Cold Atmospheric Plasma Promotes Regeneration-Associated Cell Functions of Murine Cementoblasts In Vitro
Abstract
:1. Introduction
2. Results
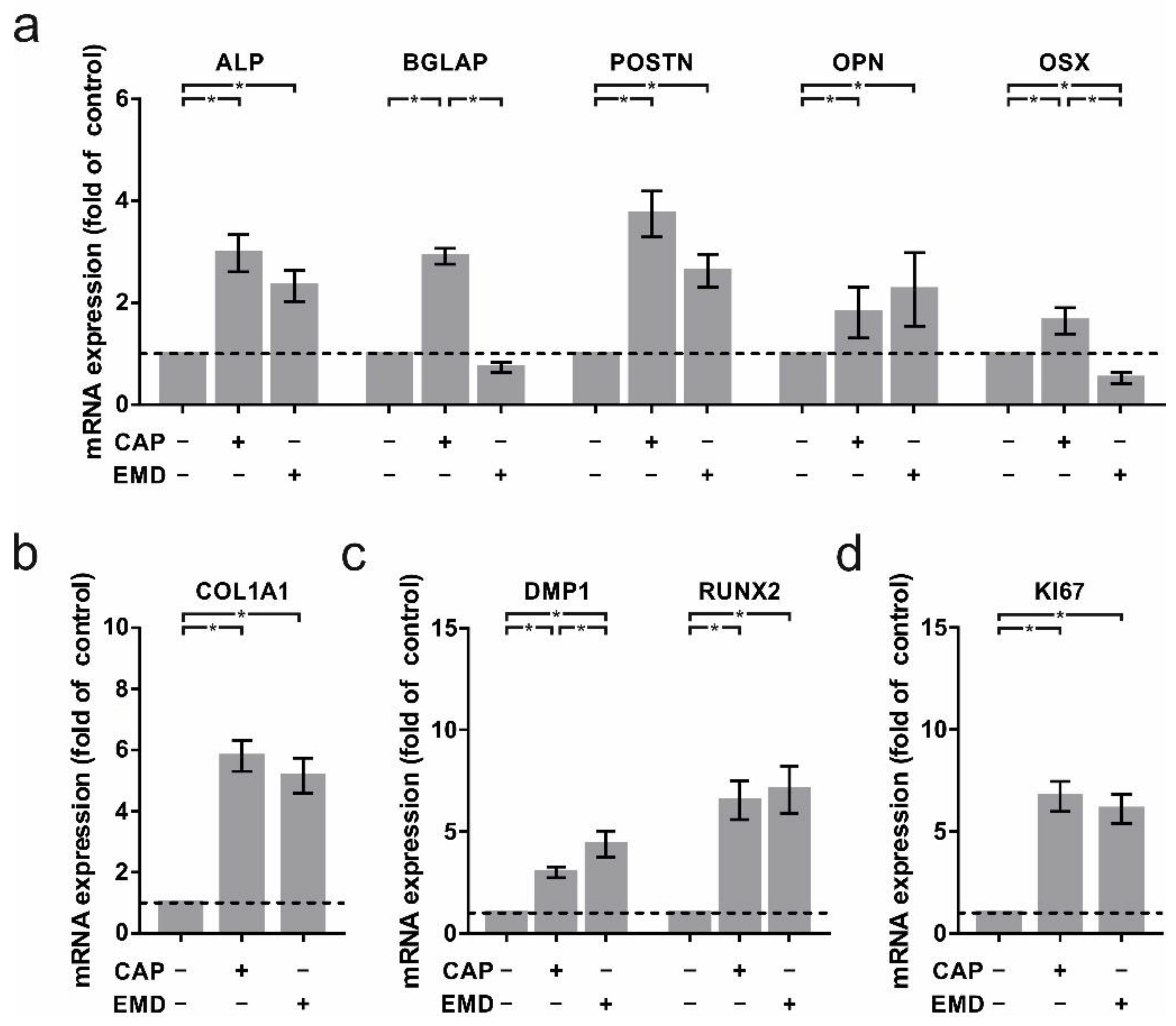
2.1. Regulation of Crucial Cementoblast Genes in CAP-Treated OCCM-30 Cells
2.2. Increased Regulation of Proteins Associated with Cementogenesis in OCCM-30 Cells
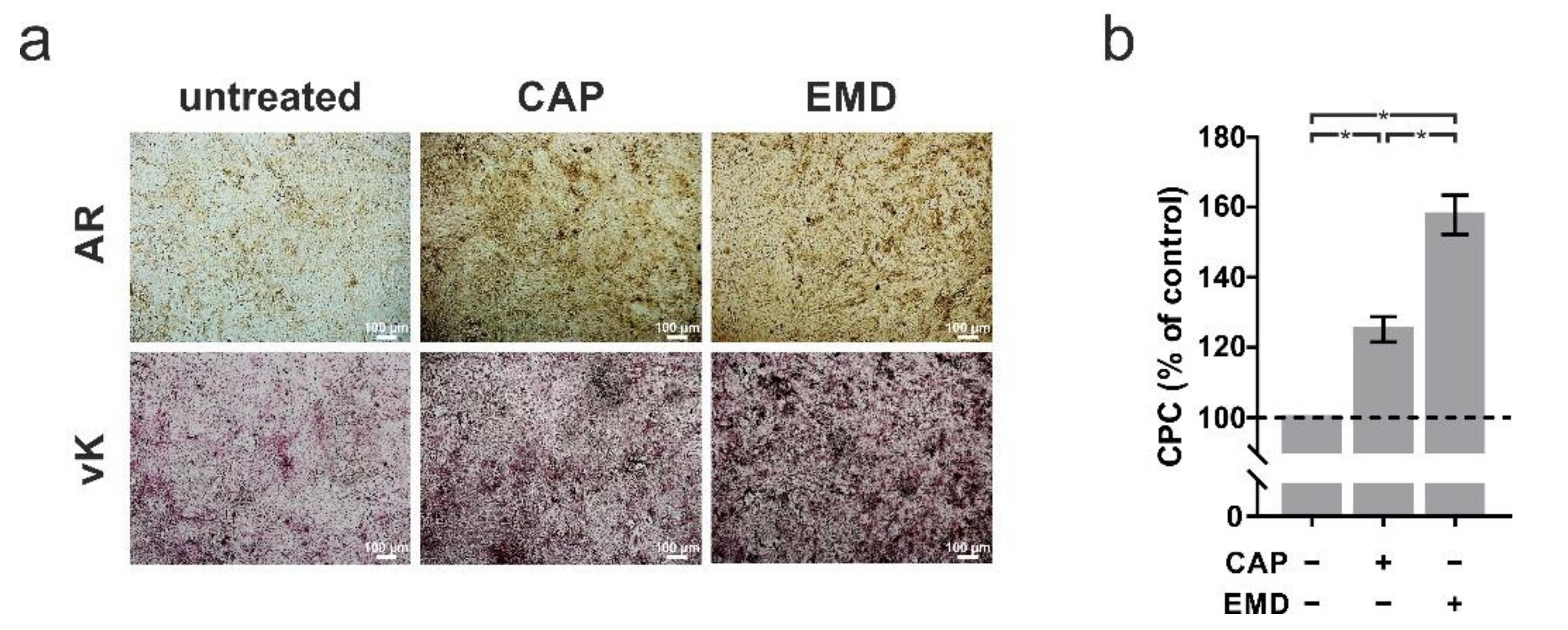
2.3. Accelerated Mineralization after Both CAP and EMD Treatment in OCCM-30 Cells
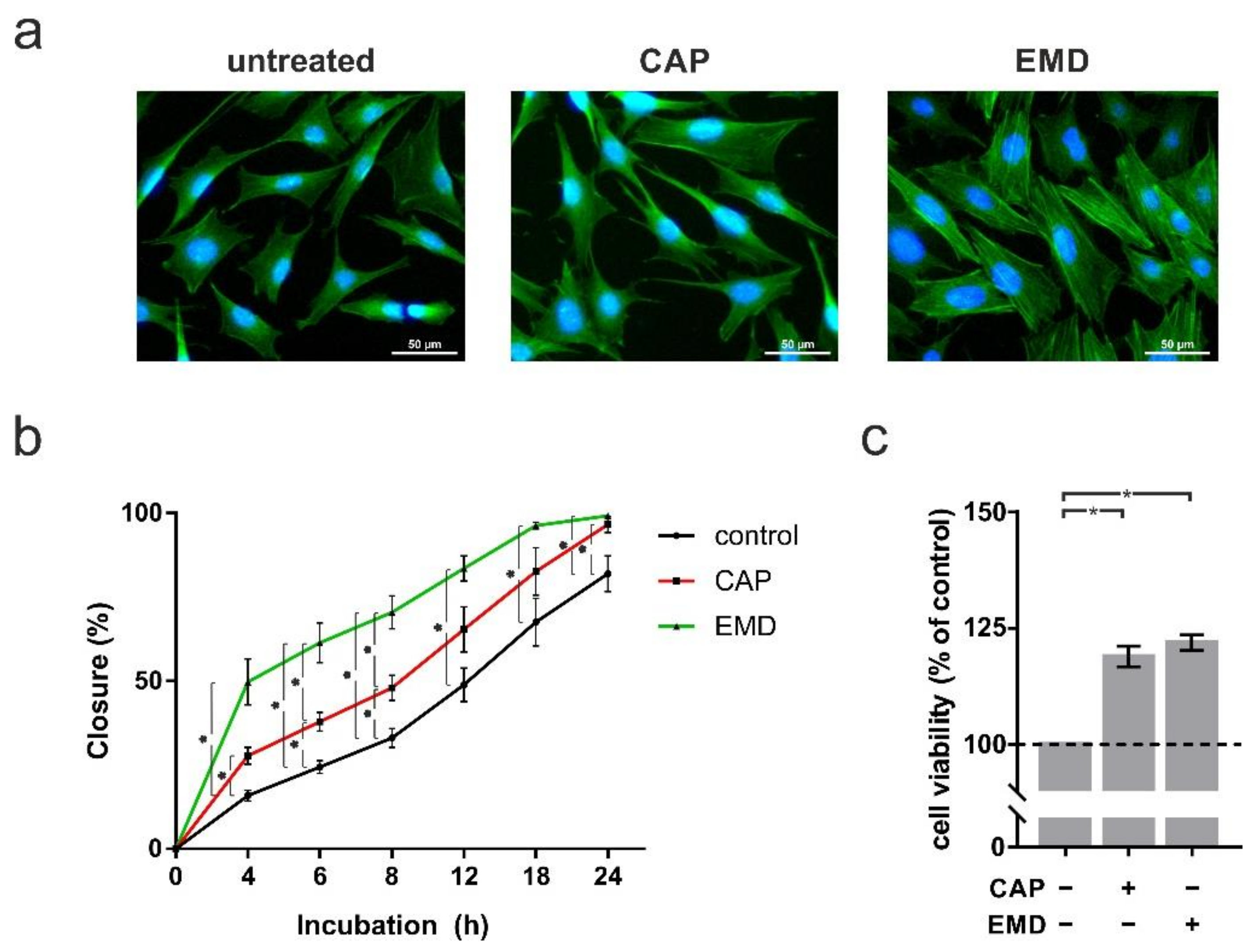
2.4. Higher Proliferation, Migration and Cell Viability after Both CAP and EMD Treatment in OCCM-30 Cells
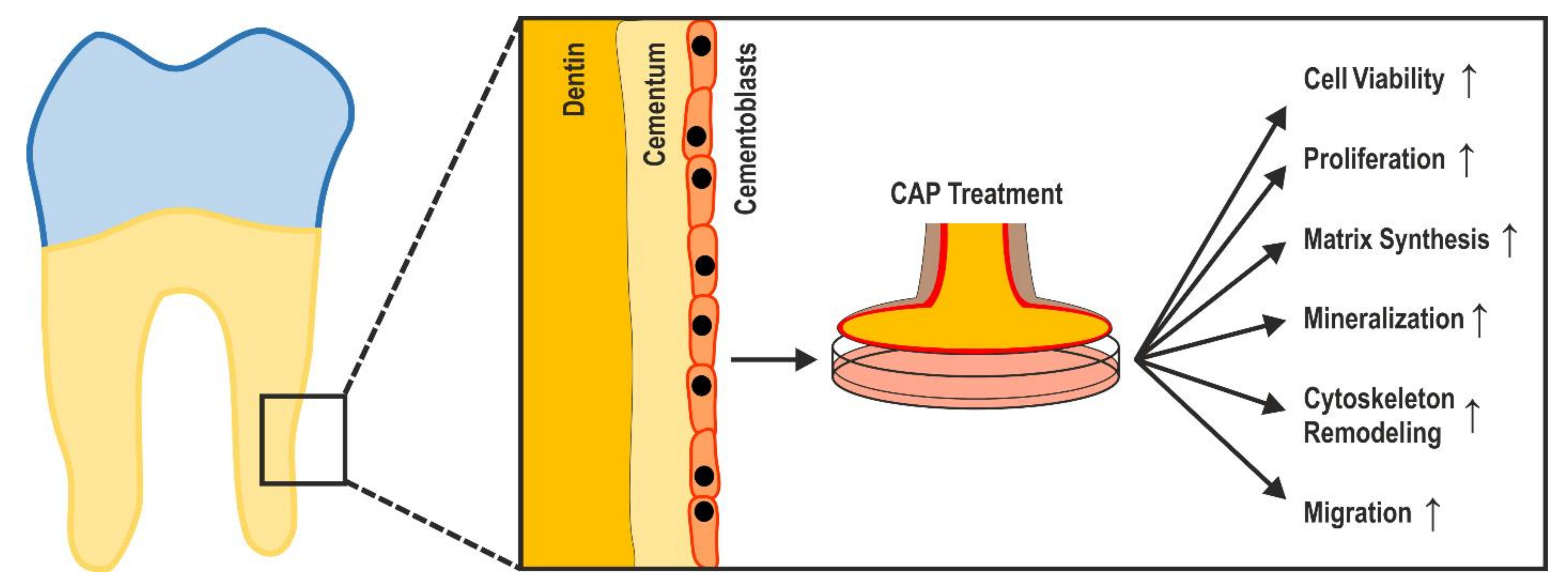
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. CAP Treatment
4.3. Analysis of Gene Expression
4.4. Immunocytochemistry
4.5. Analysis of Protein Levels
4.6. ALP Assay
4.7. Von Kossa Staining
4.8. Alizarin Red Staining
4.9. Analysis of Cell Morphology
4.10. Scratch Assay
4.11. XTT Assay
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Richards, D. One billion people have experienced a traumatic dental injury. Evid. Based Dent. 2018, 19, 34–35. [Google Scholar] [CrossRef]
- Bourguignon, C.; Cohenca, N.; Lauridsen, E.; Flores, M.T.; O’Connell, A.C.; Day, P.F.; Tsilingaridis, G.; Abbott, P.V.; Fouad, A.F.; Hicks, L.; et al. International Association of Dental Traumatology guidelines for the management of traumatic dental injuries: 1. Fractures and luxations. Dent. Traumatol. 2020, 36, 314–330. [Google Scholar] [CrossRef] [PubMed]
- Frencken, J.E.; Sharma, P.; Stenhouse, L.; Green, D.; Laverty, D.; Dietrich, T. Global epidemiology of dental caries and severe periodontitis—A comprehensive review. J. Clin. Periodontol. 2017, 44, S94–S105. [Google Scholar] [CrossRef]
- Ramadan, D.E.; Hariyani, N.; Indrawati, R.; Ridwan, R.D.; Diyatri, I. Cytokines and Chemokines in Periodontitis. Eur. J. Dent. 2020, 14, 483–495. [Google Scholar] [CrossRef]
- Hammarström, L.; Heijl, L.; Gestrelius, S. Periodontal regeneration in a buccal dehiscence model in monkeys after application of enamel matrix proteins. J. Clin. Periodontol. 1997, 24, 669–677. [Google Scholar] [CrossRef]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Berglundh, T.; Sculean, A.; Tonetti, M.S.; Aass, A.M.; Aimetti, M.; et al. Treatment of stage I–III periodontitis—The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47, 4–60. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and invivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [Green Version]
- Ayoub, S.; Berbéri, A.; Fayyad-Kazan, M. An update on human periapical cyst-mesenchymal stem cells and their potential applications in regenerative medicine. Mol. Biol. Rep. 2020, 47, 2381–2389. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Codispoti, B.; Shelton, R.M.; Scheven, B.A.; Cooper, P.R.; Tatullo, M.; Paduano, F. Dental Pulp Stem Cell Mechanoresponsiveness: Effects of Mechanical Stimuli on Dental Pulp Stem Cell Behavior. Front. Physiol. 2018, 9, 1685. [Google Scholar] [CrossRef] [PubMed]
