An Improved Vector System for Homogeneous and Stable Gene Regulation
Abstract
:1. Introduction
2. Results
2.1. Designing A New Variant of the Mammalian Inducible Expression System
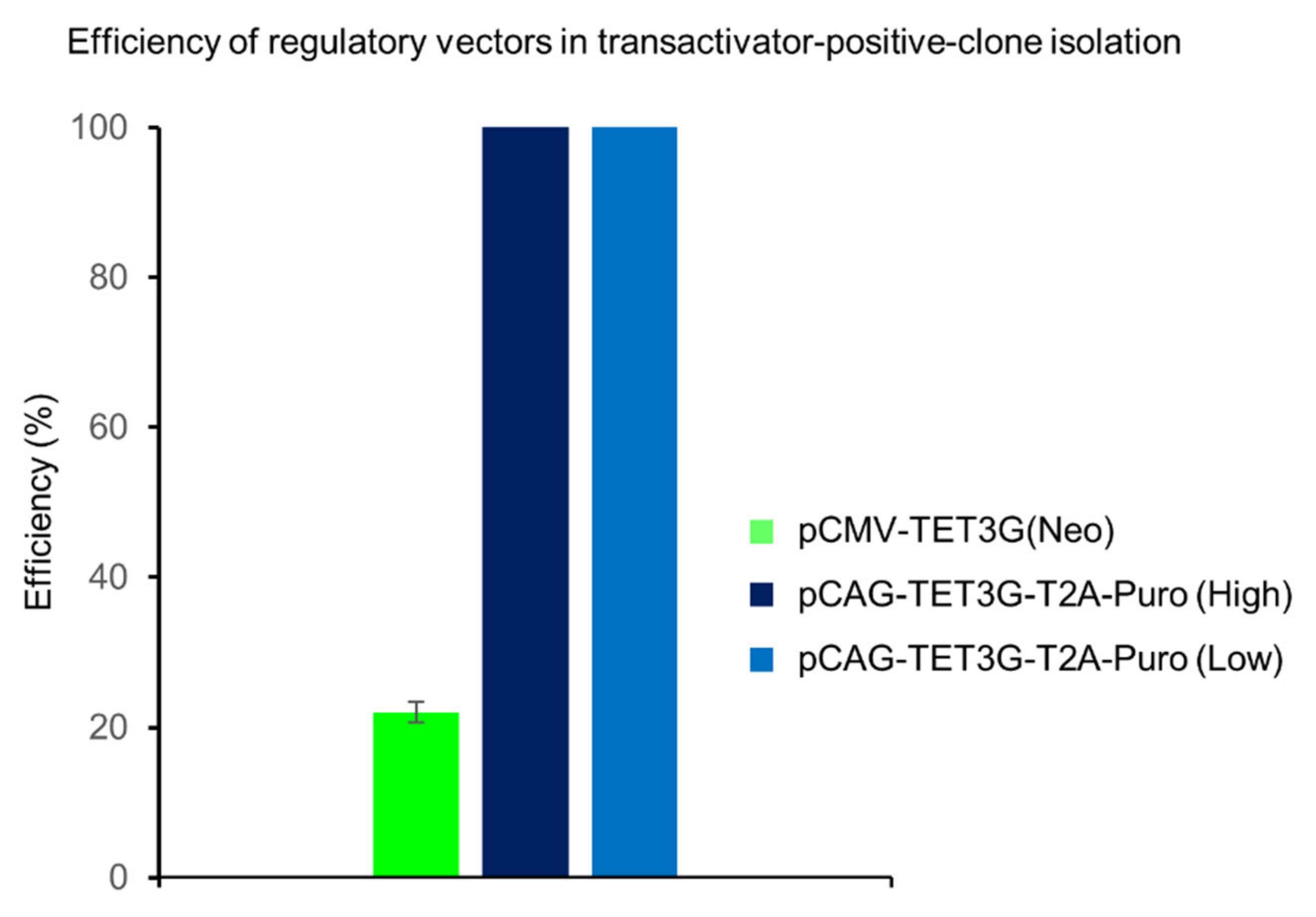
2.2. Fast Antibiotic Selection and Efficient Clone Isolation
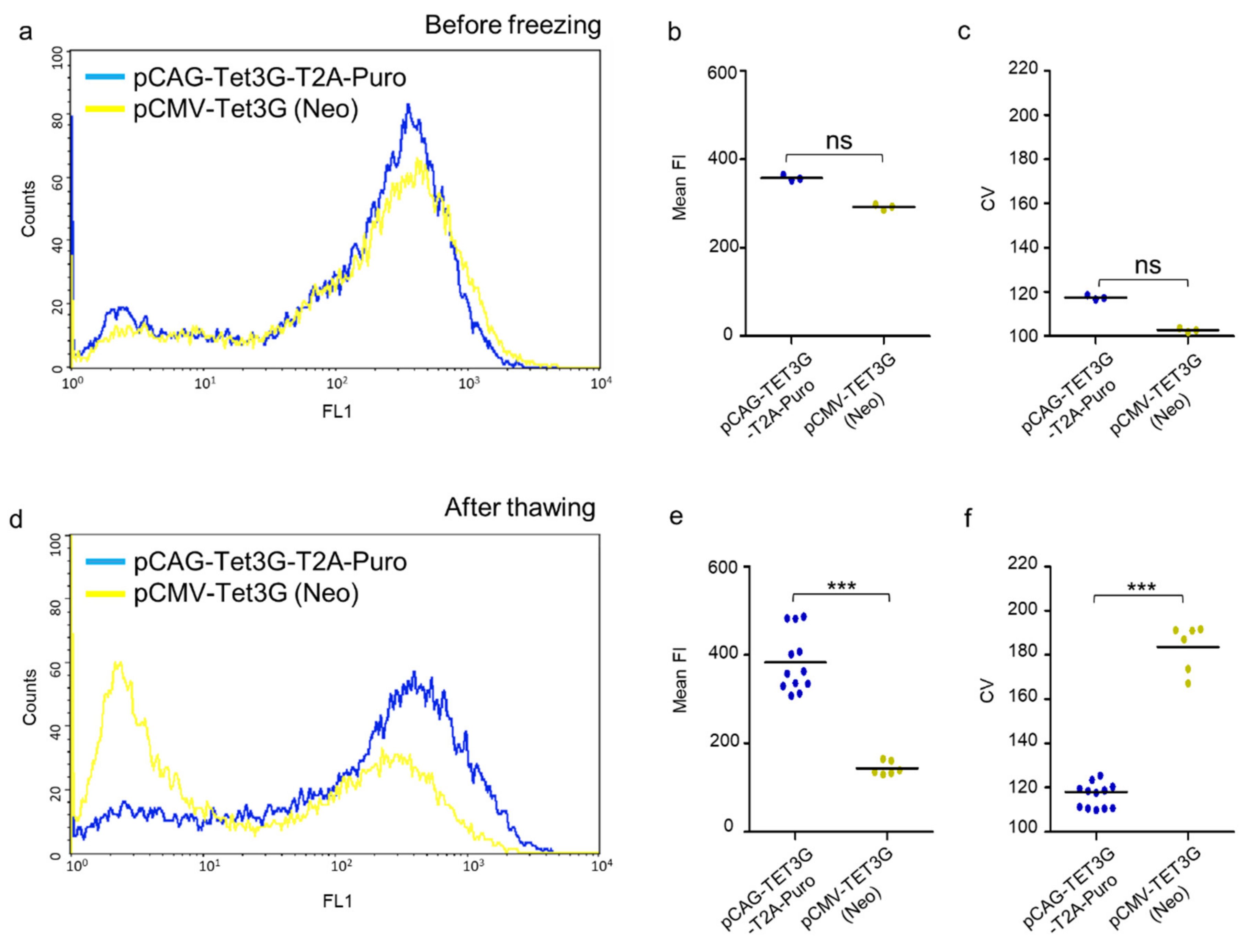
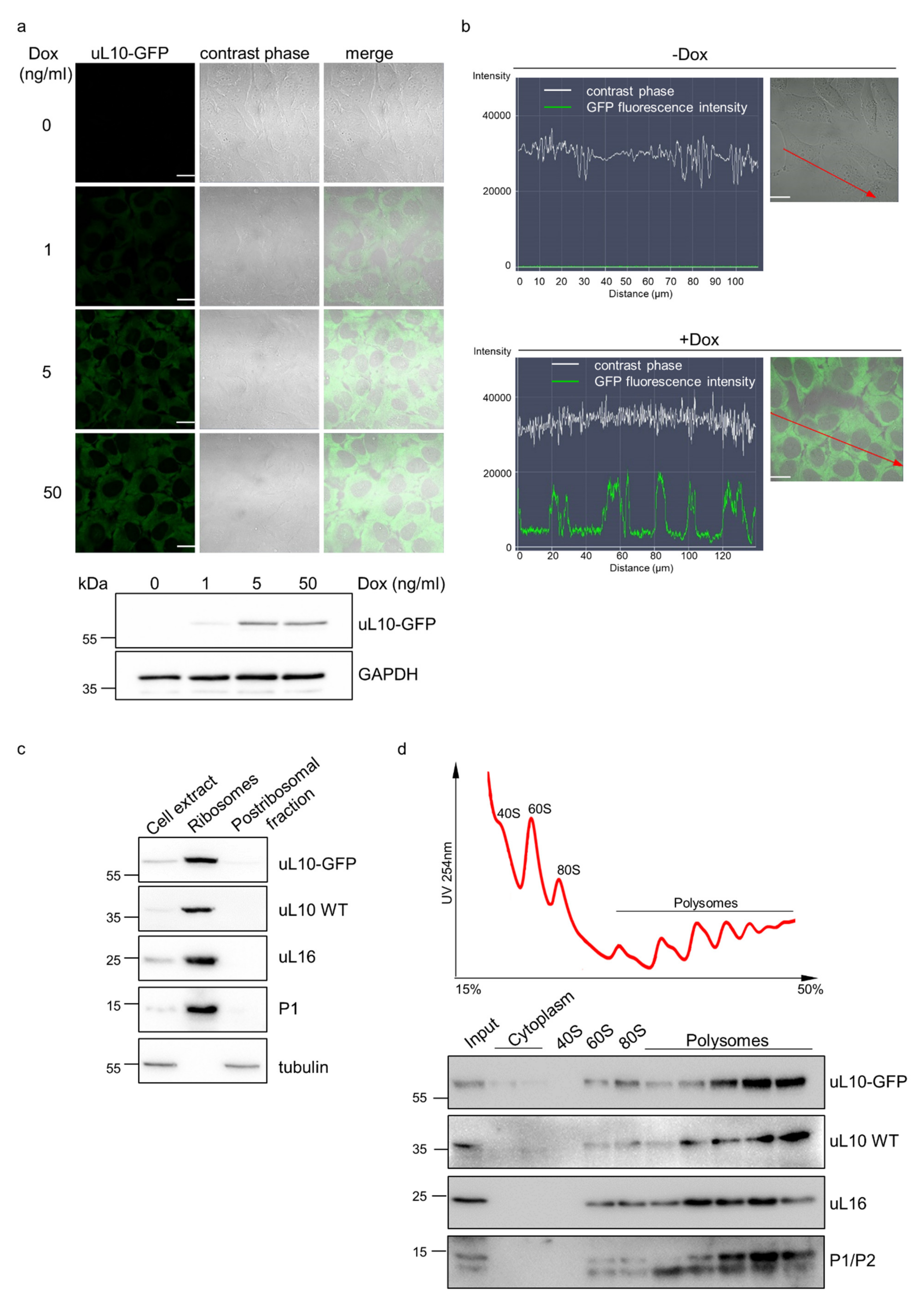
2.3. High Homogenous and Persistent Transactivator Expression
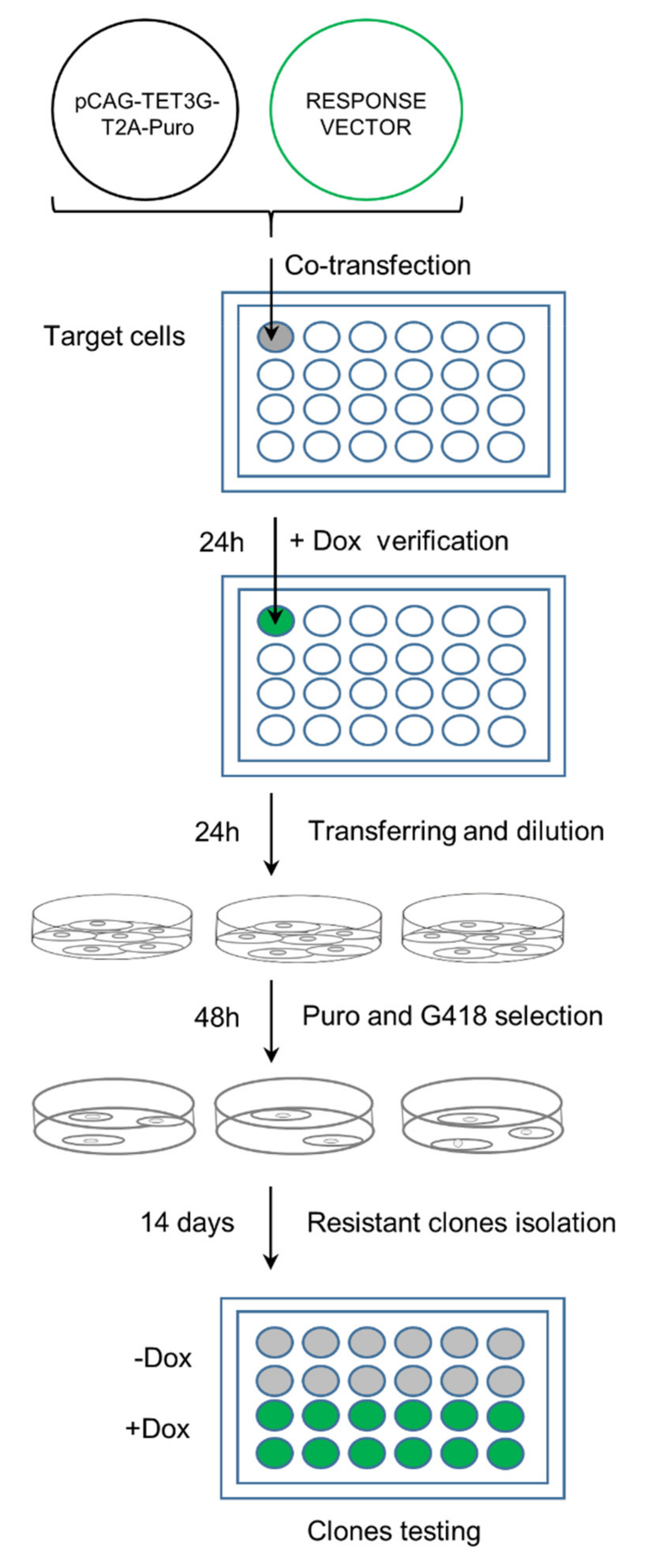
2.4. One-Step Selection of Transactivator and Regulatable Vectors
2.5. Characterization of GOI Expression in the Double Stable Cell Line Generated with the One-Step Protocol
3. Discussion
4. Materials and Methods
4.1. Genetic Manipulations
4.2. Cell Lines and Transfection
4.3. Generation of Stable Cell Lines
4.4. Confocal Microscopy
4.5. Flow Cytometry of Living Cells
4.6. Polysome Profile Analysis and Immunoblotting
4.7. Cell Fractionation and Ribosome Purification
4.8. Immunodetection
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gossen, M.; Bujard, H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. USA 1992, 89, 5547–5551. [Google Scholar] [CrossRef] [Green Version]
- Mullick, A.; Xu, Y.; Warren, R.; Koutroumanis, M.; Guilbault, C.; Broussau, S.; Malenfant, F.; Bourget, L.; Lamoureux, L.; Lo, R.; et al. The cumate gene-switch: A system for regulated expression in mammalian cells. BMC Biotechnol. 2006, 6, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivera, V.M.; Clackson, T.; Natesan, S.; Pollock, R.; Amara, J.F.; Keenan, T.; Magari, S.R.; Phillips, T.; Courage, N.L.; Cerasoli, F.; et al. A humanized system for pharmacologic control of gene expression. Nat. Med. 1996, 2, 1028–1032. