Antimicrobial Polymer−Based Assemblies: A Review
Abstract
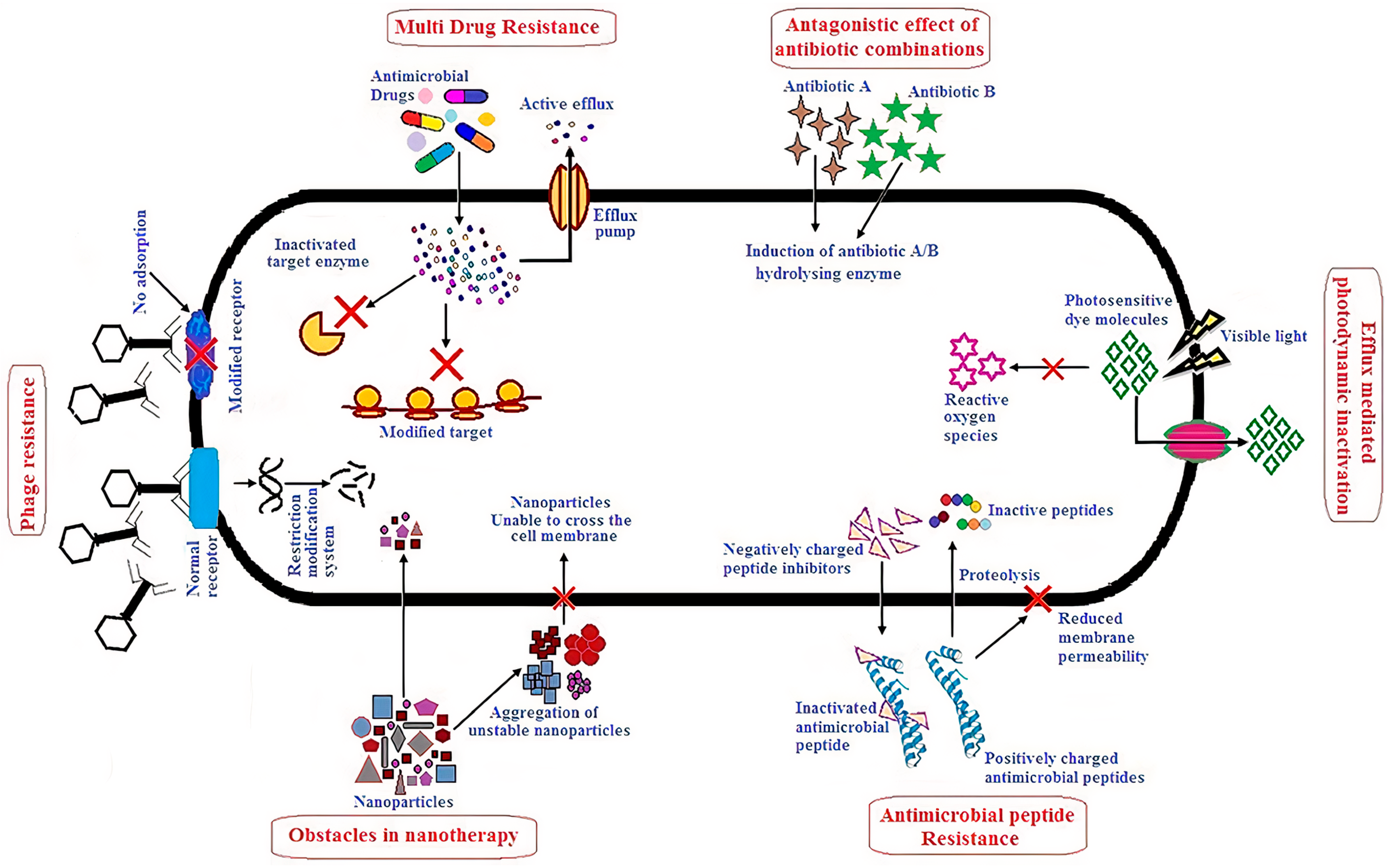
:1. Introduction
2. ASA with AMPs
2.1. Structure and Antimicrobial Activity of ASA with AMPs
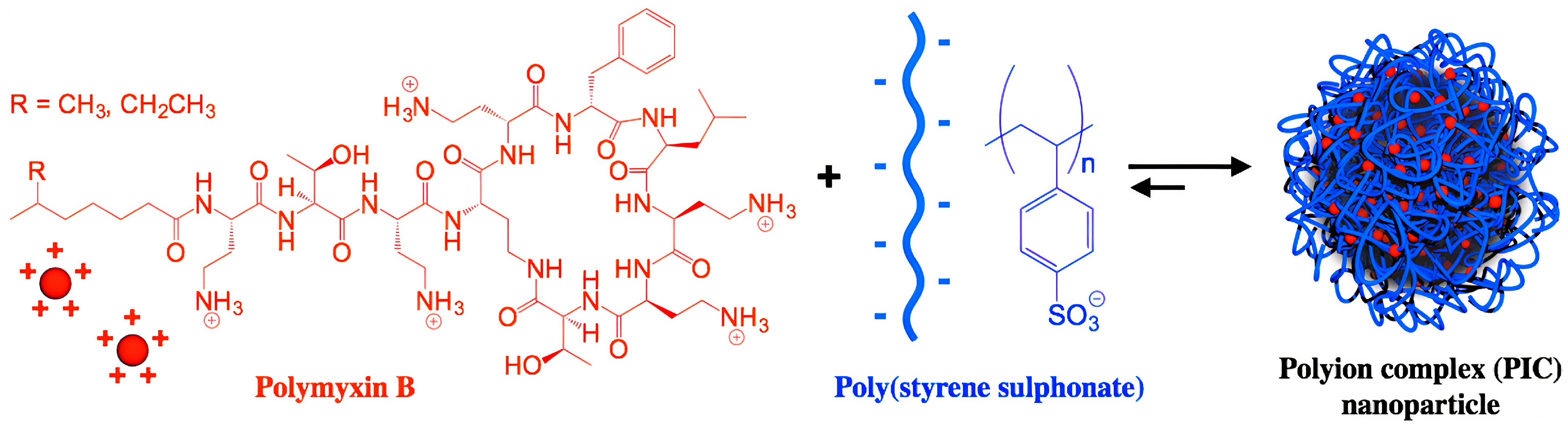
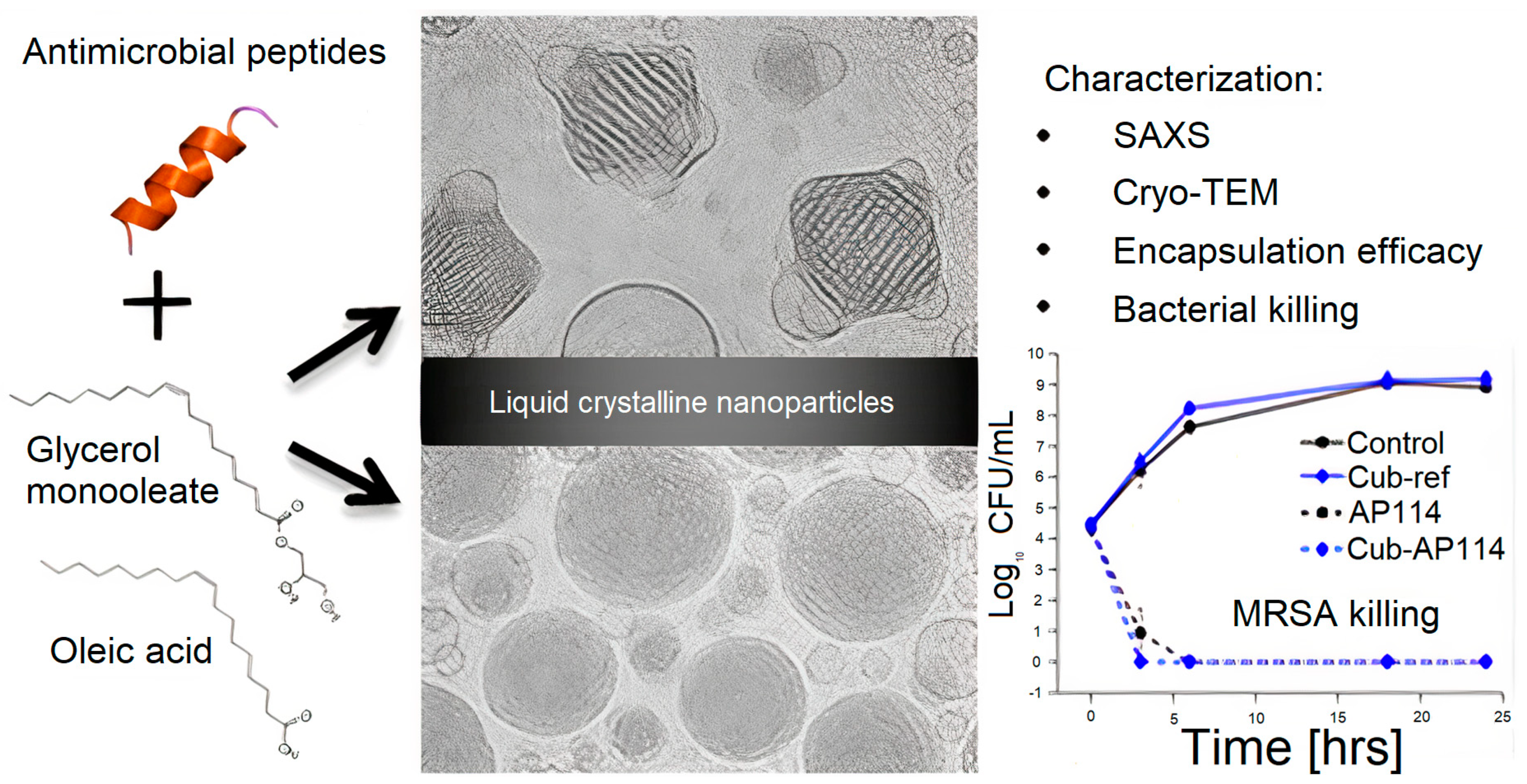
2.2. ASA with AMPs for Preserving Activity and Reducing Toxicity
3. ASAs with APs
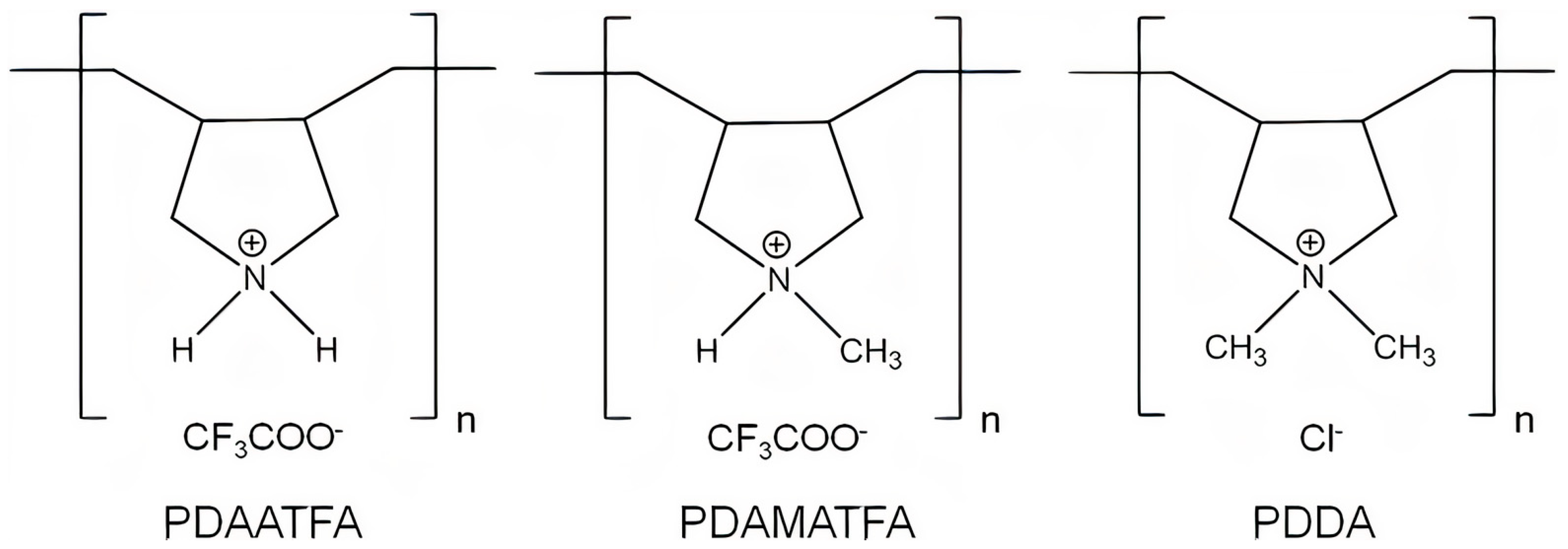
3.1. Structure and Antimicrobial Activity for ASA with APs
3.2. Biomedical Applications for ASA with APs
3.3. Other Applications for ASA with APs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| NP | Nanoparticle |
| MDR | Multidrug−resistant |
| ROS | Reactive oxygen species |
| AMP | Antimicrobial peptide |
| ASA | Antimicrobial supramolecular assembly |
| PEG | Polyethylene glycol |
| NRAMP | Nonribosomal synthetized peptide |
| RAMP | Ribosomal synthetized peptide |
| DNA | Deoxyribonucleic acid |
| Gr | Gramicidin D |
| LV | Large lipid vesicles |
| BF | Nanosized lipidic bilayer fragments |
| CD | Circular dichroism |
| DPPC | Dipalmitoylphosphatidylcholine |
| DODAB | Dioctadecyldimethylammonium bromide |
| PSS | Polystyrene sulfate |
| DX | Dextran sulfate |
| MRSA | Methicillin−resistant Staphylococcus aureus |
| IDSA | Infectious Diseases Society of America |
| LC | Liquid crystalline |
| AP | Antimicrobial polymer |
| MBC | Minimum bactericidal concentration |
| PEI | Polyethyleneimine |
| PLL | Poly−L−lysine |
| PDMAEMA | Poly [2−(N,N−dimethylamino)ethyl methacrylate] |
| PDDA | Poly (diallyldimethylammonium chloride) |
| PDAATFA | Poly (diallylammonium trifluoroacetate) |
| PDAMATFA | Poly (diallylmethylammonium trifluoroacetate) |
| PMMA | Poly (methyl methacrylate) |
| MMA | Methyl metacrylate |
| OEG | Oligo (ethylene glycol) |
| HPMC | Hydroxypropylmethylcellulose |
| QAC | Antibacterial quaternary compound |
| SNPES | Sulfonic amino poly (ether sulfone) |
| QC | Quaternized chitosan |
| PAA | Poly (acrylic acid) |
| PDA | Polydopamine |
| PDMS | Polydimethylsiloxane |
| PANI | Polyaniline |
| PDT | Photothermal therapy |
| PVPS | Poly (vinylpyrrolidone) sulfobetaine |
| PE | Polyethylene |
| CG | Chitosan/gelatin |
| SF−Mel | Melamine−modified silk fibroin |
| PCL | Polycaprolactone |
| TBAH−Alg | Tributylammonium alginate |
| CPU | Polyurethane surfaces |
| MAPG | Macroporous antimicrobial polymeric gel |
| HG | Hydrogel |
| QA | Quaternary ammonium |
| PMDETA | N,N,N′,N″,N″−pentamethyldiethylenetriamine |
| OEG−DMA | Oligoethylene glycol dimethacrylate |
| BP | Benzophenone |
| SARS−CoV−2 | Severe acute respiratory syndrome coronavirus 2 |
References
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging Strategies to Combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review. Front. Microbiol. 2019, 10, 593. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.M.; Roy, A.; Ghosh, A.K.; Hazra, T.K.; Basak, A.; Franco, O.L. Challenges and Future Prospects of Antibiotic Therapy: From Peptides to Phages Utilization. Front. Pharmacol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Kaur, I. Novel Strategies to Combat Antimicrobial Resistance. J. Infect. Dis. Ther. 2016, 4, 1–6. [Google Scholar] [CrossRef]
- León-Buitimea, A.; Garza-Cárdenas, C.R.; Garza-Cervantes, J.A.; Lerma-Escalera, J.A.; Morones-Ramírez, J.R. The Demand for New Antibiotics: Antimicrobial Peptides, Nanoparticles, and Combinatorial Therapies as Future Strategies in Antibacterial Agent Design. Front. Microbiol. 2020, 11, 1669. [Google Scholar] [CrossRef]
- Abedon, S.T.; Kuhl, S.J.; Blasdel, B.G.; Kutter, E.M. Phage Treatment of Human Infections. Bacteriophage 2011, 1, 66–85. [Google Scholar] [CrossRef] [Green Version]
- Parasuraman, P.; R. Y, T.; Shaji, C.; Sharan, A.; Bahkali, A.H.; Al-Harthi, H.F.; Syed, A.; Anju, V.T.; Dyavaiah, M.; Siddhardha, B. Biogenic Silver Nanoparticles Decorated with Methylene Blue Potentiated the Photodynamic Inactivation of Pseudomonas Aeruginosa and Staphylococcus Aureus. Pharmaceutics 2020, 12, 709. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Prasad, A.S.B.; Mehta, C.H.; Nayak, U.Y. Antimicrobial Peptide Polymers: No Escape to ESKAPE Pathogens—A Review. World J. Microbiol. Biotechnol. 2020, 36, 131. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Ribeiro, A.M.; de Melo Carrasco, L.D. Novel Formulations for Antimicrobial Peptides. Int. J. Mol. Sci. 2014, 15, 18040–18083. [Google Scholar] [CrossRef] [Green Version]
- Lei, J.; Sun, L.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q. The Antimicrobial Peptides and Their Potential Clinical Applications. Am. J. Transl. Res. 2019, 11, 3919–3931. [Google Scholar]
- Carmona-Ribeiro, A.M. Self−Assembled Antimicrobial Nanomaterials. Int. J. Environ. Res. Public. Health 2018, 15, 1408. [Google Scholar] [CrossRef] [Green Version]
- Mathiazzi, B.I.; Carmona-Ribeiro, A.M. Hybrid Nanoparticles of Poly (Methyl Methacrylate) and Antimicrobial Quaternary Ammonium Surfactants. Pharmaceutics 2020, 12, 340. [Google Scholar] [CrossRef] [PubMed]
- Vieira, D.B.; Carmona-Ribeiro, A.M. Synthetic Bilayer Fragments for Solubilization of Amphotericin B. J. Colloid Interface Sci. 2001, 244, 427–431. [Google Scholar] [CrossRef]
- Vieira, D.B.; Carmona-Ribeiro, A.M. Cationic Nanoparticles for Delivery of Amphotericin B: Preparation, Characterization and Activity in Vitro. J. Nanobiotechnol. 2008, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Melo, L.D.; Mamizuka, E.M.; Carmona-Ribeiro, A.M. Antimicrobial Particles from Cationic Lipid and Polyelectrolytes. Langmuir 2010, 26, 12300–12306. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.M.; de Melo Carrasco, L.D. Cationic Antimicrobial Polymers and Their Assemblies. Int. J. Mol. Sci. 2013, 14, 9906–9946. [Google Scholar] [CrossRef] [Green Version]
- de Melo Carrasco, L.D.; Sampaio, J.L.M.; Carmona-Ribeiro, A.M. Supramolecular Cationic Assemblies against Multidrug−Resistant Microorganisms: Activity and Mechanism of Action. Int. J. Mol. Sci. 2015, 16, 6337–6352. [Google Scholar] [CrossRef] [Green Version]
- Sanches, L.M.; Petri, D.F.S.; de Melo Carrasco, L.D.; Carmona-Ribeiro, A.M. The Antimicrobial Activity of Free and Immobilized Poly (Diallyldimethylammonium) Chloride in Nanoparticles of Poly (Methylmethacrylate). J. Nanobiotechnol. 2015, 13, 58. [Google Scholar] [CrossRef] [Green Version]
- Carrasco, L.D.D.M.; Bertolucci, R., Jr.; Ribeiro, R.T.; Sampaio, J.L.; Carmona-Ribeiro, A.M. Cationic Nanostructures against Foodborne Pathogens. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrasco, L.D.M.; Santos, H.C.A.S.; Sampaio, J.L.M.; Carmona-Ribeiro, A.M. Self−Assembled Antibiotic Nanoparticles Against Intracellular Bacteria. Drug Deliv. Lett. 2017, 7, 39–47. [Google Scholar]
