ICAN (TRPM4) Contributes to the Intrinsic Excitability of Prefrontal Cortex Layer 2/3 Pyramidal Neurons
Abstract
:1. Introduction
2. Results
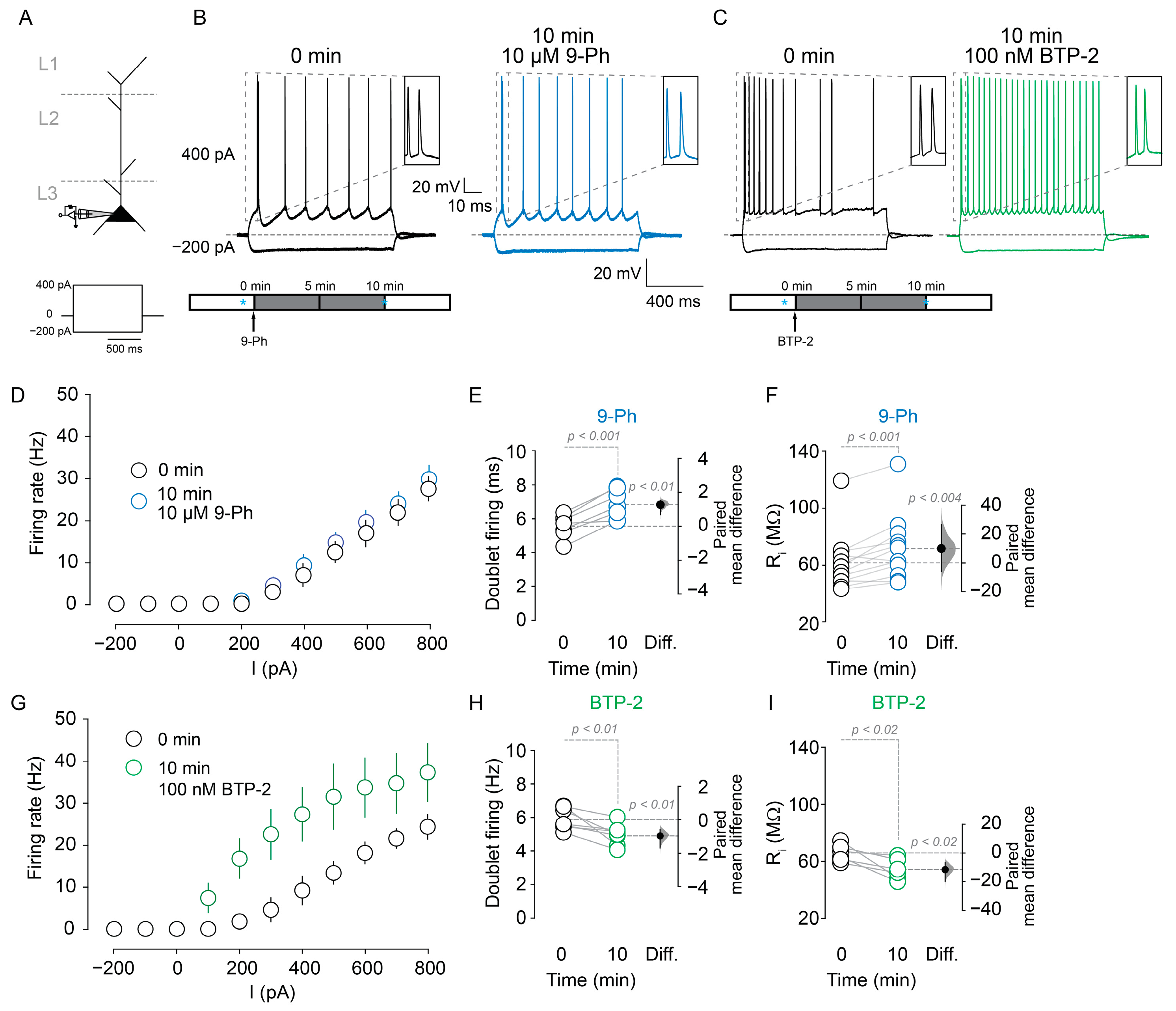
2.1. TRPM4 Participation in Pyramidal Neuron Spiking
2.2. TRPM4 Inhibition Reduces Action Potential Firing after High Frequency Stimulation
2.3. TRPM4 Silencing Reduces the Action Potential Firing after High Frequency Stimulation
2.4. TRPM4-Dependent Increase in ADP after HFS
3. Discussion
3.1. TRPM4 Activity at Resting Membrane Potential
3.2. TRPM4 Role in Intrinsic Excitability
3.3. TRPM4-Dependent ADP Activation Role in Intrinsic Excitability after Synaptic Stimulation
4. Materials and Methods
4.1. Animals
4.2. Cell Culture and Transfections
4.3. Stereotaxic Surgery
4.4. Electrophysiological Recordings
4.4.1. Voltage and Current-Clamp Experiments in Brain Slices
4.4.2. TRPM4 Current Measurements in Overexpression System
4.5. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Partridge, L.D.; Müller, T.H.; Swandulla, D. Calcium-Activated Non-Selective Channels in the Nervous System. Brain Res. Rev. 1994, 19, 319–325. [Google Scholar] [CrossRef]
- Haj-Dahmane, S.; Andrade, R. Ionic Mechanism of the Slow Afterdepolarization Induced by Muscarinic Receptor Activation in Rat Prefrontal Cortex. J. Neurophysiol. 1998, 80, 1197–1210. [Google Scholar] [CrossRef]
- Lin, E.C.; Combe, C.L.; Gasparini, S. Differential Contribution of Ca2+-Dependent Mechanisms to Hyperexcitability in Layer V Neurons of the Medial Entorhinal Cortex. Front. Cell. Neurosci. 2017, 11, 182. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.R.; Tepper, J.M. A Calcium-Activated Nonselective Cation Conductance Underlies the Plateau Potential in Rat Substantia Nigra GABAergic Neurons. J. Neurosci. 2007, 27, 6531–6541. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-Y.; Remy, S.; Varela, J.; Cooper, D.C.; Chung, S.; Kang, H.-W.; Lee, J.-H.; Spruston, N. A Post-Burst Afterdepolarization Is Mediated by Group I Metabotropic Glutamate Receptor-Dependent Upregulation of Cav2.3 R-Type Calcium Channels in CA1 Pyramidal Neurons. PLoS Biol. 2010, 8, e1000534. [Google Scholar] [CrossRef]
- Berridge, M.J. Neuronal Calcium Signaling. Neuron 1998, 21, 13–26. [Google Scholar] [CrossRef] [Green Version]
- Irie, T.; Trussell, L.O. Double-Nanodomain Coupling of Calcium Channels, Ryanodine Receptors, and BK Channels Controls the Generation of Burst Firing. Neuron 2017, 96, 856–870.e4. [Google Scholar] [CrossRef] [Green Version]
- Launay, P.; Fleig, A.; Perraud, A.-L.; Scharenberg, A.M.; Penner, R.; Kinet, J.-P. TRPM4 Is a Ca2+-Activated Nonselective Cation Channel Mediating Cell Membrane Depolarization. Cell 2002, 109, 397–407. [Google Scholar] [CrossRef] [Green Version]