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Riesterer, C.; Ayrall, A.M.; Sablitzky, F.; Littlewood, T.D.; Reth, M. Inducible site-directed recombination in mouse embryonic stem cells. Nucleic Acids Res. 1996, 24, 543–548. [Google Scholar] [CrossRef] [Green Version]
- Winkler, W.C.; Nahvi, A.; Roth, A.; Collins, J.A.; Breaker, R.R. Control of gene expression by a natural metabolite-responsive ribozyme. Nature 2004, 428, 281–286. [Google Scholar] [CrossRef]
- Kallunki, T.; Barisic, M.; Jaattela, M.; Liu, B. How to Choose the Right Inducible Gene Expression System for Mammalian Studies? Cells 2019, 8, 796. [Google Scholar] [CrossRef] [Green Version]
- Das, A.T.; Tenenbaum, L.; Berkhout, B. Tet-On Systems For Doxycycline-inducible Gene Expression. Curr. Gene Ther. 2016, 16, 156–167. [Google Scholar] [CrossRef] [Green Version]
- Choi, K.H.; Basma, H.; Singh, J.; Cheng, P.W. Activation of CMV promoter-controlled glycosyltransferase and beta -galactosidase glycogenes by butyrate, tricostatin A, and 5-aza-2′-deoxycytidine. Glycoconj J. 2005, 22, 63–69. [Google Scholar] [CrossRef]
- Krishnan, M.; Park, J.M.; Cao, F.; Wang, D.; Paulmurugan, R.; Tseng, J.R.; Gonzalgo, M.L.; Gambhir, S.S.; Wu, J.C. Effects of epigenetic modulation on reporter gene expression: Implications for stem cell imaging. FASEB J. 2006, 20, 106–108. [Google Scholar] [CrossRef] [PubMed]
