Ectopic Expression of a Heterologous Glutaredoxin Enhances Drought Tolerance and Grain Yield in Field Grown Maize
Abstract
:1. Introduction
2. Results
2.1. Upregulation of ZmGRXS17 Expression under Drought Stress
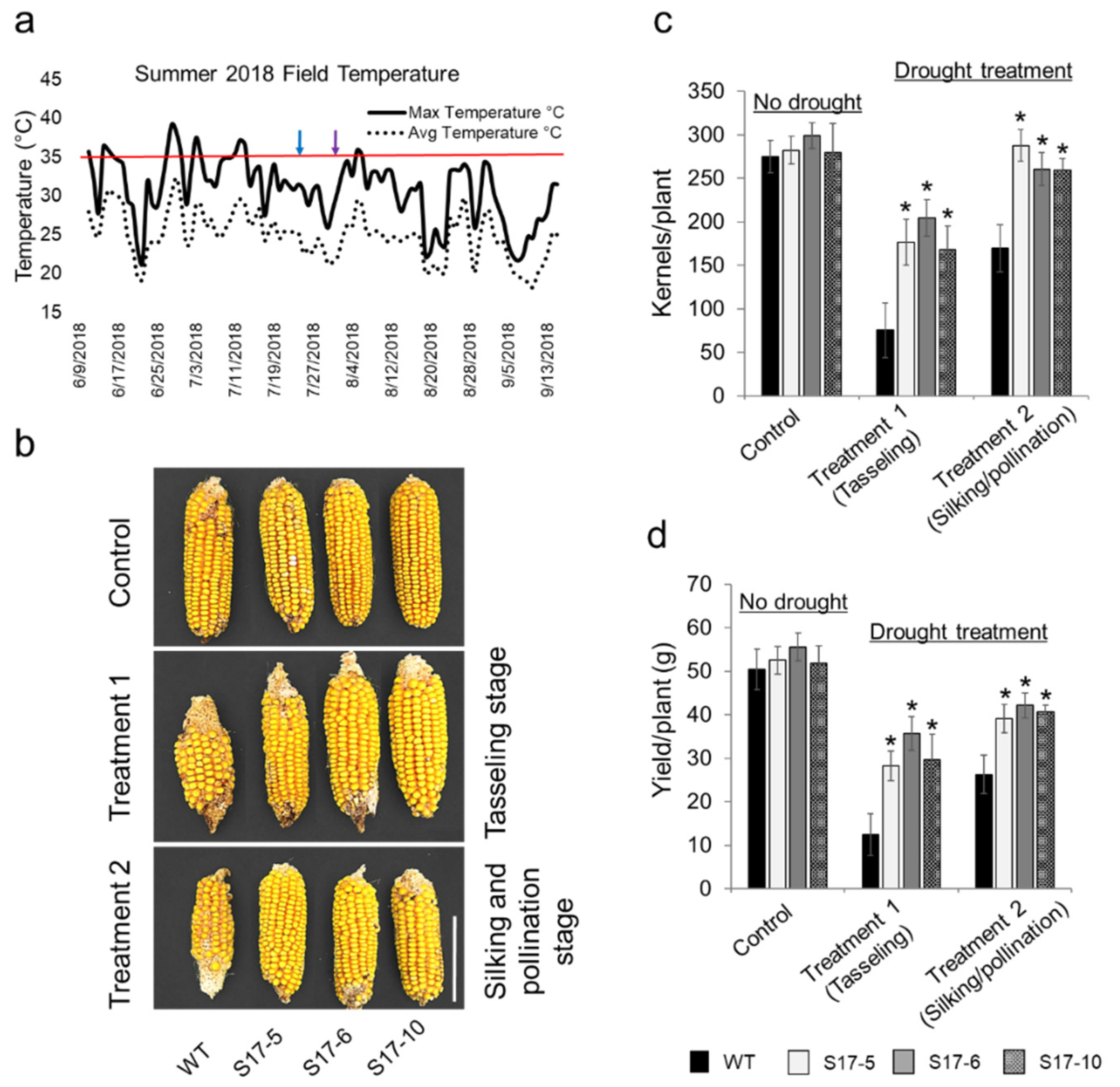
2.2. AtGRXS17-Expressing Maize Plants Increase Yield under Drought Stress Field Conditions
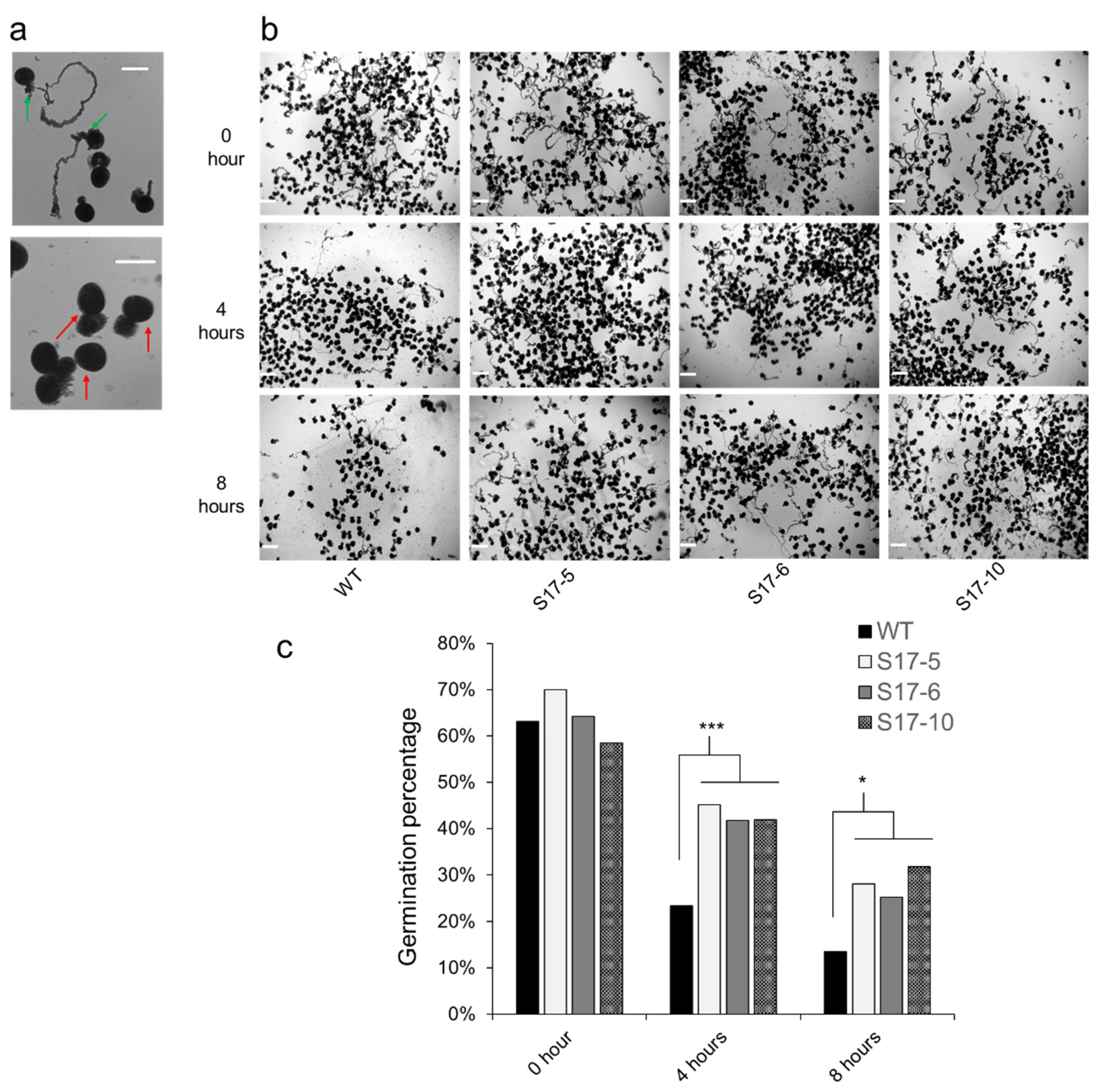
2.3. AtGRXS17-Expressing Maize Pollen Is More Drought Tolerant Than WT Pollen
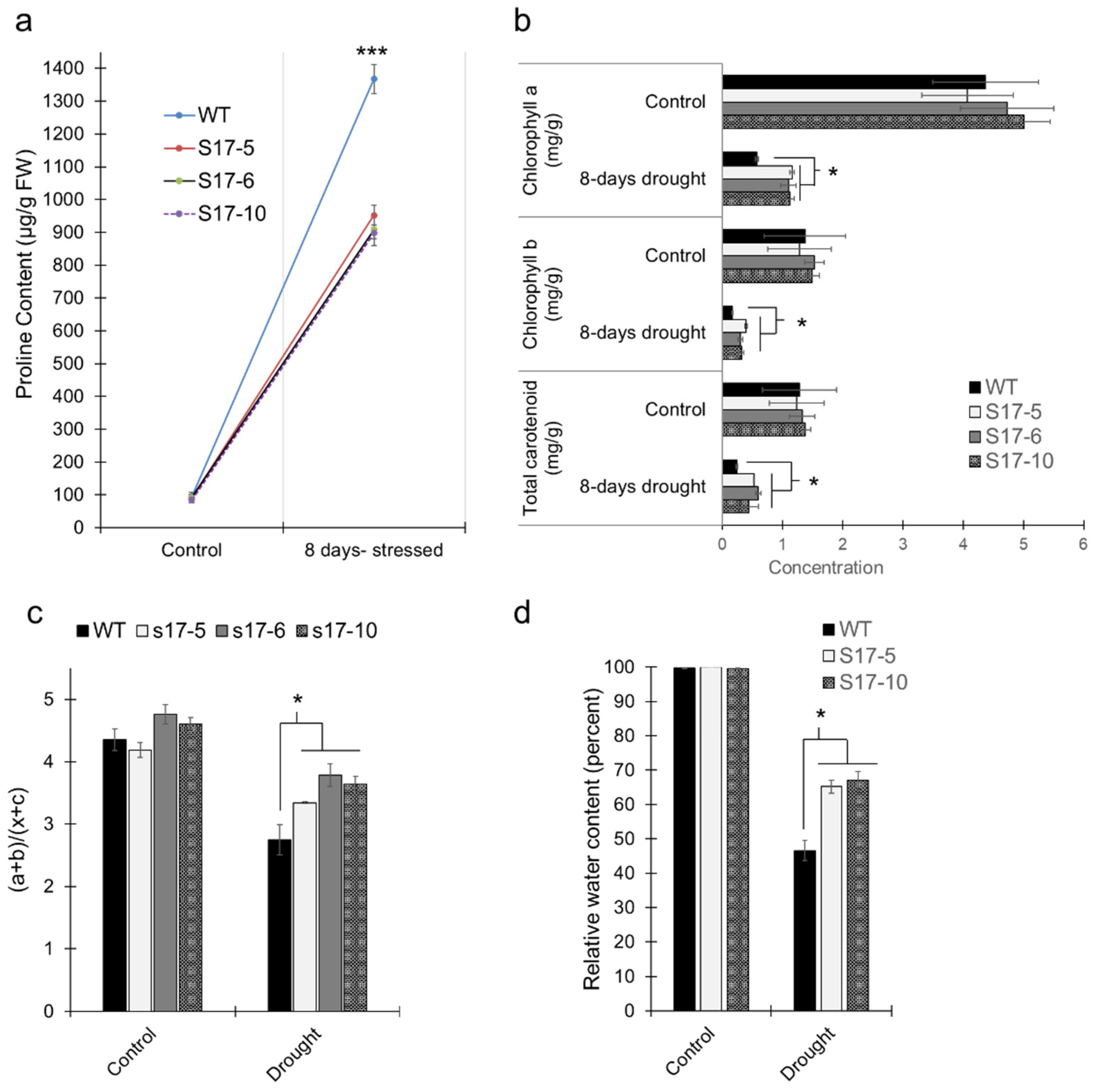
2.4. Effect of Ectopic Expression of AtGRXS17 on Proline Accumulation, Chlorophyll Content, Relative Water Content, Stomatal Conductance, and H2O2 under Drought Conditions
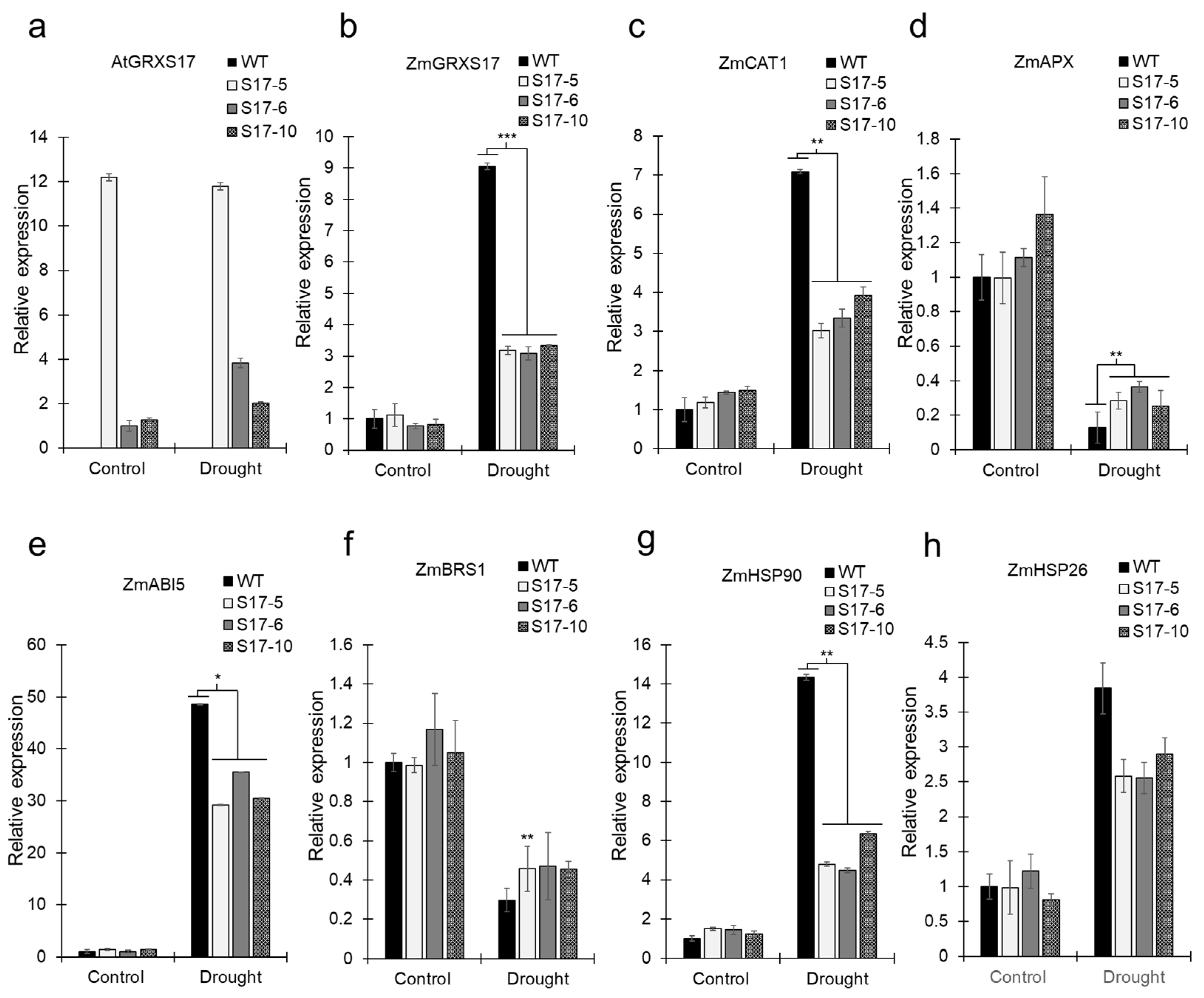
2.5. Drought Stress Results in Altered Gene Expression in Maize Plants
3. Discussion
4. Materials and Methods
4.1. Cloning AtGRXS17 and Plant Transformation
4.2. qRT-PCR Analysis of the Maize Endogenous Monothiol Glutaredoxin 17, ZmGRXS17
4.3. Field Trial
4.4. Greenhouse Experiment
4.5. Pollen Germination Analysis
4.6. Proline Content Measurement
4.7. Chlorophyll Content Measurement
4.8. Relative Water Content (RWC)
4.9. Stomatal Conductance and Chlorophyll Fluorescence Measurement
4.10. H2O2 Assay
4.11. qPCR of Leaf Tissues
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global Food Demand and the Sustainable Intensification of Agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef] [Green Version]
- United Nations; Department of Economic and Social Affairs; Population Division. World Population Prospects Highlights; 2019; ISBN 978-92-1-148316-1. Available online: https://population.un.org/wpp/Publications/Files/WPP2019_Highlights.pdf (accessed on 18 August 2020).
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of Extreme Weather Disasters on Global Crop Production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef]
- Mishra, V.; Cherkauer, K.A. Retrospective Droughts in the Crop Growing Season: Implications to Corn and Soybean Yield in the Midwestern United States. Agric. Forest Meteorol. 2010, 150, 1030–1045. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Manivannan, P.; Wahid, A.; Farooq, M.; Al-Juburi, J.; Somasundaram, R.; Panneerselvam, R. Drought Stress in Plants: A Review on Morphological Characteristics and Pigments Composition. Int. J. Agric. Biol. 2009, 11, 6. [Google Scholar]
- Xu, H.; Twine, T.E.; Girvetz, E. Climate Change and Maize Yield in Iowa. PLoS ONE 2016, 11, e0156083. [Google Scholar] [CrossRef]
