Choline-Sigma-1R as an Additional Mechanism for Potentiation of Orexin by Cocaine
Abstract
1. Introduction
2. Results
2.1. OXA (17-33) Increases Cytosolic Ca2+, [Ca2+]i, in Nucleus Accumbens Neurons via OX1 Receptor Activation
2.2. OXA (17-33) Increases [Ca2+]i via IP3-Dependent Mechanism
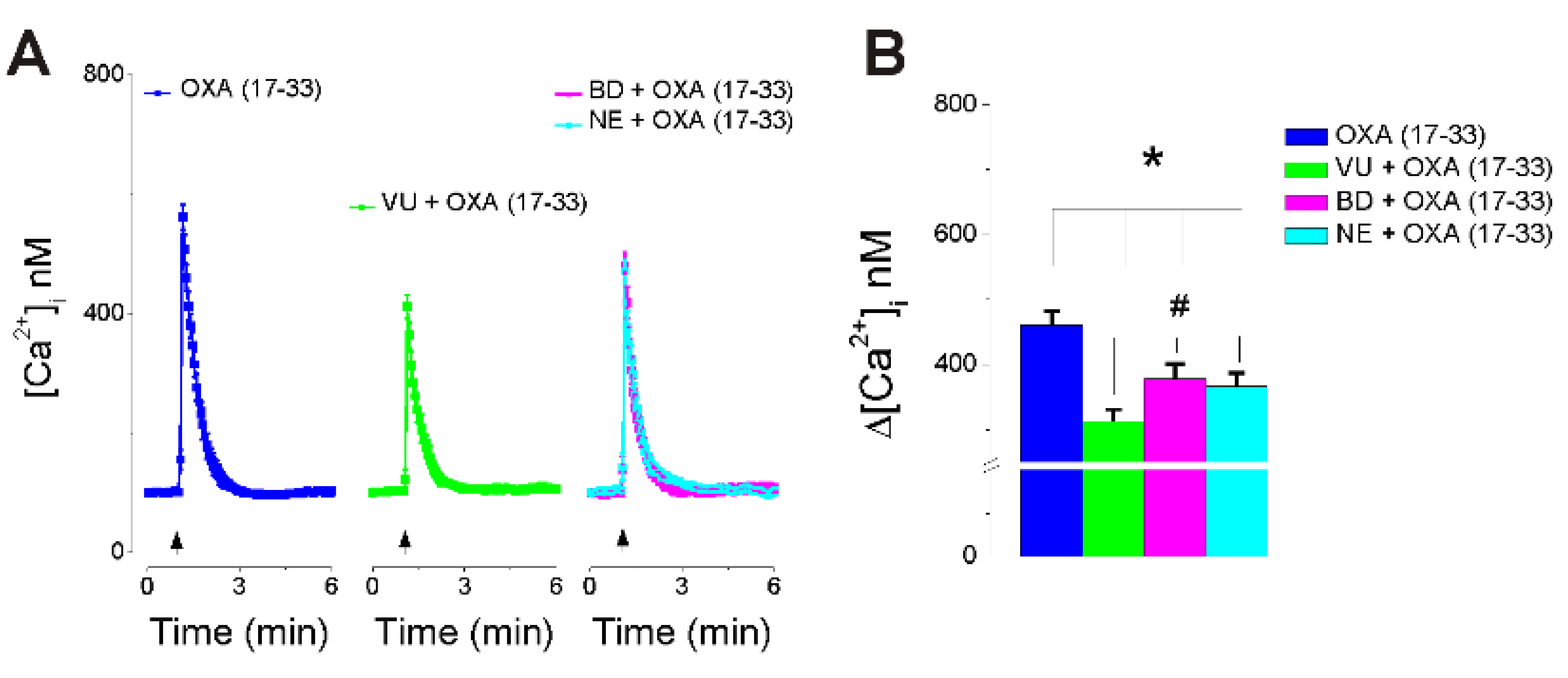
2.3. OXA (17-33) Increases [Ca2+]i via Choline-Sigma-1R-Dependent Mechanism
2.4. Cocaine Potentiates OXA (17-33)-Induced Increase in [Ca2+]i via Sigma-1R Activation
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Neuronal Cell Culture
4.3. Measurement of Cytosolic Ca2+ Concentration
4.4. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sakurai, T.; Amemiya, A.; Ishii, M.; Matsuzaki, I.; Chemelli, R.M.; Tanaka, H.; Williams, S.C.; Richardson, J.A.; Kozlowski, G.P.; Wilson, S.; et al. Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 1998, 92, 573–585. [Google Scholar] [CrossRef]
- de Lecea, L.; Kilduff, T.S.; Peyron, C.; Gao, X.; Foye, P.E.; Danielson, P.E.; Fukuhara, C.; Battenberg, E.L.; Gautvik, V.T.; Bartlett, F.S.; et al. The hypocretins: Hypothalamus-specific peptides with neuroexcitatory activity. Proc. Natl. Acad. Sci. USA 1998, 95, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Mahler, S.V.; Smith, R.J.; Moorman, D.E.; Sartor, G.C.; Aston-Jones, G. Multiple roles for orexin/hypocretin in addiction. Prog. Brain Res. 2012, 198, 79–121. [Google Scholar]
- Haghparast, A.; Fatahi, Z.; Arezoomandan, R.; Karimi, S.; Taslimi, Z.; Zarrabian, S. Functional roles of orexin/hypocretin receptors in reward circuit. Prog. Brain Res. 2017, 235, 139–154. [Google Scholar]
- Foord, S.M.; Bonner, T.I.; Neubig, R.R.; Rosser, E.M.; Pin, J.-P.; Davenport, A.P.; Spedding, M.; Harmar, A.J. International Union of Pharmacology. XLVI. G protein-coupled receptor list. Pharmacol. Rev. 2005, 57, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Kukkonen, J.P. G-protein-dependency of orexin/hypocretin receptor signalling in recombinant Chinese hamster ovary cells. Biochem. Biophys. Res. Commun. 2016, 476, 379–385. [Google Scholar] [CrossRef]
- Hopf, F.W. Recent perspectives on orexin/hypocretin promotion of addiction-related behaviors. Neuropharmacology 2020, 168, 108013. [Google Scholar] [CrossRef] [PubMed]
- Kukkonen, J.P. Lipid signaling cascades of orexin/hypocretin receptors. Biochimie 2014, 96, 158–165. [Google Scholar] [CrossRef]
- Johansson, L.; Ekholm, M.E.; Kukkonen, J.P. Multiple phospholipase activation by OX(1) orexin/hypocretin receptors. Cell. Mol. Life Sci. 2008, 65, 1948–1956. [Google Scholar] [CrossRef]
- Jantti, M.H.; Putula, J.; Somerharju, P.; Frohman, M.A.; Kukkonen, J.P. OX1 orexin/hypocretin receptor activation of phospholipase D. Br. J. Pharmacol. 2012, 165, 1109–1123. [Google Scholar] [CrossRef] [PubMed]
- Exton, J.H. Phospholipase D. Ann. N. Y. Acad. Sci. 2000, 905, 61–68. [Google Scholar] [CrossRef]