- Ban, N.; Beckmann, R.; Cate, J.H.; Dinman, J.D.; Dragon, F.; Ellis, S.R.; Lafontaine, D.L.; Lindahl, L.; Liljas, A.; Lipton, J.M.; et al. A new system for naming ribosomal proteins. Curr. Opin. Struct. Biol. 2014, 24, 165–169. [Google Scholar] [CrossRef] [Green Version]
- Derylo, K.; Michalec-Wawiorka, B.; Krokowski, D.; Wawiorka, L.; Hatzoglou, M.; Tchorzewski, M. The uL10 protein, a component of the ribosomal P-stalk, is released from the ribosome in nucleolar stress. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 34–47. [Google Scholar] [CrossRef]
- Filipek, K.; Michalec-Wawiorka, B.; Boguszewska, A.; Kmiecik, S.; Tchorzewski, M. Phosphorylation of the N-terminal domain of ribosomal P-stalk protein uL10 governs its association with the ribosome. FEBS Lett. 2020, 594, 3002–3019. [Google Scholar] [CrossRef]
- Barnard, G.F.; Staniunas, R.J.; Bao, S.; Mafune, K.; Steele, G.D., Jr.; Gollan, J.L.; Chen, L.B. Increased expression of human ribosomal phosphoprotein P0 messenger RNA in hepatocellular carcinoma and colon carcinoma. Cancer Res. 1992, 52, 3067–3072. [Google Scholar]
- Kondoh, N.; Wakatsuki, T.; Ryo, A.; Hada, A.; Aihara, T.; Horiuchi, S.; Goseki, N.; Matsubara, O.; Takenaka, K.; Shichita, M.; et al. Identification and characterization of genes associated with human hepatocellular carcinogenesis. Cancer Res. 1999, 59, 4990–4996. [Google Scholar]
- Lai, M.D.; Xu, J. Ribosomal proteins and colorectal cancer. Curr. Genom. 2007, 8, 43–49. [Google Scholar]