- Zipper, S.C.; Qiu, J.; Kucharik, C.J. Drought Effects on US Maize and Soybean Production: Spatiotemporal Patterns and Historical Changes. Environ. Res. Lett. 2016, 11, 094021. [Google Scholar] [CrossRef]
- Zheng, J.; Fu, J.; Gou, M.; Huai, J.; Liu, Y.; Jian, M.; Huang, Q.; Guo, X.; Dong, Z.; Wang, H.; et al. Genome-Wide Transcriptome Analysis of Two Maize Inbred Lines under Drought Stress. Plant Mol. Biol. 2010, 72, 407–421. [Google Scholar] [CrossRef]
- Al-Kaisi, M.M.; Elmore, R.W.; Guzman, J.G.; Hanna, H.M.; Hart, C.E.; Helmers, M.J.; Hodgson, E.W.; Lenssen, A.W.; Mallarino, A.P.; Robertson, A.E.; et al. Drought Impact on Crop Production and the Soil Environment: 2012 Experiences from Iowa. J. Soil Water Conserv. 2013, 68, 19A–24A. [Google Scholar] [CrossRef] [Green Version]
- Rippey, B.R. The U.S. Drought of 2012. Weather Clim. Extrem. 2015, 10, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Rosenzweig, C.; Iglesius, A.; Yang, X.B.; Epstein, P.R.; Chivian, E. Climate Change and Extreme Weather Events–Implications for Food Production, Plant Diseases, and Pests. Glob. Chang. 2001, 2, 16. [Google Scholar]
- Deikman, J.; Petracek, M.; Heard, J.E. Drought Tolerance through Biotechnology: Improving Translation from the Laboratory to Farmers’ Fields. Curr. Opin. Biotechnol. 2012, 23, 243–250. [Google Scholar] [CrossRef]
- Umezawa, T.; Fujita, M.; Fujita, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Engineering Drought Tolerance in Plants: Discovering and Tailoring Genes to Unlock the Future. Curr. Opin. Biotechnol. 2006, 17, 113–122. [Google Scholar] [CrossRef]
- Yang, S.; Vanderbeld, B.; Wan, J.; Huang, Y. Narrowing Down the Targets: Towards Successful Genetic Engineering of Drought-Tolerant Crops. Mol. Plant 2010, 3, 469–490. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Rupe, M.A.; Wei, J.; Winkler, C.; Goncalves-Butruille, M.; Weers, B.P.; Cerwick, S.F.; Dieter, J.A.; Duncan, K.E.; Howard, R.J.; et al. Maize ARGOS1 (ZAR1) Transgenic Alleles Increase Hybrid Maize Yield. J. Exp. Bot. 2014, 65, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.E.; Repetti, P.P.; Adams, T.R.; Creelman, R.A.; Wu, J.; Warner, D.C.; Anstrom, D.C.; Bensen, R.J.; Castiglioni, P.P.; Donnarummo, M.G.; et al. Plant Nuclear Factor Y (NF-Y) B Subunits Confer Drought Tolerance and Lead to Improved Corn Yields on Water-Limited Acres. Proc. Natl. Acad. Sci. USA 2007, 104, 