- Carmona-Ribeiro, A.M. Biomimetic Nanomaterials from the Assembly of Polymers, Lipids, and Surfactants. In Surfactants and Detergents; Dutta, K., Ed.; IntechOpen: London, UK, 2019; ISBN 978-1-78984-661-4. [Google Scholar]
- Pereira, E.M.A.; Kosaka, P.M.; Rosa, H.; Vieira, D.B.; Kawano, Y.; Petri, D.F.S.; Carmona-Ribeiro, A.M. Hybrid Materials from Intermolecular Associations between Cationic Lipid and Polymers. J. Phys. Chem. B 2008, 112, 9301–9310. [Google Scholar] [CrossRef] [PubMed]
- Cloutier, M.; Mantovani, D.; Rosei, F. Antibacterial Coatings: Challenges, Perspectives, and Opportunities. Trends Biotechnol. 2015, 33, 637–652. [Google Scholar] [CrossRef]
- Galvão, C.N.; Sanches, L.M.; Mathiazzi, B.I.; Ribeiro, R.T.; Petri, D.F.S.; Carmona-Ribeiro, A.M. Antimicrobial Coatings from Hybrid Nanoparticles of Biocompatible and Antimicrobial Polymers. Int. J. Mol. Sci. 2018, 19, 2965. [Google Scholar] [CrossRef] [Green Version]
- Krywko-Cendrowska, A.; di Leone, S.; Bina, M.; Yorulmaz-Avsar, S.; Palivan, C.G.; Meier, W. Recent Advances in Hybrid Biomimetic Polymer−Based Films: From Assembly to Applications. Polymers 2020, 12, 1003. [Google Scholar] [CrossRef] [PubMed]
- Tiller, J.C.; Liao, C.-J.; Lewis, K.; Klibanov, A.M. Designing Surfaces That Kill Bacteria on Contact. Proc. Natl. Acad. Sci. USA 2001, 98, 5981–5985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmona-Ribeiro, A.M.; Barbassa, L.; Melo, L.D. Antimicrobial Biomimetics. In Biomimetic Based Applications; George, A., Ed.; IntechOpen: Rijeka, Croatia, 2011; Volume 1, pp. 227–284. ISBN 978-953-307-195-4. [Google Scholar]
- Carmona-Ribeiro, A.M.; de Moraes Lessa, M. Interactions between Bilayer Membranes and Latex. Colloids Surf. A Physicochem. Eng. Asp. 1999, 153, 355–361. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.M. Biomimetic Nanoparticles: Preparation, Characterization and Biomedical Applications. Int. J. Nanomed. 2010, 5, 249–259. [Google Scholar] [CrossRef] [Green Version]
- Chaudhuri, R.G.; Paria, S. Core/Shell Nanoparticles: Classes, Properties, Synthesis Mechanisms, Characterization, and Applications. Chem. Rev. 2011, 112, 2373–2433. [Google Scholar] [CrossRef] [PubMed]
- Sheikhpour, M.; Barani, L.; Kasaeian, A. Biomimetics in Drug Delivery Systems: A Critical Review. J. Control. Release 2017, 253, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.T.; Galvão, C.N.; Betancourt, Y.P.; Mathiazzi, B.I.; Carmona-Ribeiro, A.M. Microbicidal Dispersions and Coatings from Hybrid Nanoparticles of Poly (Methyl Methacrylate), Poly (Diallyl Dimethyl Ammonium) Chloride, Lipids, and Surfactants. Int. J. Mol. Sci. 2019, 20, 6150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmona-Ribeiro, A.M. Biomimetic Lipid Polymer Nanoparticles for Drug Delivery. In Nanoparticles in Biology and Medicine: Methods and Protocols; Ferrari, E., Soloviev, M., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2020; pp. 45–60. ISBN 978-1-07-160319-2. [Google Scholar]
- Vieira, D.B.; Carmona-Ribeiro, A.M. Cationic Lipids and Surfactants as Antifungal Agents: Mode of Action. J. Antimicrob. Chemother. 2006, 58, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Kondaveeti, S.; Bueno, P.V.D.A.; Carmona-Ribeiro, A.M.; Esposito, F.; Lincopan, N.; Sierakowski, M.R.; Petri, D.F.S. Microbicidal Gentamicin−Alginate Hydrogels. Carbohydr. Polym. 2018, 186, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Kondaveeti, S.; Damato, T.C.; Carmona-Ribeiro, A.M.; Sierakowski, M.R.; Petri, D.F.S. Sustainable Hydroxypropyl Methylcellulose/Xyloglucan/Gentamicin Films with Antimicrobial Properties. Carbohydr. Polym. 2017, 165, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Han, Q.; Chen, B.; Zheng, Y.; Zhang, K.; Li, Q.; Wang, J. Antimicrobial Hydrogels: Promising Materials for Medical Application. Int. J. Nanomed. 2018, 13, 2217–2263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghobril, C.; Grinstaff, M.W. The Chemistry and Engineering of Polymeric Hydrogel Adhesives for Wound Closure: A Tutorial. Chem. Soc. Rev. 2015, 44, 1820–1835. [Google Scholar] [CrossRef]
- Makvandi, P.; Gu, J.T.; Zare, E.N.; Ashtari, B.; Moeini, A.; Tay, F.R.; Niu, L. Polymeric and Inorganic Nanoscopical Antimicrobial Fillers in Dentistry. Acta Biomater. 2020, 101, 69–101. [Google Scholar] [CrossRef] [PubMed]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. A Review of the Biomaterials Technologies for Infection−Resistant Surfaces. Biomaterials 2013, 34, 8533–8554. [Google Scholar] [CrossRef]
- Tapias, G.N.; Sicchierolli, S.M.; Mamizuka, E.M.; Carmona-Ribeiro, A.M. Interactions between Cationic Vesicles and Escherichia Coli. Langmuir 1994, 10, 3461–3465. [Google Scholar] [CrossRef]
- Sicchierolli, S.M.; Mamizuka, E.M.; Carmona-Ribeiro, A.M. Bacteria Flocculation and Death by Cationic Vesicles. Langmuir 1995, 11, 2991–2995. [Google Scholar] [CrossRef]
- Martins, L.M.S.; Mamizuka, E.M.; Carmona-Ribeiro, A.M. Cationic Vesicles as Bactericides. Langmuir 1997, 13, 5583–5587. [Google Scholar] [CrossRef]
- Campanhã, M.T.N.; Mamizuka, E.M.; Carmona-Ribeiro, A.M. Interactions between Cationic Liposomes and Bacteria: The Physical−Chemistry of the Bactericidal Action. J. Lipid Res. 1999, 40, 1495–1500. [Google Scholar] [CrossRef]
- Mamizuka, E.M.; Carmona-Ribeiro, A.M. Cationic Liposomes as Antimicrobial Agents. In Communicating Current Research and Educational Topics and Trends in Applied Microbiology; Méndez Vila, A., Ed.; Formatex: Badajoz, Spain, 2007; Volume 2, pp. 636–647. ISBN 13: 978-84-611-9423-0. [Google Scholar]
- Maan, A.M.C.; Hofman, A.H.; de Vos, W.M.; Kamperman, M. Recent Developments and Practical Feasibility of Polymer−Based Antifouling Coatings. Adv. Funct. Mater. 2020, 30, 2000936. [Google Scholar] [CrossRef]
- Dunne, W.M. Bacterial Adhesion: Seen Any Good Biofilms Lately? Clin. Microbiol. Rev. 2002, 15, 155–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, J.-B.D.; Fulghum, T.; Nordhaus, M.A. A Review of Immobilized Antimicrobial Agents and Methods for Testing. Biointerphases 2011, 6, MR13–MR28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamaruzzaman, N.F.; Tan, L.P.; Hamdan, R.H.; Choong, S.S.; Wong, W.K.; Gibson, A.J.; Chivu, A.; Pina, M.D.F. Antimicrobial Polymers: The Potential Replacement of Existing Antibiotics? Int. J. Mol. Sci. 2019, 20, 2747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckhard, L.H.; Houri-Haddad, Y.; Sol, A.; Zeharia, R.; Shai, Y.; Beyth, S.; Domb, A.J.; Bachrach, G.; Beyth, N. Sustained Release of Antibacterial Lipopeptides from Biodegradable Polymers against Oral Pathogens. PLoS ONE 2016, 11, e0162537. [Google Scholar] [CrossRef] [PubMed]
