.org/10.3390/ijms22105144
Sedlář A, Trávníčková M, Bojarová P, Vlachová M, Slámová K, Křen V, Bačáková L. Interaction between Galectin-3 and Integrins Mediates Cell-Matrix Adhesion in Endothelial Cells and Mesenchymal Stem Cells. International Journal of Molecular Sciences. 2021; 22(10):5144. https://doi.org/10.3390/ijms22105144
Chicago/Turabian StyleSedlář, Antonín, Martina Trávníčková, Pavla Bojarová, Miluše Vlachová, Kristýna Slámová, Vladimír Křen, and Lucie Bačáková. 2021. "Interaction between Galectin-3 and Integrins Mediates Cell-Matrix Adhesion in Endothelial Cells and Mesenchymal Stem Cells" International Journal of Molecular Sciences 22, no. 10: 5144. https://doi.org/10.3390/ijms22105144
APA StyleSedlář, A., Trávníčková, M., Bojarová, P., Vlachová, M., Slámová, K., Křen, V., & Bačáková, L. (2021). Interaction between Galectin-3 and Integrins Mediates Cell-Matrix Adhesion in Endothelial Cells and Mesenchymal Stem Cells. International Journal of Molecular Sciences, 22(10), 5144. https://doi.org/10.3390/ijms22105144