- Launay, P.; Cheng, H.; Srivatsan, S.; Penner, R.; Fleig, A.; Kinet, J.-P. TRPM4 Regulates Calcium Oscillations after T Cell Activation. Science 2004, 306, 1374–1377. [Google Scholar] [CrossRef] [Green Version]
- Lei, Y.-T.; Thuault, S.J.; Launay, P.; Margolskee, R.F.; Kandel, E.R.; Siegelbaum, S.A. Differential Contribution of TRPM4 and TRPM5 Nonselective Cation Channels to the Slow Afterdepolarization in Mouse Prefrontal Cortex Neurons. Front. Cell. Neurosci. 2014, 8, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Riquelme, D.; Silva, I.; Philp, A.M.; Huidobro-Toro, J.P.; Cerda, O.; Trimmer, J.S.; Leiva-Salcedo, E. Subcellular Localization and Activity of TRPM4 in Medial Prefrontal Cortex Layer 2/3. Front. Cell. Neurosci. 2018, 12, 12. [Google Scholar] [CrossRef] [Green Version]
- Menigoz, A.; Ahmed, T.; Sabanov, V.; Philippaert, K.; Pinto, S.; Kerselaers, S.; Segal, A.; Freichel, M.; Voets, T.; Nilius, B.; et al. TRPM4-Dependent Post-Synaptic Depolarization Is Essential for the Induction of NMDA Receptor-Dependent LTP in CA1 Hippocampal Neurons. Pflug. Arch. 2016, 468, 593–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shpak, G.; Zylbertal, A.; Yarom, Y.; Wagner, S. Calcium-Activated Sustained Firing Responses Distinguish Accessory from Main Olfactory Bulb Mitral Cells. J. Neurosci. 2012, 32, 6251–6262. [Google Scholar] [CrossRef] [Green Version]
- Teruyama, R.; Sakuraba, M.; Kurotaki, H.; Armstrong, W.E. Transient Receptor Potential Channel M4 and M5 in Magnocellular Cells in Rat Supraoptic and Paraventricular Nuclei. J. Neuroendocrinol. 2011, 23, 1204–1213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.S.; Kang, E.; Makino, Y.; Park, S.; Shin, J.H.; Song, H.; Launay, P.; Linden, D.J. Characterizing the Conductance Underlying Depolarization-Induced Slow Current in Cerebellar Purkinje Cells. J. Neurophysiol. 2013, 109, 1174–1181. [Google Scholar] [CrossRef] [Green Version]
- Mironov, S.L. Metabotropic Glutamate Receptors Activate Dendritic Calcium Waves and TRPM Channels Which Drive Rhythmic Respiratory Patterns in Mice. J. Physiol. 2008, 586, 2277–2291. [Google Scholar] [CrossRef]
- Picardo, M.C.D.; Sugimura, Y.K.; Dorst, K.E.; Kallurkar, P.S.; Akins, V.T.; Ma, X.; Teruyama, R.; Guinamard, R.; Kam, K.; Saha, M.S.; et al. Trpm4 Ion Channels in Pre-Bötzinger Complex Interneurons Are Essential for Breathing Motor Pattern but Not Rhythm. PLoS Biol. 2019, 17, e2006094. [Google Scholar] [CrossRef] [Green Version]
- Mrejeru, A.; Wei, A.; Ramirez, J.M. Calcium-Activated Non-Selective Cation Currents Are Involved in Generation of Tonic and Bursting Activity in Dopamine Neurons of the Substantia Nigra Pars Compacta: Calcium-Activated Non-Selective Cation Currents in Dopamine Neurons. J. Physiol. 2011, 589, 2497–2514. [Google Scholar] [CrossRef]
- Haj-Dahmane, S.; Andrade, R. Calcium-Activated Cation Nonselective Current Contributes to the Fast Afterdepolarization in Rat Prefrontal Cortex Neurons. J. Neurophysiol. 1997, 78, 1983–1989. [Google Scholar] [CrossRef] [Green Version]
