Curcumin at Low Doses Potentiates and at High Doses Inhibits ABT-737-Induced Platelet Apoptosis
Abstract
:1. Introduction
2. Results
2.1. Curcumin Inhibits Thrombin-Induced Platelet Activation but Does Not Stimulate Caspase 3-Dependent Apoptosis
2.2. Curcumin Inhibits P-gp Function in Platelets
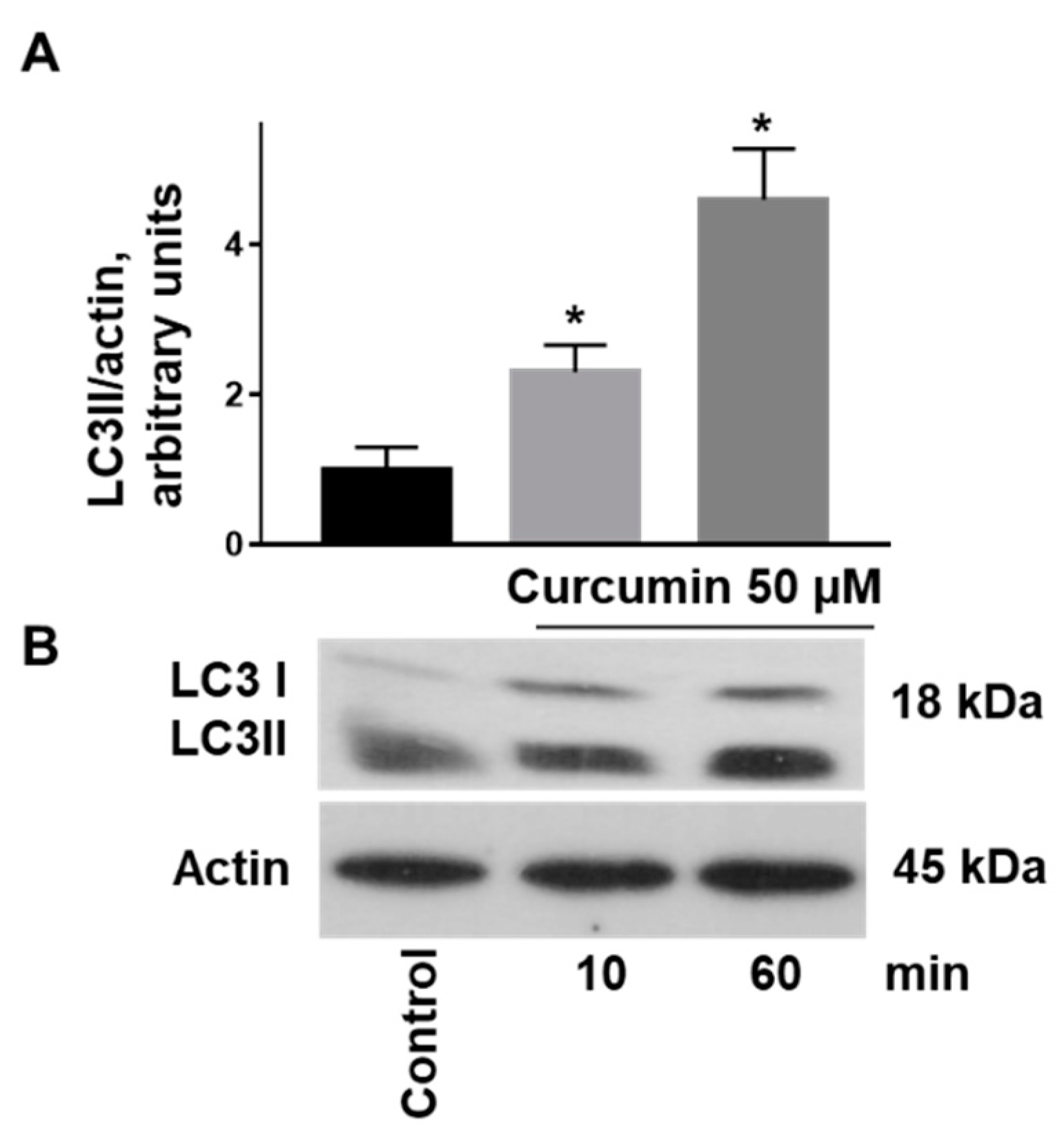
2.3. Curcumin-Induced Autophagy by Conversion of LC3I to LC3II Associated with Stimulation of AMPK and Inhibition of PKB Activity in Platelets
2.4. Curcumin Had No Effect on ABT-737-Induced Platelet Phosphatidylserine Surface Exposure
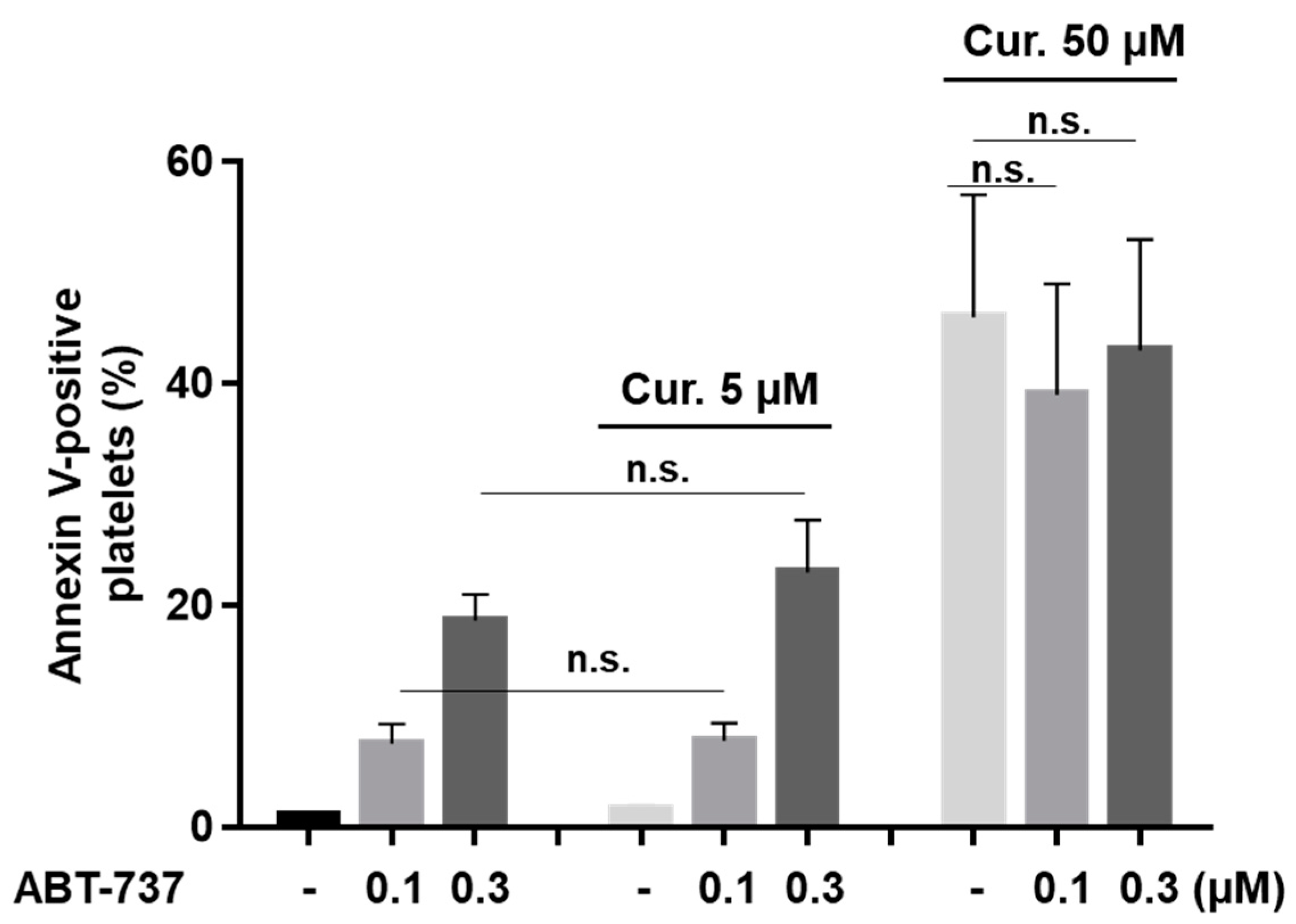
2.5. Curcumin Differentially Regulates ABT-737-Induced Platelet Apoptosis
2.6. Curcumin Inhibits Platelet-Dependent Thrombin Generation Mediated by ABT-737
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Human Platelet Preparation
Platelet-Free Plasma Preparation
4.3. Flow Cytometric Analysis of Platelets
4.3.1. Analysis of Platelet αIIbβ3 Integrin Activation, PS Surface Exposure, and Mitochondrial Membrane Potential
4.3.2. Microparticle Formation
4.3.3. Doxorubicin Accumulation
4.3.4. Platelet Ca2+-Mobilization
4.4. Western Blot Analysis
4.5. Calibrated Automated Thrombography
4.6. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baell, J.B. Feeling nature′s PAINS: Natural products, natural product drugs, and Pan Assay Interference Compounds (PAINS). J. Nat. Prod. 2016, 79, 616–628. [Google Scholar] [CrossRef]
- Reddy, A.C.; Lokesh, B.R. Effect of dietary turmeric (Curcuma longa) on iron-induced lipid peroxidation in the rat liver. Food Chem. Toxicol. 1994, 32, 279–283. [Google Scholar] [CrossRef]
- Yang, M.; Akbar, U.; Mohan, C. Curcumin in autoimmune and rheumatic diseases. Nutrients 2019, 11, 1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.; Yue, Y.; Zheng, X.; Zhang, K.; Chen, S.; Du, Z. Curcumin, inflammation, and chronic diseases: How are they linked? Molecules 2015, 20, 9183–9213. [Google Scholar] [CrossRef] [PubMed]
- Farhood, B.; Mortezaee, K.; Goradel, N.H.; Khanlarkhani, N.; Salehi, E.; Nashtaei, M.S.; Najafi, M.; Sahebkar, A. Curcumin as an anti-inflammatory agent: Implications to radiotherapy and chemotherapy. J. Cell Physiol. 2019, 234, 5728–5740. [Google Scholar] [CrossRef]