- Jue, S.-S.; Lee, W.Y.; Kwon, Y.-D.; Kim, Y.-R.; Pae, A.; Lee, B. The effects of enamel matrix derivative on the proliferation and differentiation of human mesenchymal stem cells. Clin. Oral Implant. Res. 2010, 21, 741–746. [Google Scholar] [CrossRef]
- Bosshardt, D. Are Cementoblasts a Subpopulation of Osteoblasts or a Unique Phenotype? J. Dent. Res. 2005, 84, 390–406. [Google Scholar] [CrossRef]
- Zeichner-David, M. Regeneration of periodontal tissues: Cementogenesis revisited. Periodontology 2006, 41, 196–217. [Google Scholar] [CrossRef]
- Saygin, N.E.; Giannobile, W.V.; Somerman, M.J. Molecular and cell biology of cementum. Periodontology 2000, 24, 73–98. [Google Scholar] [CrossRef]
- Beertsen, W.; McCulloch, C.A.G.; Sodek, J. The periodontal ligament: A unique, multifunctional connective tissue. Periodontology 1997, 13, 20–40. [Google Scholar] [CrossRef]
- Grandin, H.M.; Gemperli, A.C.; Dard, M. Enamel Matrix Derivative: A Review of Cellular Effects In Vitro and a Model of Molecular Arrangement and Functioning. Tissue Eng. Part B Rev. 2012, 18, 181–202. [Google Scholar] [CrossRef] [PubMed]
- Hakki, S.S.; Bozkurt, S.B.; Turkay, E.; Dard, M.; Purali, N.; Götz, W. Recombinant amelogenin regulates the bioactivity of mouse cementoblasts in vitro. Int. J. Oral Sci. 2018, 10, 15. [Google Scholar] [CrossRef]
- Miron, R.J.; Chandad, F.; Buser, D.; Sculean, A.; Cochran, D.L.; Zhang, Y. Effect of Enamel Matrix Derivative Liquid on Osteoblast and Periodontal Ligament Cell Proliferation and Differentiation. J. Periodontol. 2016, 87, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Jing, D.; Shuang, Y.; Miron, R.J. Enamel matrix derivative improves gingival fibroblast cell behavior cultured on titanium surfaces. Clin. Oral Investig. 2016, 20, 685–695. [Google Scholar] [CrossRef]
- Zeichner-David, M.; Chen, L.-S.; Hsu, Z.; Reyna, J.; Catón, J.; Bringas, P. Amelogenin and ameloblastin show growth-factor like activity in periodontal ligament cells. Eur. J. Oral Sci. 2006, 114, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Koop, R.; Merheb, J.; Quirynen, M. Periodontal Regeneration With Enamel Matrix Derivative in Reconstructive Periodontal Therapy: A Systematic Review. J. Periodontol. 2012, 83, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Sculean, A.; Cochran, D.L.; Froum, S.; Zucchelli, G.; Nemcovsky, C.; Donos, N.; Lyngstadaas, S.P.; Deschner, J.; Dard, M.; et al. Twenty years of enamel matrix derivative: The past, the present and the future. J. Clin. Periodontol. 2016, 43, 668–683. [Google Scholar] [CrossRef] [PubMed]