- Brailoiu, E.; Chakraborty, S.; Brailoiu, G.C.; Zhao, P.; Barr, J.L.; Ilies, M.A.; Unterwald, E.M.; Abood, M.E.; Taylor, C.W. Choline is an intracellular messenger linking extracellular stimuli to IP3-evoked Ca(2+) signals through Sigma-1 receptors. Cell Rep. 2019, 26, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Su, T.P. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell 2007, 131, 596–610. [Google Scholar] [CrossRef] [PubMed]
- D’Almeida, V.; Hipolide, D.C.; Raymond, R.; Barlow, K.B.L.; Parkes, J.H.; Pedrazzoli, M.; Tufik, S.; Nobrega, J.N. Opposite effects of sleep rebound on orexin OX1 and OX2 receptor expression in rat brain. Brain Res. Mol. Brain Res. 2005, 136, 148–157. [Google Scholar] [CrossRef]
- Lei, K.; Kwok, C.; Darevsky, D.; Wegner, S.A.; Yu, J.; Nakayama, L.; Pedrozo, V.; Anderson, L.; Ghotra, S.; Fouad, M.; et al. Nucleus accumbens shell Orexin-1 receptors are critical mediators of binge intake in excessive-drinking individuals. Front. Neurosci. 2019, 13, 88. [Google Scholar] [CrossRef]
- Perrey, D.A.; Zhang, Y. Therapeutics development for addiction: Orexin-1 receptor antagonists. Brain Res. 2020, 1731, 145922. [Google Scholar] [CrossRef]
- Han, Y.; Yuan, K.; Zheng, Y.; Lu, L. Orexin receptor antagonists as emerging treatments for psychiatric disorders. Neurosci. Bull. 2020, 36, 432–448. [Google Scholar] [CrossRef]
- Khoo, S.Y.; Brown, R.M. Orexin/hypocretin based pharmacotherapies for the treatment of addiction: DORA or SORA? CNS Drugs 2014, 28, 713–730. [Google Scholar] [CrossRef]
- Bentzley, B.S.; Aston-Jones, G. Orexin-1 receptor signaling increases motivation for cocaine-associated cues. Eur. J. Neurosci. 2015, 41, 1149–1156. [Google Scholar] [CrossRef]
- Zhou, L.; Ghee, S.M.; Chan, C.; Lin, L.; Cameron, M.D.; Kenny, P.J.; See, R.E. Orexin-1 receptor mediation of cocaine seeking in male and female rats. J. Pharmacol. Exp. Ther. 2012, 340, 801–809. [Google Scholar] [CrossRef]
- Prince, C.D.; Rau, A.R.; Yorgason, J.T.; Espana, R.A. Hypocretin/Orexin regulation of dopamine signaling and cocaine self-administration is mediated predominantly by hypocretin receptor 1. ACS Chem. Neurosci. 2015, 6, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Ma, H.; Jia, M.; Li, Y.; Miao, D.; Cui, C.; Wu, L. The role of the nucleus accumbens OXR1 in cocaine-induced locomotor sensitization. Behav. Brain Res. 2020, 379, 112365. [Google Scholar] [CrossRef] [PubMed]
- Martin-Fardon, R.; Weiss, F. Blockade of hypocretin receptor-1 preferentially prevents cocaine seeking: Comparison with natural reward seeking. Neuroreport 2014, 25, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Giros, B.; Jaber, M.; Jones, S.R.; Wightman, R.M.; Garon, M.G. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature 1996, 379, 606–612. [Google Scholar] [CrossRef]
- Koob, G.F.; Volkow, N.D. Neurocircuitry of addiction. Neuropsychopharmacology 2010, 35, 217–238. [Google Scholar] [CrossRef]
- Sharkey, J.; Glen, K.A.; Wolfe, S.; Kuhar, M.J. Cocaine binding at sigma receptors. Eur. J. Pharmacol. 1988, 149, 171–174. [Google Scholar] [CrossRef]
