Possible Beneficial Actions of Caffeine in SARS-CoV-2
Abstract
1. Introduction
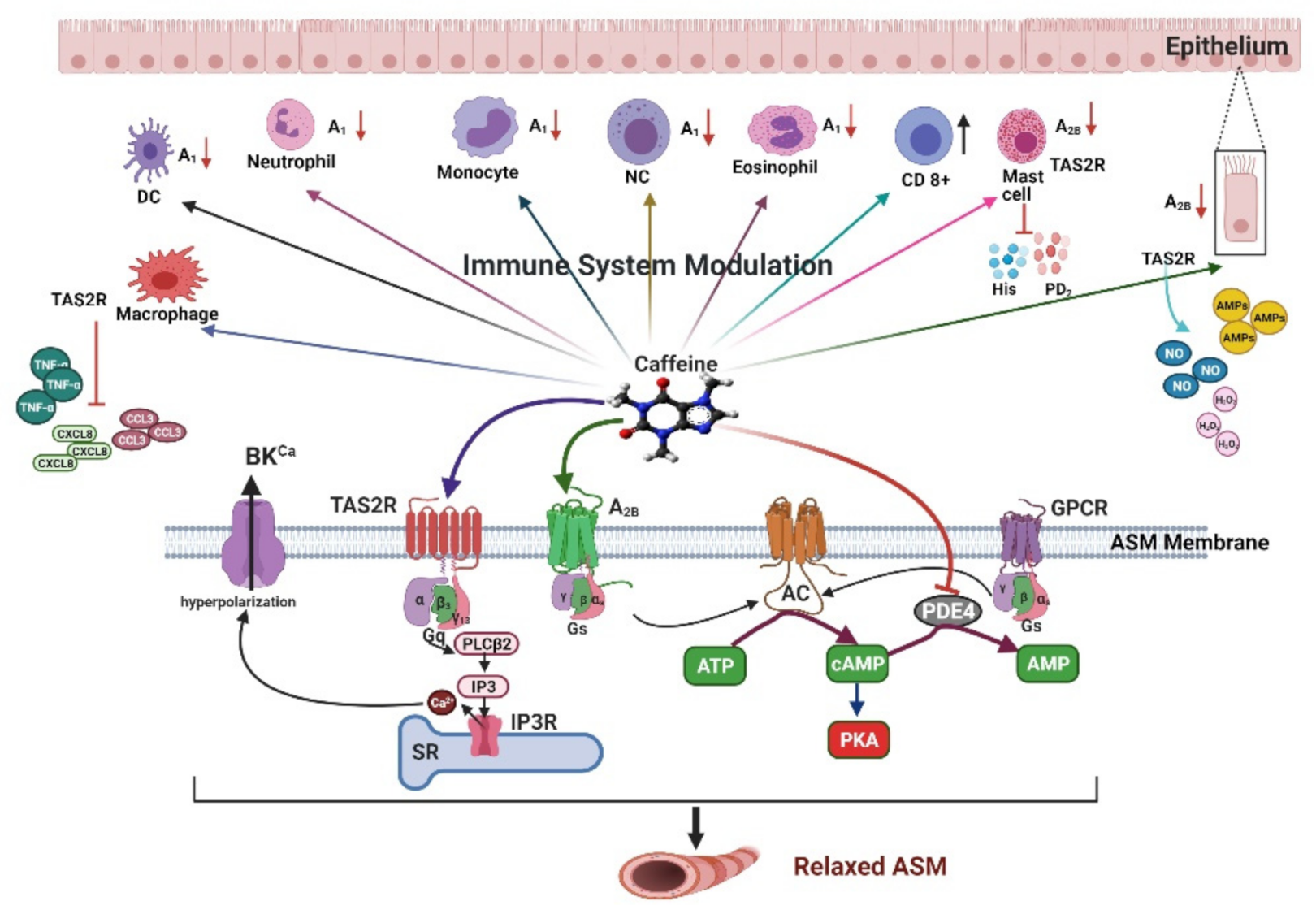
2. Pharmacological Mechanisms of Caffeine in Airway Smooth Muscle
2.1. Adenosine Receptors
2.2. Cyclic Nucleotide Phosphodiesterases
2.3. Agonist of the Type 2 Taste Receptor
3. Immunomodulatory Effects of Caffeine
3.1. Adenosine Receptors
3.2. Agonist of the Type 2 Taste Receptor
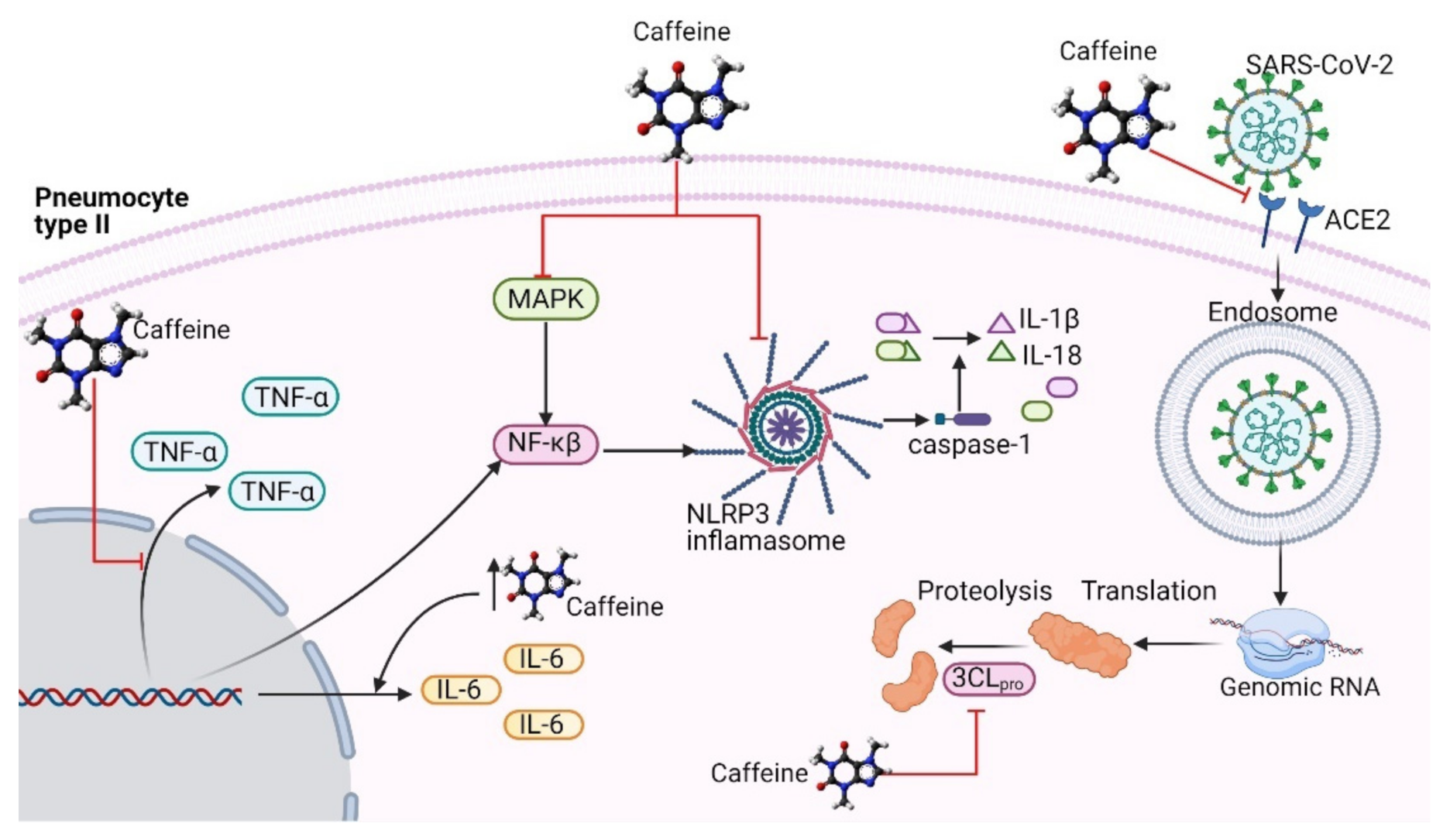
4. Probable Antiviral Activity of Caffeine in SARS-CoV-2 Infection
4.1. Caffeine Might Be an Inhibitor of the RBD–ACE2 Complex
4.2. Inhibition of 3-Chymotrypsin-Like Protease
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Cohen, J.; Normile, D. New SARS-like virus in China triggers alarm. Science 2020, 367, 234–235. [Google Scholar] [CrossRef]
- Coronavirus Disease (COVID-19) Weekly Epidemiological Update and Weekly Operational Update. 2021. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (accessed on 6 April 2021).