- Bianchi, G.; Ravera, S.; Traverso, C.; Amaro, A.; Piaggio, F.; Emionite, L.; Bachetti, T.; Pfeffer, U.; Raffaghello, L. Curcumin induces a fatal energetic impairment in tumor cells in vitro and in vivo by inhibiting ATP-synthase activity. Carcinogenesis 2018, 39, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Mapoung, S.; Pitchakarn, P.; Yodkeeree, S.; Ovatlarnporn, C.; Sakorn, N.; Limtrakul, P. Chemosensitizing effects of synthetic curcumin analogs on human multi-drug resistance leukemic cells. Chem. Biol. Interact. 2016, 244, 140–148. [Google Scholar] [CrossRef]
- Kouhpeikar, H.; Butler, A.E.; Bamian, F.; Barreto, G.E.; Majeed, M.; Sahebkar, A. Curcumin as a therapeutic agent in leukemia. J. Cell Physiol. 2019, 234, 12404–12414. [Google Scholar] [CrossRef]
- Keihanian, F.; Saeidinia, A.; Bagheri, R.K.; Johnston, T.P.; Sahebkar, A. Curcumin, hemostasis, thrombosis, and coagulation. J. Cell Physiol. 2018, 233, 4497–4511. [Google Scholar] [CrossRef]
- Shah, B.H.; Nawaz, Z.; Pertani, S.A.; Roomi, A.; Mahmood, H.; Saeed, S.A.; Gilani, A.H. Inhibitory effect of curcumin, a food spice from turmeric, on platelet-activating factor- and arachidonic acid-mediated platelet aggregation through inhibition of thromboxane formation and Ca2+ signaling. Biochem. Pharmacol. 1999, 58, 1167–1172. [Google Scholar] [CrossRef]
- Mayanglambam, A.; Dangelmaier, C.A.; Thomas, D.; Damodar Reddy, C.; Daniel, J.L.; Kunapuli, S.P. Curcumin inhibits GPVI-mediated platelet activation by interfering with the kinase activity of Syk and the subsequent activation of PLCgamma2. Platelets 2010, 21, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gu, Z.L.; Qin, Z.H.; Liang, Z.Q. Effect of curcumin on the adhesion of platelets to brain microvascular endothelial cells in vitro. Acta Pharmacol. Sin. 2008, 29, 800–8007. [Google Scholar] [CrossRef] [Green Version]
- Raghavendra, R.H.; Naidu, K.A. Spice active principles as the inhibitors of human platelet aggregation and thromboxane biosynthesis. Prostaglandins Leukot. Essent. Fatty Acids 2009, 81, 73–78. [Google Scholar] [CrossRef]
- Srivastava, K.C.; Bordia, A.; Verma, S.K. Curcumin, a major component of food spice turmeric (Curcuma longa) inhibits aggregation and alters eicosanoid metabolism in human blood platelets. Prostaglandins Leukot. Essent. Fatty Acids 1995, 52, 223–227. [Google Scholar] [CrossRef]
- Maheswaraiah, A.; Rao, L.J.; Naidu, K.A. Anti-platelet activity of water dispersible curcuminoids in rat platelets. Phytother. Res. 2015, 29, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, J.; Prasad, S.; Aggarwal, B.B. Curcumin and cancer cells: How many ways can curry kill tumor cells selectively? AAPS J. 2009, 11, 495–510. [Google Scholar] [CrossRef]