- Chang, T.W.; Chen, C.C.; Chen, K.Y.; Su, J.H.; Chang, J.H.; Chang, M.C. Ribosomal phosphoprotein P0 interacts with GCIP and overexpression of P0 is associated with cellular proliferation in breast and liver carcinoma cells. Oncogene 2008, 27, 332–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Artero-Castro, A.; Castellvi, J.; Garcia, A.; Hernandez, J.; Cajal, S.R.; Lleonart, M.E. Expression of the ribosomal proteins Rplp0, Rplp1, and Rplp2 in gynecologic tumors. Hum. Pathol. 2011, 42, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Teller, A.; Jechorek, D.; Hartig, R.; Adolf, D.; Reissig, K.; Roessner, A.; Franke, S. Dysregulation of apoptotic signaling pathways by interaction of RPLP0 and cathepsin X/Z in gastric cancer. Pathol. Res. Pract. 2015, 211, 62–70. [Google Scholar] [CrossRef]
- Hitoshi, N.; Ken-ichi, Y.; Jun-ichi, M. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 1991, 108, 193–199. [Google Scholar] [CrossRef]
- Alexopoulou, A.N.; Couchman, J.R.; Whiteford, J.R. The CMV early enhancer/chicken beta actin (CAG) promoter can be used to drive transgene expression during the differentiation of murine embryonic stem cells into vascular progenitors. BMC Cell Biol. 2008, 9, 2. [Google Scholar] [CrossRef] [Green Version]
- Trehan, A.; Kielbus, M.; Czapinski, J.; Stepulak, A.; Huhtaniemi, I.; Rivero-Muller, A. REPLACR-mutagenesis, a one-step method for site-directed mutagenesis by recombineering. Sci. Rep. 2016, 6, 19121. [Google Scholar] [CrossRef] [Green Version]
- Tchorzewski, M.; Krokowski, D.; Rzeski, W.; Issinger, O.G.; Grankowski, N. The subcellular distribution of the human ribosomal “stalk” components: P1, P2 and P0 proteins. Int. J. Biochem. Cell Biol. 2003, 35, 203–211. [Google Scholar] [CrossRef]