- Rojas, M.A.; Marini, L.; Pilloni, A.; Sahrmann, P. Early wound healing outcomes after regenerative periodontal surgery with enamel matrix derivatives or guided tissue regeneration: A systematic review. BMC Oral Health 2019, 19, 76. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Dard, M.; Weinreb, M. Enamel matrix derivative, inflammation and soft tissue wound healing. J. Periodontal Res. 2014, 50, 555–569. [Google Scholar] [CrossRef]
- Duchesne, C.; Banzet, S.; Lataillade, J.; Rousseau, A.; Frescaline, N. Cold atmospheric plasma modulates endothelial nitric oxide synthase signalling and enhances burn wound neovascularisation. J. Pathol. 2019, 249, 368–380. [Google Scholar] [CrossRef]
- Gao, J.; Wang, L.; Xia, C.; Yang, X.; Cao, Z.; Zheng, L.; Ko, R.; Shen, C.; Yang, C.; Cheng, C. Cold atmospheric plasma promotes different types of superficial skin erosion wounds healing. Int. Wound J. 2019, 16, 1103–1111. [Google Scholar] [CrossRef]
- He, R.; Li, Q.; Shen, W.; Wang, T.; Lu, H.; Lu, J.; Lu, F.; Luo, M.; Zhang, J.; Gao, H.; et al. The efficacy and safety of cold atmospheric plasma as a novel therapy for diabetic wound in vitro and in vivo. Int. Wound J. 2020, 17, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Dezest, M.; Chavatte, L.; Bourdens, M.; Quinton, D.; Camus, M.; Garrigues, L.; Descargues, P.; Arbault, S.; Burlet-Schiltz, O.; Casteilla, L.; et al. Mechanistic insights into the impact of Cold Atmospheric Pressure Plasma on human epithelial cell lines. Sci. Rep. 2017, 7, srep41163. [Google Scholar] [CrossRef] [Green Version]
- Dijksteel, G.S.; Ulrich, M.M.W.; Vlig, M.; Sobota, A.; Middelkoop, E.; Boekema, B.K.H.L. Safety and bactericidal efficacy of cold atmospheric plasma generated by a flexible surface Dielectric Barrier Discharge device against Pseudomonas aeruginosa in vitro and in vivo. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 1–10. [Google Scholar] [CrossRef]
- Kluge, S.; Bekeschus, S.; Bender, C.; Benkhai, H.; Sckell, A.; Below, H.; Stope, M.B.; Kramer, A. Investigating the Mutagenicity of a Cold Argon-Plasma Jet in an HET-MN Model. PLoS ONE 2016, 11, e0160667. [Google Scholar] [CrossRef] [Green Version]
- Weltmann, K.-D.; Kindel, E.; Brandenburg, R.; Meyer, C.; Bussiahn, R.; Wilke, C.; Von Woedtke, T. Atmospheric Pressure Plasma Jet for Medical Therapy: Plasma Parameters and Risk Estimation. Contrib. Plasma Phys. 2009, 49, 631–640. [Google Scholar] [CrossRef]
- Gan, L.; Zhang, S.; Poorun, D.; Liu, D.; Lu, X.; He, M.; Duan, X.; Chen, H. Medical applications of nonthermal atmospheric pressure plasma in dermatology. J. der Dtsch. Dermatol. Ges. 2018, 16, 7–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arndt, S.; Unger, P.; Wacker, E.; Shimizu, T.; Heinlin, J.; Li, Y.-F.; Thomas, H.; Morfill, G.E.; Zimmermann, J.L.; Bosserhoff, A.-K.; et al. Cold Atmospheric Plasma (CAP) Changes Gene Expression of Key Molecules of the Wound Healing Machinery and Improves Wound Healing In Vitro and In Vivo. PLoS ONE 2013, 8, e79325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilmore, B.F.; Flynn, P.B.; O’Brien, S.; Hickok, N.; Freeman, T.; Bourke, P. Cold Plasmas for Biofilm Control: Opportunities and Challenges. Trends Biotechnol. 2018, 36, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Nasir, N.M.; Lee, B.K.; Yap, S.S.; Thong, K.L. Cold plasma inactivation of chronic wound bacteria. Arch. Biochem. Biophys. 2016, 605, 76–85. [Google Scholar] [CrossRef]