- Roberts, C.B.; O’Boyle, M.P.; Suter, K.J. Dendrites Determine the Contribution of after Depolarization Potentials (ADPs) to Generation of Repetitive Action Potentials in Hypothalamic Gonadotropin Releasing-Hormone (GnRH) Neurons. J. Comput. Neurosci. 2009, 26, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Zilles, K. Anatomy of the Neocortex: Cytoarchitecture and Myeloarchitecture; MIT: Cambridge, MA, USA, 1990. [Google Scholar]
- Cruikshank, S.J.; Ahmed, O.J.; Stevens, T.R.; Patrick, S.L.; Gonzalez, A.N.; Elmaleh, M.; Connors, B.W. Thalamic Control of Layer 1 Circuits in Prefrontal Cortex. J. Neurosci. 2012, 32, 17813–17823. [Google Scholar] [CrossRef] [Green Version]
- Fuster, J. The Prefrontal Cortex, 5th ed.; Academic Press: Cambridge, MA, USA, 2015; ISBN 978-0-12-407815-4. [Google Scholar]
- Sieveritz, B.; García-Muñoz, M.; Arbuthnott, G.W. Thalamic Afferents to Prefrontal Cortices from Ventral Motor Nuclei in Decision-Making. Eur. J. Neurosci. 2019, 49, 646–657. [Google Scholar] [CrossRef] [Green Version]
- Arnsten, A.F.T.; Paspalas, C.D.; Gamo, N.J.; Yang, Y.; Wang, M. Dynamic Network Connectivity: A New Form of Neuroplasticity. Trends Cogn. Sci. 2010, 14, 365–375. [Google Scholar] [CrossRef] [Green Version]
- Constantinidis, C.; Funahashi, S.; Lee, D.; Murray, J.D.; Qi, X.-L.; Wang, M.; Arnsten, A.F.T. Persistent Spiking Activity Underlies Working Memory. J. Neurosci. 2018, 38, 7020–7028. [Google Scholar] [CrossRef]
- Miller, E.K.; Lundqvist, M.; Bastos, A.M. Working Memory 2.0. Neuron 2018, 100, 463–475. [Google Scholar] [CrossRef] [Green Version]
- Larkum, M.E.; Waters, J.; Sakmann, B.; Helmchen, F. Dendritic Spikes in Apical Dendrites of Neocortical Layer 2/3 Pyramidal Neurons. J. Neurosci. 2007, 27, 8999–9008. [Google Scholar] [CrossRef]
- Aizenman, C.D.; Linden, D.J. Rapid, Synaptically Driven Increases in the Intrinsic Excitability of Cerebellar Deep Nuclear Neurons. Nat. Neurosci. 2000, 3, 109–111. [Google Scholar] [CrossRef]
- Frick, A.; Johnston, D. Plasticity of Dendritic Excitability. J. Neurobiol. 2005, 64, 100–115. [Google Scholar] [CrossRef]
- Huang, W.C.; Xiao, S.; Huang, F.; Harfe, B.D.; Jan, Y.N.; Jan, L.Y. Calcium-Activated Chloride Channels (CaCCs) Regulate Action Potential and Synaptic Response in Hippocampal Neurons. Neuron 2012, 74, 179–192. [Google Scholar] [CrossRef] [Green Version]
- Jones, S.L.; To, M.-S.; Stuart, G.J. Dendritic Small Conductance Calcium-Activated Potassium Channels Activated by Action Potentials Suppress EPSPs and Gate Spike-Timing Dependent Synaptic Plasticity. eLife 2017, 6, e30333. [Google Scholar] [CrossRef]
