Molecular Structure of Cefuroxime Axetil Complexes with α-, β-, γ-, and 2-Hydroxypropyl-β-Cyclodextrins: Molecular Simulations and Raman Spectroscopic and Imaging Studies
Abstract
:1. Introduction
2. Results
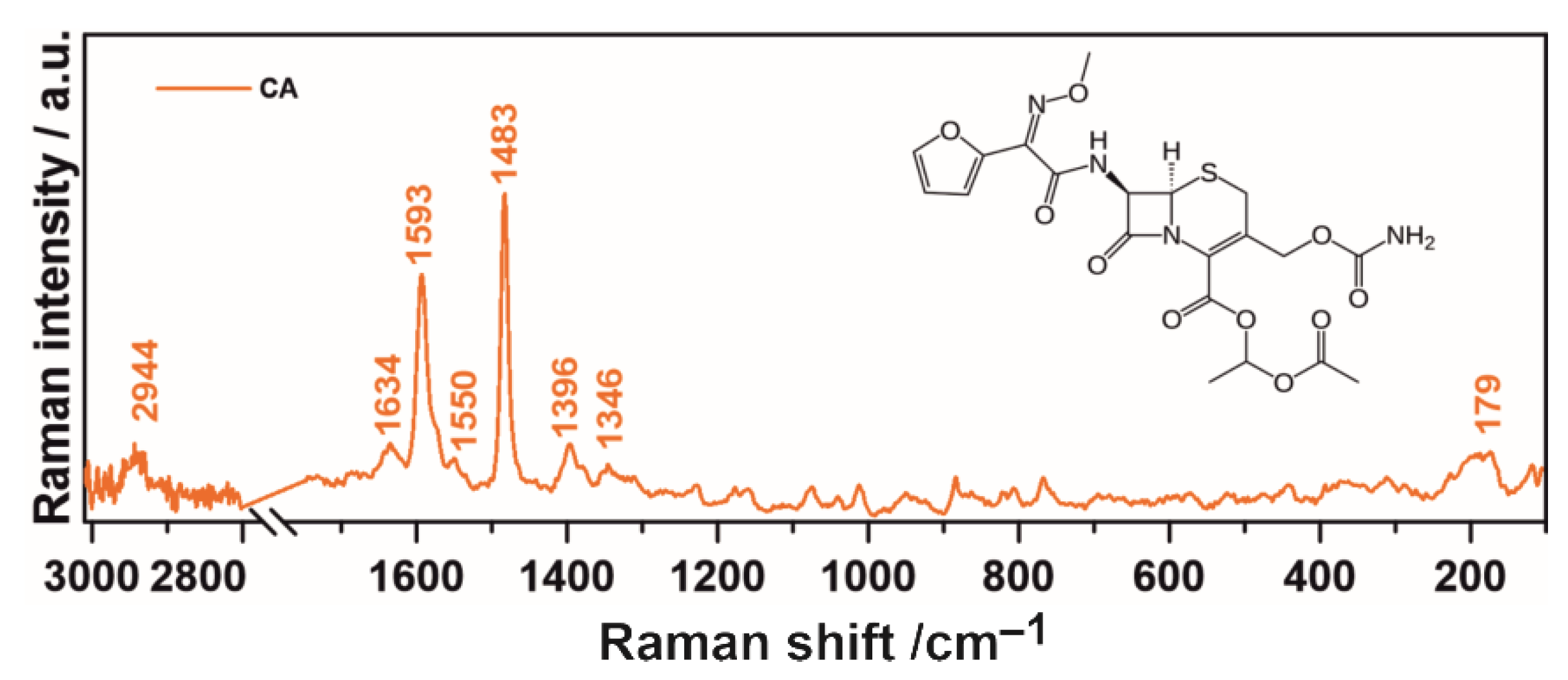
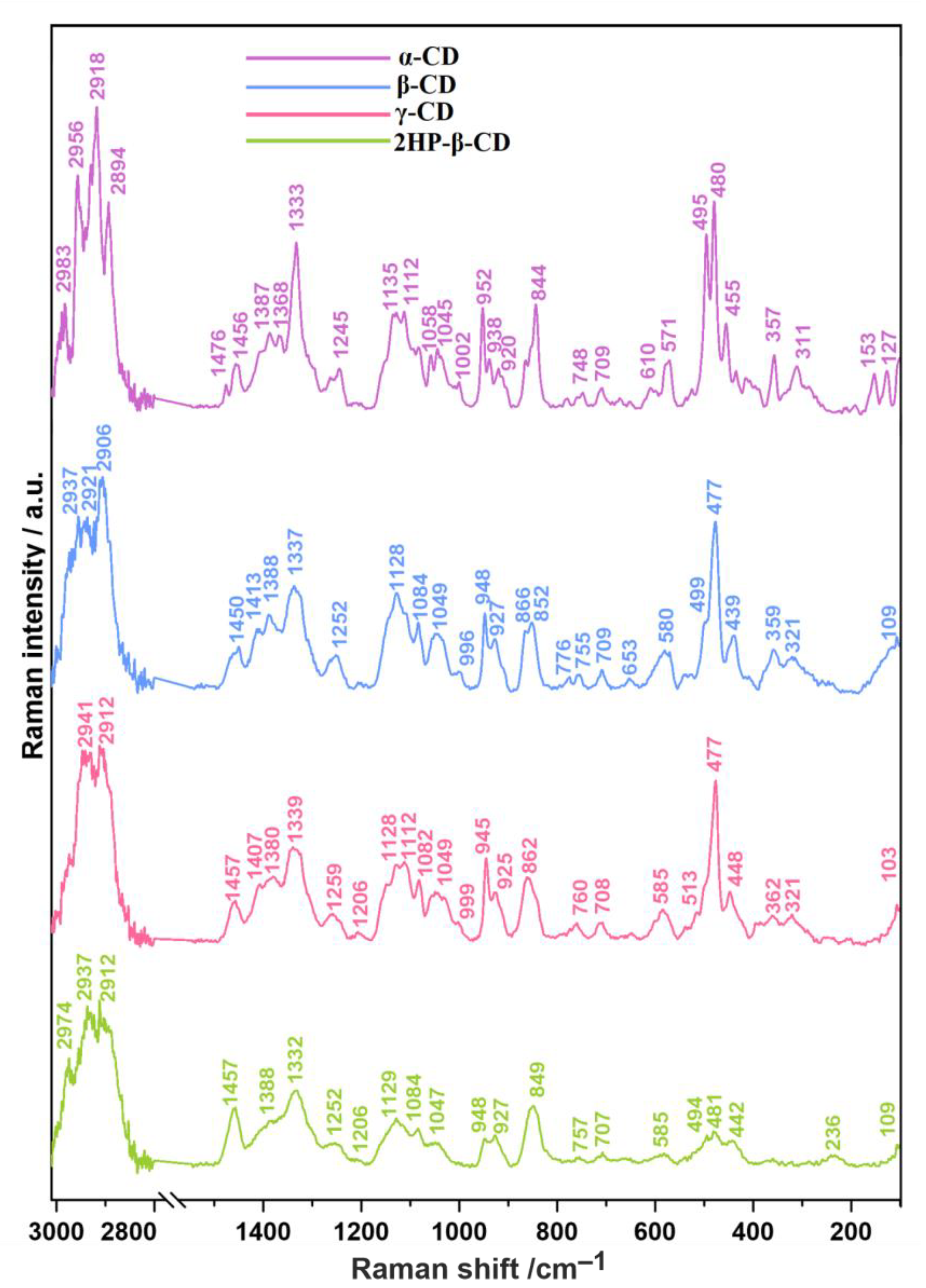
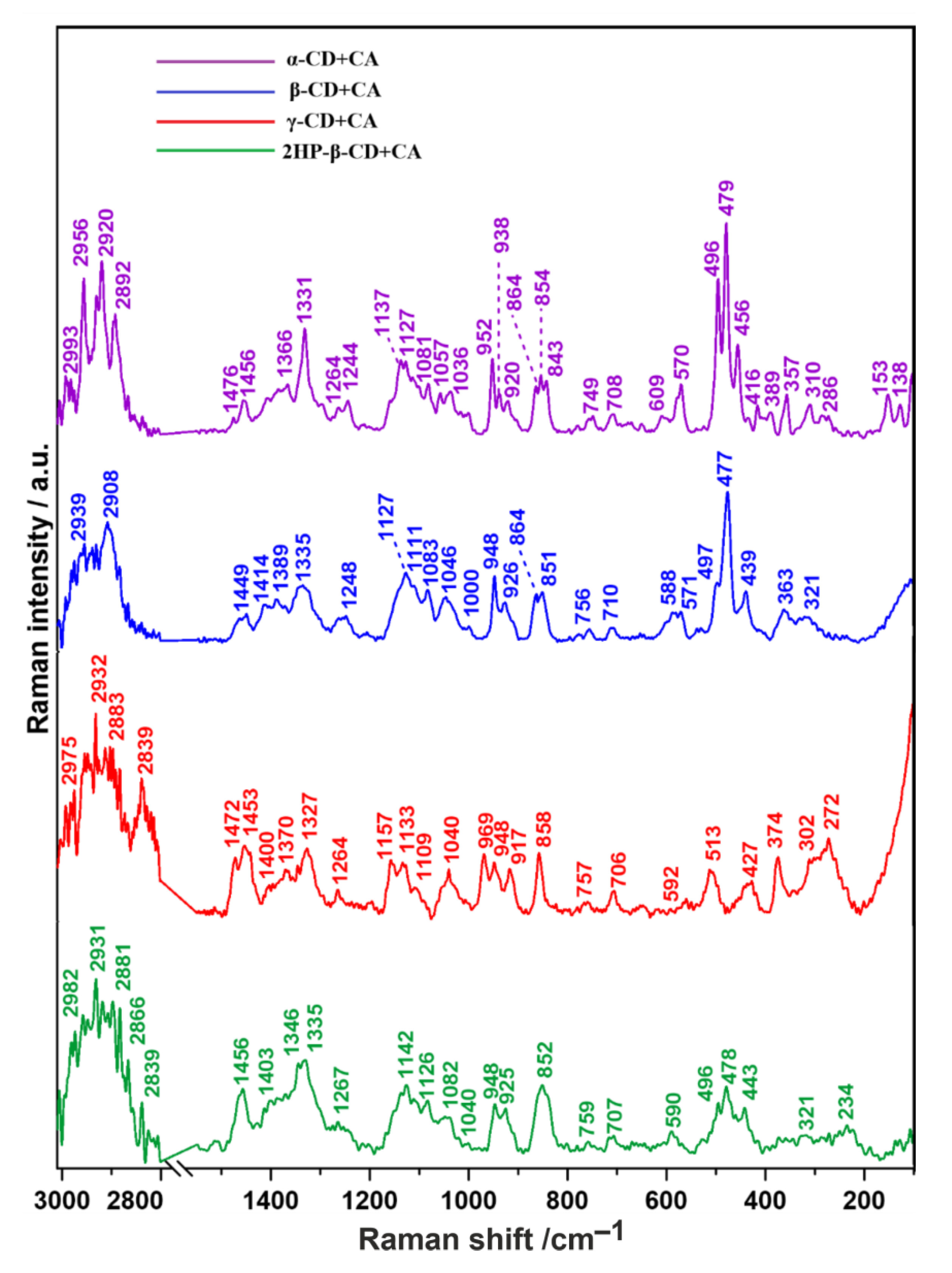
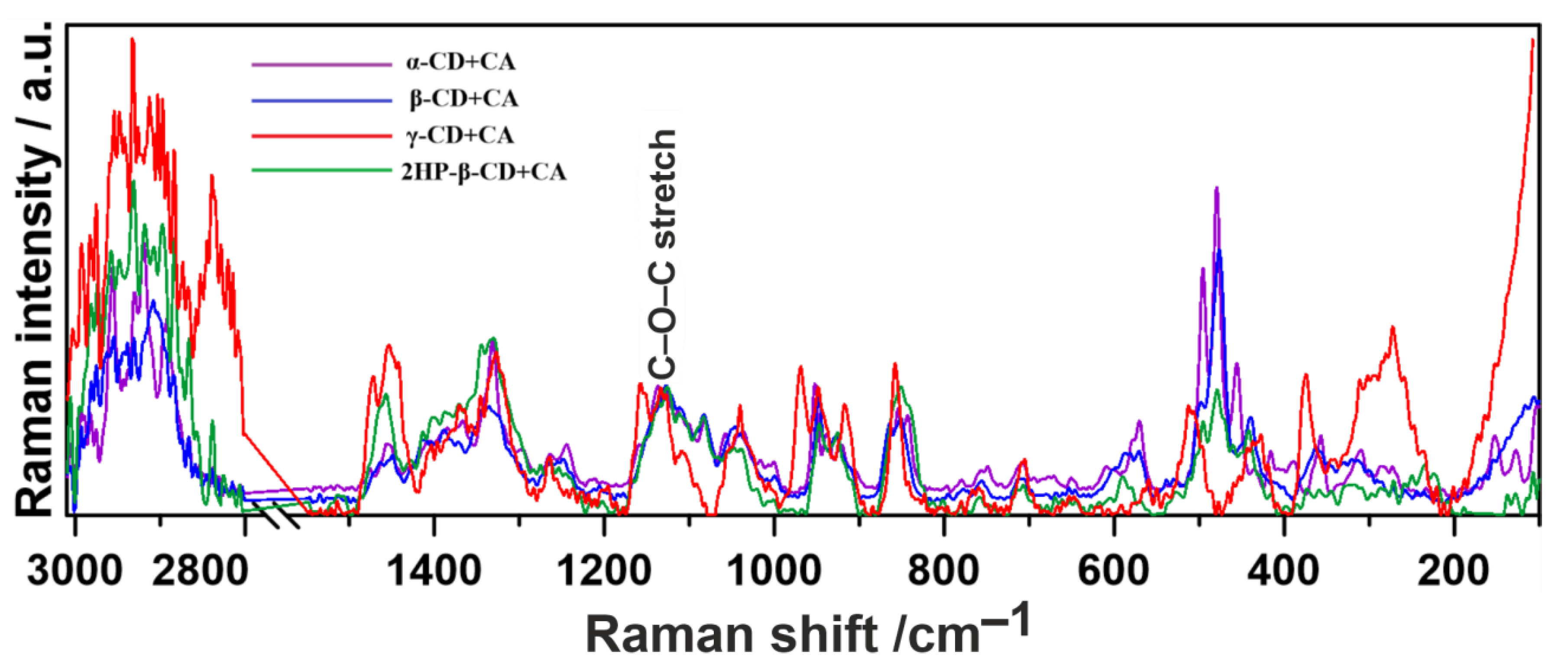
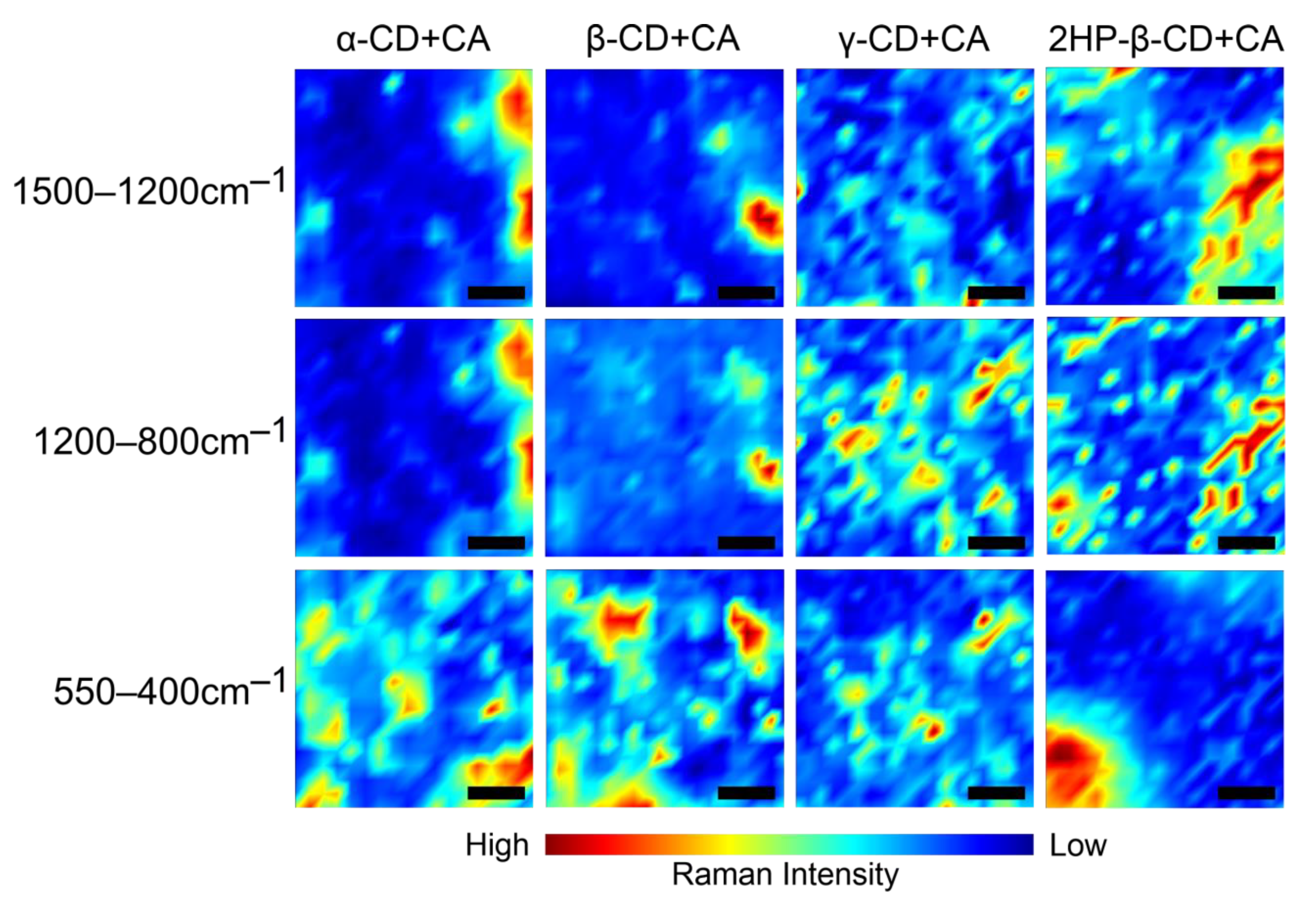
2.1. Raman Spectroscopy