- Rupf, S.; Idlibi, A.N.; Al Marrawi, F.; Hannig, M.; Schubert, A.; Von Mueller, L.; Spitzer, W.; Holtmann, H.; Lehmann, A.; Rueppell, A.; et al. Removing Biofilms from Microstructured Titanium Ex Vivo: A Novel Approach Using Atmospheric Plasma Technology. PLoS ONE 2011, 6, e25893. [Google Scholar] [CrossRef]
- Schneider, C.; Gebhardt, L.; Arndt, S.; Karrer, S.; Zimmermann, J.L.; Fischer, M.J.M.; Bosserhoff, A.-K. Acidification is an Essential Process of Cold Atmospheric Plasma and Promotes the Anti-Cancer Effect on Malignant Melanoma Cells. Cancers 2019, 11, 671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaquero, J.; Judée, F.; Vallette, M.; Decauchy, H.; Arbelaiz, A.; Aoudjehane, L.; Scatton, O.; Gonzalez-Sanchez, E.; Merabtene, F.; Augustin, J.; et al. Cold-Atmospheric Plasma Induces Tumor Cell Death in Preclinical In Vivo and In Vitro Models of Human Cholangiocarcinoma. Cancers 2020, 12, 1280. [Google Scholar] [CrossRef] [PubMed]
- Kleineidam, B.; Nokhbehsaim, M.; Deschner, J.; Wahl, G. Effect of cold plasma on periodontal wound healing—an in vitro study. Clin. Oral Investig. 2019, 23, 1941–1950. [Google Scholar] [CrossRef] [PubMed]
- Hammarstrom, L. Enamel matrix, cementum development and regeneration. J. Clin. Periodontol. 1997, 24, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Sculean, A.; Alessandri, R.; Miron, R.; Salvi, G.E.; Bosshardt, D.D. Enamel Matrix Proteins and Periodontal Wound Healing and Regeneration. Clin. Adv. Periodontics 2011, 1, 101–117. [Google Scholar] [CrossRef]
- Eggers, B.; Marciniak, J.; Memmert, S.; Kramer, F.J.; Deschner, J.; Nokhbehsaim, M. The beneficial effect of cold atmospheric plasma on parameters of molecules and cell function involved in wound healing in human osteoblast-like cells in vitro. Odontology 2020, 108, 607–616. [Google Scholar] [CrossRef] [Green Version]
- Zweifler, L.; Patel, M.K.; Jr, F.H.N.; Wimer, H.F.; Millan, J.L.; Somerman, M.J.; Foster, B.L. Counter-regulatory phosphatases TNAP and NPP1 temporally regulate tooth root cementogenesis. Int. J. Oral Sci. 2015, 7, 27–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herasse, M.; Spentchian, M.; Taillandier, A.; Keppler-Noreuil, K.; Fliorito, A.N.M.; Bergoffen, J.; Wallerstein, R.; Muti, C.; Simon-Bouy, B.; Mornet, E. Molecular study of three cases of odontohypophosphatasia resulting from heterozygosity for mutations in the tissue non-specific alkaline phosphatase gene. J. Med Genet. 2003, 40, 605–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boskey, A.; Maresca, M.; Ullrich, W.; Doty, S.B.; Butler, W.T.; Prince, C.W. Osteopontin-hydroxyapatite interactions in vitro: Inhibition of hydroxyapatite formation and growth in a gelatin-gel. Bone Miner. 1993, 22, 147–159. [Google Scholar] [CrossRef]