- Michalec, B.; Krokowski, D.; Grela, P.; Wawiorka, L.; Sawa-Makarska, J.; Grankowski, N.; Tchorzewski, M. Subcellular localization of ribosomal P0-like protein MRT4 is determined by its N-terminal domain. Int. J. Biochem. Cell Biol. 2010, 42, 736–748. [Google Scholar] [CrossRef] [PubMed]
- Gossen, M.; Freundlieb, S.; Bender, G.; Muller, G.; Hillen, W.; Bujard, H. Transcriptional activation by tetracyclines in mammalian cells. Science 1995, 268, 1766–1769. [Google Scholar] [CrossRef]
- Loew, R.; Heinz, N.; Hampf, M.; Bujard, H.; Gossen, M. Improved Tet-responsive promoters with minimized background expression. BMC Biotechnol. 2010, 10, 81. [Google Scholar] [CrossRef] [Green Version]
- Urlinger, S.; Baron, U.; Thellmann, M.; Hasan, M.T.; Bujard, H.; Hillen, W. Exploring the sequence space for tetracycline-dependent transcriptional activators: Novel mutations yield expanded range and sensitivity. Proc. Natl. Acad. Sci. USA 2000, 97, 7963–7968. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Vink, M.; Klaver, B.; Berkhout, B.; Das, A.T. Optimization of the Tet-On system for regulated gene expression through viral evolution. Gene Ther. 2006, 13, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
- Szymczak-Workman, A.L.; Vignali, K.M.; Vignali, D.A. Design and construction of 2A peptide-linked multicistronic vectors. Cold Spring Harb. Protoc. 2012, 2012, 199–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, K.; Jeong, J.; Chung, B.H. Live imaging of cellular dynamics using a multi-imaging vector in single cells. Chem. Commun. 2014, 50, 10734–10736. [Google Scholar] [CrossRef] [PubMed]