- Cheng, V.C.C.; Lau, S.K.P.; Woo, P.C.Y.; Yuen, K.Y. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin. Microbiol. Rev. 2007, 20, 660–694. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Müller, M.A.; et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef]
- Li, Q.; Kang, C. Progress in developing inhibitors of SARS-CoV-2 3C-like protease. Microorganisms 2020, 8, 1250. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Cao, B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensiv. Care Med. 2020, 46, 846–848. [Google Scholar] [CrossRef]
- Wang, L.; He, W.; Yu, X.; Hu, D.; Bao, M.; Liu, H.; Zhou, J.; Jiang, H. Coronavirus disease 2019 in elderly patients: Characteristics and prognostic factors based on 4-week follow-up. J. Infect. 2020, 80, 639–645. [Google Scholar] [CrossRef]
- Rothan, H.A.; Byrareddy, S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020, 109, 102433. [Google Scholar] [CrossRef]
- Velavan, T.P.; Meyer, C.G. The COVID-19 epidemic. Trop. Med. Int. Health 2020, 25, 278–280. [Google Scholar] [CrossRef]
- Lin, L.; Lu, L.; Cao, W.; Li, T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection—A review of immune changes in patients with viral pneumonia. Emerg. Microbes Infect. 2020, 9, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Wadman, M.; Couzin-Frankel, J.; Kaiser, J.; Matacic, C. How does coronavirus kill? Clinicians trace a ferocious rampage through the body, from brain to toes. Sci. Biol. Coronavirus 2020. [Google Scholar] [CrossRef]
- Conti, P.; Ronconi, G.; Caraffa, A.L.; Gallenga, C.E.; Ross, R.; Frydas, I.; Kritas, S.K. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): Anti-inflammatory strategies. J. Biol. Regul. Homeost Agents 2020, 34, 327–331. [Google Scholar] [PubMed]
- Dong, E.; Du, H.; Gardner, L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020, 20, 533–534. [Google Scholar] [CrossRef]
- Ramanathan, K.; Antognini, D.; Combes, A.; Paden, M.; Zakhary, B.; Ogino, M.; MacLaren, G.; Brodie, D.; Shekar, K. Planning and provision of ECMO services for severe ARDS during the COVID-19 pandemic and other outbreaks of emerging infectious diseases. Lancet Respir. Med. 2020, 8, 518–526. [Google Scholar] [CrossRef]
- Pan, A.; Liu, L.; Wang, C.; Guo, H.; Hao, X.; Wang, Q.; Huang, J.; He, N.; Yu, H.; Lin, X.; et al. Association of Public Health Interventions with the Epidemiology of the COVID-19 Outbreak in Wuhan, China. JAMA 2020, 323, 1915. [Google Scholar] [CrossRef]
- Stasi, C.; Fallani, S.; Voller, F.; Silvestri, C. Treatment for COVID-19: An overview. Eur. J. Pharmacol. 2020, 889, 173644. [Google Scholar] [CrossRef]
- WHO Updates Clinical Care Guidance with Corticosteroid Recommendations. 2020. Available online: https://www.who.int/news-room/feature-stories/detail/who-updates-clinical-care-guidance-with-corticosteroid-recommendations (accessed on 6 April 2021).