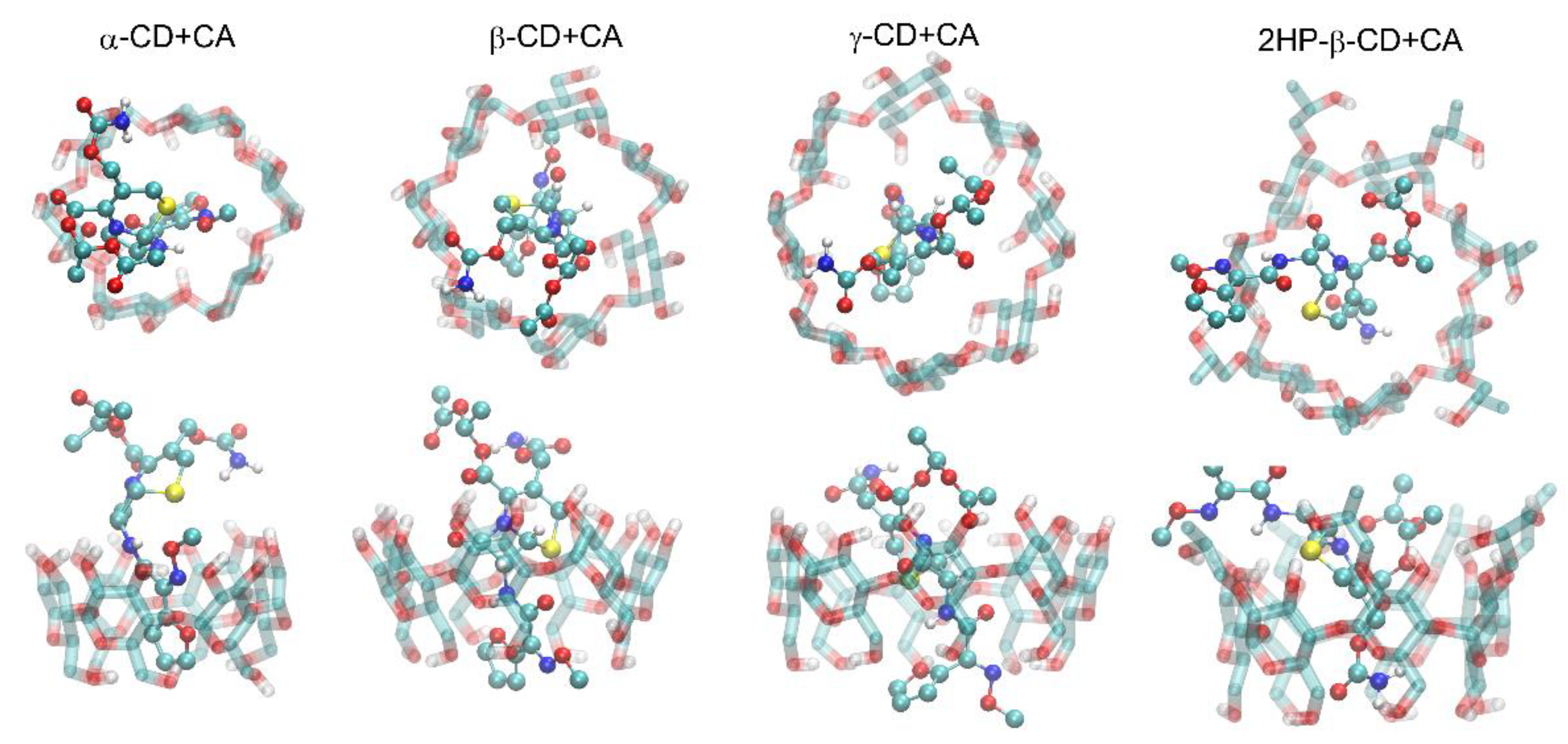
2.2. Molecular Modeling
3. Discussion
- The most fundamental observation regarding the guest–host type of complexation of CA exploiting the inner cavity of CDs is common for both methods.
