Recombinant ?Y278H Fibrinogen Showed Normal Secretion from CHO Cells, but a Corresponding Heterozygous Patient Showed Hypofibrinogenemia
Abstract
:1. Introduction
2. Results
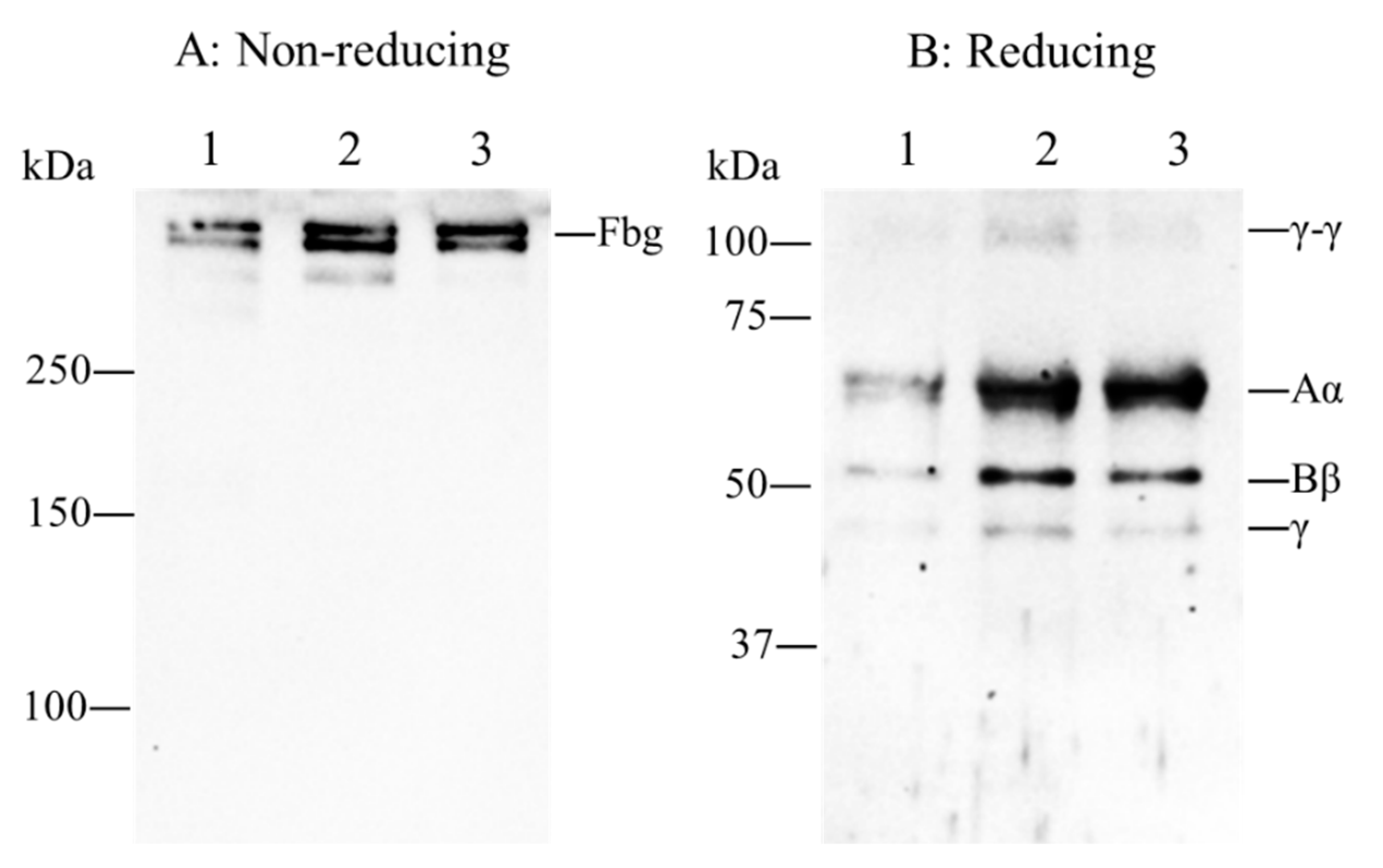
2.1. Patient’s Coagulation Tests, DNA Sequence Analysis and Immunoblotting Analysis
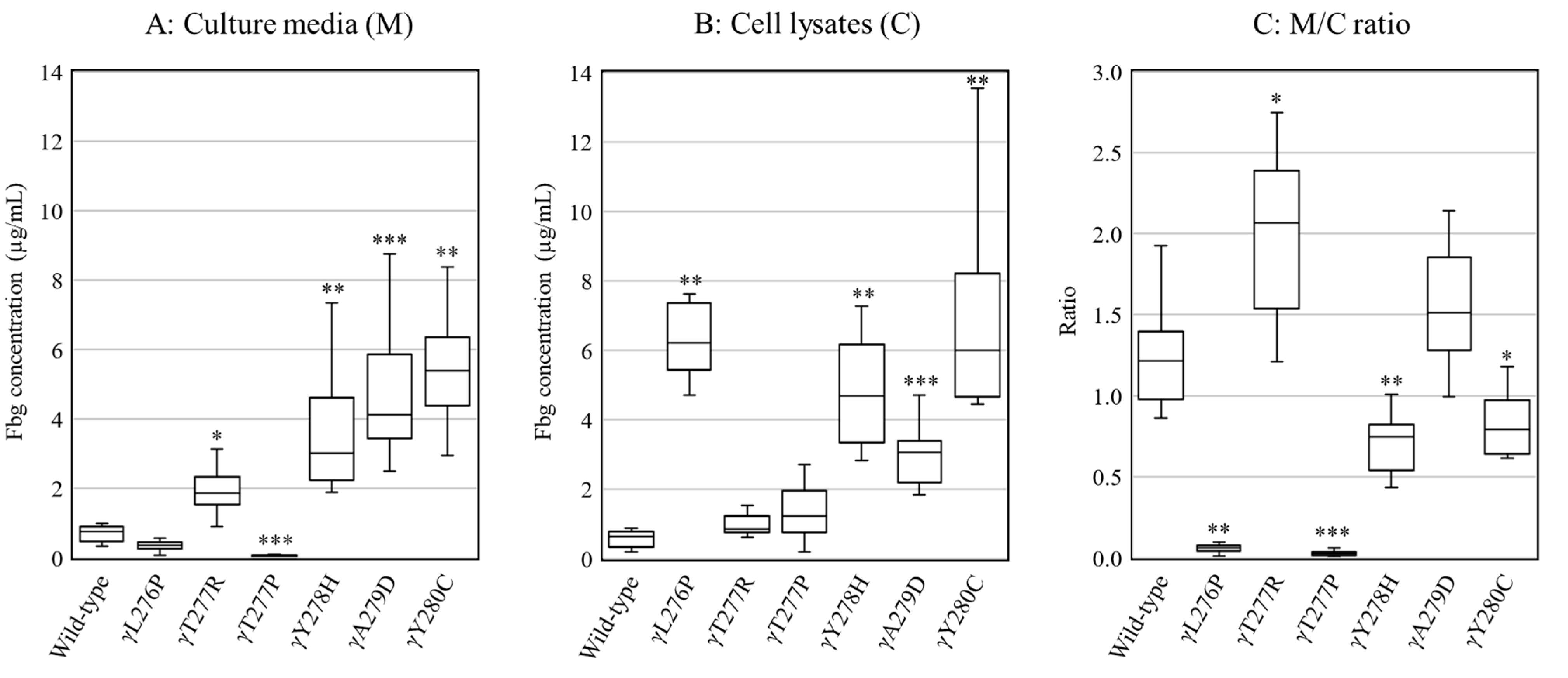
2.2. Secretion and Synthesis of Variant Fibrinogens in CHO Cells
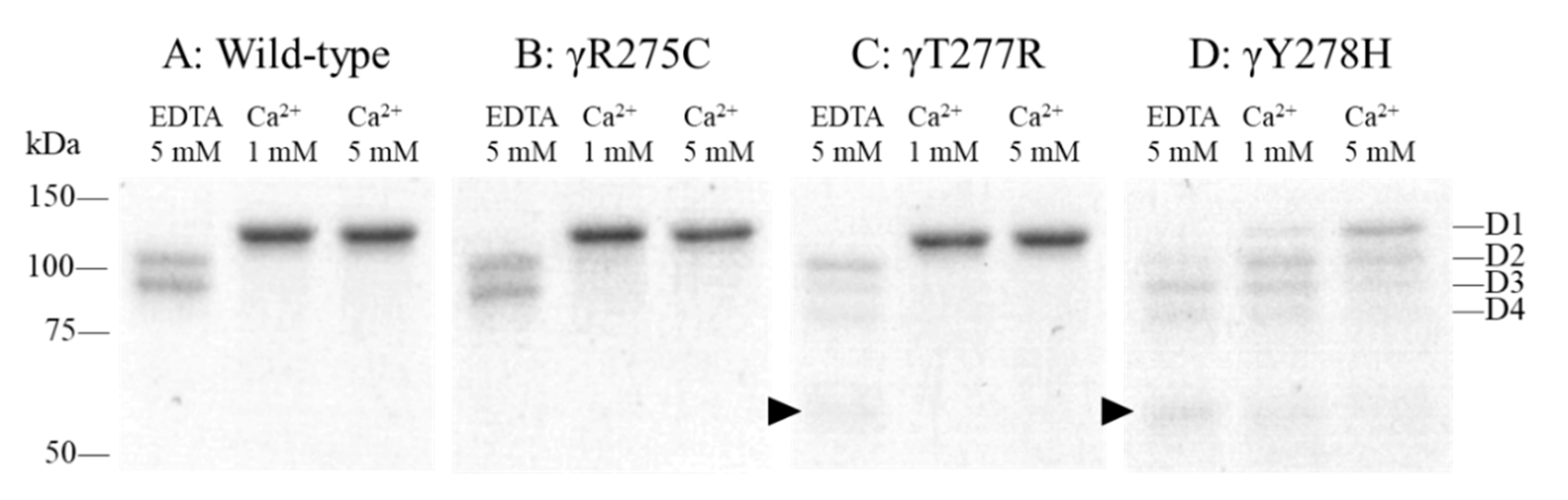
2.3. Fibrinogen Degradation Assay Using Plasmin
2.4. Thrombin-Catalyzed Fibrin Polymerization
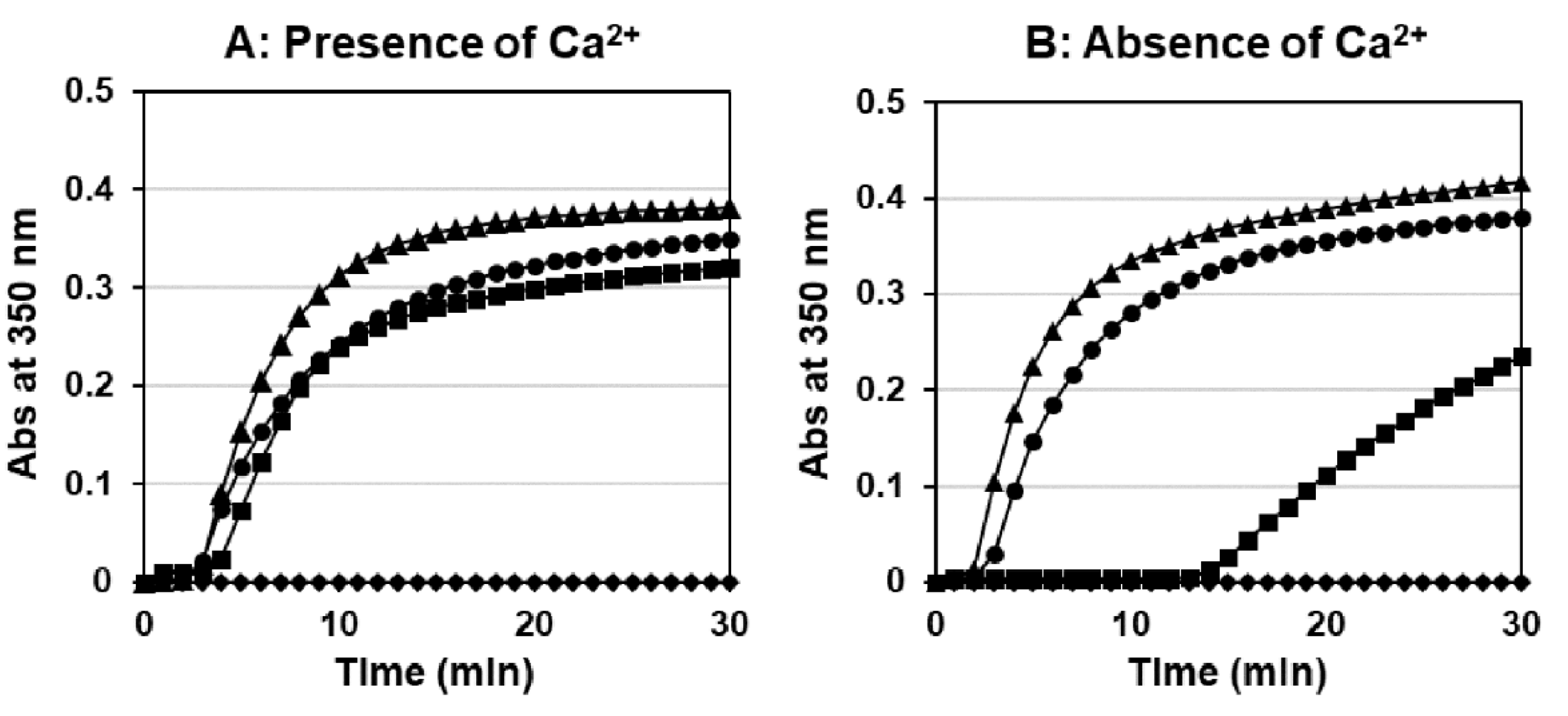
2.5. Protection Assay for the Plasmin Digestion of Fibrinogen
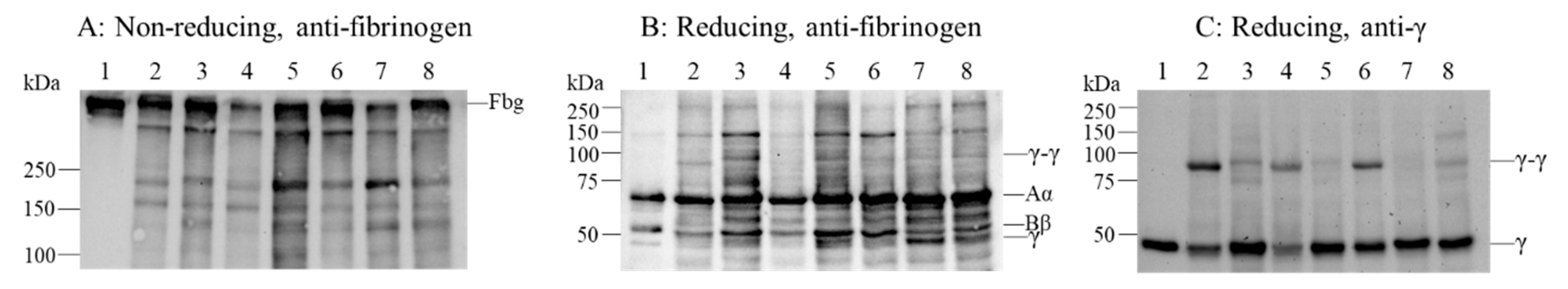
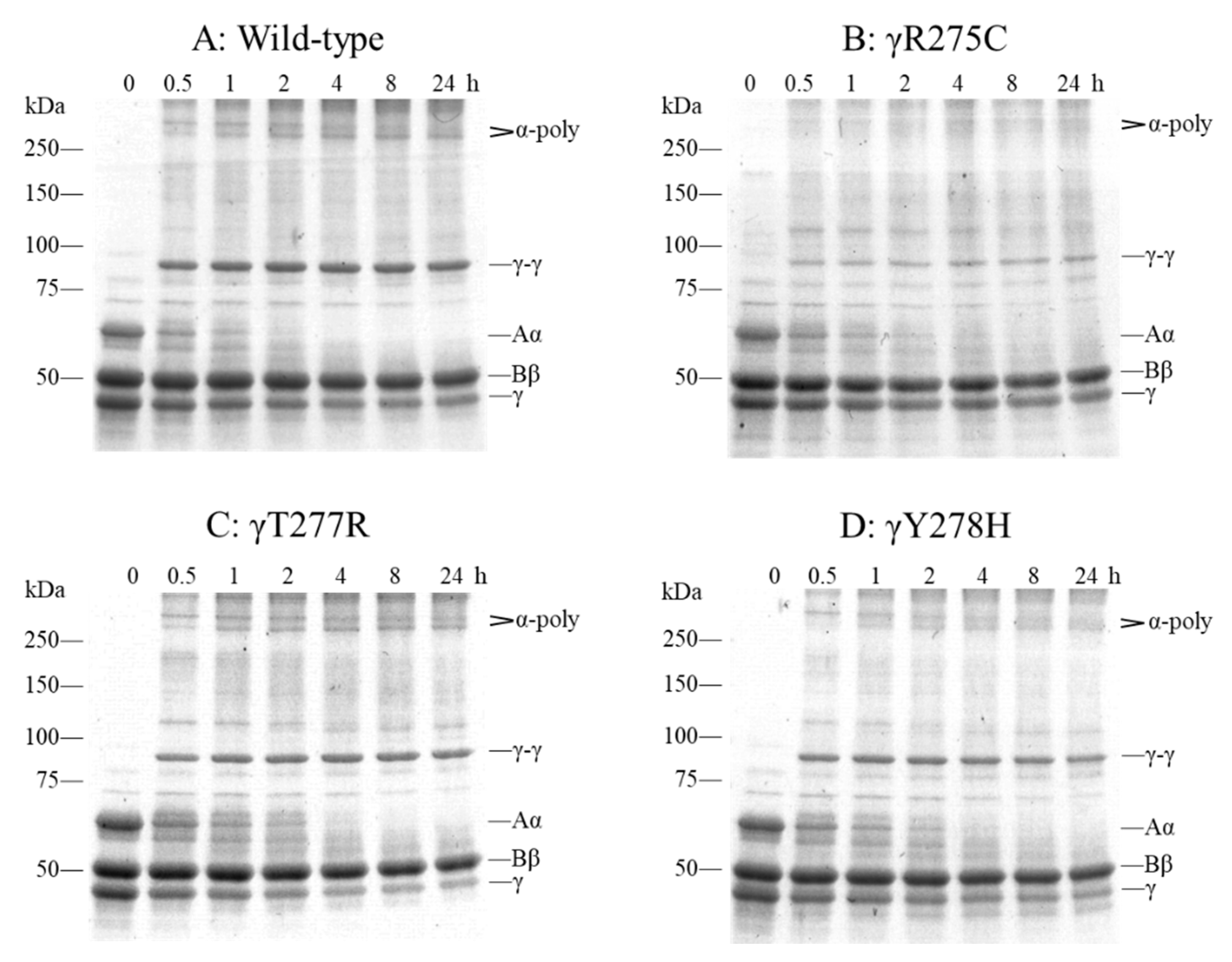
2.6. Factor (F) XIIIa-Catalyzed Cross-Linking of Fibrinogen
3. Discussion
4. Materials and Methods
4.1. Patient and Coagulation Tests
4.2. DNA Sequence Analysis
4.3. Immunoblotting Analysis of Plasma Fibrinogen
4.4. Preparation and Production of Recombinant Fibrinogen Variants
4.5. ELISA
4.6. Immunoblotting Analysis of Recombinant Fibrinogen
4.7. Fibrinogen Degradation Assays Using Plasmin
4.8. Thrombin-Catalyzed Fibrin Polymerization
4.9. Protection Assay for the Plasmin Digestion of Fibrinogen
4.10. FXIIIa-Catalyzed Cross-Linking of Fibrinogen
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Weisel, J.W. Fibrinogen and fibrin. Adv. Protein. Chem. 2005, 70, 247–299. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Cao, Z.; Chung, D.W.; Davie, E.W. The role of βγ and αγ complexes in the assembly of human fibrinogen. J. Biol. Chem. 1996, 271, 27942–27947. [Google Scholar] [CrossRef] [Green Version]
- Medved, L.; Weisel, J.W. Fibrinogen and Factor XIII Subcommittee of Scientific Standardization Committee of International Society on Thrombosis and Haemostasis. Recommendations for nomenclature on fibrinogen and fibrin. J. Thromb. Haemost. 2009, 7, 355–359. [Google Scholar] [CrossRef] [Green Version]
- Simurda, T.; Snahnicanova, Z.; Loderer, D.; Sokol, J.; Stasko, J.; Lasabova, Z.; Kubisz, P. Fibrinogen Martin: A Novel Mutation in FGB (Gln180Stop) Causing Congenital Afibrinogenemia. Semin. Thromb. Hemost. 2016, 42, 455–458. [Google Scholar] [CrossRef] [Green Version]
- Doolittle, R.F. Fibrinogen and fibrin. Annu. Rev. Biochem. 1984, 53, 195–229. [Google Scholar] [CrossRef] [PubMed]