- Shabir, U.; Ali, S.; Magray, A.R.; Ganai, B.A.; Firdous, P.; Hassan, T.; Nazir, R. Fish Antimicrobial Peptides (AMP’s) as Essential and Promising Molecular Therapeutic Agents: A Review. Microb. Pathog. 2018, 114, 50–56. [Google Scholar] [CrossRef]
- Teixeira, M.C.; Carbone, C.; Sousa, M.C.; Espina, M.; Garcia, M.L.; Sanchez-Lopez, E.; Souto, E.B. Nanomedicines for the Delivery of Antimicrobial Peptides (AMPs). Nanomaterials 2020, 10, 560. [Google Scholar] [CrossRef] [Green Version]
- Rai, M.; Pandit, R.; Gaikwad, S.; Kövics, G. Antimicrobial Peptides as Natural Bio−Preservative to Enhance the Shelf−Life of Food. J. Food Sci. Technol. 2016, 53, 3381–3394. [Google Scholar] [CrossRef] [Green Version]
- Biswas, G.R.; Majee, S.B. Niosomes in Ocular Drug Delivery. Eur. J. Pharm. Med. Res. 2017, 4, 813–819. [Google Scholar]
- Faya, M.; Kalhapure, R.S.; Kumalo, H.M.; Waddad, A.Y.; Omolo, C.; Govender, T. Conjugates and Nano−Delivery of Antimicrobial Peptides for Enhancing Therapeutic Activity. J. Drug Deliv. Sci. Technol. 2018, 44, 153–171. [Google Scholar] [CrossRef]
- Memariani, H.; Memariani, M.; Shahidi-Dadras, M.; Nasiri, S.; Akhavan, M.M.; Moravvej, H. Melittin: From Honeybees to Superbugs. Appl. Microbiol. Biotechnol. 2019, 103, 3265–3276. [Google Scholar] [CrossRef] [PubMed]
- Wang, G. Improved Methods for Classification, Prediction and Design of Antimicrobial Peptides. Methods Mol. Biol. 2015, 1268, 43–66. [Google Scholar] [CrossRef]
- Epand, R.M.; Vogel, H.J. Diversity of Antimicrobial Peptides and Their Mechanisms of Action. Biochim. Biophys. Acta BBA Biomembr. 1999, 1462, 11–28. [Google Scholar] [CrossRef] [Green Version]
- Koehbach, J.; Craik, D.J. The Vast Structural Diversity of Antimicrobial Peptides. Trends Pharmacol. Sci. 2019, 40, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Patočka, J.; Kuča, K. Insect Antimicrobial Peptides, a Mini Review. Toxins 2018, 10, 461. [Google Scholar] [CrossRef] [PubMed]
- Raheem, N.; Straus, S.K. Mechanisms of Action for Antimicrobial Peptides With Antibacterial and Antibiofilm Functions. Front. Microbiol. 2019, 10, 2866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nordström, R. Polymeric Nanoparticles as Carriers for Antimicrobial Peptides: Factors Affecting Peptide and Membrane Interactions. Ph.D. Thesis, Uppsala University, Upsala, Sweden, 2019. [Google Scholar]
- Kumar, P.; Kizhakkedathu, J.N.; Straus, S.K. Antimicrobial Peptides: Diversity, Mechanism of Action and Strategies to Improve the Activity and Biocompatibility In Vivo. Biomolecules 2018, 8, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Koh, J.-J.; Liu, S.; Lakshminarayanan, R.; Verma, C.S.; Beuerman, R.W. Membrane Active Antimicrobial Peptides: Translating Mechanistic Insights to Design. Front. Neurosci. 2017, 11, 73. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, C.A.; Olivares-Ortega, C.; Soto-Arriaza, M.A.; Carmona-Ribeiro, A.M. Interaction of Gramicidin with DPPC/DODAB Bilayer Fragments. Biochim. Biophys. Acta BBA Biomembr. 2012, 1818, 3064–3071. [Google Scholar] [CrossRef]
- Akaike, N. Chapter 8—Gramicidin Perforated Patch. In Physiology and Pathology of Chloride Transporters and Channels in the Nervous System; Alvarez-Leefmans, F.J., Delpire, E., Eds.; Academic Press: San Diego, CA, USA, 2010; pp. 141–147. ISBN 978-0-12-374373-2. [Google Scholar]
- Mishra, B.; Reiling, S.; Zarena, D.; Wang, G. Host Defense Antimicrobial Peptides as Antibiotics: Design and Application Strategies. Curr. Opin. Chem. Biol. 2017, 38, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Ragioto, D.A.; Carrasco, L.D.; Carmona-Ribeiro, A.M. Novel Gramicidin Formulations in Cationic Lipid as Broad−Spectrum Microbicidal Agents. Int. J. Nanomed. 2014, 9, 3183–3192. [Google Scholar] [CrossRef] [Green Version]
- Xavier, G.R.S.; Carmona-Ribeiro, A.M. Cationic Biomimetic Particles of Polystyrene/Cationic Bilayer/Gramicidin for Optimal Bactericidal Activity. Nanomaterials 2017, 7, 422. [Google Scholar] [CrossRef] [Green Version]
- Riool, M.; de Breij, A.; de Boer, L.; Kwakman, P.H.S.; Cordfunke, R.A.; Cohen, O.; Malanovic, N.; Emanuel, N.; Lohner, K.; Drijfhout, J.W.; et al. Controlled Release of LL−37−Derived Synthetic Antimicrobial and Anti−Biofilm Peptides SAAP−145 and SAAP−276 Prevents Experimental Biomaterial−Associated Staphylococcus Aureus Infection. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef]
- Patrick, J.W.; Gamez, R.C.; Russell, D.H. The Influence of Lipid Bilayer Physicochemical Properties on Gramicidin A Conformer Preferences. Biophys. J. 2016, 110, 1826–1835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fael, H.; Demirel, A.L. Nisin/Polyanion Layer−by−Layer Films Exhibiting Different Mechanisms in Antimicrobial Efficacy. RSC Adv. 2020, 10, 10329–10337. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Choi, H.; Weisshaar, J.C. Melittin−Induced Permeabilization, Re−Sealing, and Re−Permeabilization of E. Coli Membranes. Biophys. J. 2018, 114, 368–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamasbi, E.; Mularski, A.; Separovic, F. Model Membrane and Cell Studies of Antimicrobial Activity of Melittin Analogues. Curr. Top. Med. Chem. 2016, 16, 40–45. [Google Scholar] [CrossRef]
- Jo, M.; Park, M.H.; Kollipara, P.S.; An, B.J.; Song, H.S.; Han, S.B.; Kim, J.H.; Song, M.J.; Hong, J.T. Anti−Cancer Effect of Bee Venom Toxin and Melittin in Ovarian Cancer Cells through Induction of Death Receptors and Inhibition of JAK2/STAT3 Pathway. Toxicol. Appl. Pharmacol. 2012, 258, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.-J.; Choi, Y.; Shin, J.-M.; Cho, H.-J.; Kang, J.-H.; Park, K.-K.; Choe, J.-Y.; Bae, Y.-S.; Han, S.-M.; Kim, C.-H.; et al. Melittin Suppresses EGF−Induced Cell Motility and Invasion by Inhibiting PI3K/Akt/MTOR Signaling Pathway in Breast Cancer Cells. Food Chem. Toxicol. 2014, 68, 218–225. [Google Scholar] [CrossRef]
- Choi, K.E.; Hwang, C.J.; Gu, S.M.; Park, M.H.; Kim, J.H.; Park, J.H.; Ahn, Y.J.; Kim, J.Y.; Song, M.J.; Song, H.S.; et al. Cancer Cell Growth Inhibitory Effect of Bee Venom via Increase of Death Receptor 3 Expression and Inactivation of NF−Kappa B in NSCLC Cells. Toxins 2014, 6, 2210–2228. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.-F.; Jie, M.-M.; Li, B.-S.; Hu, C.-J.; Xie, R.; Tang, B.; Yang, S.-M. Peptide−Based Treatment: A Promising Cancer Therapy. Available online: https://www.hindawi.com/journals/jir/2015/761820/ (accessed on 7 December 2020).