16450–16455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habben, J.E.; Bao, X.; Bate, N.J.; DeBruin, J.L.; Dolan, D.; Hasegawa, D.; Helentjaris, T.G.; Lafitte, R.H.; Lovan, N.; Mo, H.; et al. Transgenic Alteration of Ethylene Biosynthesis Increases Grain Yield in Maize under Field Drought-Stress Conditions. Plant Biotechnol. J. 2014, 12, 685–693. [Google Scholar] [CrossRef]
- Li, B.; Wei, A.; Song, C.; Li, N.; Zhang, J. Heterologous Expression of the TsVP Gene Improves the Drought Resistance of Maize. Plant Biotechnol. J. 2008, 6, 146–159. [Google Scholar] [CrossRef]
- Nuccio, M.L.; Wu, J.; Mowers, R.; Zhou, H.-P.; Meghji, M.; Primavesi, L.F.; Paul, M.J.; Chen, X.; Gao, Y.; Haque, E.; et al. Expression of Trehalose-6-Phosphate Phosphatase in Maize Ears Improves Yield in Well-Watered and Drought Conditions. Nat. Biotechnol. 2015, 33, 862–869. [Google Scholar] [CrossRef]
- Quan, R.; Shang, M.; Zhang, H.; Zhao, Y.; Zhang, J. Engineering of Enhanced Glycine Betaine Synthesis Improves Drought Tolerance in Maize: Glycine Betaine Improves Maize Drought Tolerance. Plant Biotechnol. J. 2004, 2, 477–486. [Google Scholar] [CrossRef]
- Shou, H. Expression of the Nicotiana Protein Kinase (NPK1) Enhanced Drought Tolerance in Transgenic Maize. J. Exp. Bot. 2004, 55, 1013–1019. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.X. Barley HVA1 Gene Confers Drought and Salt Tolerance in Transgenic Maize Zea mays L. Adv. Crop. Sci. Tech. 2013, 1. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Li, N.; Gao, F.; Yang, A.; Zhang, J. Over-Expression of TsCBF1 Gene Confers Improved Drought Tolerance in Transgenic Maize. Mol. Breed. 2010, 26, 455–465. [Google Scholar] [CrossRef]
- Thannickal, V.J.; Fanburg, B.L. Reactive Oxygen Species in Cell Signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 279, L1005–L1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sofo, A.; Scopa, A.; Nuzzaci, M.; Vitti, A. Ascorbate Peroxidase and Catalase Activities and Their Genetic Regulation in Plants Subjected to Drought and Salinity Stresses. Int. J. Mol. Sci. 2015, 16, 13561–13578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mashamaite, L.N.; Rohwer, J.M.; Pillay, C.S. The Glutaredoxin Mono- and Di-Thiol Mechanisms for Deglutathionylation Are Functionally Equivalent: Implications for Redox Systems Biology. Biosci. Rep. 2015, 35, e00173. [Google Scholar] [CrossRef]