- Mosesson, M.W.; Siebenlist, K.R.; DiOrio, J.P.; Matsuda, M.; Hainfeld, J.F.; Wall, J.S. The role of fibrinogen D domain intermolecular association sites in the polymerization of fibrin and fibrinogen Tokyo II (γ 275 Arg→Cys). J. Clin. Investig. 1995, 96, 1053–1058. [Google Scholar] [CrossRef]
- Spraggon, G.; Everse, S.J.; Doolittle, R.F. Crystal structures of fragment D from human fibrinogen and its crosslinked counterpart from fibrin. Nature 1997, 389, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Mochalkin, I.; Doolittle, R.F. A model of fibrin formation based on crystal structures of fibrinogen and fibrin fragments complexed with synthetic peptides. Proc. Natl. Acad. Sci. USA 2000, 97, 14156–14161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosesson, M.W.; DiOrio, J.P.; Siebenlist, K.R.; Wall, J.S.; Hainfeld, J.F. Evidence for a second type of fibril branch point in fibrin polymer networks, the trimolecular junction. Blood 1993, 82, 1517–1521. [Google Scholar] [CrossRef] [Green Version]
- Mosesson, M.W. Fibrinogen and fibrin structure and functions. J. Thromb. Haemost. 2005, 3, 1894–1904. [Google Scholar] [CrossRef]
- Lord, S.T. Molecular mechanisms affecting fibrin structure and stability. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 494–499. [Google Scholar] [CrossRef] [Green Version]
- GFHT Web Site. Available online: https://site.geht.org/base-de-donnees-fibrinogene/ (accessed on 22 March 2021).
- Simurda, T.; Zolkova, J.; Kolkova, Z.; Loderer, D.; Dobrotova, M.; Skornova, I.; Brunclíkova, M.; Grendar, M.; Lasabova, Z.; Stasko, J.; et al. Comparison of clinical phenotype with genetic and laboratory results in 31 patients with congenital dysfibrinogenemia in northern Slovakia. Int. J. Hematol. 2020, 111, 795–802. [Google Scholar] [CrossRef]
- de Moerloose, P.; Casini, A.; Neerman-Arbez, M. Congenital fibrinogen disorders: An update. Semin. Thromb. Hemost. 2013, 39, 585–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirota-Kawadobora, M.; Terasawa, F.; Suzuki, T.; Tozuka, M.; Sano, K.; Okumura, N. Comparison of thrombin-catalyzed fibrin polymerization and factor XIIIa-catalyzed cross-linking of fibrin among three recombinant variant fibrinogens, γ 275C, γ 275H, and γ 275A. J. Thromb. Haemost. 2004, 2, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, E.W.; Fretto, L.J.; McKee, P.A. A re-examination of the cleavage of fibrinogen and fibrin by plasmin. J. Biol. Chem. 1975, 250, 7210–7218. [Google Scholar] [CrossRef]