- Moghtaderi, H.; Sepehri, H.; Attari, F. Combination of arabinogalactan and curcumin induces apoptosis in breast cancer cells in vitro and inhibits tumor growth via overexpression of p53 level in vivo. Biomed. Pharm. 2017, 88, 582–594. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Yang, X.; Chen, Y.; Jiang, Y.; Wang, S.J.; Li, Y.; Wang, X.Q.; Meng, Y.; Zhu, M.M.; Ma, X.; et al. Curcumin Suppresses Lung Cancer Stem Cells via Inhibiting Wnt/beta-catenin and Sonic Hedgehog Pathways. Phytother. Res. 2017, 31, 680–688. [Google Scholar] [CrossRef]
- Schwertheim, S.; Wein, F.; Lennartz, K.; Worm, K.; Schmid, K.W.; Sheu-Grabellus, S.Y. Curcumin induces G2/M arrest, apoptosis, NF-kappaB inhibition, and expression of differentiation genes in thyroid carcinoma cells. J. Cancer Res. Clin. Oncol. 2017, 143, 1143–1154. [Google Scholar] [CrossRef]
- Alexandru, O.; Georgescu, A.M.; Ene, L.; Purcaru, S.O.; Serban, F.; Popescu, A.; Brindusa, C.; Tataranu, L.G.; Ciubotaru, V.; Dricu, A. The effect of curcumin on low-passage glioblastoma cells in vitro. J. Cancer Res. Ther. 2016, 12, 1025–1032. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, W.; Li, P.; Zheng, Z.; Tu, Y.; Zhang, Y.; You, T. Curcumin enhances the effects of 5-Fluorouracil and oxaliplatin in inducing gastric cancer cell apoptosis both in vitro and in vivo. Oncol. Res. 2016, 23, 29–34. [Google Scholar] [CrossRef]
- Lv, Z.D.; Liu, X.P.; Zhao, W.J.; Dong, Q.; Li, F.N.; Wang, H.B.; Kong, B. Curcumin induces apoptosis in breast cancer cells and inhibits tumor growth in vitro and in vivo. Int. J. Clin. Exp. Pathol. 2014, 7, 2818–2824. [Google Scholar] [PubMed]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic roles of curcumin: Lessons learned from clinical trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosseini, M.; Hassanian, S.M.; Mohammadzadeh, E.; ShahidSales, S.; Maftouh, M.; Fayazbakhsh, H.; Khazaei, M.; Avan, A. Therapeutic potential of curcumin in treatment of pancreatic cancer: Current status and future perspectives. J. Cell Biochem. 2017, 118, 1634–1638. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Pramanik, D.; Campbell, N.R.; Das, S.; Gupta, S.; Chenna, V.; Bisht, S.; Sysa-Shah, P.; Bedja, D.; Karikari, C.; Steenbergen, C.; et al. A composite polymer nanoparticle overcomes multidrug resistance and ameliorates doxorubicin-associated cardiomyopathy. Oncotarget 2012, 3, 640–650. [Google Scholar] [CrossRef] [Green Version]