- Rouhier, N. Plant Glutaredoxins: Pivotal Players in Redox Biology and Iron-Sulphur Centre Assembly. New Phytol. 2010, 186, 365–372. [Google Scholar] [CrossRef]
- Wu, Q.; Yang, J.; Cheng, N.; Hirschi, K.D.; White, F.F.; Park, S. Glutaredoxins in Plant Development, Abiotic Stress Response, and Iron Homeostasis: From Model Organisms to Crops. Environ. Exp. Bot. 2017, 139, 91–98. [Google Scholar] [CrossRef]
- Cheng, N.-H.; Liu, J.-Z.; Liu, X.; Wu, Q.; Thompson, S.M.; Lin, J.; Chang, J.; Whitham, S.A.; Park, S.; Cohen, J.D.; et al. Arabidopsis Monothiol Glutaredoxin, AtGRXS17, Is Critical for Temperature-Dependent Postembryonic Growth and Development via Modulating Auxin Response. J. Biol. Chem. 2011, 286, 20398–20406. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Wu, Q.; Sprague, S.A.; Park, J.; Oh, M.; Rajashekar, C.B.; Koiwa, H.; Nakata, P.A.; Cheng, N.; Hirschi, K.D.; et al. Tomato Expressing Arabidopsis Glutaredoxin Gene AtGRXS17 Confers Tolerance to Chilling Stress via Modulating Cold Responsive Components. Hortic. Res. 2015, 2, 15051. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Lin, J.; Liu, J.-Z.; Wang, X.; Lim, W.; Oh, M.; Park, J.; Rajashekar, C.B.; Whitham, S.A.; Cheng, N.-H.; et al. Ectopic Expression of Arabidopsis Glutaredoxin AtGRXS17 Enhances Thermotolerance in Tomato: Ectopic Expression of AtGRXS17 in Tomato. Plant Biotechnol. J. 2012, 10, 945–955. [Google Scholar] [CrossRef]
- Wu, Q.; Hu, Y.; Sprague, S.A.; Kakeshpour, T.; Park, J.; Nakata, P.A.; Cheng, N.; Hirschi, K.D.; White, F.F.; Park, S. Expression of a Monothiol Glutaredoxin, AtGRXS17, in Tomato (Solanum lycopersicum) Enhances Drought Tolerance. Biochem. Biophys. Res. Commun. 2017, 491, 1034–1039. [Google Scholar] [CrossRef]
- Yu, H.; Yang, J.; Shi, Y.; Donelson, J.; Thompson, S.M.; Sprague, S.; Roshan, T.; Wang, D.-L.; Liu, J.; Park, S.; et al. Arabidopsis Glutaredoxin S17 Contributes to Vegetative Growth, Mineral Accumulation, and Redox Balance during Iron Deficiency. Front. Plant Sci. 2017, 8, 1045. [Google Scholar] [CrossRef] [Green Version]
- USDA Foreign Agricultural Service. World Agricultural Production. Ag Data Commons. 2020. Available online: https://data.nal.usda.gov/dataset/world-agricultural-production (accessed on 15 November 2020).
- Çakir, R. Effect of Water Stress at Different Development Stages on Vegetative and Reproductive Growth of Corn. Field Crop. Res. 2004, 89, 1–16. [Google Scholar] [CrossRef]