- Kamijyo, Y.; Hirota-Kawadobora, M.; Fujihara, N.; Wakabayashi, S.; Matsuda, K.; Yamauchi, K.; Terasawa, F.; Okumura, N.; Honda, T. Functional analysis of heterozygous plasma dysfibrinogens derived from two families of γArg275Cys and three families of γArg275His, and haplotype analysis for these families. Rinsho Byori 2009, 57, 651–658. [Google Scholar]
- Terasawa, F.; Kamijyo, Y.; Fujihara, N.; Okumura, N. Assembly and secretion of mutant fibrinogens with variant γ-chain C terminal region (γ313-γ345). Rinsho Byori 2010, 58, 772–778. [Google Scholar] [PubMed]
- Brennan, S.O.; Wyatt, J.; Medicina, D.; Callea, F.; George, P.M. Fibrinogen brescia: Hepatic endoplasmic reticulum storage and hypofibrinogenemia because of a γ284 Gly→Arg mutation. Am. J. Pathol. 2000, 157, 189–196. [Google Scholar] [CrossRef]
- Fellowes, A.P.; Brennan, S.O.; Ridgway, H.J.; Heaton, D.C.; George, P.M. Electrospray ionization mass spectrometry identification of fibrinogen Banks Peninsula (γ280Tyr→Cys): A new variant with defective polymerization. Br. J. Haematol. 1998, 101, 24–31. [Google Scholar] [CrossRef]
- Asselta, R.; Robusto, M.; Platé, M.; Santoro, C.; Peyvandi, F.; Duga, S. Molecular characterization of 7 patients affected by dys- or hypo-dysfibrinogenemia: Identification of a novel mutation in the fibrinogen Bβ chain causing a gain of glycosylation. Thromb. Res. 2015, 136, 168–174. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, M.; Xie, H.; Jin, Y.; Yang, L.; Xu, P. A novel fibrinogen mutation (γ Thr277Arg) causes hereditary hypofibrinogenemia in a Chinese family. Blood Coagul. Fibrinolysis 2013, 24, 642–644. [Google Scholar] [CrossRef]
- Terasawa, F.; Okumura, N.; Kitano, K.; Hayashida, N.; Shimosaka, M.; Okazaki, M.; Lord, S.T. Hypofibrinogenemia associated with a heterozygous missense mutation γ153Cys to arg (Matsumoto IV): In vitro expression demonstrates defective secretion of the variant fibrinogen. Blood 1999, 94, 4122–4131. [Google Scholar] [CrossRef] [PubMed]