- Wester, J.C.; Mahadevan, V.; Rhodes, C.T.; Calvigioni, D.; Venkatesh, S.; Maric, D.; Hunt, S.; Yuan, X.; Zhang, Y.; Petros, T.J.; et al. Neocortical Projection Neurons Instruct Inhibitory Interneuron Circuit Development in a Lineage-Dependent Manner. Neuron 2019, 102, 960–975. [Google Scholar] [CrossRef]
- Jung, H.; Staff, N.P.; Spruston, N. Action Potential Bursting in Subicular Pyramidal Neurons Is Driven by a Calcium Tail Current. J. Neurosci. 2001, 21, 3312–3321. [Google Scholar] [CrossRef] [Green Version]
- Wojda, U.; Salinska, E.; Kuznicki, J. Calcium Ions in Neuronal Degeneration. IUBMB Life 2008, 60, 575–590. [Google Scholar] [CrossRef]
- Nilius, B.; Prenen, J.; Janssens, A.; Voets, T.; Droogmans, G. Decavanadate Modulates Gating of TRPM4 Cation Channels. J. Physiol. 2004, 560, 753–765. [Google Scholar] [CrossRef] [Green Version]
- Nilius, B.; Mahieu, F.; Prenen, J.; Janssens, A.; Owsianik, G.; Vennekens, R.; Voets, T. The Ca2+-Activated Cation Channel TRPM4 Is Regulated by Phosphatidylinositol 4,5-Biphosphate. EMBO J. 2006, 25, 467–478. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Okawa, H.; Wang, Y.; Liman, E.R. Phosphatidylinositol 4,5-Bisphosphate Rescues TRPM4 Channels from Desensitization. J. Biol. Chem. 2005, 280, 39185–39192. [Google Scholar] [CrossRef] [Green Version]
- Bousova, K.; Jirku, M.; Bumba, L.; Bednarova, L.; Sulc, M.; Franek, M.; Vyklicky, L.; Vondrasek, J.; Teisinger, J. PIP2 and PIP3 Interact with N-Terminus Region of TRPM4 Channel. Biophys. Chem. 2015, 205, 24–32. [Google Scholar] [CrossRef]
- Srivastava, S.; Choudhury, P.; Li, Z.; Liu, G.; Nadkarni, V.; Ko, K.; Coetzee, W.A.; Skolnik, E.Y. Phosphatidylinositol 3-Phosphate Indirectly Activates KCa3.1 via 14 Amino Acids in the Carboxy Terminus of KCa3.1. Mol. Biol. Cell 2006, 17, 146–154. [Google Scholar] [CrossRef] [Green Version]
- Veress, R.; Baranyai, D.; Hegyi, B.; Kistamás, K.; Dienes, C.; Magyar, J.; Bányász, T.; Nánási, P.P.; Szentandrássy, N.; Horváth, B. Transient Receptor Potential Melastatin 4 Channel Inhibitor 9-Phenanthrol Inhibits K + but Not Ca 2+ Currents in Canine Ventricular Myocytes. Can. J. Physiol. Pharmacol. 2018, 96, 1022–1029. [Google Scholar] [CrossRef]
- Burris, S.K.; Wang, Q.; Bulley, S.; Neeb, Z.P.; Jaggar, J.H. 9-Phenanthrol Inhibits Recombinant and Arterial Myocyte TMEM16A Channels: 9-Phenanthrol Inhibits Arterial Myocyte TMEM16A Channels. Br. J. Pharmacol. 2015, 172, 2459–2468. [Google Scholar] [CrossRef] [Green Version]
- Boffi, J.C.; Knabbe, J.; Kaiser, M.; Kuner, T. KCC2-Dependent Steady-State Intracellular Chloride Concentration and PH in Cortical Layer 2/3 Neurons of Anesthetized and Awake Mice. Front. Cell. Neurosci. 2018, 12, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Takezawa, R.; Cheng, H.; Beck, A.; Ishikawa, J.; Launay, P.; Kubota, H.; Kinet, J.; Fleig, A.; Yamada, T.; Penner, R. A Pyrazole Derivative Potently Inhibits Lymphocyte Ca2+ Influx and Cytokine Production by Facilitating Transient Receptor Potential Melastatin 4 Channel Activity. Mol. Pharmacol. 2006, 69, 1413–1420. [Google Scholar] [CrossRef]