- Rady, I.; Siddiqui, I.A.; Rady, M.; Mukhtar, H. Melittin, a Major Peptide Component of Bee Venom, and Its Conjugates in Cancer Therapy. Cancer Lett. 2017, 402, 16–31. [Google Scholar] [CrossRef] [Green Version]
- Mahadevappa, R.; Ma, R.; Kwok, H.F. Venom Peptides: Improving Specificity in Cancer Therapy. Trends Cancer 2017, 3, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Światły-Błaszkiewicz, A.; Mrówczyńska, L.; Matuszewska, E.; Lubawy, J.; Urbański, A.; Kokot, Z.J.; Rosiński, G.; Matysiak, J. The Effect of Bee Venom Peptides Melittin, Tertiapin, and Apamin on the Human Erythrocytes Ghosts: A Preliminary Study. Metabolites 2020, 10, 191. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Liu, J.; Gou, L.; Li, J.; Yuan, B.; Yang, K.; Ma, Y. Designing Melittin−Graphene Hybrid Complexes for Enhanced Antibacterial Activity. Adv. Healthc. Mater. 2019, 8, 1801521. [Google Scholar] [CrossRef] [PubMed]
- Pletzer, D.; Mansour, S.C.; Hancock, R.E.W. Synergy between Conventional Antibiotics and Anti−Biofilm Peptides in a Murine, Sub−Cutaneous Abscess Model Caused by Recalcitrant ESKAPE Pathogens. PLoS Pathog. 2018, 14, e1007084. [Google Scholar] [CrossRef] [PubMed]
- Magana, M.; Sereti, C.; Ioannidis, A.; Mitchell, C.A.; Ball, A.R.; Magiorkinis, E.; Chatzipanagiotou, S.; Hamblin, M.R.; Hadjifrangiskou, M.; Tegos, G.P. Options and Limitations in Clinical Investigation of Bacterial Biofilms. Clin. Microbiol. Rev. 2018, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talapko, J.; Škrlec, I. The Principles, Mechanisms, and Benefits of Unconventional Agents in the Treatment of Biofilm Infection. Pharmaceuticals 2020, 13, 299. [Google Scholar] [CrossRef]
- Barreto-Santamaría, A.; Patarroyo, M.E.; Curtidor, H. Designing and Optimizing New Antimicrobial Peptides: All Targets Are Not the Same. Crit. Rev. Clin. Lab. Sci. 2019, 56, 351–373. [Google Scholar] [CrossRef] [PubMed]
- Kiani, M.H.; Imran, M.; Raza, A.; Shahnaz, G. Multi−Functionalized Nanocarriers Targeting Bacterial Reservoirs to Overcome Challenges of Multi Drug−Resistance. DARU J. Pharm. Sci. 2020, 28, 319–332. [Google Scholar] [CrossRef]
- Yeom, J.-H.; Lee, B.; Kim, D.; Lee, J.; Kim, S.; Bae, J.; Park, Y.; Lee, K. Gold Nanoparticle−DNA Aptamer Conjugate−Assisted Delivery of Antimicrobial Peptide Effectively Eliminates Intracellular Salmonella Enterica Serovar Typhimurium. Biomaterials 2016, 104, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Dubashynskaya, N.V.; Skorik, Y.A. Polymyxin Delivery Systems: Recent Advances and Challenges. Pharmaceuticals 2020, 13, 83. [Google Scholar] [CrossRef] [PubMed]
- Insua, I.; Zizmare, L.; Peacock, A.F.A.; Krachler, A.M.; Fernandez-Trillo, F. Polymyxin B Containing Polyion Complex (PIC) Nanoparticles: Improving the Antimicrobial Activity by Tailoring the Degree of Polymerisation of the Inert Component. Sci. Rep. 2017, 7, 9396. [Google Scholar] [CrossRef]
- Insua, I.; Majok, S.; Peacock, A.F.A.; Krachler, A.M.; Fernandez-Trillo, F. Preparation and Antimicrobial Evaluation of Polyion Complex (PIC) Nanoparticles Loaded with Polymyxin B. Eur. Polym. J. 2017, 87, 478–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGuinness, W.A.; Malachowa, N.; DeLeo, F.R. Vancomycin Resistance in Staphylococcus Aureus. Yale J. Biol. Med. 2017, 90, 269–281. [Google Scholar] [PubMed]
- Claeys, K.C.; Lagnf, A.M.; Hallesy, J.A.; Compton, M.T.; Gravelin, A.L.; Davis, S.L.; Rybak, M.J. Pneumonia Caused by Methicillin−Resistant Staphylococcus Aureus: Does Vancomycin Heteroresistance Matter? Antimicrob. Agents Chemother. 2016, 60, 1708–1716. [Google Scholar] [CrossRef] [Green Version]
- Hassan, D.; Omolo, C.A.; Gannimani, R.; Waddad, A.Y.; Mocktar, C.; Rambharose, S.; Agrawal, N.; Govender, T. Delivery of Novel Vancomycin Nanoplexes for Combating Methicillin Resistant Staphylococcus Aureus (MRSA) Infections. Int. J. Pharm. 2019, 558, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Hajiahmadi, F.; Alikhani, M.Y.; Shariatifar, H.; Arabestani, M.R.; Ahmadvand, D. The Bactericidal Effect of Liposomal Vancomycin as a Topical Combating System against Methicillin−Resistant Staphylococcus Aureus Skin Wound Infection in Mice. Med. J. Islam. Repub. Iran 2019, 33, 941–947. [Google Scholar] [CrossRef]
- Ciociola, T.; Giovati, L.; Conti, S.; Magliani, W.; Santinoli, C.; Polonelli, L. Natural and Synthetic Peptides with Antifungal Activity. Future Med. Chem. 2016, 8, 1413–1433. [Google Scholar] [CrossRef]
- Huang, K.-S.; Yang, C.-H.; Huang, S.-L.; Chen, C.-Y.; Lu, Y.-Y.; Lin, Y.-S. Recent Advances in Antimicrobial Polymers: A Mini−Review. Int. J. Mol. Sci. 2016, 17, 1578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magana, M.; Pushpanathan, M.; Santos, A.L.; Leanse, L.; Fernandez, M.; Ioannidis, A.; Giulianotti, M.A.; Apidianakis, Y.; Bradfute, S.; Ferguson, A.L.; et al. The Value of Antimicrobial Peptides in the Age of Resistance. Lancet Infect. Dis. 2020, 20, e216–e230. [Google Scholar] [CrossRef]
- Gajdács, M. The Concept of an Ideal Antibiotic: Implications for Drug Design. Molecules 2019, 24, 892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rafferty, J.; Nagaraj, H.; P. McCloskey, A.; Huwaitat, R.; Porter, S.; Albadr, A.; Laverty, G. Peptide Therapeutics and the Pharmaceutical Industry: Barriers Encountered Translating from the Laboratory to Patients. Curr. Med. Chem. 2016, 23, 4231–4259. [Google Scholar] [CrossRef] [PubMed]
- Vukomanović, M.; Žunič, V.; Kunej, Š.; Jančar, B.; Jeverica, S.; Podlipec, R.; Suvorov, D. Nano−Engineering the Antimicrobial Spectrum of Lantibiotics: Activity of Nisin against Gram Negative Bacteria. Sci. Rep. 2017, 7, 4324. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.; McClements, D.J.; Udenigwe, C.C. Encapsulation of Bioactive Whey Peptides in Soy Lecithin−Derived Nanoliposomes: Influence of Peptide Molecular Weight. Food Chem. 2016, 213, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Mai, S.; Mauger, M.T.; Niu, L.; Barnes, J.B.; Kao, S.; Bergeron, B.E.; Ling, J.; Tay, F.R. Potential Applications of Antimicrobial Peptides and Their Mimics in Combating Caries and Pulpal Infections. Acta Biomater. 2017, 49, 16–35. [Google Scholar] [CrossRef] [PubMed]