- Arai, S.; Ogiwara, N.; Mukai, S.; Takezawa, Y.; Sugano, M.; Honda, T.; Okumura, N. The fibrous form of intracellular inclusion bodies in recombinant variant fibrinogen-producing cells is specific to the hepatic fibrinogen storage disease-inducible variant fibrinogen. Int. J. Hematol. 2017, 105, 758–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brennan, S.O.; Wyatt, J.M.; Ockelford, P.; George, P.M. Defective fibrinogen polymerization associated with a novel γ279Ala→Asp mutation. Br. J. Haematol. 2000, 108, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Kaido, T.; Yoda, M.; Kamijo, T.; Taira, C.; Higuchi, Y.; Okumura, N. Heterozygous variant fibrinogen γA289V (Kanazawa III) was confirmed as hypodysfibrinogenemia by plasma and recombinant fibrinogens. Int. J. Lab. Hematol. 2020, 42, 190–197. [Google Scholar] [CrossRef]
- Bantia, S.; Mane, S.M.; Bell, W.R.; Dang, C.V. Fibrinogen Baltimore I: Polymerization defect associated with a γ 292Gly----Val (GGC----GTC) mutation. Blood 1990, 76, 2279–2283. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, T.; Takezawa, Y.; Terasawa, F.; Okumura, N. Comparison of fibrinogen synthesis and secretion between novel variant fibrinogen, nagakute (γ305Thr→Ala), and other variants located in γ305-308 residues. Rinsho Byori 2012, 60, 831–838. [Google Scholar]
- Dear, A.; Dempfle, C.E.; Brennan, S.O.; Kirschstein, W.; George, P.M. Fibrinogen Mannheim II: A novel γ307 His→Tyr substitution in the γD domain causes hypofibrinogenemia. J. Thromb. Haemost. 2004, 2, 2194–2199. [Google Scholar] [CrossRef]
- Okumura, N.; Furihata, K.; Terasawa, F.; Nakagoshi, R.; Ueno, I.; Katsuyama, T. Fibrinogen Matsumoto I: A γ 364 Asp→His (GAT→CAT) substitution associated with defective fibrin polymerization. Thromb. Haemost. 1996, 75, 887–891. [Google Scholar] [CrossRef]
- Okumura, N.; Terasawa, F.; Fujita, K.; Tozuka, M.; Ota, H.; Katsuyama, T. Difference in electrophoretic mobility and plasmic digestion profile between four recombinant fibrinogens, γ 308K, γ 308I, γ 308A, and wild type (γ 308N). Electrophoresis 2000, 21, 2309–2315. [Google Scholar] [CrossRef]
- Ebert, R.F.; Bell, W.R. Fibrinogen Baltimore III: Congenital dysfibrinogenemia with a shortened gamma-subunit. Thromb. Res. 1988, 51, 251–258. [Google Scholar] [CrossRef]