- Batra, H.; Pawar, S.; Bahl, D. Curcumin in combination with anti-cancer drugs: A nanomedicine review. Pharm. Res. 2018, 39, 91–105. [Google Scholar] [CrossRef]
- Ismail, N.I.; Othman, I.; Abas, F.; Lajis, N.H.L.; Naidu, R. Mechanism of apoptosis induced by curcumin in colorectal cancer. Int. J. Mol. Sci. 2019, 20, 2454. [Google Scholar] [CrossRef] [Green Version]
- Trotta, T.; Panaro, M.A.; Prifti, E.; Porro, C. Modulation of biological activities in glioblastoma mediated by curcumin. Nutr. Cancer 2019, 71, 1241–1253. [Google Scholar] [CrossRef]
- Kang, B.; Park, H.; Kim, B. Anticancer activity and underlying mechanism of phytochemicals against multiple myeloma. Int. J. Mol. Sci. 2019, 9, 2302. [Google Scholar] [CrossRef] [Green Version]
- Tabeshpour, J.; Hashemzaei, M.; Sahebkar, A. The regulatory role of curcumin on platelet functions. J. Cell Biochem. 2018, 119, 8713–8722. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.D.; Pang, Y.X.; Zhao, X.R.; Li, R.; Jin, C.J.; Xue, J.; Dong, R.Y.; Liu, P.S. Curcumin induces apoptotic cell death and protective autophagy by inhibiting AKT/mTOR/p70S6K pathway in human ovarian cancer cells. Arch. Gynecol. Obstet. 2019, 299, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Rukoyatkina, N.; Butt, E.; Subramanian, H.; Nikolaev, V.O.; Mindukshev, I.; Walter, U.; Gambaryan, S.; Benz, P.M. Protein kinase A activation by the anti-cancer drugs ABT-737 and thymoquinone is caspase-3-dependent and correlates with platelet inhibition and apoptosis. Cell Death Dis. 2017, 8, e2898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogler, M.; Hamali, H.A.; Sun, X.M.; Bampton, E.T.; Dinsdale, D.; Snowden, R.T.; Dyer, M.J.; Goodall, A.H.; Cohen, G.M. BCL2/BCL-X(L) inhibition induces apoptosis, disrupts cellular calcium homeostasis, and prevents platelet activation. Blood 2011, 117, 7145–7154. [Google Scholar] [CrossRef] [Green Version]
- Rywaniak, J.; Luzak, B.; Podsedek, A.; Dudzinska, D.; Rozalski, M.; Watala, C. Comparison of cytotoxic and anti-platelet activities of polyphenolic extracts from Arnica montana flowers and Juglans regia husks. Platelets 2015, 26, 168–176. [Google Scholar] [CrossRef]