- The contribution of guest–host hydrogen bonding can be identified as originating from the interactions of either hydroxyl (α-CD), both hydroxyl and hydroxymethyl groups (γ-CD) or hydroxypropyl groups (2HP-β-CD) with hydrogen and acceptors located in the CA molecule.
- The crucial role of the furanyl ring is confirmed in the case of binding by most CDs; additionally, the involvement of the lactam ring is characteristic for γ-CD, which is interpreted in sterical terms.
- The larger number of CA moieties involved in binding to 2HP-β-CD in comparison to other CDs is confirmed as well.
- Overall, the MD results explain the differences between binding modes observed for various complexes. Specifically, the similarities of the binding modes exhibited by α-CD and β-CD result from the analogous arrangement of the CA molecule in the binding cavities of CDs, including the interactions with the same furanyl group. The different binding mechanism found for γ-CD originates from the contribution of the condensed ring interacting with the inner cavity of CD. Finally, the spectra for 2HP-β-CD, differing to the largest extent in comparison to those for remaining systems, are the results of reorientation of the CA molecule in the CD binding cavity and the intensive interactions with the hydroxypropyl groups, which are absent in alternative CDs.
4. Materials and Methods
4.1. Preparation of Cefuroxime Axetil Complexes with Cyclodextrins
4.2. Raman Spectroscopy and Imaging
4.3. Molecular Modeling
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CD | cyclodextrin |
| α-CD | α-cyclodextrin |
| β-CD | β-cyclodextrin |
| γ-CD | γ-cyclodextrin |
| CA | cefuroxime axetil |
| 2HP-β-CD | 2-hydroxypropyl-β cyclodextrin |
References
- Crini, G. Review: A History of Cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef]
- Khalid, Q.; Ahmad, M.; Minhas, M.U. Synthesis of β-cyclodextrin hydrogel nanoparticles for improving the solubility of dexibuprofen: Characterization and toxicity evaluation. Drug Dev. Ind. Pharm. 2017, 43, 1873–1884. [Google Scholar] [CrossRef] [PubMed]
- Wimmer, T. Cyclodextrins. In ULLMANN’S Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2012. [Google Scholar] [CrossRef]
- Wimmer, R.; Aachmann, F.L.; Larsen, K.L.; Petersen, S.B. NMR diffusion as a novel tool for measuring the association constant between cyclodextrin and guest molecules. Carbohydr. Res. 2002, 337, 841–849. [Google Scholar]
- Kurkov, S.V.; Ukhatskaya, E.V.; Loftsson, T. Drug/cyclodextrin: Beyond inclusion complexation. J. Incl. Phenom. Macrocycl. Chem. 2011, 69, 297–301. [Google Scholar] [CrossRef]
- Brewster, M.E.; Loftsson, T. Cyclodextrins as pharmaceutical solubilizers. Adv. Drug Deliv. Rev. 2007, 59, 645–666. [Google Scholar] [CrossRef]
- Jin, L.; Liu, Q.; Sun, Z.; Ni, X.; Wei, M. Preparation of 5-fluorouracil/β-cyclodextrin complex intercalated in layered double hydroxide and the controlled drug release properties. Ind. Eng. Chem. Res. 2010, 49, 11176–11181. [Google Scholar] [CrossRef]
- Duchene, D.; Wouessidjewe, D.; Denis, W. Industrial Uses of Cyclodextrins and Their Derivatives. J. Coord. Chem. 1992, 27, 223–236. [Google Scholar] [CrossRef]
- Braga, S.S. Cyclodextrins: Emerging Medicines of the New Millennium. Biomolecules 2019, 9, 801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loftsson, T.; Jarho, P.; Másson, M.; Järvinen, T. Cyclodextrins in drug delivery. Expert Opin. Drug Deliv. 2005, 2, 335–351. [Google Scholar] [CrossRef]
- Tiwari, G.; Tiwari, R.; Rai, A. Cyclodextrins in delivery systems: Applications. J. Pharm. Bioallied Sci. 2010, 2, 72. [Google Scholar] [CrossRef]
- Stella, V.J.; He, Q. Cyclodextrins. Toxicol. Pathol. 2008, 36, 30–42. [Google Scholar] [CrossRef]
- Irie, T.; Uekama, K. Pharmaceutical Applications of Cyclodextrins. III. Toxicological Issues and Safety Evaluation. J. Pharm. Sci. 1997, 86, 147–162. [Google Scholar] [CrossRef]
- Vyas, A.; Saraf, S.S.; Saraf, S.S. Cyclodextrin based novel drug delivery systems. J. Incl. Phenom. Macrocycl. Chem. 2008, 62, 23–42. [Google Scholar] [CrossRef]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of Cyclodextrins and Drug/Cyclodextrin Complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef] [Green Version]
- Yong, C.W.; Washington, C.; Smith, W. Structural Behaviour of 2-Hydroxypropyl-β-Cyclodextrin in Water: Molecular Dynamics Simulation Studies. Pharm. Res. 2008, 25, 1092–1099. [Google Scholar] [CrossRef]
- Miranda, J.C.; Martins, T.E.A.; Veiga, F.; Ferraz, H.G. Cyclodextrins and ternary complexes: Technology to improve solubility of poorly soluble drugs. Braz. J. Pharm. Sci. 2011, 47, 665–681. [Google Scholar] [CrossRef] [Green Version]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef]
- Jambhekar, S.S.; Breen, P. Cyclodextrins in pharmaceutical formulations I: Structure and physicochemical properties, formation of complexes, and types of complex. Drug Discov. Today 2016, 21, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Uekama, K.; Hirayama, F.; Irie, T. Cyclodextrin Drug Carrier Systems. Chem. Rev. 1998, 98, 2045–2076. [Google Scholar] [CrossRef] [PubMed]
- Malanga, M.; Szemán, J.; Fenyvesi, É.; Puskás, I.; Csabai, K.; Gyémánt, G.; Fenyvesi, F.; Szente, L. “Back to the Future”: A New Look at Hydroxypropyl Beta-Cyclodextrins. J. Pharm. Sci. 2016, 105, 2921–2931. [Google Scholar] [CrossRef] [Green Version]