- Meyer, M.; Bergmann, F.; Brennan, S.O. Novel fibrinogen mutation (γ 313 Ser→Asn) associated with hypofibrinogenemia in two unrelated families. Blood Coagul. Fibrinolysis 2006, 17, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Brennan, S.O.; Davis, R.L.; Conard, K.; Savo, A.; Furuya, K.N. Novel fibrinogen mutation γ314Thr→Pro (fibrinogen AI duPont) associated with hepatic fibrinogen storage disease and hypofibrinogenaemia. Liver Int. 2010, 30, 1541–1547. [Google Scholar] [CrossRef]
- Mukai, S.; Ikeda, M.; Takezawa, Y.; Sugano, M.; Honda, T.; Okumura, N. Differences in the function and secretion of congenital aberrant fibrinogenemia between heterozygous γD320G (Okayama II) and γΔN319-ΔD320 (Otsu I). Thromb. Res. 2015, 136, 1318–1324. [Google Scholar] [CrossRef] [Green Version]
- Lounes, K.C.; Soria, C.; Valognes, A.; Turchini, M.F.; Soria, J.; Koopman, J. Fibrinogen Bastia (γ 318 Asp→Tyr) a novel abnormal fibrinogen characterized by defective fibrin polymerization. Thromb. Haemost. 1999, 82, 1639–1643. [Google Scholar] [CrossRef] [PubMed]
- Brennan, S.O.; Davis, R.L.; Mosesson, M.W.; Hernandez, I.; Lowen, R.; Alexander, S.J. Congenital hypodysfibrinogenaemia (Fibrinogen Des Moines) due to a γ320Asp deletion at the Ca2+ binding site. Thromb. Haemost. 2007, 98, 467–469. [Google Scholar] [PubMed]
- Brennan, S.O.; Laurie, A. Functionally compromised FGG variant (γ320Asp→Glu) expressed at low level in plasma fibrinogen. Thromb. Res. 2014, 134, 744–746. [Google Scholar] [CrossRef]
- Haneishi, A.; Terasawa, F.; Fujihara, N.; Yamauchi, K.; Okumura, N.; Katsuyama, T. Recombinant variant fibrinogens substituted at residues γ326Cys and γ339Cys demonstrated markedly impaired secretion of assembled fibrinogen. Thromb. Res. 2009, 124, 368–372. [Google Scholar] [CrossRef] [Green Version]
- Guglielmone, H.A.; Sanchez, M.C.; Abate Daga, D.; Bocco, J.L. A new heterozygous mutation in gamma fibrinogen gene leading to 326 Cys→Ser substitution in fibrinogen Córdoba is associated with defective polymerization and familial hypodysfibrinogenemia. J. Thromb. Haemost. 2004, 2, 352–354. [Google Scholar] [CrossRef]
- Meyer, M.; Franke, K.; Richter, W.; Steiniger, F.; Seyfert, U.T.; Schenk, J.; Treuner, J.; Haberbosch, W.; Eisert, R.; Barthels, M. New molecular defects in the gamma subdomain of fibrinogen D-domain in four cases of (hypo)dysfibrinogenemia: Fibrinogen variants Hannover VI, Homburg VII, Stuttgart and Suhl. Thromb. Haemost. 2003, 89, 637–646. [Google Scholar]