- Gundlach, A.L.; Largent, B.L.; Snyder, S.H. Autoradiographic localization of sigma receptor binding sites in guinea pig and rat central nervous system with (+)3H-3-(3-hydroxyphenyl)-N-(1-propyl)piperidine. J. Neurosci. 1986, 6, 1757–1770. [Google Scholar] [CrossRef]
- Alonso, G.; Phan, V.; Guillemain, I.; Saunier, M.; Legrand, A.; Anoal, M.; Maurice, T. Immunocytochemical localization of the sigma(1) receptor in the adult rat central nervous system. Neuroscience 2000, 97, 155–170. [Google Scholar] [CrossRef]
- Barr, J.L.; Deliu, E.; Brailoiu, C.G.; Zhao, P.; Yan, G.; Abood, M.E.; Unterwald, E.M.; Brailoiu, E. Mechanisms of activation of nucleus accumbens neurons by cocaine via sigma-1 receptor-inositol 1,4,5-trisphosphate-transient receptor potential canonical channel pathways. Cell Calcium 2015, 58, 196–207. [Google Scholar] [CrossRef]
- Feng, X.M.; Mi, W.L.; Xia, F.; Mao-Ying, W.L.; Jiang, J.W.; Xiao, S.; Wang, Z.F.; Wang, Y.Q.; Wu, G.C. Involvement of spinal orexin A in the electroacupuncture analgesia in a rat model of post-laparotomy pain. BMC Complement. Altern. Med. 2012, 12, 225. [Google Scholar] [CrossRef]
- Smart, D.; Sabido-David, C.; Brough, S.J.; Jewitt, F.; Johns, A.; Porter, R.A.; Jerman, J.C. SB-334867-A: The first selective orexin-1 receptor antagonist. Br. J. Pharmacol. 2001, 132, 1179–1182. [Google Scholar] [CrossRef]
- Maruyama, T.; Kanaji, T.; Nakade, S.; Kanno, T.; Mikoshiba, K. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J. Biochem. 1997, 122, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Bowman, E.J.; Siebers, A.; Altendorf, K. Bafilomycins: A class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc. Natl. Acad. Sci. USA 1988, 85, 7972–7976. [Google Scholar] [CrossRef]
- Scott, S.A.; Selvy, P.E.; Buck, J.R.; Cho, H.P.; Criswell, T.L.; Thomas, A.L.; Armstrong, M.D.; Arteaga, C.L.; Lindsley, C.W.; Brown, H.A. Design of isoform-selective phospholipase D inhibitors that modulate cancer cell invasiveness. Nat. Chem. Biol. 2009, 5, 108–117. [Google Scholar] [CrossRef]
- Matsumoto, R.R.; Bowen, W.D.; Tom, M.A.; Vo, V.N.; Truong, D.D.; de Costa, B.R. Characterization of two novel sigma receptor ligands: Antidystonic effects in rats suggest sigma receptor antagonism. Eur. J. Pharmacol. 1995, 280, 301–310. [Google Scholar] [CrossRef]
- Chaki, S.; Okuyama, S.; Ogawa, S.; Tanaka, M.; Muramatsu, M.; Nakazato, A.; Tomisawa, K. Solubilization and characterization of binding sites for [3H]NE-100, a novel and potent sigma 1 ligand, from guinea pig brain. Life Sci. 1996, 59, 1331–1340. [Google Scholar] [CrossRef]
- Bjornstrom, K.; Turina, D.; Strid, T.; Sundqvist, T.; Eintrei, C. Orexin A inhibits propofol-induced neurite retraction by a phospholipase D/protein kinase Cepsilon-dependent mechanism in neurons. PLoS ONE 2014, 9, e97129. [Google Scholar] [CrossRef]
- Berridge, M.J. The Inositol Trisphosphate/Calcium signaling pathway in health and disease. Physiol. Rev. 2016, 96, 1261–1296. [Google Scholar] [CrossRef]