- Miyake, K.; Arima, H.; Hirayama, F.; Yamamoto, M.; Horikawa, T.; Sumiyoshi, H.; Noda, S.; Uekama, K. Improvement of Solubility and Oral Bioavailability of Rutin by Complexation with 2-Hydroxypropyl-β-cyclodextrin. Pharm. Dev. Technol. 2000, 5, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.; Scott, R.C. 2-Hydroxypropyl-β-cyclodextrin (HP-β-CD): A toxicology review. Food Chem. Toxicol. 2005, 43, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Mohit, V.; Harshal, G.; Neha, D.; Vilasrao, K.; Rajashree, H. Effect of preparation method on complexation of Cefdinir with β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2010, 67, 39–47. [Google Scholar] [CrossRef]
- Leder, R.D.; Carson, D.S. Cefuroxime Axetil (Ceftin®): A Brief Review. Infect. Dis. Obstet. Gynecol. 1997, 5, 211–214. [Google Scholar] [CrossRef] [Green Version]
- Harding, S.M.; Williams, P.E.; Ayrton, J. Pharmacology of Cefuroxime as the 1-acetoxyethyl ester in volunteers. Antimicrob. Agents Chemother. 1984, 25, 78–82. [Google Scholar] [CrossRef] [Green Version]
- Chaudhry, S.B.; Veve, M.P.; Wagner, J.L. Cephalosporins: A Focus on Side Chains and β-Lactam Cross-Reactivity. Pharmacy 2019, 7, 103. [Google Scholar] [CrossRef] [Green Version]
- Ginsburg, C.M.; McCracken, G.H.; Petruska, M.; Olson, K. Pharmacokinetics and bactericidal activity of cefuroxime axetil. Antimicrob. Agents Chemother. 1985, 28, 504–507. [Google Scholar] [CrossRef] [Green Version]
- Pichichero, M.E. Use of selected cephalosporins in penicillin-allergic patients: A paradigm shift. Diagn. Microbiol. Infect. Dis. 2007, 57, S13–S18. [Google Scholar] [CrossRef] [PubMed]
- Sader, H.S.; Jacobs, M.R.; Fritsche, T.R. Review of the spectrum and potency of orally administered cephalosporins and amoxicillin/clavulanate. Diagn. Microbiol. Infect. Dis. 2007, 57, S5–S12. [Google Scholar] [CrossRef]
- Jun, S.W.; Kim, M.-S.; Jo, G.H.; Lee, S.; Woo, J.S.; Park, J.-S.; Hwang, S.-J. Cefuroxime axetil solid dispersions prepared using solution enhanced dispersion by supercritical fluids. J. Pharm. Pharmacol. 2010, 57, 1529–1537. [Google Scholar] [CrossRef]
- Shah, M.; Shah, V.; Ghosh, A.; Zhang, Z.; Minko, T. Molecular Inclusion Complexes of β-Cyclodextrin Derivatives Enhance Aqueous Solubility and Cellular Internalization of Paclitaxel: Preformulation and In vitro Assessments. J. Pharm. Pharmacol. 2015, 3, 8. [Google Scholar] [CrossRef] [Green Version]
- Loftsson, T. Cyclodextrins and the Biopharmaceutics classification system of drugs. J. Incl. Phenom. Macrocycl. Chem. 2002, 44, 63–67. [Google Scholar] [CrossRef]
- Skoog, D.A.; Holler, F.J.; Croush, S.R. Principles of Instrumental Analysis, 6th ed.; Thomson Brooks/Cole: Belmont, CA, USA, 2007. [Google Scholar]
- Rohman, A.; Windarsih, A.; Lukitaningsih, E.; Rafi, M.; Betania, K.; Fadzillah, N.A. The use of FTIR and Raman spectroscopy in combination with chemometrics for analysis of biomolecules in biomedical fluids: A review. Biomed. Spectrosc. Imaging 2020, 8, 55–71. [Google Scholar] [CrossRef] [Green Version]
- Gordon, K.C.; McGoverin, C.M. Raman mapping of pharmaceuticals. Int. J. Pharm. 2011, 417, 151–162. [Google Scholar] [CrossRef]
- Khan, G.M.; Wazir, F.; Zhu, J.B. Ibuprofen-Cyclodextrin Inclusion Complexes: Evaluation of Different Complexation Methods. J. Med. Sci. 2001, 1, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Mizera, M.; Szymanowska, D.; Stasiłowicz, A.; Siąkowska, D.; Lewandowska, K.; Miklaszewski, A.; Plech, T.; Tykarska, E.; Cielecka-Piontek, J. Computer-Aided Design of Cefuroxime Axetil/Cyclodextrin System with Enhanced Solubility and Antimicrobial Activity. Biomolecules 2019, 10, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sapte, S.; Pore, Y. Inclusion complexes of cefuroxime axetil with β-cyclodextrin: Physicochemical characterization, molecular modeling and effect of l-arginine on complexation. J. Pharm. Anal. 2016, 6, 300–306. [Google Scholar] [CrossRef] [Green Version]
- Shah, M.; Pore, Y.; Dhawale, S.; Burade, K.; Kuchekar, B. Physicochemical characterization of spray dried ternary micro-complexes of cefuroxime axetil with hydroxypropyl-β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2013, 76, 391–401. [Google Scholar] [CrossRef]
- Talaczynska, A.; Mizera, M.; Szybowicz, M.; Nowicka, A.B.; Garbacki, P.; Paczkowska, M.; Zalewski, P.; Kozak, M.; Oszczapowicz, I.; Jelinska, A.; et al. Studies of the crystalline form of cefuroxime axetil: Implications for its compatibility with excipients. Acta Pol. Pharm. 2016, 73, 1299–1309. [Google Scholar] [PubMed]
- Talaczyńska, A.; Lewandowska, K.; Jelińska, A.; Garbacki, P.; Podborska, A.; Zalewski, P.; Oszczapowicz, I.; Sikora, A.; Kozak, M.; Cielecka-Piontek, J. Application of Vibrational Spectroscopy Supported by Theoretical Calculations in Identification of Amorphous and Crystalline Forms of Cefuroxime Axetil. Sci. World J. 2015, 2015, 921049. [Google Scholar] [CrossRef] [PubMed]
- Venuti, V.; Crupi, V.; Fazio, B.; Majolino, D.; Acri, G.; Testagrossa, B.; Stancanelli, R.; De Gaetano, F.; Gagliardi, A.; Paolino, D.; et al. Physicochemical Characterization and Antioxidant Activity Evaluation of Idebenone/Hydroxypropyl-β-Cyclodextrin Inclusion Complex. Biomolecules 2019, 9, 531. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira, V.E.; Almeida, E.W.C.; Castro, H.V.; Edwards, H.G.M.; Dos Santos, H.F.; de Oliveira, L.F.C. Carotenoids and β-Cyclodextrin Inclusion Complexes: Raman Spectroscopy and Theoretical Investigation. J. Phys. Chem. A 2011, 115, 8511–8519. [Google Scholar] [CrossRef]