- Hartley, P.S.; Savill, J.; Brown, S.B. The death of human platelets during incubation in citrated plasma involves shedding of CD42b and aggregation of dead platelets. Thromb. Haemost. 2006, 95, 100–106. [Google Scholar]
- Burkhart, J.M.; Vaudel, M.; Gambaryan, S.; Radau, S.; Walter, U.; Martens, L.; Geiger, J.; Sickmann, A.; Zahedi, R.P. The first comprehensive and quantitative analysis of human platelet protein composition allows the comparative analysis of structural and functional pathways. Blood 2012, 120, e73–e82. [Google Scholar] [CrossRef] [Green Version]
- Shen, F.; Chu, S.; Bence, A.K.; Bailey, B.; Xue, X.; Erickson, P.A.; Montrose, M.H.; Beck, W.T.; Erickson, L.C. Quantitation of doxorubicin uptake, efflux, and modulation of multidrug resistance (MDR) in MDR human cancer cells. J. Pharm. Exp. Ther. 2008, 324, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Wang, J.; Xie, R.; Liu, R.; Lu, Y. Mitochondria-derived reactive oxygen species play an important role in Doxorubicin-induced platelet apoptosis. Int. J. Mol. Sci. 2015, 16, 11087–11100. [Google Scholar] [CrossRef] [Green Version]
- Nanayakkara, A.K.; Follit, C.A.; Chen, G.; Williams, N.S.; Vogel, P.D.; Wise, J.G. Targeted inhibitors of P-glycoprotein increase chemotherapeutic-induced mortality of multidrug resistant tumor cells. Sci. Rep. 2018, 8, 967. [Google Scholar] [CrossRef] [Green Version]
- Beevers, C.S.; Chen, L.; Liu, L.; Luo, Y.; Webster, N.J.; Huang, S. Curcumin disrupts the Mammalian target of rapamycin-raptor complex. Cancer Res. 2009, 69, 1000–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zenkov, N.K.; Chechushkov, A.V.; Kozhin, P.M.; Kandalintseva, N.V.; Martinovich, G.G.; Menshchikova, E.B. Plant phenols and autophagy. Biochemistry 2016, 81, 297–314. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, A.; Cicero, A.F.G.; Panahi, Y.; Mohajeri, M.; Sahebkar, A. Curcumin: A naturally occurring autophagy modulator. J. Cell Physiol. 2019, 234, 5643–5654. [Google Scholar] [CrossRef]
- Saha, S.; Panigrahi, D.P.; Patil, S.; Bhutia, S.K. Autophagy in health and disease: A comprehensive review. Biomed. Pharm. 2018, 104, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Tanida, I.; Ueno, T.; Kominami, E. LC3 and Autophagy. Methods Mol. Biol. 2008, 445, 77–88. [Google Scholar] [CrossRef]