- Izadi, N.; Keikha, M.; Ghazvini, K.; Karbalaei, M. Oral Antimicrobial Peptides and New Therapeutic Strategies for Plaque−Mediated Diseases. Gene Rep. 2020, 21, 100811. [Google Scholar] [CrossRef]
- Lewies, A.; Wentzel, J.F.; Jordaan, A.; Bezuidenhout, C.; Du Plessis, L.H. Interactions of the Antimicrobial Peptide Nisin Z with Conventional Antibiotics and the Use of Nanostructured Lipid Carriers to Enhance Antimicrobial Activity. Int. J. Pharm. 2017, 526, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Boge, L.; Bysell, H.; Ringstad, L.; Wennman, D.; Umerska, A.; Cassisa, V.; Eriksson, J.; Joly-Guillou, M.-L.; Edwards, K.; Andersson, M. Lipid−Based Liquid Crystals As Carriers for Antimicrobial Peptides: Phase Behavior and Antimicrobial Effect. Langmuir 2016, 32, 4217–4228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piras, A.M.; Maisetta, G.; Sandreschi, S.; Gazzarri, M.; Bartoli, C.; Grassi, L.; Esin, S.; Chiellini, F.; Batoni, G. Chitosan Nanoparticles Loaded with the Antimicrobial Peptide Temporin B Exert a Long−Term Antibacterial Activity in Vitro against Clinical Isolates of Staphylococcus Epidermidis. Front. Microbiol. 2015, 6, 372. [Google Scholar] [CrossRef] [Green Version]
- Niemirowicz, K.; Surel, U.; Wilczewska, A.Z.; Mystkowska, J.; Piktel, E.; Gu, X.; Namiot, Z.; Kułakowska, A.; Savage, P.B.; Bucki, R. Bactericidal Activity and Biocompatibility of Ceragenin−Coated Magnetic Nanoparticles. J. Nanobiotechnol. 2015, 13, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bueno, P.V.A.; Hilamatu, K.C.P.; Carmona-Ribeiro, A.M.; Petri, D.F.S. Magnetically Triggered Release of Amoxicillin from Xanthan/Fe3O4/Albumin Patches. Int. J. Biol. Macromol. 2018, 115, 792–800. [Google Scholar] [CrossRef]
- Agbale, C.M.; Cardoso, M.H.; Galyuon, I.K.; Franco, O.L. Designing Metallodrugs with Nuclease and Protease Activity. Metallomics 2016, 8, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Ahire, J.J.; Neveling, D.P.; Dicks, L.M.T. Co−Spinning of Silver Nanoparticles with Nisin Increases the Antimicrobial Spectrum of PDLLA: PEO Nanofibers. Curr. Microbiol. 2015, 71, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Kong, E.F.; Tsui, C.; Boyce, H.; Ibrahim, A.; Hoag, S.W.; Karlsson, A.J.; Meiller, T.F.; Jabra-Rizk, M.A. Development and In Vivo Evaluation of a Novel Histatin−5 Bioadhesive Hydrogel Formulation against Oral Candidiasis. Antimicrob. Agents Chemother. 2016, 60, 881–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakia, M.; Koo, J.M.; Kim, D.; Ji, K.; Huh, P.; Yoon, J.; Yoo, S.I. Development of Silver Nanoparticle−Based Hydrogel Composites for Antimicrobial Activity. Green Chem. Lett. Rev. 2020, 13, 34–40. [Google Scholar] [CrossRef] [Green Version]
- Saleem, H.; Zaidi, S.J. Sustainable Use of Nanomaterials in Textiles and Their Environmental Impact. Materials 2020, 13, 5134. [Google Scholar] [CrossRef]
- Deshmukh, S.P.; Patil, S.M.; Mullani, S.B.; Delekar, S.D. Silver Nanoparticles as an Effective Disinfectant: A Review. Mater. Sci. Eng. C 2019, 97, 954–965. [Google Scholar] [CrossRef]
- Attia, N.F.; Morsy, M.S. Facile Synthesis of Novel Nanocomposite as Antibacterial and Flame Retardant Material for Textile Fabrics. Mater. Chem. Phys. 2016, 180, 364–372. [Google Scholar] [CrossRef]
- Faya, M.; Hazzah, H.A.; Omolo, C.A.; Agrawal, N.; Maji, R.; Walvekar, P.; Mocktar, C.; Nkambule, B.; Rambharose, S.; Albericio, F.; et al. Novel Formulation of Antimicrobial Peptides Enhances Antimicrobial Activity against Methicillin−Resistant Staphylococcus Aureus (MRSA). Amino Acids 2020, 52, 1439–1457. [Google Scholar] [CrossRef]
- Yu, Z.; Cowan, J.A. Metal Complexes Promoting Catalytic Cleavage of Nucleic Acids—Biochemical Tools and Therapeutics. Curr. Opin. Chem. Biol. 2018, 43, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Di Natale, C.; De Benedictis, I.; De Benedictis, A.; Marasco, D. Metal–Peptide Complexes as Promising Antibiotics to Fight Emerging Drug Resistance: New Perspectives in Tuberculosis. Antibiotics 2020, 9, 337. [Google Scholar] [CrossRef]
- Agbale, C.M.; Sarfo, J.K.; Galyuon, I.K.; Juliano, S.A.; Silva, G.G.O.; Buccini, D.F.; Cardoso, M.H.; Torres, M.D.T.; Angeles-Boza, A.M.; de la Fuente-Nunez, C.; et al. Antimicrobial and Antibiofilm Activities of Helical Antimicrobial Peptide Sequences Incorporating Metal−Binding Motifs. Biochemistry 2019, 58, 3802–3812. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Lu, M.; Yang, T.; Liu, H.; Sun, Y.; Zhao, X.; Xu, H.; Yang, L.; Ding, P. Structure−Function Relationships of Nonviral Gene Vectors: Lessons from Antimicrobial Polymers. Acta Biomater. 2019, 86, 15–40. [Google Scholar] [CrossRef] [PubMed]
- Kyzioł, A.; Khan, W.; Sebastian, V.; Kyzioł, K. Tackling Microbial Infections and Increasing Resistance Involving Formulations Based on Antimicrobial Polymers. Chem. Eng. J. 2020, 385, 123888. [Google Scholar] [CrossRef]
- Alfei, S.; Schito, A.M. Positively Charged Polymers as Promising Devices against Multidrug Resistant Gram−Negative Bacteria: A Review. Polymers 2020, 12, 1195. [Google Scholar] [CrossRef] [PubMed]
- Michl, T.D.; Hibbs, B.; Hyde, L.; Postma, A.; Tran, D.T.T.; Zhalgasbaikyzy, A.; Vasilev, K.; Meagher, L.; Griesser, H.J.; Locock, K.E.S. Bacterial Membrane Permeability of Antimicrobial Polymethacrylates: Evidence for a Complex Mechanism from Super−Resolution Fluorescence Imaging. Acta Biomater. 2020, 108, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Feng, J.; Li, S.; Sun, T.; Shi, Q.; Xie, X. Synthesis, Characterization, and Antimicrobial Evaluation of Random Poly(Ester−Carbonate)s Bearing Pendant Primary Amine in the Main Chain. Polymers 2020, 12, 2640. [Google Scholar] [CrossRef] [PubMed]
- Kamenieva, T.; Tarasyuk, O.; Derevianko, K.; Aksenovska, O.; Shybyryn, O.; Metelytsia, L.; Rogalsky, S. Antioxidant Activity of Polymeric Biocide Polyhexamethylene Guanidine Hydrochloride. Catal. Petrochem. 2020, 30, 73–82. [Google Scholar] [CrossRef]