- Panel, C.-T.G. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. 2019. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 6 April 2021).
- Fredholm, B.B.; Bättig, K.; Holmén, J.; Nehlig, A.; Zvartau, E.E. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol. Rev. 1999, 51, 83–133. [Google Scholar]
- Institute of Medicine Committee on Military Nutrition, R. Caffeine for the Sustainment of Mental Task Performance: Formulations for Military Operations; National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Nawrot, P.; Jordan, S.; Eastwood, J.; Rotstein, J.; Hugenholtz, A.; Feeley, M. Effects of caffeine on human health. Food Addit. Contam. 2003, 20, 1–30. [Google Scholar] [CrossRef]
- Kreutzer, K.; Bassler, D. Caffeine for Apnea of Prematurity: A Neonatal Success Story. Neonatology 2014, 105, 332–336. [Google Scholar] [CrossRef]
- Hedner, T.; Hedner, J.; Bergman, B.; Mueller, R.; Jonason, J. Characterization of adenosine-induced respiratory depression in the preterm rabbit. Neonatology 1985, 47, 323–332. [Google Scholar] [CrossRef]
- Vyas-Read, S.; Kanaan, U.; Shankar, P.; Stremming, J.; Travers, C.; Carlton, D.P.; Fitzpatrick, A. Early characteristics of infants with pulmonary hypertension in a referral neonatal intensive care unit. BMC Pediatr. 2017, 17, 163. [Google Scholar] [CrossRef] [PubMed]
- Welsh, E.J.; Bara, A.; Barley, E.; Cates, C.J. Caffeine for asthma. Cochrane Database Syst. Rev. 2010, 2010, CD001112. [Google Scholar] [CrossRef]
- Kivity, S.; Ben Aharon, Y.; Man, A.; Topilsky, M. The effect of caffeine on exercise-induced bronchoconstriction. Chest 1990, 97, 1083–1085. [Google Scholar] [CrossRef] [PubMed]
- Tilley, S.L. Methylxanthines in asthma. Methylxanthines 2011, 200, 439–456. [Google Scholar]
- Willson, C. The clinical toxicology of caffeine: A review and case study. Toxicol. Rep. 2018, 5, 1140–1152. [Google Scholar] [CrossRef]
- Campbell, M.E.; Grant, D.M.; Inaba, T.; Kalow, W. Biotransformation of caffeine, paraxanthine, theophylline, and theobromine by polycyclic aromatic hydrocarbon-inducible cytochrome(s) P-450 in human liver microsomes. Drug Metab. Dispos. 1987, 15, 237–249. [Google Scholar]
- Ajjampur, K.; Subramaniam, A. The importance of early use of beta blockers and gastric decontamination in caffeine overdose: A case report. Aust. Crit. Care 2020. [Google Scholar] [CrossRef] [PubMed]
- Montaño, L.M.; Carbajal, V.; Arreola, J.L.; Barajas-López, C.; Flores-Soto, E.; Vargas, M.H. Acetylcholine and tachykinins involvement in the caffeine-induced biphasic change in intracellular Ca2+ in bovine airway smooth muscle. Br. J. Pharmacol. 2003, 139, 1203–1211. [Google Scholar] [CrossRef]