- Marino, G.; Niso-Santano, M.; Baehrecke, E.H.; Kroemer, G. Self-consumption: The interplay of autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2014, 15, 81–94. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Feng, K.; Li, J.; Yu, D.; Fan, Q.; Tang, T.; Yao, X.; Wang, X. Curcumin inhibits apoptosis of chondrocytes through activation ERK1/2 signaling pathways induced autophagy. Nutrients 2017, 9, 414. [Google Scholar] [CrossRef]
- Wang, J.L.; Wang, J.J.; Cai, Z.N.; Xu, C.J. The effect of curcumin on the differentiation, apoptosis and cell cycle of neural stem cells is mediated through inhibiting autophagy by the modulation of Atg7 and p62. Int. J. Mol. Med. 2018, 42, 2481–2488. [Google Scholar] [CrossRef]
- Wang, C.Y.; Ma, S.; Bi, S.J.; Su, L.; Huang, S.Y.; Miao, J.Y.; Ma, C.H.; Gao, C.J.; Hou, M.; Peng, J. Enhancing autophagy protects platelets in immune thrombocytopenia patients. Ann. Transl. Med. 2019, 7, 134. [Google Scholar] [CrossRef]
- Rukoyatkina, N.; Mindukshev, I.; Walter, U.; Gambaryan, S. Dual role of the p38 MAPK/cPLA2 pathway in the regulation of platelet apoptosis induced by ABT-737 and strong platelet agonists. Cell Death Dis. 2013, 4, e931. [Google Scholar] [CrossRef] [Green Version]
- Lopes-Rodrigues, V.; Sousa, E.; Vasconcelos, M.H. Curcumin as a modulator of P-glycoprotein in cancer: Challenges and perspectives. Pharmaceuticals 2016, 9, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, J.; Shin, D.; Lim, K.S.; Lee, S.; Jung, K.H.; Chu, K.; Hong, K.S.; Shin, K.H.; Cho, J.Y.; Yoon, S.H.; et al. Aspirin decreases systemic exposure to clopidogrel through modulation of P-glycoprotein but does not alter its antithrombotic activity. Clin. Pharm. Ther. 2014, 95, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, A. Autophagy and cell death: No longer at odds. Cell 2007, 131, 1032–1034. [Google Scholar] [CrossRef] [Green Version]
- Maiuri, M.C.; Zalckvar, E.; Kimchi, A.; Kroemer, G. Self-eating and self-killing: Crosstalk between autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2007, 8, 741–752. [Google Scholar] [CrossRef]
- Ouseph, M.M.; Huang, Y.; Banerjee, M.; Joshi, S.; MacDonald, L.; Zhong, Y.; Liu, H.; Li, X.; Xiang, B.; Zhang, G.; et al. Autophagy is induced upon platelet activation and is essential for hemostasis and thrombosis. Blood 2015, 126, 1224–1233. [Google Scholar] [CrossRef] [Green Version]