- Lanza, A.M.; Kim, D.S.; Alper, H.S. Evaluating the influence of selection markers on obtaining selected pools and stable cell lines in human cells. Biotechnol. J. 2013, 8, 811–821. [Google Scholar] [CrossRef]
- Miyazaki, J.; Takaki, S.; Araki, K.; Tashiro, F.; Tominaga, A.; Takatsu, K.; Yamamura, K. Expression vector system based on the chicken beta-actin promoter directs efficient production of interleukin-5. Gene 1989, 79, 269–277. [Google Scholar]
- Bailey, L.A.; Hatton, D.; Field, R.; Dickson, A.J. Determination of Chinese hamster ovary cell line stability and recombinant antibody expression during long-term culture. Biotechnol. Bioeng. 2012, 109, 2093–2103. [Google Scholar] [CrossRef]
- He, L.; Winterrowd, C.; Kadura, I.; Frye, C. Transgene copy number distribution profiles in recombinant CHO cell lines revealed by single cell analyses. Biotechnol. Bioeng. 2012, 109, 1713–1722. [Google Scholar] [CrossRef]
- Hsu, C.C.; Li, H.P.; Hung, Y.H.; Leu, Y.W.; Wu, W.H.; Wang, F.S.; Lee, K.D.; Chang, P.J.; Wu, C.S.; Lu, Y.J.; et al. Targeted methylation of CMV and E1A viral promoters. Biochem. Biophys. Res. Commun. 2010, 402, 228–234. [Google Scholar] [CrossRef]