- Pérez-Betancourt, Y.; Távora, B.D.C.L.F.; Colombini, M.; Faquim-Mauro, E.L.; Carmona-Ribeiro, A.M. Simple Nanoparticles from the Assembly of Cationic Polymer and Antigen as Immunoadjuvants. Vaccines 2020, 8, 105. [Google Scholar] [CrossRef] [Green Version]
- Carmona-Ribeiro, A.M.; Pérez-Betancourt, Y. Cationic Nanostructures for Vaccines Design. Biomimetics 2020, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Jung, K.J.; Yoon, S.; Lee, K.; Kim, B. Polyhexamethyleneguanidine Phosphate Induces Cytotoxicity through Disruption of Membrane Integrity. Toxicology 2019, 414, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Betancourt, Y.; Távora, B.D.C.L.F.; Faquim-Mauro, E.L.; Carmona-Ribeiro, A.M. Biocompatible Lipid Polymer Cationic Nanoparticles for Antigen Presentation. Polymers 2021, 13, 185. [Google Scholar] [CrossRef] [PubMed]
- Samal, S.K.; Dash, M.; Van Vlierberghe, S.; Kaplan, D.L.; Chiellini, E.; van Blitterswijk, C.; Moroni, L.; Dubruel, P. Cationic Polymers and Their Therapeutic Potential. Chem. Soc. Rev. 2012, 41, 7147–7194. [Google Scholar] [CrossRef] [PubMed]
- Nazir, F.; Iqbal, M. Synthesis, Characterization and Cytotoxicity Studies of Aminated Microcrystalline Cellulose Derivatives against Melanoma and Breast Cancer Cell Lines. Polymers 2020, 12, 2634. [Google Scholar] [CrossRef]
- Ergene, C.; Yasuhara, K.; Palermo, E.F. Biomimetic Antimicrobial Polymers: Recent Advances in Molecular Design. Polym. Chem. 2018, 9, 2407–2427. [Google Scholar] [CrossRef] [Green Version]
- Timofeeva, L.; Kleshcheva, N. Antimicrobial Polymers: Mechanism of Action, Factors of Activity, and Applications. Appl. Microbiol. Biotechnol. 2011, 89, 475–492. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, S.; Ahmad, N.M.; Mahmood, A.; Iqbal, M. Antibacterial Amphiphilic Copolymers of Dimethylamino Ethyl Methacrylate and Methyl Methacrylate to Control Biofilm Adhesion for Antifouling Applications. Polymers 2021, 13, 216. [Google Scholar] [CrossRef] [PubMed]
- Jelkmann, M.; Menzel, C.; Baus, R.A.; Ausserhofer, P.; Baecker, D.; Gust, R.; Bernkop-Schnürch, A. Chitosan: The One and Only? Aminated Cellulose as an Innovative Option for Primary Amino Groups Containing Polymers. Biomacromolecules 2018, 19, 4059–4067. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.F.; Facchi, S.P.; Follmann, H.D.M.; Pereira, A.G.B.; Rubira, A.F.; Muniz, E.C. Antimicrobial Activity of Chitosan Derivatives Containing N−Quaternized Moieties in Its Backbone: A Review. Int. J. Mol. Sci. 2014, 15, 20800–20832. [Google Scholar] [CrossRef]
- Yang, Y.; Cai, Z.; Huang, Z.; Tang, X.; Zhang, X. Antimicrobial Cationic Polymers: From Structural Design to Functional Control. Polym. J. 2018, 50, 33–44. [Google Scholar] [CrossRef]
- Timofeeva, L.M.; Kleshcheva, N.A.; Moroz, A.F.; Didenko, L.V. Secondary and Tertiary Polydiallylammonium Salts: Novel Polymers with High Antimicrobial Activity. Biomacromolecules 2009, 10, 2976–2986. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, A.; Ikeda, T.; Endo, T. Antibacterial Activity of Polymeric Sulfonium Salts. J. Polym. Sci. Part A Polym. Chem. 1993, 31, 2873–2876. [Google Scholar] [CrossRef]
- Zhang, B.; Li, M.; Lin, M.; Yang, X.; Sun, J. A Convenient Approach for Antibacterial Polypeptoids Featuring Sulfonium and Oligo(Ethylene Glycol) Subunits. Biomater. Sci. 2020, 8, 6969–6977. [Google Scholar] [CrossRef] [PubMed]
- Garcia, I.M.; Rodrigues, S.B.; Leitune, V.C.B.; Collares, F.M.; Garcia, I.M.; Rodrigues, S.B.; Leitune, V.C.B.; Collares, F.M. Antibacterial, Chemical and Physical Properties of Sealants with Polyhexamethylene Guanidine Hydrochloride. Braz. Oral Res. 2019, 33. [Google Scholar] [CrossRef]
- Cowan, M.G.; Masuda, M.; McDanel, W.M.; Kohno, Y.; Gin, D.L.; Noble, R.D. Phosphonium−Based Poly(Ionic Liquid) Membranes: The Effect of Cation Alkyl Chain Length on Light Gas Separation Properties and Ionic Conductivity. J. Membr. Sci. 2016, 498, 408–413. [Google Scholar] [CrossRef]
- Kanazawa, A.; Ikeda, T.; Endo, T. Polymeric Phosphonium Salts as a Novel Class of Cationic Biocides. IV. Synthesis and Antibacterial Activity of Polymers with Phosphonium Salts in the Main Chain. J. Polym. Sci. Part Polym. Chem. 1993, 31, 3031–3038. [Google Scholar] [CrossRef]
- Kim, S.-H.; Semenya, D.; Castagnolo, D. Antimicrobial Drugs Bearing Guanidine Moieties: A Review. Eur. J. Med. Chem. 2021, 216, 113293. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Xiao, H.; Zhang, Y. Antimicrobial Polymeric Materials with Quaternary Ammonium and Phosphonium Salts. Int. J. Mol. Sci. 2015, 16, 3626–3655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bieser, A.M.; Tiller, J.C. Mechanistic Considerations on Contact−Active Antimicrobial Surfaces with Controlled Functional Group Densities. Macromol. Biosci. 2011, 11, 526–534. [Google Scholar] [CrossRef]
- Chen, Y.; Tan, W.; Li, Q.; Dong, F.; Gu, G.; Guo, Z. Synthesis of Inulin Derivatives with Quaternary Phosphonium Salts and Their Antifungal Activity. Int. J. Biol. Macromol. 2018, 113, 1273–1278. [Google Scholar] [CrossRef] [PubMed]
- Leong, J.; Yang, C.; Tan, J.; Tan, B.Q.; Hor, S.; Hedrick, J.L.; Yang, Y.Y. Combination of Guanidinium and Quaternary Ammonium Polymers with Distinctive Antimicrobial Mechanisms Achieving a Synergistic Antimicrobial Effect. Biomater. Sci. 2020, 8, 6920–6929. [Google Scholar] [CrossRef] [PubMed]