- Feng, W.; Chang, C.; Luo, D.; Su, H.; Yu, S.; Hua, W.; Chen, Z.; Hu, H.; Liu, W. Dissection of autophagy in human platelets. Autophagy 2014, 10, 642–651. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Du, J.; Stitham, J.; Atteya, G.; Lee, S.; Xiang, Y.; Wang, D.; Jin, Y.; Leslie, K.L.; Spollett, G.; et al. Inducing mitophagy in diabetic platelets protects against severe oxidative stress. EMBO Mol. Med. 2016, 8, 779–795. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Hemshekhar, M.; Kemparaju, K.; Girish, K.S. Aggregation is impaired in starved platelets due to enhanced autophagy and cellular energy depletion. Platelets 2019, 30, 487–497. [Google Scholar] [CrossRef]
- Schoenwaelder, S.M.; Yuan, Y.; Josefsson, E.C.; White, M.J.; Yao, Y.; Mason, K.D.; O′Reilly, L.A.; Henley, K.J.; Ono, A.; Hsiao, S.; et al. Two distinct pathways regulate platelet phosphatidylserine exposure and procoagulant function. Blood 2009, 114, 663–666. [Google Scholar] [CrossRef] [Green Version]
- Döhrmann, M.; Makhoul, S.; Gross, K.; Krause, M.; Pillitteri, D.; von Auer, C.; Walter, U.; Lutz, J.; Volf, I.; Kehrel, B.E.; et al. CD36-fibrin interaction propagates FXI-dependent thrombin generation of human platelets. FASEB J. 2020, 34, 9337–9357. [Google Scholar] [CrossRef]
- Gambaryan, S.; Geiger, J.; Schwarz, U.R.; Butt, E.; Begonja, A.; Obergfell, A.; Walter, U. Potent inhibition of human platelets by cGMP analogs independent of cGMP-dependent protein kinase. Blood 2004, 103, 2593–2600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gambaryan, S.; Kobsar, A.; Rukoyatkina, N.; Herterich, S.; Geiger, J.; Smolenski, A.; Lohmann, S.M.; Walter, U. Thrombin and collagen induce a feedback inhibitory signaling pathway in platelets involving dissociation of the catalytic subunit of protein kinase A from an NFkappaB-IkappaB complex. J. Biol. Chem. 2010, 285, 18352–18363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rukoyatkina, N.; Begonja, A.J.; Geiger, J.; Eigenthaler, M.; Walter, U.; Gambaryan, S. Phosphatidylserine surface expression and integrin alpha IIb beta 3 activity on thrombin/convulxin stimulated platelets/particles of different sizes. Br. J. Haematol. 2009, 144, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Makhoul, S.; Dorschel, S.; Gambaryan, S.; Walter, U.; Jurk, K. Feedback Regulation of Syk by Protein Kinase C in Human Platelets. Int. J. Mol. Sci. 2019, 21, 176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jurk, K.; Lahav, J.; Van Aken, H.; Brodde, M.F.; Nofer, J.R.; Kehrel, B.E. Extracellular protein disulfide isomerase regulates feedback activation of platelet thrombin generation via modulation of coagulation factor binding. J. Thromb. Haemost. 2011, 9, 2278–2290. [Google Scholar] [CrossRef] [PubMed]