- Tijunelyte, I.; Dupont, N.; Milosevic, I.; Barbey, C.; Rinnert, E.; Lidgi-Guigui, N.; Guenin, E.; de la Chapelle, M.L. Investigation of aromatic hydrocarbon inclusion into cyclodextrins by Raman spectroscopy and thermal analysis. Environ. Sci. Pollut. Res. 2017, 24, 27077–27089. [Google Scholar] [CrossRef]
- Barron, L.D.; Gargaro, A.R.; Wen, Z.Q.; MacNicol, D.D.; Butters, C. Vibrational Raman optical activity of cyclodextrins. Tetrahedron Asymmetry 1990, 1, 513–516. [Google Scholar] [CrossRef]
- De Gelder, J.; De Gussem, K.; Vandenabeele, P.; Moens, L. Reference database of Raman spectra of biological molecules. J. Raman Spectrosc. 2007, 38, 1133–1147. [Google Scholar] [CrossRef]
- Paczkowska, M.; Szymanowska-Powałowska, D.; Mizera, M.; Siąkowska, D.; Błaszczak, W.; Piotrowska-Kempisty, H.; Cielecka-Piontek, J. Cyclodextrins as multifunctional excipients: Influence of inclusion into β-cyclodextrin on physicochemical and biological properties of tebipenem pivoxil. PLoS ONE 2019, 14, e0210694. [Google Scholar] [CrossRef] [Green Version]
- Fan, D.; Ma, W.; Wang, L.; Huang, J.; Zhao, J.; Zhang, H.; Chen, W. Determination of structural changes in microwaved rice starch using Fourier transform infrared and Raman spectroscopy. Starch Stärke 2012, 64, 598–606. [Google Scholar] [CrossRef]
- Fechner, P.M.; Wartewig, S.; Kleinebudde, P.; Neubert, R.H.H. Studies of the retrogradation process for various starch gels using Raman spectroscopy. Carbohydr. Res. 2005, 340, 2563–2568. [Google Scholar] [CrossRef] [PubMed]
- Egyed, O. Spectroscopic studies on β-cyclodextrin. Vib. Spectrosc. 1990, 1, 225–227. [Google Scholar] [CrossRef]
- Wiercigroch, E.; Szafraniec, E.; Czamara, K.; Pacia, M.Z.; Majzner, K.; Kochan, K.; Kaczor, A.; Baranska, M.; Malek, K. Raman and infrared spectroscopy of carbohydrates: A review. Spectrochim. Acta A. Mol. Biomol. Spectrosc. 2017, 185, 317–335. [Google Scholar] [CrossRef] [PubMed]
- França de Sá, S.; Ferreira, J.L.; Matos, A.S.; Macedo, R.; Ramos, A.M. A new insight into polyurethane foam deterioration-The use of Raman microscopy for the evaluation of long-term storage conditions. J. Raman Spectrosc. 2016, 47, 1494–1504. [Google Scholar] [CrossRef]
- Morzyk-Ociepa, B.; Nowak, M.J.; Michalska, D. Vibrational spectra of 1-methylthymine: Matrix isolation, solid state and theoretical studies. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2004, 60, 2113–2123. [Google Scholar] [CrossRef]
- Podstawka, E.; Światłowska, M.; Borowiec, E.; Proniewicz, L.M. Food additives characterization by infrared, Raman, and surface-enhanced Raman spectroscopies. J. Raman Spectrosc. 2007, 38, 356–363. [Google Scholar] [CrossRef]
- Bauer, A.J.R. Raman Spectroscopic Study of Sugars in Common Liquid Sweeteners. Spectroscopy 2014, 29, 98. [Google Scholar]
- Cozar, O.; Cioica, N.; Coţa, C.; Nagy, E.M.; Fechete, R. Plasticizers effect on native biodegradable package materials. In Proceedings of the Tim15-16 Physics Conference, Timisoara, Romania, 26–28 May 2016. [Google Scholar]
- Vázquez, M.; Oliva, M.; Téllez-Luis, S.J.; Ramírez, J.A. Hydrolysis of sorghum straw using phosphoric acid: Evaluation of furfural production. Bioresour. Technol. 2007, 98, 3053–3060. [Google Scholar] [CrossRef] [PubMed]
- Madan, J.; Dhiman, N.; Parmar, V.K.; Sardana, S.; Bharatam, P.V.; Aneja, R.; Chandra, R.; Katyal, A. Inclusion complexes of noscapine in β-cyclodextrin offer better solubility and improved pharmacokinetics. Cancer Chemother. Pharmacol. 2010, 65, 537–548. [Google Scholar] [CrossRef]
- Saha, S.; Roy, A.; Roy, K.; Roy, M.N. Study to explore the mechanism to form inclusion complexes of β-cyclodextrin with vitamin molecules. Sci. Rep. 2016, 6, 35764. [Google Scholar] [CrossRef] [PubMed]
- Esmonde-White, K. Raman Spectroscopy of Soft Musculoskeletal Tissues. Appl. Spectrosc. 2014, 68, 1203–1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sergeeva, A.V.; Zhitova, E.S.; Nuzhdaev, A.A.; Zolotarev, A.A.; Bocharov, V.N.; Ismagilova, R.M. Infrared and Raman Spectroscopy of Ammoniovoltaite, (NH4)2Fe2+5Fe3+3Al(SO4)12(H2O)18. Minerals 2020, 10, 781. [Google Scholar] [CrossRef]
- Chan, J.W.; Taylor, D.S.; Zwerdling, T.; Lane, S.M.; Ihara, K.; Huser, T. Micro-Raman Spectroscopy Detects Individual Neoplastic and Normal Hematopoietic Cells. Biophys. J. 2006, 90, 648–656. [Google Scholar] [CrossRef] [Green Version]
- Chylińska, M.; Szymańska-Chargot, M.; Zdunek, A. Imaging of polysaccharides in the tomato cell wall with Raman microspectroscopy. Plant Methods 2014, 10, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueiras, A.; Ribeiro, L.; Vieira, M.T.; Veiga, F. Preparation and physicochemical characterization of omeprazole:methyl-beta-cyclodextrin inclusion complex in solid state. J. Incl. Phenom. Macrocycl. Chem. 2007, 57, 173–177. [Google Scholar] [CrossRef] [Green Version]
- Sinha, V.R.; Anitha, R.; Ghosh, S.; Nanda, A.; Kumria, R. Complexation of celecoxib with β-cyclodextrin: Characterization of the interaction in solution and in solid state. J. Pharm. Sci. 2005, 94, 676–687. [Google Scholar] [CrossRef]
- Badr-Eldin, S.M.; Elkheshen, S.A.; Ghorab, M.M. Inclusion complexes of tadalafil with natural and chemically modified β-cyclodextrins. I: Preparation and In-Vitro evaluation. Eur. J. Pharm. Biopharm. 2008, 70, 819–827. [Google Scholar] [CrossRef]