- Moritz, B.; Becker, P.B.; Gopfert, U. CMV promoter mutants with a reduced propensity to productivity loss in CHO cells. Sci. Rep. 2015, 5, 16952. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Yu, C.; Daley, T.P.; Wang, F.; Cao, W.S.; Bhate, S.; Lin, X.; Still, I.I.C.; Liu, H.; Zhao, D.; et al. CRISPR Activation Screens Systematically Identify Factors that Drive Neuronal Fate and Reprogramming. Cell Stem Cell 2018, 23, 758–771. [Google Scholar] [CrossRef] [Green Version]
- Ran, F.A.; Hsu, P.D.; Wright, J.; Agarwala, V.; Scott, D.A.; Zhang, F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013, 8, 2281–2308. [Google Scholar] [CrossRef] [Green Version]
- Potorac, I.; Rivero-Muller, A.; Trehan, A.; Kielbus, M.; Jozwiak, K.; Pralong, F.; Hafidi, A.; Thiry, A.; Ménagé, J.J.; Huhtaniemi, I.T.; et al. A vital region for human glycoprotein hormone trafficking revealed by an LHB mutation. J. Endocrinol. 2016, 231, 197–207. [Google Scholar] [CrossRef] [Green Version]
- Belin, S.; Hacot, S.; Daudignon, L.; Therizols, G.; Pourpe, S.; Mertani, H.C.; Rosa-Calatrava, M.; Diaz, J.J. Purification of ribosomes from human cell lines. Curr. Protoc. Cell Biol. 2010, 49, 3–40. [Google Scholar] [CrossRef] [PubMed]
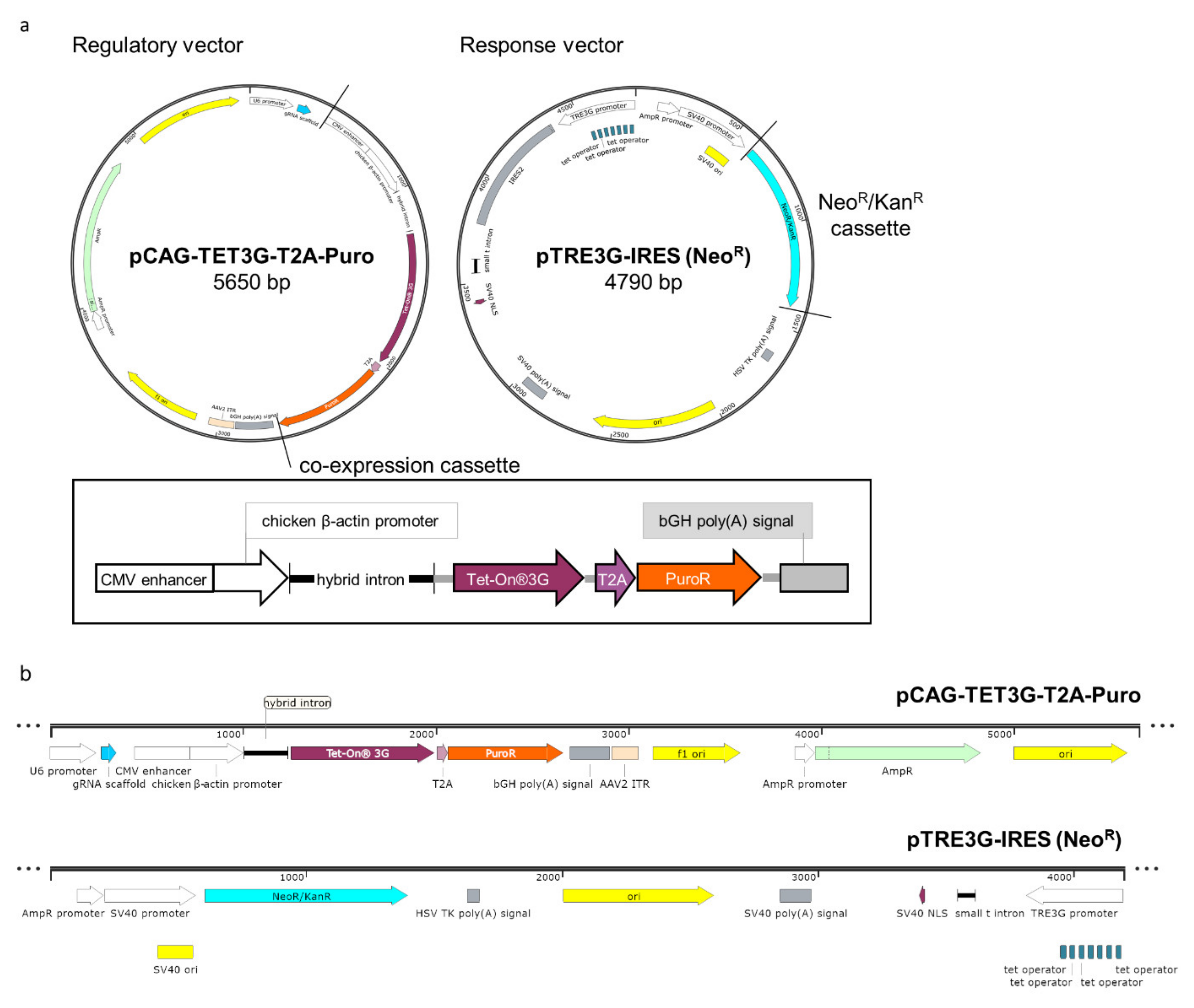
| Feature | Tet-On 3G | Tet-On 3G-T2A-Puro | Advantages of New System Tet-On 3G-T2A-Puro |
|---|---|---|---|
| REGULATORY PLASMID | |||
| Promotor | CMV | CAG | Less prone to silencing through methylationPersistent expression of transactivator |
| Selection cassette | Kan/Neo | Puro | Fast selection of resistant clones |
| Days of selection | >14 | 4–8 | |
| Co-expression of selection cassette and transactivator | − | + | Every resistant clonal cell expresses transactivator (higher efficiency) |