- Kuroki, A.; Tchoupa, A.K.; Hartlieb, M.; Peltier, R.; Locock, K.E.S.; Unnikrishnan, M.; Perrier, S. Targeting Intracellular, Multi−Drug Resistant Staphylococcus Aureus with Guanidinium Polymers by Elucidating the Structure−Activity Relationship. Biomaterials 2019, 217, 119249. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cai, P.; Tong, Z.-F.; Xiao, H.; Pan, Y. Preparation of Copolymer−Based Nanoparticles with Broad−Spectrum Antimicrobial Activity. Polymers 2017, 9, 717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Chen, J.; Cao, W.; Wei, D.; Zheng, A.; Guan, Y. Permanent Antimicrobial Cotton Fabrics Obtained by Surface Treatment with Modified Guanidine. Carbohydr. Polym. 2018, 180, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.; Hasanin, M.; Hesemann, P. Synthesis and Antimicrobial Properties of New Chitosan Derivatives Containing Guanidinium Groups. Carbohydr. Polym. 2020, 241, 116363. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Xia, Q.; Xiao, H. Cationic Polymers with Tailored Structures for Rendering Polysaccharide−Based Materials Antimicrobial: An Overview. Polymers 2019, 11, 1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rauschenbach, M.; Lawrenson, S.B.; Taresco, V.; Pearce, A.K.; O’Reilly, R.K. Antimicrobial Hyperbranched Polymer–Usnic Acid Complexes through a Combined ROP−RAFT Strategy. Macromol. Rapid Commun. 2020, 41, 2000190. [Google Scholar] [CrossRef]
- Olmo, J.A.-D.; Ruiz-Rubio, L.; Pérez-Alvarez, L.; Sáez-Martínez, V.; Vilas-Vilela, J.L. Antibacterial Coatings for Improving the Performance of Biomaterials. Coatings 2020, 10, 139. [Google Scholar] [CrossRef] [Green Version]
- Murrieta-Rico, F.N. Prospects for Further Development of Face Masks to Minimize Pandemics—Functionalization of Textile Materials with Biocide Inorganic Nanoparticles: A Review. IEEE Lat. Am. Trans. 2021, 19, 1010–1023. [Google Scholar]
- Kumar, L.; Brice, J.; Toberer, L.; Klein-Seetharaman, J.; Knauss, D.; Sarkar, S.K. Antimicrobial Biopolymer Formation from Sodium Alginate and Algae Extract Using Aminoglycosides. PLoS ONE 2019, 14, e0214411. [Google Scholar] [CrossRef]
- Alzagameem, A.; Klein, S.E.; Bergs, M.; Do, X.T.; Korte, I.; Dohlen, S.; Hüwe, C.; Kreyenschmidt, J.; Kamm, B.; Larkins, M.; et al. Antimicrobial Activity of Lignin and Lignin−Derived Cellulose and Chitosan Composites against Selected Pathogenic and Spoilage Microorganisms. Polymers 2019, 11, 670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivakanthan, S.; Rajendran, S.; Gamage, A.; Madhujith, T.; Mani, S. Antioxidant and Antimicrobial Applications of Biopolymers: A Review. Food Res. Int. 2020, 136, 109327. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.T.; Lindsay, A.C.; Chen, X.; Gözaydin, G.; Yan, N.; Sperry, J. Transferring the Biorenewable Nitrogen Present in Chitin to Several N−Functional Groups. Sustain. Chem. Pharm. 2019, 13, 100143. [Google Scholar] [CrossRef]
- Sipponen, M.H.; Lange, H.; Crestini, C.; Henn, A.; Österberg, M. Lignin for Nano- and Microscaled Carrier Systems: Applications, Trends, and Challenges. Chemsuschem 2019, 12, 2039–2054. [Google Scholar] [CrossRef] [PubMed]
- Karim, N.; Afroj, S.; Lloyd, K.; Oaten, L.C.; Andreeva, D.V.; Carr, C.; Farmery, A.D.; Kim, I.-D.; Novoselov, K.S. Sustainable Personal Protective Clothing for Healthcare Applications: A Review. ACS Nano 2020, 14, 12313–12340. [Google Scholar] [CrossRef] [PubMed]
- Wo, Y.; Brisbois, E.J.; Bartlett, R.H.; Meyerhoff, M.E. Recent Advances in Thromboresistant and Antimicrobial Polymers for Biomedical Applications: Just Say Yes to Nitric Oxide (NO). Biomater. Sci. 2016, 4, 1161–1183. [Google Scholar] [CrossRef] [Green Version]
- Christman, K.L.; Vázquez-Dorbatt, V.; Schopf, E.; Kolodziej, C.M.; Li, R.C.; Broyer, R.M.; Chen, Y.; Maynard, H.D. Nanoscale Growth Factor Patterns by Immobilization on a Heparin−Mimicking Polymer. J. Am. Chem. Soc. 2008, 130, 16585–16591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sangaj, N.; Kyriakakis, P.; Yang, D.; Chang, C.-W.; Arya, G.; Varghese, S. Heparin Mimicking Polymer Promotes Myogenic Differentiation of Muscle Progenitor Cells. Biomacromolecules 2010, 11, 3294–3300. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Li, H.; Chen, S.; Nie, C.; Cheng, C.; Zhao, C. Interfacial Self−Assembly of Heparin−Mimetic Multilayer on Membrane Substrate as Effective Antithrombotic, Endothelialization, and Antibacterial Coating. ACS Biomater. Sci. Eng. 2015, 1, 1183–1193. [Google Scholar] [CrossRef]
- Smith, R.J.; Moule, M.G.; Sule, P.; Smith, T.; Cirillo, J.D.; Grunlan, J.C. Polyelectrolyte Multilayer Nanocoating Dramatically Reduces Bacterial Adhesion to Polyester Fabric. ACS Biomater. Sci. Eng. 2017, 3, 1845–1852. [Google Scholar] [CrossRef]
- Batul, R.; Tamanna, T.; Khaliq, A.; Yu, A. Recent Progress in the Biomedical Applications of Polydopamine Nanostructures. Biomater. Sci. 2017, 5, 1204–1229. [Google Scholar] [CrossRef] [PubMed]
- Karkhanechi, H.; Takagi, R.; Matsuyama, H. Biofouling Resistance of Reverse Osmosis Membrane Modified with Polydopamine. Desalination 2014, 336, 87–96. [Google Scholar] [CrossRef]
- Su, L.; Yu, Y.; Zhao, Y.; Liang, F.; Zhang, X. Strong Antibacterial Polydopamine Coatings Prepared by a Shaking−Assisted Method. Sci. Rep. 2016, 6, 24420. [Google Scholar] [CrossRef]
- Sun, H.; Hong, Y.; Xi, Y.; Zou, Y.; Gao, J.; Du, J. Synthesis, Self−Assembly, and Biomedical Applications of Antimicrobial Peptide−Polymer Conjugates. Biomacromolecules 2018, 19, 1701–1720. [Google Scholar] [CrossRef] [PubMed]
- Sahariah, P.; Sørensen, K.K.; Hjálmarsdóttir, M.Á.; Sigurjónsson, Ó.E.; Jensen, K.J.; Másson, M.; Thygesen, M.B. Antimicrobial Peptide Shows Enhanced Activity and Reduced Toxicity upon Grafting to Chitosan Polymers. Chem. Commun. 2015, 51, 11611–11614. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.; Chua, R.R.Y.; Bow, H.; Tambyah, P.A.; Hadinoto, K.; Leong, S.S.J. Development of a Catheter Functionalized by a Polydopamine Peptide Coating with Antimicrobial and Antibiofilm Properties. Acta Biomater. 2015, 15, 127–138. [Google Scholar] [CrossRef]
- Zare, E.N.; Jamaledin, R.; Naserzadeh, P.; Afjeh-Dana, E.; Ashtari, B.; Hosseinzadeh, M.; Vecchione, R.; Wu, A.; Tay, F.R.; Borzacchiello, A.; et al. Metal−Based Nanostructures/PLGA Nanocomposites: Antimicrobial Activity, Cytotoxicity, and Their Biomedical Applications. ACS Appl. Mater. Interfaces 2020, 12, 3279–3300. [Google Scholar] [CrossRef] [PubMed]