- Foster, B.; Ao, M.; Millán, J.L.; Nociti, F.H.; Somerman, M.J.; Salmon, C.R.; Chavez, M.B.; Kolli, T.N.; Tran, A.B.; Chu, E.Y.; et al. Osteopontin regulates dentin and alveolar bone development and mineralization. Bone 2018, 107, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, K.; Amizuka, N.; Takeshita, S.; Takamatsu, H.; Katsuura, M.; Ozawa, H.; Toyama, Y.; Bonewald, L.F.; Kudo, A. Identification and Characterization of a Novel Protein, Periostin, with Restricted Expression to Periosteum and Periodontal Ligament and Increased Expression by Transforming Growth Factor β. J. Bone Miner. Res. 1999, 14, 1239–1249. [Google Scholar] [CrossRef]
- Romanos, G.E.; Asnani, K.P.; Hingorani, D.; Deshmukh, V.L. Periostin: Role in formation and maintenance of dental tissues. J. Cell. Physiol. 2013, 229, 1–5. [Google Scholar] [CrossRef]
- He, G.; George, A. Dentin Matrix Protein 1 Immobilized on Type I Collagen Fibrils Facilitates Apatite Deposition in Vitro. J. Biol. Chem. 2004, 279, 11649–11656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, L.; MacDougall, M.; Zhang, S.; Xie, Y.; Zhang, J.; Li, Z.; Lu, Y.; Mishina, Y.; Feng, J.Q. Deletion of Dentin Matrix Protein-1 Leads to a Partial Failure of Maturation of Predentin into Dentin, Hypomineralization, and Expanded Cavities of Pulp and Root Canal during Postnatal Tooth Development. J. Biol. Chem. 2004, 279, 19141–19148. [Google Scholar] [CrossRef] [Green Version]
- De Almeida, A.B.; Dos Santos, E.J.L.; Abuna, G.F.; Ribeiro, C.S.; Casati, M.Z.; Ruiz, K.G.S.; Junior, F.H.N. Isolation and characterization of a human cementocyte-like cell line, HCY-23. Braz. Oral Res. 2019, 33, e058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Errico, J.; Macneil, R.; Takata, T.; Berry, J.; Strayhorn, C.; Somerman, M. Expression of bone associated markers by tooth root lining cells, in situ and in vitro. Bone 1997, 20, 117–126. [Google Scholar] [CrossRef]
- Hirata, A.; Sugahara, T.; Nakamura, H. Localization of Runx2, Osterix, and Osteopontin in Tooth Root Formation in Rat Molars. J. Histochem. Cytochem. 2008, 57, 397–403. [Google Scholar] [CrossRef]
- Yamamoto, T.; Takahashi, S. Hertwig’s epithelial root sheath cells do not transform into cementoblasts in rat molar cementogenesis. Ann. Anat. Anat. Anz. 2009, 191, 547–555. [Google Scholar] [CrossRef] [Green Version]
- Boabaid, F.; Gibson, C.W.; Kuehl, M.A.; Berry, J.E.; Snead, M.L.; Nociti, F.H.; Katchburian, E.; Somerman, M.J. Leucine-Rich Amelogenin Peptide: A Candidate Signaling Molecule During Cementogenesis. J. Periodontol. 2004, 75, 1126–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chun, Y.-H.P.; Foster, B.L.; Lukasavage, P.A.; Berry, J.E.; Zhao, M.; Tenenbaum, H.C.; Somerman, M.J. Bisphosphonate Modulates Cementoblast Behavior In Vitro. J. Periodontol. 2005, 76, 1890–1900. [Google Scholar] [CrossRef]