- Srivats, S.; Balasuriya, D.; Pasche, M.; Vistal, G.; Edwardson, J.M.; Taylor, C.W.; Murell-Lagnado, R.D. Sigma1 receptors inhibit store-operated Ca2+ entry by attenuating coupling of STIM1 to Orai1. J. Cell Biol. 2016, 213, 65–79. [Google Scholar] [CrossRef]
- Maurice, T.; Su, T.P. The pharmacology of sigma-1 receptors. Pharmacol. Ther. 2009, 124, 195–206. [Google Scholar] [CrossRef]
- Katz, J.L.; Hong, W.C.; Hiranita, T.; Su, T.P. A role for sigma receptors in stimulant self-administration and addiction. Pharmaceuticals 2011, 4, 880–914. [Google Scholar] [CrossRef]
- Hayashi, T.; Tsai, S.Y.; Mori, T.; Fujimoto, M.; Su, T.P. Targeting ligand-operated chaperone sigma-1 receptors in the treatment of neuropsychiatric disorders. Expert Opin. Ther. Targets 2011, 15, 557–577. [Google Scholar] [CrossRef] [PubMed]
- Gentile, T.A.; Simmons, S.J.; Watson, M.N.; Connelly, K.L.; Brailoiu, E.; Zhang, Y.; Muschamp, J.W. Effects of suvorexant, a dual Orexin/Hypocretin receptor antagonist, on impulsive behavior associated with cocaine. Neuropsychopharmacology 2018, 43, 1001–1009. [Google Scholar] [CrossRef]
- Cox, C.D.; Breslin, M.J.; Whitman, D.B.; Schreier, J.D.; McGaughey, G.B.; Bogusky, M.J.; Roecker, A.J.; Mercer, S.P.; Bednar, R.A.; Lemaire, W.; et al. Discovery of the dual orexin receptor antagonist [(7R)-4-(5-chloro-1,3-benzoxazol-2-yl)-7-methyl-1,4-diazepan-1-yl][5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl]methanone (MK-4305) for the treatment of insomnia. J. Med. Chem. 2010, 53, 5320–5332. [Google Scholar] [CrossRef]
- Brailoiu, G.C.; Deliu, E.; Barr, J.L.; Console-Bram, L.M.; Ciuciu, A.M.; Abood, M.E.; Unterwald, E.M.; Brailoiu, E. HIV Tat excites D1 receptor-like expressing neurons from rat nucleus accumbens. Drug Alcohol Depend. 2017, 178, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Grynkiewicz, G.; Poenie, M.; Tsien, R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985, 260, 3440–3450. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barr, J.L.; Zhao, P.; Brailoiu, G.C.; Brailoiu, E. Choline-Sigma-1R as an Additional Mechanism for Potentiation of Orexin by Cocaine. Int. J. Mol. Sci. 2021, 22, 5160. https://doi.org/10.3390/ijms22105160
Barr JL, Zhao P, Brailoiu GC, Brailoiu E. Choline-Sigma-1R as an Additional Mechanism for Potentiation of Orexin by Cocaine. International Journal of Molecular Sciences. 2021; 22(10):5160. https://doi.org/10.3390/ijms22105160
Chicago/Turabian StyleBarr, Jeffrey L., Pingwei Zhao, G. Cristina Brailoiu, and Eugen Brailoiu. 2021. "Choline-Sigma-1R as an Additional Mechanism for Potentiation of Orexin by Cocaine" International Journal of Molecular Sciences 22, no. 10: 5160. https://doi.org/10.3390/ijms22105160
APA StyleBarr, J. L., Zhao, P., Brailoiu, G. C., & Brailoiu, E. (2021). Choline-Sigma-1R as an Additional Mechanism for Potentiation of Orexin by Cocaine. International Journal of Molecular Sciences, 22(10), 5160. https://doi.org/10.3390/ijms22105160