- Zare, E.N.; Makvandi, P.; Ashtari, B.; Rossi, F.; Motahari, A.; Perale, G. Progress in Conductive Polyaniline−Based Nanocomposites for Biomedical Applications: A Review. J. Med. Chem. 2020, 63, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Korupalli, C.; Kalluru, P.; Nuthalapati, K.; Kuthala, N.; Thangudu, S.; Vankayala, R. Recent Advances of Polyaniline−Based Biomaterials for Phototherapeutic Treatments of Tumors and Bacterial Infections. Bioengineering 2020, 7, 94. [Google Scholar] [CrossRef]
- Kim, S.H.; Kang, E.B.; Jeong, C.J.; Sharker, S.M.; In, I.; Park, S.Y. Light Controllable Surface Coating for Effective Photothermal Killing of Bacteria. ACS Appl. Mater. Interfaces 2015, 7, 15600–15606. [Google Scholar] [CrossRef]
- Hsiao, Y.-C.; Jheng, P.-R.; Nguyen, H.T.; Chen, Y.-H.; Manga, Y.B.; Lu, L.-S.; Rethi, L.; Chen, C.-H.; Huang, T.-W.; Lin, J.-D.; et al. Photothermal−Irradiated Polyethyleneimine−Polypyrrole Nanopigment Film−Coated Polyethylene Fabrics for Infrared−Inspired with Pathogenic Evaluation. ACS Appl. Mater. Interfaces 2021, 13, 2483–2495. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.-T.; Hsu, C.-C. Development of a 3D Porous Chitosan/Gelatin Liver Scaffold for a Bioartificial Liver Device. J. Biosci. Bioeng. 2020, 129, 741–748. [Google Scholar] [CrossRef] [PubMed]
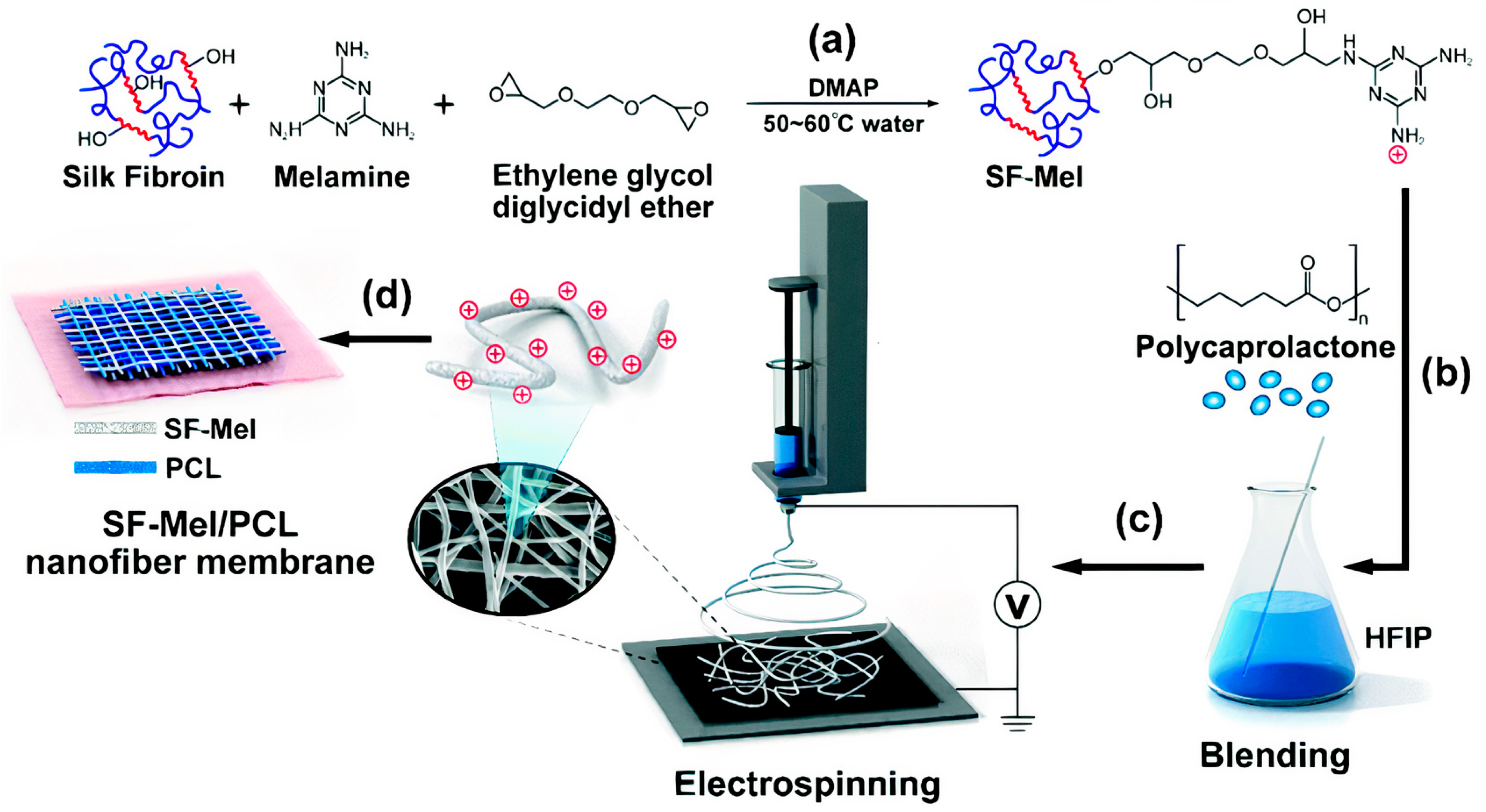
- Hu, W.; Wang, Z.; Xu, Y.; Wang, X.; Xiao, Y.; Zhang, S.; Wang, J. Remodeling of Inherent Antimicrobial Nanofiber Dressings with Melamine−Modified Fibroin into Neoskin. J. Mater. Chem. B 2019, 7, 3412–3423. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Z.; Liang, Y.; He, J.; Guo, B. Multifunctional Tissue−Adhesive Cryogel Wound Dressing for Rapid Nonpressing Surface Hemorrhage and Wound Repair. ACS Appl. Mater. Interfaces 2020, 12, 35856–35872. [Google Scholar] [CrossRef] [PubMed]
- Salekdeh, S.S.H.; Daemi, H.; Zare-Gachi, M.; Rajabi, S.; Bazgir, F.; Aghdami, N.; Nourbakhsh, M.S.; Baharvand, H. Assessment of the Efficacy of Tributylammonium Alginate Surface−Modified Polyurethane as an Antibacterial Elastomeric Wound Dressing for Both Noninfected and Infected Full−Thickness Wounds. ACS Appl. Mater. Interfaces 2020, 12, 3393–3406. [Google Scholar] [CrossRef] [PubMed]
- Milazzo, M.; Gallone, G.; Marcello, E.; Mariniello, M.D.; Bruschini, L.; Roy, I.; Danti, S. Biodegradable Polymeric Micro/Nano−Structures with Intrinsic Antifouling/Antimicrobial Properties: Relevance in Damaged Skin and Other Biomedical Applications. J. Funct. Biomater. 2020, 11, 60. [Google Scholar] [CrossRef]
- Gu, J.; Clegg, J.R.; Heersema, L.A.; Peppas, N.A.; Smyth, H.D.C. Optimization of Cationic Nanogel PEGylation to Achieve Mammalian Cytocompatibility with Limited Loss of Gram−Negative Bactericidal Activity. Biomacromolecules 2020, 21, 1528–1538. [Google Scholar] [CrossRef]
- Gao, H.; Zhong, Z.; Xia, H.; Hu, Q.; Ye, Q.; Wang, Y.; Chen, L.; Du, Y.; Shi, X.; Zhang, L. Construction of Cellulose Nanofibers/Quaternized Chitin/Organic Rectorite Composites and Their Application as Wound Dressing Materials. Biomater. Sci. 2019, 7, 2571–2581. [Google Scholar] [CrossRef]
- Bolto, B.; Gregory, J. Organic Polyelectrolytes in Water Treatment. Water Res. 2007, 41, 2301–2324. [Google Scholar] [CrossRef]
- Abiola, O.N. Polymers for Coagulation and Flocculation in Water Treatment. In Polymeric Materials for Clean Water; Das, R., Ed.; Springer Series on Polymer and Composite Materials; Springer International Publishing: Cham, Switzerland, 2019; pp. 77–92. ISBN 978-3-030-00743-0. [Google Scholar]
- Carmona-Ribeiro, A.; Carrasco, L. Fungicidal Assemblies and Their Mode of Action. OA Biotechnol. 2013, 2. [Google Scholar] [CrossRef]
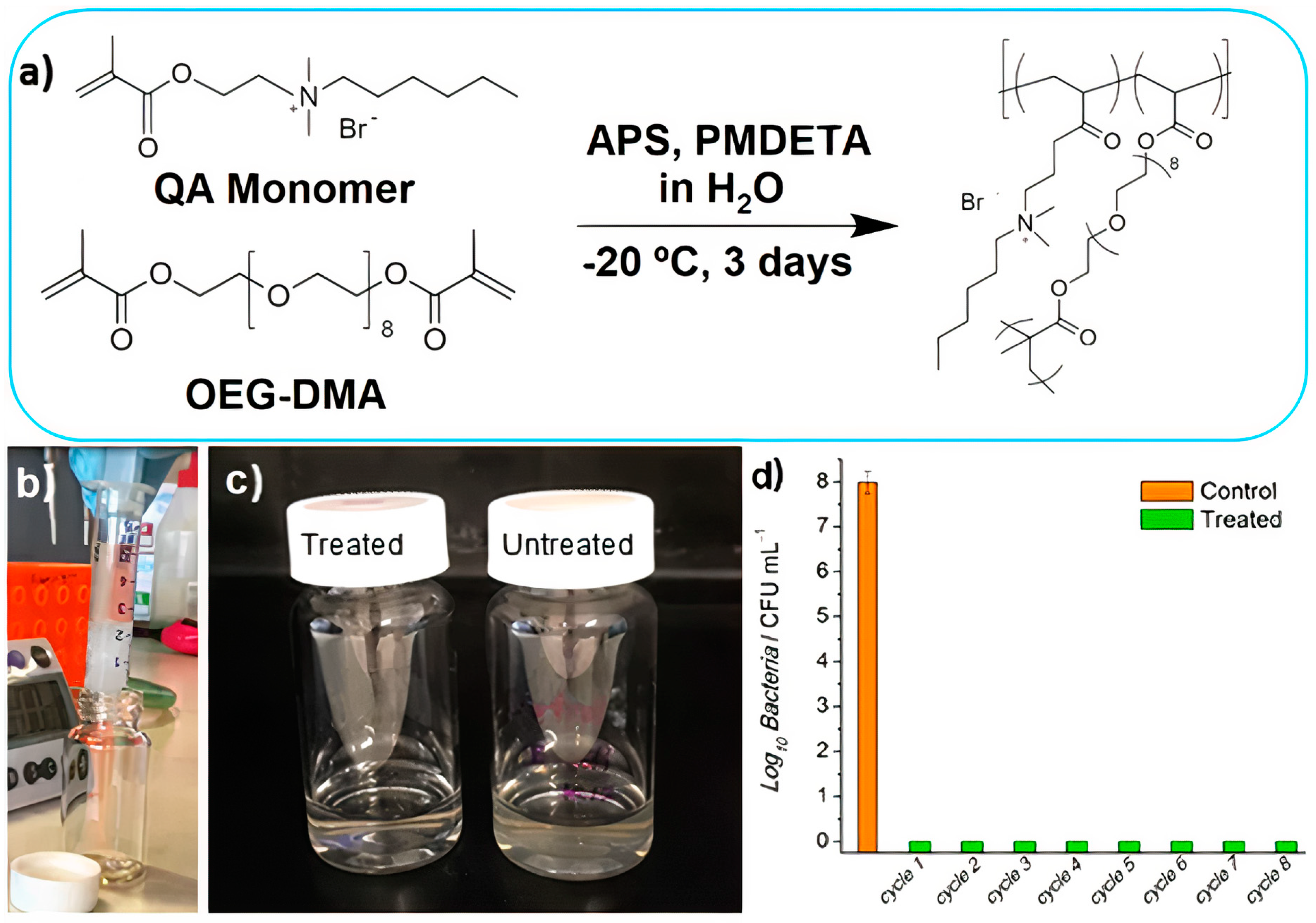
- Kumar, A.; Boyer, C.; Nebhani, L.; Wong, E.H.H. Highly Bactericidal Macroporous Antimicrobial Polymeric Gel for Point−of−Use Water Disinfection. Sci. Rep. 2018, 8, 7965. [Google Scholar] [CrossRef] [PubMed]
- Pullangott, G.; Kannan, U.; Gayathri, S.; Kiran, D.V.; Maliyekkal, S.M. A Comprehensive Review on Antimicrobial Face Masks: An Emerging Weapon in Fighting Pandemics. RSC Adv. 2021, 11, 6544–6576. [Google Scholar] [CrossRef]
- Majchrzycka, K.; Okrasa, M.; Szulc, J.; Jachowicz, A.; Gutarowska, B. Survival of Microorganisms on Nonwovens Used for the Construction of Filtering Facepiece Respirators. Int. J. Environ. Res. Public. Health 2019, 16, 1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demir, B.; Cerkez, I.; Worley, S.D.; Broughton, R.M.; Huang, T.-S. N−Halamine−Modified Antimicrobial Polypropylene Nonwoven Fabrics for Use against Airborne Bacteria. ACS Appl. Mater. Interfaces 2015, 7, 1752–1757. [Google Scholar] [CrossRef] [PubMed]
- Monge, F.A.; Jagadesan, P.; Bondu, V.; Donabedian, P.L.; Ista, L.; Chi, E.Y.; Schanze, K.S.; Whitten, D.G.; Kell, A.M. Highly Effective Inactivation of SARS−CoV−2 by Conjugated Polymers and Oligomers. ACS Appl. Mater. Interfaces 2020, 12, 55688–55695. [Google Scholar] [CrossRef] [PubMed]
- Martí, M.; Tuñón-Molina, A.; Aachmann, F.L.; Muramoto, Y.; Noda, T.; Takayama, K.; Serrano-Aroca, Á. Protective Face Mask Filter Capable of Inactivating SARS−CoV−2, and Methicillin−Resistant Staphylococcus Aureus and Staphylococcus Epidermidis. Polymers 2021, 13, 207. [Google Scholar] [CrossRef] [PubMed]



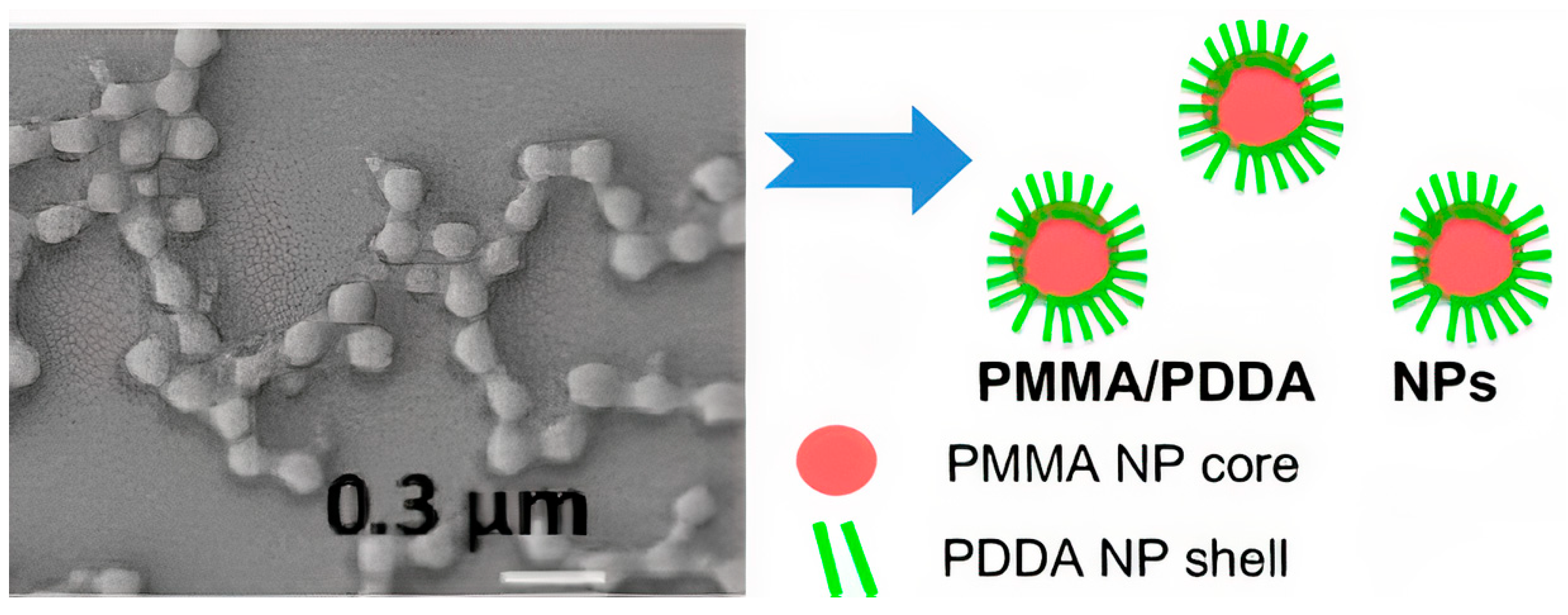

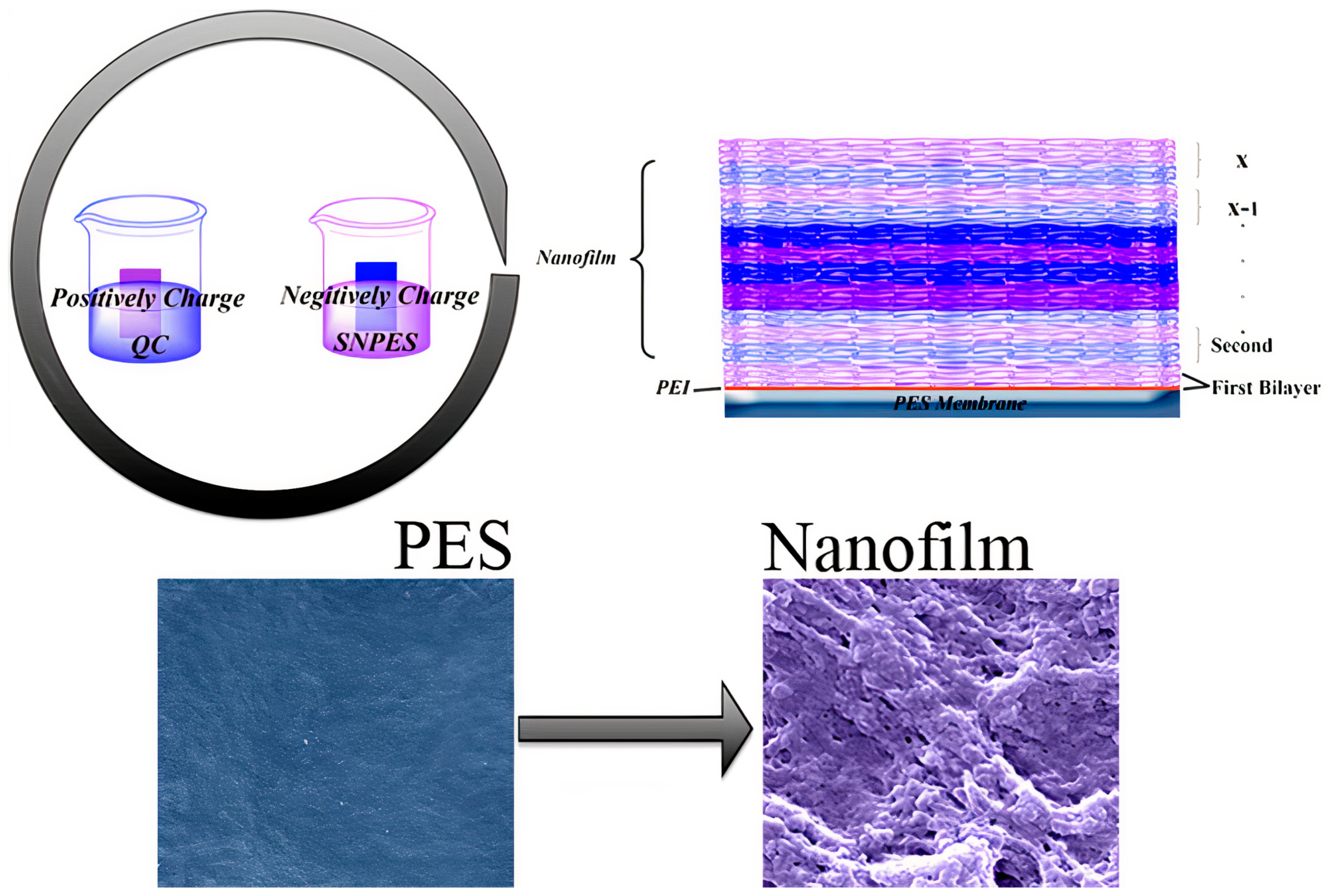
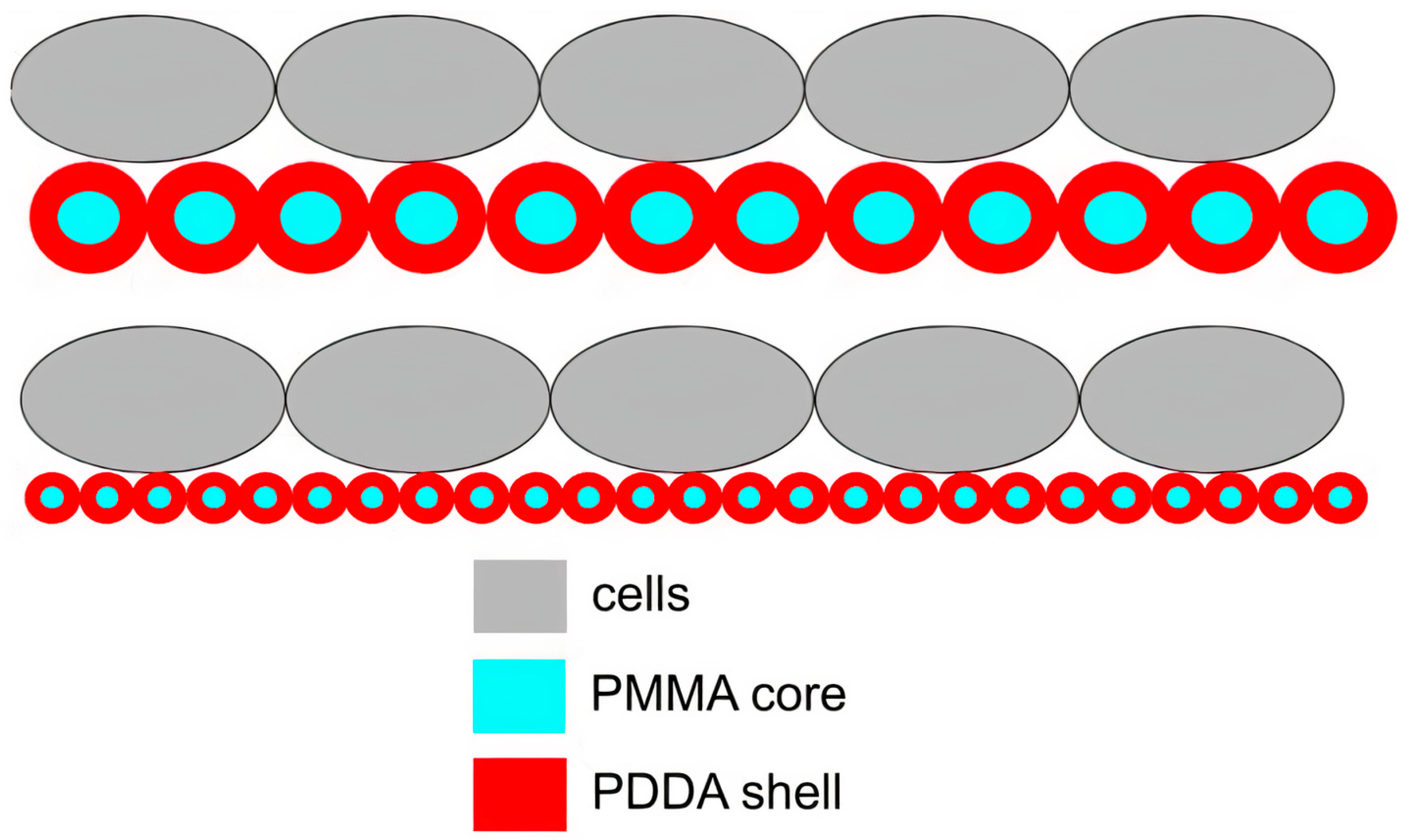
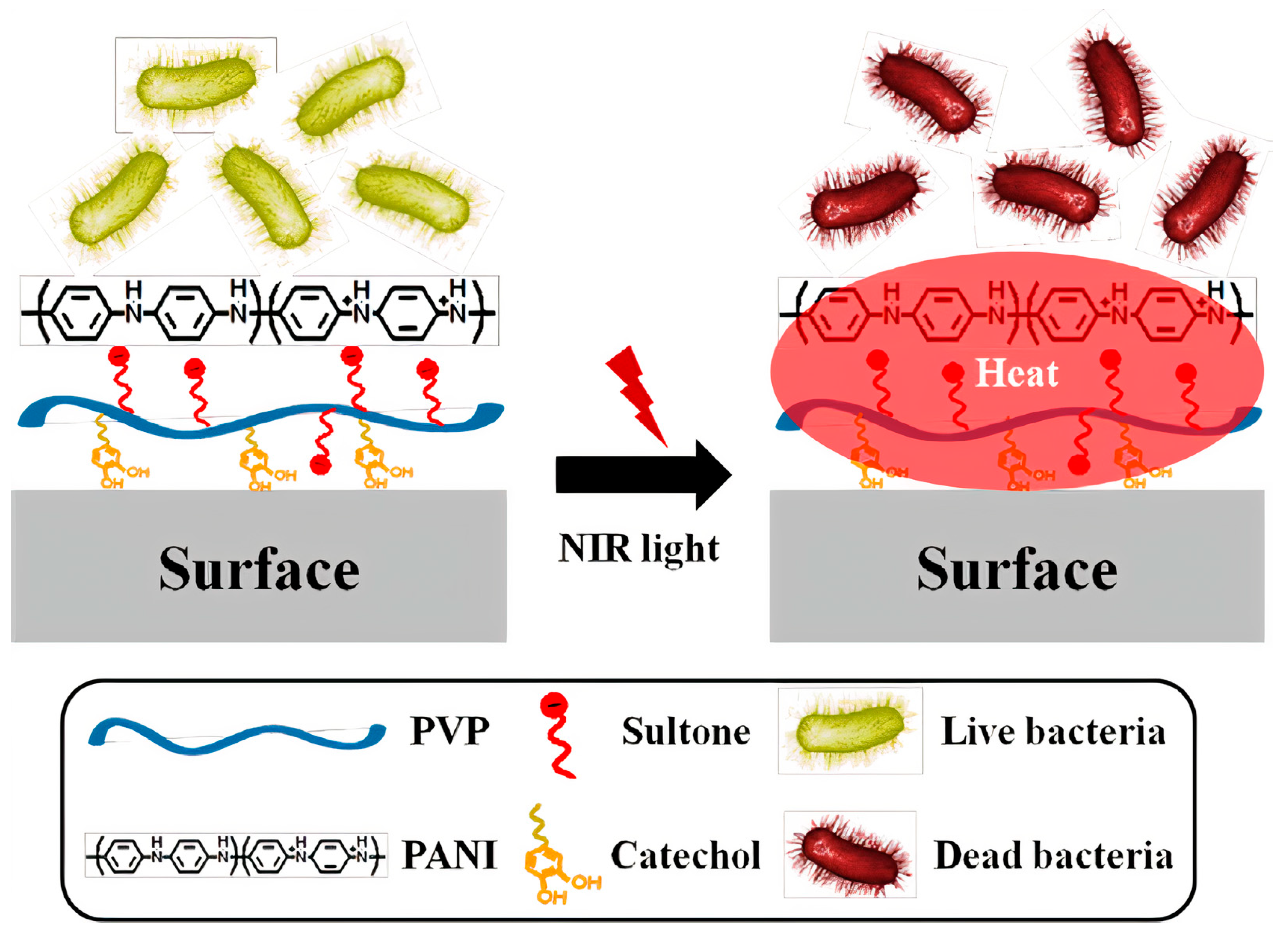
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carmona-Ribeiro, A.M.; Araújo, P.M. Antimicrobial Polymer−Based Assemblies: A Review. Int. J. Mol. Sci. 2021, 22, 5424. https://doi.org/10.3390/ijms22115424
Carmona-Ribeiro AM, Araújo PM. Antimicrobial Polymer−Based Assemblies: A Review. International Journal of Molecular Sciences. 2021; 22(11):5424. https://doi.org/10.3390/ijms22115424
Chicago/Turabian StyleCarmona-Ribeiro, Ana Maria, and Péricles Marques Araújo. 2021. "Antimicrobial Polymer−Based Assemblies: A Review" International Journal of Molecular Sciences 22, no. 11: 5424. https://doi.org/10.3390/ijms22115424
APA StyleCarmona-Ribeiro, A. M., & Araújo, P. M. (2021). Antimicrobial Polymer−Based Assemblies: A Review. International Journal of Molecular Sciences, 22(11), 5424. https://doi.org/10.3390/ijms22115424