- Hakki, S.S.; Berry, J.E.; Somerman, M.J. The Effect of Enamel Matrix Protein Derivative on Follicle Cells In Vitro. J. Periodontol. 2001, 72, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Tokiyasu, Y.; Takata, T.; Saygin, E.; Somerman, M. Enamel Factors Regulate Expression of Genes Associated With Cementoblasts. J. Periodontol. 2000, 71, 1829–1839. [Google Scholar] [CrossRef]
- Wu, S.-M.; Chiu, H.-C.; Chin, Y.-T.; Lin, H.-Y.; Chiang, C.-Y.; Tu, H.-P.; Fu, M.M.J.; Fu, E. Effects of enamel matrix derivative on the proliferation and osteogenic differentiation of human gingival mesenchymal stem cells. Stem Cell Res. Ther. 2014, 5, 52. [Google Scholar] [CrossRef] [Green Version]
- Miron, R.J.; Caluseru, O.M.; Guillemette, V.; Zhang, Y.; Gemperli, A.C.; Chandad, F.; Sculean, A. Influence of Enamel Matrix Derivative on Cells at Different Maturation Stages of Differentiation. PLoS ONE 2013, 8, e71008. [Google Scholar] [CrossRef] [Green Version]
- Hisanaga, Y.; Suzuki, E.; Aoki, H.; Sato, M.; Saito, A.; Azuma, T. Effect of the combined use of enamel matrix derivative and atelocollagen sponge scaffold on osteoblastic differentiation of mouse induced pluripotent stem cells in vitro. J. Periodontal Res. 2017, 53, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Azmaz, N.T.; Bozkurt, S.B.; Hakki, S.S.; Belli, S. Warm Gutta-Percha Techniques Regulate Cell Viability, Heat Shock, and Mineralized Tissue–associated Proteins of Cementoblasts. J. Endod. 2020, 46, 957–963. [Google Scholar] [CrossRef]
- Božić, D.; Grgurevic, L.; Erjavec, I.; Brkljačić, J.; Orlić, I.; Razdorov, G.; Grgurevic, I.; Vukicevic, S.; Plančak, D. The proteome and gene expression profile of cementoblastic cells treated by bone morphogenetic protein-7 in vitro. J. Clin. Periodontol. 2011, 39, 80–90. [Google Scholar] [CrossRef]
- Li, T.; Wang, H.; Lv, C.; Huang, L.; Zhang, C.; Zhou, C.; Zou, S.; Duan, P. Intermittent parathyroid hormone promotes cementogenesis via ephrinB2-EPHB4 forward signaling. J. Cell. Physiol. 2021, 236, 2070–2086. [Google Scholar] [CrossRef] [PubMed]
- Lou, B.-S.; Hsieh, J.-H.; Chen, C.-M.; Hou, C.-W.; Wu, H.-Y.; Chou, P.-Y.; Lai, C.-H.; Lee, J.-W. Helium/Argon-Generated Cold Atmospheric Plasma Facilitates Cutaneous Wound Healing. Front. Bioeng. Biotechnol. 2020, 8, 683. [Google Scholar] [CrossRef]
- Maisch, T.; Bosserhoff, A.K.; Unger, P.; Heider, J.; Shimizu, T.; Zimmermann, J.L.; Morfill, G.E.; Landthaler, M.; Karrer, S. Investigation of toxicity and mutagenicity of cold atmospheric argon plasma. Environ. Mol. Mutagen. 2017, 58, 172–177. [Google Scholar] [CrossRef]