- Atteya, A.M. Alteration of water relations and yield of corn genotypes in response to drought stress. Bulg. J. Plant Physiol. 2003, 29, 63–67. [Google Scholar]
- Aylor, D.E. Rate of Dehydration of Corn (Zea mays L.) Pollen in the Air. J. Exp. Bot. 2003, 54, 2307–2312. [Google Scholar] [CrossRef] [Green Version]
- Luna, V.S.; Figueroa, M.J.; Baltazar, M.B.; Gomez, L.R.; Townsend, R.; Schoper, J.B. Maize Pollen Longevity and Distance Isolation Requirements for Effective Pollen Control. Crop Sci. 2001, 41, 1551–1557. [Google Scholar] [CrossRef]
- Fonseca, A.E.; Westgate, M.E. Relationship between Desiccation and Viability of Maize Pollen. Field Crop. Res. 2005, 94, 114–125. [Google Scholar] [CrossRef]
- Ouyang, W.; Struik, P.C.; Yin, X.; Yang, J. Stomatal Conductance, Mesophyll Conductance, and Transpiration Efficiency in Relation to Leaf Anatomy in Rice and Wheat Genotypes under Drought. J. Exp. Bot. 2017, 68, 5191–5205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz de Carvalho, M.H. Drought Stress and Reactive Oxygen Species: Production, Scavenging and Signaling. Plant Signal. Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef] [Green Version]
- Kar, R.K. Plant Responses to Water Stress: Role of Reactive Oxygen Species. Plant Signal. Behav. 2011, 6, 1741–1745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merah, O. Potential Importance of Water Status Traits for Durum Wheat Improvement under Mediterranean Conditions. J. Agric. Sci. 2001, 137, 139–145. [Google Scholar] [CrossRef]
- Khayatnezhad, M.; Gholamin, R.; Jamaati-e-Somarin, S.; Zabihi-e, R. The Leaf Chlorophyll Content and Stress Resistance Relationship Considering in Corn Cultivars (Zea. mays). Adv. Environ. Biol. 2011, 5, 118–122. [Google Scholar]
- Keyvan, S. The Effects of Drought Stress on Yield, Relative Water Content, Proline, Soluble Carbohydrates and Chlorophyll of Bread Wheat Cultivars. J. Anim. Plant Sci. 2010, 8, 1051–1060. [Google Scholar]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Ibarra-Caballero, J.; Villanueva-Verduzco, C.; Molina-Galán, J.; Sánchez-De-Jiménez, E. Proline Accumulation as a Symptom of Drought Stress in Maize: A Tissue Differentiation Requirement. J. Exp. Bot. 1988, 39, 889–897. [Google Scholar] [CrossRef]
- Verslues, P.E.; Kim, Y.-S.; Zhu, J.-K. Altered ABA, Proline and Hydrogen Peroxide in an Arabidopsis Glutamate:Glyoxylate Aminotransferase Mutant. Plant Mol. Biol. 2007, 64, 205–217. [Google Scholar] [CrossRef]