- Song, K.S.; Park, N.J.; Choi, J.R.; Doh, H.J.; Chung, K.H. Fibrinogen Seoul (FGG Ala341Asp): A novel mutation associated with hypodysfibrinogenemia. Clin. Appl. Thromb. Hemost. 2006, 12, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Okumura, N.; Gorkun, O.V.; Lord, S.T. Severely impaired polymerization of recombinant fibrinogen gamma-364 Asp → His, the substitution discovered in a heterozygous individual. J. Biol. Chem. 1997, 272, 29596–29601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, T.; Arai, S.; Ogiwara, N.; Takezawa, Y.; Nanya, M.; Terasawa, F.; Okumura, N. γ375W fibrinogen-synthesizing CHO cells indicate the accumulation of variant fibrinogen within endoplasmic reticulum. Thromb. Res. 2014, 133, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, N.; Hirata, H.; Morigami, Y.; Imaoka, S.; Matsuda, M.; Yamazumi, K.; Asakura, S. Characterization of an abnormal fibrinogen Osaka V with the replacement of gamma-arginine 375 by glycine. The lack of high affinity calcium binding to D-domains and the lack of protective effect of calcium on fibrinolysis. J. Biol. Chem. 1992, 267, 2753–2759. [Google Scholar] [CrossRef]
- Ikeda, M.; Kobayashi, T.; Arai, S.; Mukai, S.; Takezawa, Y.; Terasawa, F.; Okumura, N. Recombinant γT305A fibrinogen indicates severely impaired fibrin polymerization due to the aberrant function of hole ‘A’ and calcium binding sites. Thromb. Res. 2014, 134, 518–525. [Google Scholar] [CrossRef] [Green Version]
- Appel, I.M.; Grimminck, B.; Geerts, J.; Stigter, R.; Cnossen, M.H.; Beishuizen, A. Age dependency of coagulation parameters during childhood and puberty. J. Thromb. Haemost. 2012, 10, 2254–2263. [Google Scholar] [CrossRef]
- Duga, S.; Braidotti, P.; Asselta, R.; Maggioni, M.; Santagostino, E.; Pellegrini, C.; Coggi, G.; Malcovati, M.; Tenchini, M.L. Liver histology of an afibrinogenemic patient with the Bβ-L353R mutation showing no evidence of hepatic endoplasmic reticulum storage disease (ERSD); comparative study in COS-1 cells of the intracellular processing of the Bβ-L353R fibrinogen vs. the ERSD-associated γ-G284R mutant. J. Thromb. Haemost. 2005, 3, 724–732. [Google Scholar] [CrossRef]