- Flores-Soto, E.; Reyes-García, J.; Sommer, B.; Chavez, J.; Barajas-López, C.; Montaño, L.M. PPADS, a P2X receptor antagonist, as a novel inhibitor of the reverse mode of the Na+/Ca2+ exchanger in guinea pig airway smooth muscle. Eur. J. Pharmacol. 2012, 674, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Soto, E.F.; Reyes-García, J.; Sommer, B.; Montaño, L.M. Sarcoplasmic reticulum Ca2+ refilling is determined by L-type Ca2+ and store operated Ca2+ channels in guinea pig airway smooth muscle. Eur. J. Pharmacol. 2013, 721, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, C.; Chen, J.; Li, X. Rediscovery of caffeine: An excellent drug for improving patient outcomes while fighting WARS. Curr. Med. Chem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Kalidhindi, R.S.R.; Borkar, N.A.; Ambhore, N.S.; Pabelick, C.M.; Prakash, Y.S.; Sathish, V. Sex steroids skew ACE2 expression in human airway: A contributing factor to sex differences in COVID-19? Am. J. Physiol. Cell. Mol. Physiol. 2020, 319, L843–L847. [Google Scholar] [CrossRef] [PubMed]
- Cushley, M.J.; Tattersfield, A.E.; Holgate, S.T. Adenosine antagonism as an alternative mechanism of action of methylxanthines in asthma. Agents Actions Suppl. 1983, 13, 109–113. [Google Scholar] [PubMed]
- Crimi, N.; Palermo, F.; Oliveri, R.; Maccarrone, C.; Palermo, B.; Vancheri, C.; Polosa, R.; Mistretta, A. Enhancing effect of dipyridamole inhalation on adenosine-induced bronchospasm in asthmatic patients. Allergy 1988, 43, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Bucchioni, E.; Csoma, Z.; Allegra, L.; Chung, K.; Barnes, P.; Kharitonov, S. Adenosine 5′-monophosphate increases levels of leukotrienes in breath condensate in asthma. Respir. Med. 2004, 98, 651–655. [Google Scholar] [CrossRef][Green Version]
- Polosa, R. Adenosine-receptor subtypes: Their relevance to adenosine-mediated responses in asthma and chronic obstructive pulmonary disease. Eur. Respir. J. 2002, 20, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Holgate, S.T. Adenosine provocation: A new test for allergic type airway inflammation. Am. J. Respir. Crit. Care Med. 2002, 165, 317–318. [Google Scholar] [CrossRef]
- Driver, A.G.; Kukoly, C.A.; Ali, S.; Mustafa, S.J. Adenosine in bronchoalveolar lavage fluid in asthma. Am. Rev. Respir. Dis. 1993, 148, 91–97. [Google Scholar] [CrossRef]
- Csoma, Z.; Huszár, É.; Vizi, É.; Vass, G.; Szabó, Z.; Herjavecz, I.; Kollai, M.; Horvath, I. Adenosine level in exhaled breath increases during exercise-induced bronchoconstriction. Eur. Respir. J. 2005, 25, 873–878. [Google Scholar] [CrossRef]
- Huszár, É.; Vass, G.; Vizi, É.; Csoma, Z.; Barát, E.; Világos, G.M.; Herjavecz, I.; Horvath, I. Adenosine in exhaled breath condensate in healthy volunteers and in patients with asthma. Eur. Respir. J. 2002, 20, 1393–1398. [Google Scholar] [CrossRef]
- Ali, S.; Mustafa, S.J.; Metzger, W.J. Adenosine receptor-mediated bronchoconstriction and bronchial hyperresponsiveness in allergic rabbit model. Am. J. Physiol. Content 1994, 266, 271–277. [Google Scholar] [CrossRef]
- Dahlen, S.E.; Hansson, G.; Hedqvist, P.; Bjorck, T.; Granstrom, E.; Dahlen, B. Allergen challenge of lung tissue from asthmatics elicits bronchial contraction that correlates with the release of leukotrienes C4, D4, and E4. Proc. Natl. Acad. Sci. USA 1983, 80, 1712–1716. [Google Scholar] [CrossRef]