- Vishwakarma, K.; Upadhyay, N.; Kumar, N.; Yadav, G.; Singh, J.; Mishra, R.K.; Kumar, V.; Verma, R.; Upadhyay, R.G.; Pandey, M.; et al. Abscisic Acid Signaling and Abiotic Stress Tolerance in Plants: A Review on Current Knowledge and Future Prospects. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beardsell, M.F.; Cohen, D. Relationships between Leaf Water Status, Abscisic Acid Levels, and Stomatal Resistance in Maize and Sorghum. Plant Physiol. 1975, 56, 207–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Yang, A.; Yin, H.; Zhang, J. Influence of Water Stress on Endogenous Hormone Contents and Cell Damage of Maize Seedlings. J. Integr. Plant Biol. 2008, 50, 427–434. [Google Scholar] [CrossRef]
- Brocard, I.M.; Lynch, T.J.; Finkelstein, R.R. Regulation and Role of the Arabidopsis Abscisic Acid-Insensitive 5 Gene in Abscisic Acid, Sugar, and Stress Response. Plant Physiol. 2002, 129, 1533–1543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waterland, N.L.; Campbell, C.A.; Finer, J.J.; Jones, M.L. Abscisic Acid Application Enhances Drought Stress Tolerance in Bedding Plants. HortScience 2010, 45, 409–413. [Google Scholar] [CrossRef] [Green Version]
- Wei, L.; Wang, L.; Yang, Y.; Wang, P.; Guo, T.; Kang, G. Abscisic Acid Enhances Tolerance of Wheat Seedlings to Drought and Regulates Transcript Levels of Genes Encoding Ascorbate-Glutathione Biosynthesis. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, F.; Li, M.; Liang, D.; Zou, J. Physiological Responses of Kiwifruit Plants to Exogenous ABA under Drought Conditions. Plant Growth Regul. 2011, 64, 63–74. [Google Scholar] [CrossRef]
- Finkelstein, R.R.; Lynch, T.J. The Arabidopsis Abscisic Acid Response Gene ABI5 Encodes a Basic Leucine Zipper Transcription Factor. Plant Cell 2000, 12, 599–609. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Molina, L.; Mongrand, S.; Chua, N.-H. A Postgermination Developmental Arrest Checkpoint Is Mediated by Abscisic Acid and Requires the ABI5 Transcription Factor in Arabidopsis. Proc. Natl. Acad. Sci. USA 2001, 98, 4782–4787. [Google Scholar] [CrossRef] [Green Version]
- Skubacz, A.; Daszkowska-Golec, A.; Szarejko, I. The Role and Regulation of ABI5 (ABA-Insensitive 5) in Plant Development, Abiotic Stress Responses and Phytohormone Crosstalk. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Yan, F.; Deng, W.; Wang, X.; Yang, C.; Li, Z. Maize (Zea mays L.) Homologue of ABA-Insensitive (ABI) 5 Gene Plays a Negative Regulatory Role in Abiotic Stresses Response. Plant Growth Regul. 2012, 68, 383–393. [Google Scholar] [CrossRef]