- Ishikawa, J.; Ohga, K.; Yoshino, T.; Takezawa, R.; Ichikawa, A.; Kubota, H.; Yamada, T. A Pyrazole Derivative, YM-58483, Potently Inhibits Store-Operated Sustained Ca 2+ Influx and IL-2 Production in T Lymphocytes. J. Immunol. 2003, 170, 4441–4449. [Google Scholar] [CrossRef] [Green Version]
- Parekh, A.B.; Fleig, A.; Penner, R. The Store-Operated Calcium Current ICRAC: Nonlinear Activation by InsP3 and Dissociation from Calcium Release. Cell 1997, 89, 973–980. [Google Scholar] [CrossRef] [Green Version]
- Parekh, A.B.; Penner, R. Store Depletion and Calcium Influx. Physiol. Rev. 1997, 77, 901–930. [Google Scholar] [CrossRef]
- Anastasiades, P.G.; Collins, D.P.; Carter, A.G. Mediodorsal and Ventromedial Thalamus Engage Distinct L1 Circuits in the Prefrontal Cortex. Neuron 2021, 109, 314–330.e4. [Google Scholar] [CrossRef]
- Jones, S.L.; Stuart, G.J. Different Calcium Sources Control Somatic versus Dendritic SK Channel Activation during Action Potentials. J. Neurosci. 2013, 33, 19396–19405. [Google Scholar] [CrossRef] [Green Version]
- Iyer, R.; Ungless, M.A.; Faisal, A.A. Calcium-Activated SK Channels Control Firing Regularity by Modulating Sodium Channel Availability in Midbrain Dopamine Neurons. Sci. Rep. 2017, 7, 5248. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Huang, H. SK Channels Regulate Resting Properties and Signaling Reliability of a Developing Fast-Spiking Neuron. J. Neurosci. 2017, 37, 10738–10747. [Google Scholar] [CrossRef] [Green Version]
- Lee, U.S.; Cui, J. BK Channel Activation: Structural and Functional Insights. Trends Neurosci. 2010, 33, 415–423. [Google Scholar] [CrossRef] [Green Version]
- Bock, T.; Stuart, G.J. The Impact of BK Channels on Cellular Excitability Depends on Their Subcellular Location. Front. Cell. Neurosci. 2016, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Malley, J.J.; Seibt, F.; Chin, J.; Beierlein, M. TRPM4 Conductances in Thalamic Reticular Nucleus Neurons Generate Persistent Firing during Slow Oscillations. J. Neurosci. 2020, 40, 4813–4823. [Google Scholar] [CrossRef]
- Nilius, B.; Prenen, J.; Tang, J.; Wang, C.; Owsianik, G.; Janssens, A.; Voets, T.; Zhu, M.X. Regulation of the Ca2+ Sensitivity of the Nonselective Cation Channel TRPM4. J. Biol. Chem. 2005, 280, 6423–6433. [Google Scholar] [CrossRef] [Green Version]
- Magee, J.C.; Carruth, M. Dendritic Voltage-Gated Ion Channels Regulate the Action Potential Firing Mode of Hippocampal CA1 Pyramidal Neurons. J. Neurophysiol. 1999, 82, 1895–1901. [Google Scholar] [CrossRef]
- Metz, A.E.; Jarsky, T.; Spruston, N. R-Type Calcium Channels Contribute to Afterdepolarization and Bursting in Hippocampal CA1 Pyramidal Neurons. J. Neurosci. 2005, 25, 5763–5773. [Google Scholar] [CrossRef]