- Kreaz, R.M.; Dombi, G.Y.; Kata, M. Increasing The Solubility of Furosemide with Cyclodextrins. In Proceedings of the Eighth International Symposium on Cyclodextrins; Springer: Dordrecht, The Netherlands, 1996; pp. 341–344. [Google Scholar]
- Yang, Z.; Chai, K.; Ji, H. Selective inclusion and separation of cinnamaldehyde and benzaldehyde by insoluble β-cyclodextrin polymer. Sep. Purif. Technol. 2011, 80, 209–216. [Google Scholar] [CrossRef]
- Bratu, I.; Hernanz, A.; Gavira, J.M.; Bora, G.H. FT-IR spectroscopy of inclusion complexes of β-cyclodextrin with fenbuten and ibuprofen. Rom. J. Phys. 2005, 50, 1063–1069. [Google Scholar]
- Mangolim, C.S.; Moriwaki, C.; Nogueira, A.C.; Sato, F.; Baesso, M.L.; Neto, A.M.; Matioli, G. Curcumin–β-cyclodextrin inclusion complex: Stability, solubility, characterisation by FT-IR, FT-Raman, X-ray diffraction and photoacoustic spectroscopy, and food application. Food Chem. 2014, 153, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Witte, F.; Hoffmann, H. Aggregation Behavior of Hydrophobically Modified β-Cyclodextrins In Aqueous Solution. In Proceedings of the Eighth International Symposium on Cyclodextrins; Springer: Dordrecht, The Netherlands, 1996; pp. 37–40. [Google Scholar]
- Challa, R.; Ahuja, A.; Ali, J.; Khar, R.K. Cyclodextrins in drug delivery: An updated review. AAPS PharmSciTech 2005, 6, E329–E357. [Google Scholar] [CrossRef]
- Borrego-Sánchez, A.; Hernández-Laguna, A.; Sainz-Díaz, C.I. Molecular modeling and infrared and Raman spectroscopy of the crystal structure of the chiral antiparasitic drug Praziquantel. J. Mol. Model. 2017, 23, 106. [Google Scholar] [CrossRef] [PubMed]
- Sathyanarayanmoorthi, V.; Karunathan, R.; Kannappan, V. Molecular Modeling and Spectroscopic Studies of Benzothiazole. J. Chem. 2013, 2013, 258519. [Google Scholar] [CrossRef] [Green Version]
- Prasad, R.; Gupta, K.M.; Poornachary, S.K.; Dalvi, S.V. Elucidating the polymorphic behavior of curcumin during antisolvent crystallization: Insights from Raman spectroscopy and molecular modeling. Cryst. Growth Des. 2020, 20, 6008–6023. [Google Scholar] [CrossRef]
- Miskovsky, P.; Hritz, J.; Sanchez-Cortes, S.; Fabriciova, G.; Ulicny, J.; Chinsky, L. Interaction of Hypericin with Serum Albumins: Surface-enhanced Raman Spectroscopy, Resonance Raman Spectroscopy and Molecular Modeling Study. Photochem. Photobiol. 2007, 74, 172–183. [Google Scholar] [CrossRef]
- Mosier-Boss, P.A.; Lieberman, S.H. Surface-Enhanced Raman Spectroscopy (SERS) and Molecular Modeling of the Chromate Interaction with 4-(2-Mercaptoethyl)Pyridinium. Langmuir 2003, 19, 6826–6836. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; van Gunsteren, W.F.; Hermans, J. Interaction Models for Water in Relation to Protein Hydration. In Intermolecular Forces; Springer: Dordrecht, The Netherlands, 1981; pp. 331–342. [Google Scholar]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindah, E. Gromacs: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Hansen, H.S.; Hünenberger, P.H. A reoptimized GROMOS force field for hexopyranose-based carbohydrates accounting for the relative free energies of ring conformers, anomers, epimers, hydroxymethyl rotamers, and glycosidic linkage conformers. J. Comput. Chem. 2011, 32, 998–1032. [Google Scholar] [CrossRef] [PubMed]
- Plazinski, W.; Lonardi, A.; Hünenberger, P.H. Revision of the GROMOS 56A6 CARBO force field: Improving the description of ring-conformational equilibria in hexopyranose-based carbohydrates chains. J. Comput. Chem. 2016, 37, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, J.; Kleist, C.; Jakobtorweihen, S.; Hansen, N. Validation and Comparison of Force Fields for Native Cyclodextrins in Aqueous Solution. J. Phys. Chem. B 2018, 122, 1608–1626. [Google Scholar] [CrossRef]
- Stroet, M.; Caron, B.; Visscher, K.M.; Geerke, D.P.; Malde, A.K.; Mark, A.E. Automated Topology Builder Version 3.0: Prediction of Solvation Free Enthalpies in Water and Hexane. J. Chem. Theory Comput. 2018, 14, 5834–5845. [Google Scholar] [CrossRef]
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007, 126, 014101. [Google Scholar] [CrossRef] [Green Version]
- Parrinello, M.; Rahman, A. Polymorphic transitions in single crystals: A new molecular dynamics method. J. Appl. Phys. 1981, 52, 7182–7190. [Google Scholar] [CrossRef]
- Hockney, R.W. The potential calculation and some applications. Methods Comput. Phys. 1970, 9, 135–211. [Google Scholar]
- Hess, B. P-LINCS: A Parallel Linear Constraint Solver for Molecular Simulation. J. Chem. Theory Comput. 2008, 4, 116–122. [Google Scholar] [CrossRef]
- Miyamoto, S.; Kollman, P.A. Settle: An analytical version of the SHAKE and RATTLE algorithm for rigid water models. J. Comput. Chem. 1992, 13, 952–962. [Google Scholar] [CrossRef]
- Barker, J.A.; Watts, R.O. Monte Carlo studies of the dielectric properties of water-like models. Mol. Phys. 1973, 26, 789–792. [Google Scholar] [CrossRef]
- Heinz, T.N.; van Gunsteren, W.F.; Hünenberger, P.H. Comparison of four methods to compute the dielectric permittivity of liquids from molecular dynamics simulations. J. Chem. Phys. 2001, 115, 1125–1136. [Google Scholar] [CrossRef]
- Bennett, C.H. Efficient estimation of free energy differences from Monte Carlo data. J. Comput. Phys. 1976, 22, 245–268. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R.J. Avogadro: An advanced semantic chemical editor, visualization and analysis platform. Cheminformatics 2012, 4, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Cyclodextrin | Number of Glucopyranose Units | Molecular Weight [g/mol] | Cavity Inner Diameter [Å] | Cavity Outer Diameter [Å] | Cavity Height [Å] | Cavity Volume [Å] | Specific Rotation, Optical Activity [α]D20 [H2O, 1%] | Solubility in H2O [g/100 mL, 25 °C] |