- Assadian, O.; Ousey, K.J.; Daeschlein, G.; Kramer, A.; Parker, C.; Tanner, J.; Leaper, D.J. Effects and safety of atmospheric low-temperature plasma on bacterial reduction in chronic wounds and wound size reduction: A systematic review and meta-analysis. Int. Wound J. 2019, 16, 103–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, A.; Bekeschus, S.; Wende, K.; Vollmar, B.; Von Woedtke, T. A cold plasma jet accelerates wound healing in a murine model of full-thickness skin wounds. Exp. Dermatol. 2017, 26, 156–162. [Google Scholar] [CrossRef]
- Mateu-Sanz, M.; Tornín, J.; Brulin, B.; Khlyustova, A.; Ginebra, M.-P.; Layrolle, P.; Canal, C. Cold Plasma-Treated Ringer’s Saline: A Weapon to Target Osteosarcoma. Cancers 2020, 12, 227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gjika, E.; Pal-Ghosh, S.; Tang, A.; Kirschner, M.; Tadvalkar, G.; Canady, J.; Stepp, M.A.; Keidar, M. Adaptation of Operational Parameters of Cold Atmospheric Plasma for in Vitro Treatment of Cancer Cells. ACS Appl. Mater. Interfaces 2018, 10, 9269–9279. [Google Scholar] [CrossRef]
- Haralambiev, L.; Bandyophadyay, A.; Suchy, B.; Weiss, M.; Kramer, A.; Bekeschus, S.; Ekkernkamp, A.; Mustea, A.; Kaderali, L.; Stope, M.B. Determination of Immediate vs. Kinetic Growth Retardation in Physically Plasma-treated Cells by Experimental and Modelling Data. Anticancer. Res. 2020, 40, 3743–3749. [Google Scholar] [CrossRef] [PubMed]
- Dubuc, A.; Monsarrat, P.; Virard, F.; Merbahi, N.; Sarrette, J.-P.; Laurencin-Dalicieux, S.; Cousty, S. Use of cold-atmospheric plasma in oncology: A concise systematic review. Ther. Adv. Med Oncol. 2018, 10, 1758835918786475. [Google Scholar] [CrossRef] [PubMed]
- Guida, L.; Annunziata, M.; Carinci, F.; Di Feo, A.; Passaro, I.; Oliva, A. In Vitro Biologic Response of Human Bone Marrow Stromal Cells to Enamel Matrix Derivative. J. Periodontol. 2007, 78, 2190–2196. [Google Scholar] [CrossRef] [PubMed]
- Heng, N.H.; N’Guessan, P.D.; Kleber, B.-M.; Bernimoulin, J.-P.; Pischon, N. Enamel Matrix Derivative Induces Connective Tissue Growth Factor Expression in Human Osteoblastic Cells. J. Periodontol. 2007, 78, 2369–2379. [Google Scholar] [CrossRef]
- Kletschkus, K.; Haralambiev, L.; Nitsch, A.; Pfister, F.; Klinkmann, G.; Kramer, A.; Bekeschus, S.; Mustea, A.; Stope, M.B. The Application of a Low-temperature Physical Plasma Device Operating Under Atmospheric Pressure Leads to the Production of Toxic NO2. Anticancer. Res. 2020, 40, 2591–2599. [Google Scholar] [CrossRef]
- Weiss, M.; Barz, J.; Ackermann, M.; Utz, R.; Ghoul, A.; Weltmann, K.-D.; Stope, M.B.; Wallwiener, D.; Schenke-Layland, K.; Oehr, C.; et al. Dose-Dependent Tissue-Level Characterization of a Medical Atmospheric Pressure Argon Plasma Jet. ACS Appl. Mater. Interfaces 2019, 11, 19841–19853. [Google Scholar] [CrossRef] [PubMed]