- Zou, M.; Guan, Y.; Ren, H.; Zhang, F.; Chen, F. A BZIP Transcription Factor, OsABI5, Is Involved in Rice Fertility and Stress Tolerance. Plant Mol. Biol. 2008, 66, 675–683. [Google Scholar] [CrossRef]
- Anjum, S.A.; Ashraf, U.; Tanveer, M.; Khan, I.; Hussain, S.; Shahzad, B.; Zohaib, A.; Abbas, F.; Saleem, M.F.; Ali, I.; et al. Drought Induced Changes in Growth, Osmolyte Accumulation and Antioxidant Metabolism of Three Maize Hybrids. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Q.; Wu, J.; Zheng, X.; Zheng, S.; Sun, X.; Qiu, Q.; Lu, T. Gene Knockout Study Reveals That Cytosolic Ascorbate Peroxidase 2(OsAPX2) Plays a Critical Role in Growth and Reproduction in Rice under Drought, Salt and Cold Stresses. PLoS ONE 2013, 8, e57472. [Google Scholar] [CrossRef]
- Zhang, J.; Kirkham, M.B. Antioxidant Responses to Drought in Sunflower and Sorghum Seedlings. New Phytol. 1996, 132, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive Oxygen Species: Metabolism, Oxidative Stress, and Signal Transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouhier, N.; Villarejo, A.; Srivastava, M.; Gelhaye, E.; Keech, O.; Droux, M.; Finkemeier, I.; Samuelsson, G.; Dietz, K.J.; Jacquot, J.-P.; et al. Identification of Plant Glutaredoxin Targets. Antioxid. Redox Signal. 2005, 7, 919–929. [Google Scholar] [CrossRef]
- Priya, M.; Dhanker, O.P.; Siddique, K.H.M.; HanumanthaRao, B.; Nair, R.M.; Pandey, S.; Singh, S.; Varshney, R.K.; Prasad, P.V.V.; Nayyar, H. Drought and Heat Stress-Related Proteins: An Update about Their Functional Relevance in Imparting Stress Tolerance in Agricultural Crops. Theor. Appl. Genet. 2019, 132, 1607–1638. [Google Scholar] [CrossRef]
- Augustine, S.M. Function of Heat-Shock Proteins in Drought Tolerance Regulation of Plants. In Drought Stress Tolerance in Plants, Vol 1: Physiology and Biochemistry; Hossain, M.A., Wani, S.H., Bhattacharjee, S., Burritt, D.J., Tran, L.-S.P., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 163–185. ISBN 978-3-319-28899-4. [Google Scholar]