- Platè, M.; Asselta, R.; Spena, S.; Spreafico, M.; Fagoonee, S.; Peyvandi, F.; Tenchini, M.L.; Duga, S. Congenital hypofibrinogenemia: Characterization of two missense mutations affecting fibrinogen assembly and secretion. Blood Cells Mol. Dis. 2008, 41, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Vu, D.; Di Sanza, C.; Neerman-Arbez, M. Manipulating the quality control pathway in transfected cells: Low temperature allows rescue of secretion-defective fibrinogen mutants. Haematologica 2008, 93, 224–231. [Google Scholar] [CrossRef] [Green Version]
- Rooney, M.M.; Parise, L.V.; Lord, S.T. Dissecting clot retraction and platelet aggregation. Clot retraction does not require an intact fibrinogen gamma chain C terminus. J. Biol. Chem. 1996, 271, 8553–8555. [Google Scholar] [CrossRef] [Green Version]

| Variant | Recombinant Fibrinogen Production | Patients’ Plasma Fibrinogen Level | Disagreement | ||
|---|---|---|---|---|---|
| M/C (M/C of Wild-Type) | Reference | Antigenic (g/L) | Reference | ||
| γC153R | NA (2.09 ± 1.38) | [23] | 0.87 | [23] | ◯ |
| γR275C | Not reduced | [15] | 2.91 | [17] and 26 cases | ◯ |
| γR275H | Not reduced | [24] | 2.02 | [17] and 31 cases | ◯ |
| γT277P | 0.03 ± 0.02 (1.24 ± 0.31) | This manuscript | 1.18 | [21] | ◯ |
| γT277R | 2.03 ± 0.50 (1.24 ± 0.31) | 0.79 | [22] | ✕ | |
| γY278H | 0.72 ± 0.17 (1.24 ± 0.31) | 1.16 | This manuscript | ✕ | |
| γA279D | 1.62 ± 0.48 (1.24 ± 0.31) | 2.7 | [25] | ◯ | |
| γY280C | 0.82 ± 0.20 (1.24 ± 0.31) | NA * | [20] and 1 case | ◯ | |
| γG284R | 0.05 ± 0.01 (0.77 ± 0.22) | [24] | 1.00 | [19] | ◯ |
| γA289V | 0.07 ± 0.02 (0.88 ± 0.17) | [26] | 0.50 | [26] and 1 case | ◯ |
| γG292V | 0.35 ± 0.12 (0.88 ± 0.17) | [26] | 1.20 | [27] | ◯ |
| γT305A | 1.12 ± 0.14 (1.66 ± 0.40) | [28] | 1.12 | [28] | ✕ |
| γH307Y | 0.31 ± 0.09 (1.66 ± 0.40) | [28] | 0.86 | [29] | ◯ |
| γN308K | 1.74 ± 0.62 (1.66 ± 0.40) | [28] | 2.46 | [30] and 4 cases | ◯ |
| γN308I | Not reduced | [31] | NA * | [32] | ◯ |
| γS313N | 0.06 ± 0.01 (1.05 ± 0.17) | [18] | 0.58 | [33] | ◯ |
| γT314P | 0.08 ± 0.04 (0.77 ± 0.22) | [24] | 0.47 | [34] | ◯ |
| γD316N | 0.26 ± 0.05 (0.77 ± 0.22) | [24] | 1.36 | [21] and 1 case | ◯ |
| γD318Y | 0.70 ± 0.17 (1.30 ± 0.19) | [35] | 2.9 | [36] | ◯ |