- Jablonowski, L.; Kocher, T.; Schindler, A.; Müller, K.; Dombrowski, F.; Von Woedtke, T.; Arnold, T.; Lehmann, A.; Rupf, S.; Evert, M.; et al. Side effects by oral application of atmospheric pressure plasma on the mucosa in mice. PLoS ONE 2019, 14, e0215099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacoby, J.M.; Strakeljahn, S.; Nitsch, A.; Bekeschus, S.; Hinz, P.; Mustea, A.; Ekkernkamp, A.; Tzvetkov, M.V.; Haralambiev, L.; Stope, M.B. An Innovative Therapeutic Option for the Treatment of Skeletal Sarcomas: Elimination of Osteo- and Ewing’s Sarcoma Cells Using Physical Gas Plasma. Int. J. Mol. Sci. 2020, 21, 4460. [Google Scholar] [CrossRef]
- D’Errico, J.A.; Berry, J.E.; Ouyang, H.; Strayhorn, C.L.; Windle, J.J.; Somerman, M.J. Employing a Transgenic Animal Model to Obtain Cementoblasts In Vitro. J. Periodontol. 2000, 71, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Crowe, A.R.; Yue, W. Semi-quantitative Determination of Protein Expression Using Immunohistochemistry Staining and Analysis: An Integrated Protocol. BIO-PROTOCOL 2019, 9, e3465. [Google Scholar] [CrossRef]
- Reinholz, G.G.; Getz, B.; Pederson, L.; Sanders, E.S.; Subramaniam, M.; Ingle, J.N.; Spelsberg, T.C. Bisphosphonates directly regulate cell proliferation, differentiation, and gene expression in human osteoblasts. Cancer Res. 2000, 60, 6001–6007. [Google Scholar]
- Memmert, S.; Nokhbehsaim, M.; Jäger, A.; Deschner, J.; Damanaki, A.; Nogueira, A.V.B.; Papadopoulou, A.K.; Piperi, C.; Basdra, E.K.; Rath-Deschner, B.; et al. Role of cathepsin S In periodontal wound healing—An in vitro study on human PDL cells. BMC Oral Health 2018, 18, 1–7. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eggers, B.; Marciniak, J.; Deschner, J.; Stope, M.B.; Mustea, A.; Kramer, F.-J.; Nokhbehsaim, M. Cold Atmospheric Plasma Promotes Regeneration-Associated Cell Functions of Murine Cementoblasts In Vitro. Int. J. Mol. Sci. 2021, 22, 5280. https://doi.org/10.3390/ijms22105280
Eggers B, Marciniak J, Deschner J, Stope MB, Mustea A, Kramer F-J, Nokhbehsaim M. Cold Atmospheric Plasma Promotes Regeneration-Associated Cell Functions of Murine Cementoblasts In Vitro. International Journal of Molecular Sciences. 2021; 22(10):5280. https://doi.org/10.3390/ijms22105280
Chicago/Turabian StyleEggers, Benedikt, Jana Marciniak, James Deschner, Matthias Bernhard Stope, Alexander Mustea, Franz-Josef Kramer, and Marjan Nokhbehsaim. 2021. "Cold Atmospheric Plasma Promotes Regeneration-Associated Cell Functions of Murine Cementoblasts In Vitro" International Journal of Molecular Sciences 22, no. 10: 5280. https://doi.org/10.3390/ijms22105280
APA StyleEggers, B., Marciniak, J., Deschner, J., Stope, M. B., Mustea, A., Kramer, F.-J., & Nokhbehsaim, M. (2021). Cold Atmospheric Plasma Promotes Regeneration-Associated Cell Functions of Murine Cementoblasts In Vitro. International Journal of Molecular Sciences, 22(10), 5280. https://doi.org/10.3390/ijms22105280