- Li, K.; Abbott, S.B.G.; Shi, Y.; Eggan, P.; Gonye, E.C.; Bayliss, D.A. TRPM4 Mediates a Subthreshold Membrane Potential Oscillation in Respiratory Chemoreceptor Neurons That Drives Pacemaker Firing and Breathing. Cell Rep. 2021, 34. [Google Scholar] [CrossRef]
- Raman, I.M.; Bean, B.P. Resurgent Sodium Current and Action Potential Formation in Dissociated Cerebellar Purkinje Neurons. J. Neurosci. 1997, 17, 4517–4526. [Google Scholar] [CrossRef] [Green Version]
- Chu, Z.; Moenter, S.M. Physiologic Regulation of a Tetrodotoxin-Sensitive Sodium Influx That Mediates a Slow Afterdepolarization Potential in Gonadotropin-Releasing Hormone Neurons: Possible Implications for the Central Regulation of Fertility. J. Neurosci. 2006, 26, 11961–11973. [Google Scholar] [CrossRef] [Green Version]
- Aman, T.K.; Grieco-Calub, T.M.; Chen, C.; Rusconi, R.; Slat, E.A.; Isom, L.L.; Raman, I.M. Regulation of Persistent Na Current by Interactions between Beta Subunits of Voltage-Gated Na Channels. J. Neurosci. 2009, 29, 2027–2042. [Google Scholar] [CrossRef] [Green Version]
- Leiva-Salcedo, E.; Riquelme, D.; Cerda, O.; Stutzin, A. TRPM4 Activation by Chemically- and Oxygen Deprivation-Induced Ischemia and Reperfusion Triggers Neuronal Death. Channels 2017, 11, 624–635. [Google Scholar] [CrossRef]
- Korn, S.J.; Marty, A.; Connor, J.A.; Horn, R. [22]—Perforated Patch Recording. In Methods in Neurosciences; Conn, P.M., Ed.; Electrophysiology and Microinjection; Academic Press: Cambridge, MA, USA, 1991; Volume 4, pp. 364–373. [Google Scholar]
- Spruston, N.; Johnston, D. Perforated Patch-Clamp Analysis of the Passive Membrane Properties of Three Classes of Hippocampal Neurons. J. Neurophysiol. 1992, 67, 508–529. [Google Scholar] [CrossRef] [PubMed]
- Rothman, J.S.; Silver, R.A. NeuroMatic: An Integrated Open-Source Software Toolkit for Acquisition, Analysis and Simulation of Electrophysiological Data. Front. Neuroinform. 2018, 12, 14. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riquelme, D.; Peralta, F.A.; Navarro, F.D.; Moreno, C.; Leiva-Salcedo, E. ICAN (TRPM4) Contributes to the Intrinsic Excitability of Prefrontal Cortex Layer 2/3 Pyramidal Neurons. Int. J. Mol. Sci. 2021, 22, 5268. https://doi.org/10.3390/ijms22105268
Riquelme D, Peralta FA, Navarro FD, Moreno C, Leiva-Salcedo E. ICAN (TRPM4) Contributes to the Intrinsic Excitability of Prefrontal Cortex Layer 2/3 Pyramidal Neurons. International Journal of Molecular Sciences. 2021; 22(10):5268. https://doi.org/10.3390/ijms22105268
Chicago/Turabian StyleRiquelme, Denise, Francisco A. Peralta, Franco D. Navarro, Claudio Moreno, and Elias Leiva-Salcedo. 2021. "ICAN (TRPM4) Contributes to the Intrinsic Excitability of Prefrontal Cortex Layer 2/3 Pyramidal Neurons" International Journal of Molecular Sciences 22, no. 10: 5268. https://doi.org/10.3390/ijms22105268