- Song, H.; Zhao, R.; Fan, P.; Wang, X.; Chen, X.; Li, Y. Overexpression of AtHsp90.2, AtHsp90.5 and AtHsp90.7 in Arabidopsis Thaliana Enhances Plant Sensitivity to Salt and Drought Stresses. Planta 2009, 229, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhu, J.; Li, X.; Li, Z.; Han, L.; Luo, H. AsHSP26.8a, a Creeping Bentgrass Small Heat Shock Protein Integrates Different Signaling Pathways to Modulate Plant Abiotic Stress Response. BMC Plant Biol. 2020, 20, 184. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.; Knuesting, J.; Bariat, L.; Dard, A.; Freibert, S.A.; Marchand, C.H.; Young, D.; Dung, N.H.T.; Voth, W.; Debures, A.; et al. Redox Modification of the Iron-Sulfur Glutaredoxin GRXS17 Activates Holdase Activity and Protects Plants from Heat Stress. Plant Physiol. 2020, 184, 676–692. [Google Scholar] [CrossRef]
- Sprague, S.A. Ectopic Expression of an Arabidopsis Glutaredoxin Increases Thermotolerance in Maize during Reproductive Developmental Stages. Ph.D. Thesis, Kansas State University, Manhattan, KS, USA, 2018. [Google Scholar]
- Hu, Y.; Wu, Q.; Peng, Z.; Sprague, S.A.; Wang, W.; Park, J.; Akhunov, E.; Jagadish, K.S.V.; Nakata, P.A.; Cheng, N.; et al. Silencing of OsGRXS17 in Rice Improves Drought Stress Tolerance by Modulating ROS Accumulation and Stomatal Closure. Sci. Rep. 2017, 7, 15950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Barrs, H.D.; Weatherley, P.E. A Re-examination of the Relative Turgidity Technique for Estimating Water Deficits in Leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamang, T.M.; Sprague, S.A.; Kakeshpour, T.; Liu, S.; White, F.F.; Park, S. Ectopic Expression of a Heterologous Glutaredoxin Enhances Drought Tolerance and Grain Yield in Field Grown Maize. Int. J. Mol. Sci. 2021, 22, 5331. https://doi.org/10.3390/ijms22105331
Tamang TM, Sprague SA, Kakeshpour T, Liu S, White FF, Park S. Ectopic Expression of a Heterologous Glutaredoxin Enhances Drought Tolerance and Grain Yield in Field Grown Maize. International Journal of Molecular Sciences. 2021; 22(10):5331. https://doi.org/10.3390/ijms22105331
Chicago/Turabian StyleTamang, Tej Man, Stuart A. Sprague, Tayebeh Kakeshpour, Sanzhen Liu, Frank F. White, and Sunghun Park. 2021. "Ectopic Expression of a Heterologous Glutaredoxin Enhances Drought Tolerance and Grain Yield in Field Grown Maize" International Journal of Molecular Sciences 22, no. 10: 5331. https://doi.org/10.3390/ijms22105331
APA StyleTamang, T. M., Sprague, S. A., Kakeshpour, T., Liu, S., White, F. F., & Park, S. (2021). Ectopic Expression of a Heterologous Glutaredoxin Enhances Drought Tolerance and Grain Yield in Field Grown Maize. International Journal of Molecular Sciences, 22(10), 5331. https://doi.org/10.3390/ijms22105331