- Mindukshev, I.; Gambaryan, S.; Kehrer, L.; Schuetz, C.; Kobsar, A.; Rukoyatkina, N.; Nikolaev, V.O.; Krivchenko, A.; Watson, S.P.; Walter, U.; et al. Low angle light scattering analysis: A novel quantitative method for functional characterization of human and murine platelet receptors. Clin Chem Lab Med. 2012, 50, 1253–1262. [Google Scholar] [CrossRef]
- Reiss, C.; Mindukshev, I.; Bischoff, V.; Subramanian, H.; Kehrer, L.; Friebe, A.; Stasch, J.P.; Gambaryan, S.; Walter, U. The sGC stimulator riociguat inhibits platelet function in washed platelets but not in whole blood. Br. J. Pharmacol. 2015, 172, 5199–5210. [Google Scholar] [CrossRef]
- Rukoyatkina, N.; Shpakova, V.; Panteleev, M.; Kharazova, A.; Gambaryan, S.; Geiger, J. Multifaceted effects of arachidonic acid and interaction with cyclic nucleotides in human platelets. Thromb. Res. 2018, 171, 22–30. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rukoyatkina, N.; Shpakova, V.; Sudnitsyna, J.; Panteleev, M.; Makhoul, S.; Gambaryan, S.; Jurk, K. Curcumin at Low Doses Potentiates and at High Doses Inhibits ABT-737-Induced Platelet Apoptosis. Int. J. Mol. Sci. 2021, 22, 5405. https://doi.org/10.3390/ijms22105405
Rukoyatkina N, Shpakova V, Sudnitsyna J, Panteleev M, Makhoul S, Gambaryan S, Jurk K. Curcumin at Low Doses Potentiates and at High Doses Inhibits ABT-737-Induced Platelet Apoptosis. International Journal of Molecular Sciences. 2021; 22(10):5405. https://doi.org/10.3390/ijms22105405
Chicago/Turabian StyleRukoyatkina, Natalia, Valentina Shpakova, Julia Sudnitsyna, Michael Panteleev, Stephanie Makhoul, Stepan Gambaryan, and Kerstin Jurk. 2021. "Curcumin at Low Doses Potentiates and at High Doses Inhibits ABT-737-Induced Platelet Apoptosis" International Journal of Molecular Sciences 22, no. 10: 5405. https://doi.org/10.3390/ijms22105405
APA StyleRukoyatkina, N., Shpakova, V., Sudnitsyna, J., Panteleev, M., Makhoul, S., Gambaryan, S., & Jurk, K. (2021). Curcumin at Low Doses Potentiates and at High Doses Inhibits ABT-737-Induced Platelet Apoptosis. International Journal of Molecular Sciences, 22(10), 5405. https://doi.org/10.3390/ijms22105405