|---|---|---|---|---|---|---|---|---|
| α-CD | 6 | 973 | 4.7–5.3 | 14.6 | 7.9 | 174 | +150.5° | 14.5 |
| β-CD | 7 | 1135 | 6.0–6.5 | 15.4 | 7.9 | 262 | +162.5° | 1.85 |
| γ-CD | 8 | 1297 | 7.0–8.3 | 17.5 | 7.9 | 427 | +177.4° | 23.20 |
| 2HP-β-CD | 7 | 1460 | 6.0 | - | - | - | +135.0° | 33 |
| Raman Shift/cm−1 | Band Assignments | |||
|---|---|---|---|---|
| Sample | ||||
| α-CD+CA | β-CD+CA | γ-CD+CA | 2HP-β-CD+CA | |
| 2993 | - | - | - | CH3 antisymmetric stretching |
| - | - | 2975 | 2982 | CH stretching |
| 2920 | - | - | - | |
| 2892 | 2908 | 2883 | 2881 | |
| 2956 | - | - | - | CH stretching or wagging |
| - | 2939 | 2932 | 2931 | CH2 antisymmetric stretching, CH3 symmetric stretching |
| 2918 | - | - | - | CH stretching or wagging |
| - | - | - | 2866 | CH2 symmetric stretching |
| - | - | 2839 | 2839 | CH3 stretching |
| 1476 | - | 1472 | - | CH deformational |
| 1456 | 1449 | 1453 | 1456 | CH2 deformational |
| - | 1414 | 1400 | 1403 | C–O–C symmetric and antisymmetric stretching |
| - | 1389 | - | - | C–H stretching or wagging |
| 1366 | - | 1370 | - | C–C stretching and ring deformation |
| - | - | - | 1346 * | Deformations of the CH2OH group, C–O, C–N stretching |
| 1331 | 1335 | 1327 | 1335 | CH2 deformational |
| 1264 | - | 1264 | 1267 | C=O stretching |
| 1244 | 1248 | - | - | C=O stretching, CH in plane bending of the aromatic rings, OH in plane bending, CH2 stretching |
| - | - | 1157 * | - | –C=C–H antisymmetric angular deformation in plane, furanyl ring in CA |
| 1137 | - | 1133 | 1142 | C-O-C stretching |
| 1127 | 1127 | - | 1126 | C–O–C symmetric stretching |
| - | 1111 | 1109 | - | C–O–C symmetric and antisymmetric stretching of glycosidic bonds |
| 1081 | 1083 | - | 1082 | |
| 1057 1036 | 1046 | 1040 | 1040 | C–O stretching |
| - | 1000 * | - | - | “breathing mode” of the aromatic carbon ring, C=C–H stretching |
| - | - | 969 * | - | C–H and C–OH deformational |
| 952 | 948 | 948 | 948 | Skeletal mode of α-(1–4) linkage (delocalized mode), C–O stretching |
| 938 864 | 926 864 | 917 - | 925 - | Glucopyranose (C–O–C) skeletal mode of α-anomers |
| 854 | 851 | 858 | 852 | Skeletal vibrations, OCH side group deformational of d-glucopyranose units, CNC symmetric stretching of the imide |
| 843 | - | - | - | C–O–C antisymmetric stretching |
| 749 | 756 | 757 | 759 | d-glucopyranose ring breathing mode and C–C central stretch |
| 708 | 710 | 706 | 707 | C–H out-of-plane bending of CA |
| 609 * | - | - | - | C–C–C ring in-plane bending of CA |
| - | 588 | 592 | 590 | Symmetric oxygen breathing vibration, C–O bending |
| 570 | 571 | - | - | OH wagging |
| - | - | 513 * | - | In-plane C–C stretching and ring deformation of CA |
| 496 | 497 | - | 496 | C–C–C bending |
| 479 | 477 | - | 478 | Skeletal vibrations, amylose |
| 456 | 439 | - | 443 | CH stretching |
| 416 357 | - 363 | 427 - | - 234 | OH stretching |
| 389 | - | 374 | - | C–C stretching |
| - | 321 * | - | External C–OH out of plane bending of glucopyranose units | |
| 310 | - | 302 | - | C–C antisymmetric stretching |
| 286 | - | 272 | - | C–O stretching |
| 153 * | - | - | - | Breathing motions of oxygen atoms in the macrocyclic ring |
| 138 * | - | - | - | Stretching or bending vibrations of hydrogen bonds |
| The Raman Intensity Ratio | Sample | |||
|---|---|---|---|---|
| α-CD+CA * | β-CD+CA ** | γ-CD+CA *** | 2HP-β-CD+CA **** | |
| IC=O/CH2 | 0.249 ± 0.055 | 0.330 ± 0.056 | 0.394 ± 0.100 | 0.238 ± 0.081 |
| IOH/CH2 | 0.492 ± 0.081 | 0.574 ± 0.084 | 0.495 ± 0.133 | 0.132 ± 0.086 |
| IC=O/OH | 0.505 ± 0.094 | 0.588 ± 0.138 | 0.837 ± 0.250 | 1.770 ± 0.990 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gieroba, B.; Kalisz, G.; Sroka-Bartnicka, A.; Płazińska, A.; Płaziński, W.; Starek, M.; Dąbrowska, M. Molecular Structure of Cefuroxime Axetil Complexes with α-, β-, γ-, and 2-Hydroxypropyl-β-Cyclodextrins: Molecular Simulations and Raman Spectroscopic and Imaging Studies. Int. J. Mol. Sci. 2021, 22, 5238. https://doi.org/10.3390/ijms22105238
Gieroba B, Kalisz G, Sroka-Bartnicka A, Płazińska A, Płaziński W, Starek M, Dąbrowska M. Molecular Structure of Cefuroxime Axetil Complexes with α-, β-, γ-, and 2-Hydroxypropyl-β-Cyclodextrins: Molecular Simulations and Raman Spectroscopic and Imaging Studies. International Journal of Molecular Sciences. 2021; 22(10):5238. https://doi.org/10.3390/ijms22105238
Chicago/Turabian StyleGieroba, Barbara, Grzegorz Kalisz, Anna Sroka-Bartnicka, Anita Płazińska, Wojciech Płaziński, Małgorzata Starek, and Monika Dąbrowska. 2021. "Molecular Structure of Cefuroxime Axetil Complexes with α-, β-, γ-, and 2-Hydroxypropyl-β-Cyclodextrins: Molecular Simulations and Raman Spectroscopic and Imaging Studies" International Journal of Molecular Sciences 22, no. 10: 5238. https://doi.org/10.3390/ijms22105238
APA StyleGieroba, B., Kalisz, G., Sroka-Bartnicka, A., Płazińska, A., Płaziński, W., Starek, M., & Dąbrowska, M. (2021). Molecular Structure of Cefuroxime Axetil Complexes with α-, β-, γ-, and 2-Hydroxypropyl-β-Cyclodextrins: Molecular Simulations and Raman Spectroscopic and Imaging Studies. International Journal of Molecular Sciences, 22(10), 5238. https://doi.org/10.3390/ijms22105238