| γΔ319 | 0.03 ± 0.01 (1.30 ± 0.19) | [35] | NA * | Czwalinna A, 2004 [12] (on-line submission) | ◯ |
| γN319K | 0.05 ± 0.01 (1.30 ± 0.19) | [35] | NA | Meyer M, 2000 [12] (congress abstract) | NA |
| γΔ320 | 0.09 ± 0.03 (1.30 ± 0.19) | [35] | 0.80 | [37] and 1 case | ◯ |
| γD320E | 0.06 ± 0.03 (1.30 ± 0.19) | [35] | 1.30 | [38] | ◯ |
| γD320G | 0.11 ± 0.02 (1.30 ± 0.19) | [35] | 0.85 | [35] and 1 case | ◯ |
| γC326S | 0.03 ± 0.02 (2.17 ± 0.57) | [39] | 0.81 | [40] and 3 cases | ◯ |
| γC326Y | 0.02 ± 0.03 (2.17 ± 0.57) | [39] | 1.50 | [41] and 1 case | ◯ |
| γM336I | 0.05 ± 0.01 (1.05 ± 0.17) | [18] | NA * | [41] and 1 case | ◯ |
| γA341D | 0.15 ± 0.06 (1.05 ± 0.17) | [18] | 1.37 | [42] | ◯ |
| γN345D | 0.09 ± 0.02 (1.05 ± 0.17) | [18] | <0.5 | [41] | ◯ |
| γD364H | Not reduced | [43] | 3.40 | [22] | ◯ |
| γG366S | 0.03 ± 0.02 (0.77 ± 0.22) | [24] | 0.95 | [21] | ◯ |
| γR375G | 1.10 ± 0.25 (0.94 ± 0.10) | [44] | 2.50 | [45] | ◯ |
| γR375W | 0.09 ± 0.10 (0.94 ± 0.10) | [44] | 1.10 | 13 cases | ◯ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamijo, T.; Kaido, T.; Yoda, M.; Arai, S.; Yamauchi, K.; Okumura, N. Recombinant ?Y278H Fibrinogen Showed Normal Secretion from CHO Cells, but a Corresponding Heterozygous Patient Showed Hypofibrinogenemia. Int. J. Mol. Sci. 2021, 22, 5218. https://doi.org/10.3390/ijms22105218
Kamijo T, Kaido T, Yoda M, Arai S, Yamauchi K, Okumura N. Recombinant ?Y278H Fibrinogen Showed Normal Secretion from CHO Cells, but a Corresponding Heterozygous Patient Showed Hypofibrinogenemia. International Journal of Molecular Sciences. 2021; 22(10):5218. https://doi.org/10.3390/ijms22105218
Chicago/Turabian StyleKamijo, Tomu, Takahiro Kaido, Masahiro Yoda, Shinpei Arai, Kazuyoshi Yamauchi, and Nobuo Okumura. 2021. "Recombinant ?Y278H Fibrinogen Showed Normal Secretion from CHO Cells, but a Corresponding Heterozygous Patient Showed Hypofibrinogenemia" International Journal of Molecular Sciences 22, no. 10: 5218. https://doi.org/10.3390/ijms22105218
APA StyleKamijo, T., Kaido, T., Yoda, M., Arai, S., Yamauchi, K., & Okumura, N. (2021). Recombinant ?Y278H Fibrinogen Showed Normal Secretion from CHO Cells, but a Corresponding Heterozygous Patient Showed Hypofibrinogenemia. International Journal of Molecular Sciences, 22(10), 5218. https://doi.org/10.3390/ijms22105218