| RESPONSE PLASMID | |||
| Selection cassette | external (linear for co-transfection) | internal (within plasmid backbone) | Double stable cell line establishment with regulated GOI expression according to ‘fast-track’ protocol |
| Procedure | two-step | two-step or one-step | Alternative protocols possible: standard or rapid ‘fast-track’ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michalec-Wawiórka, B.; Czapiński, J.; Filipek, K.; Rulak, P.; Czerwonka, A.; Tchórzewski, M.; Rivero-Müller, A. An Improved Vector System for Homogeneous and Stable Gene Regulation. Int. J. Mol. Sci. 2021, 22, 5206. https://doi.org/10.3390/ijms22105206
Michalec-Wawiórka B, Czapiński J, Filipek K, Rulak P, Czerwonka A, Tchórzewski M, Rivero-Müller A. An Improved Vector System for Homogeneous and Stable Gene Regulation. International Journal of Molecular Sciences. 2021; 22(10):5206. https://doi.org/10.3390/ijms22105206
Chicago/Turabian StyleMichalec-Wawiórka, Barbara, Jakub Czapiński, Kamil Filipek, Patrycja Rulak, Arkadiusz Czerwonka, Marek Tchórzewski, and Adolfo Rivero-Müller. 2021. "An Improved Vector System for Homogeneous and Stable Gene Regulation" International Journal of Molecular Sciences 22, no. 10: 5206. https://doi.org/10.3390/ijms22105206
APA StyleMichalec-Wawiórka, B., Czapiński, J., Filipek, K., Rulak, P., Czerwonka, A., Tchórzewski, M., & Rivero-Müller, A. (2021). An Improved Vector System for Homogeneous and Stable Gene Regulation. International Journal of Molecular Sciences, 22(10), 5206. https://doi.org/10.3390/ijms22105206