- Olah, M.E.; Stiles, G.L. Adenosine receptor subtypes: Characterization and therapeutic regulation. Annu. Rev. Pharmacol. Toxicol. 1995, 35, 581–606. [Google Scholar] [CrossRef]
- Fredholm, B.B.; Ijzerman, A.P.; Jacobson, K.A.; Linden, J.; Müller, C.E. International union of basic and clinical pharmacology. LXXXI. Nomenclature and classification of adenosine receptors—An update. Pharmacol. Rev. 2011, 63, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Conde, S.V.; Obeso, A.; Vicario, I.; Rigual, R.; Rocher, A.; Gonzalez, C. Caffeine inhibition of rat carotid body chemoreceptors is mediated by A2A and A2B adenosine receptors. J. Neurochem. 2006, 98, 616–628. [Google Scholar] [CrossRef] [PubMed]
- Antonioli, L.; Blandizzi, C.; Pacher, P.; Haskó, G. The purinergic system as a pharmacological target for the treatment of immune-iediated inflammatory diseases. Pharmacol. Rev. 2019, 71, 345–382. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.N.; Nadeem, A.; Spina, D.; Brown, R.; Page, C.P.; Mustafa, S.J. Adenosine receptors and asthma. Handb. Exp. Pharmacol. 2009, 193, 329–362. [Google Scholar]
- Ukena, D.; Shamim, M.; Padgett, W.; Daly, J. Analogs of caffeine: Antagonists with selectivity for A2 adenosine receptors. Life Sci. 1986, 39, 743–750. [Google Scholar] [CrossRef]
- Seale, T.W.; Abla, K.A.; Shamim, M.T.; Carney, J.M.; Daly, J.W. 3,7-Dimethyl-1-propargylxanthine: A potent and selective in vivo antagonist of adenosine analogs. Life Sci. 1988, 43, 1671–1684. [Google Scholar] [CrossRef]
- Baraldi, P.G.; Romagnoli, R.; Preti, D.; Fruttarolo, F.; Carrion, M.D.; Tabrizi, M.A. Ligands for A2B adenosine receptor subtype. Curr. Med. Chem. 2006, 13, 3467–3482. [Google Scholar] [CrossRef]
- Akkari, R.; Burbiel, J.C.; Hockemeyer, J.; Müller, C.E. Recent progress in the development of adenosine receptor ligands as antiinflammatory drugs. Curr. Top. Med. Chem. 2006, 6, 1375–1399. [Google Scholar] [CrossRef]
- Maurice, D.H.; Ke, H.; Ahmad, F.; Wang, Y.; Chung, J.; Manganiello, V.C. Advances in targeting cyclic nucleotide phosphodiesterases. Nat. Rev. Drug Discov. 2014, 13, 290–314. [Google Scholar] [CrossRef]
- Zhang, K.Y.; Ibrahim, P.N.; Gillette, S.; Bollag, G. Phosphodiesterase-4 as a potential drug target. Expert Opin. Ther. Targets 2005, 9, 1283–1305. [Google Scholar] [CrossRef] [PubMed]
- Choi, O.H.; Shamim, M.T.; Padgett, W.L.; Daly, J.W. Caffeine and theophylline analogues: Correlation of behavioral effects with activity as adenosine receptor antagonists and as phosphodiesterase inhibitors. Life Sci. 1988, 43, 387–398. [Google Scholar] [CrossRef]
- Rabe, K.F.; Magnussen, H.; Dent, G. Theophylline and selective PDE inhibitors as bronchodilators and smooth muscle relaxants. Eur. Respir. J. 1995, 8, 637–642. [Google Scholar]
- Travadi, J.; Patole, S. Phosphodiesterase inhibitors for persistent pulmonary hypertension of the newborn: A review. Pediatr. Pulmonol. 2003, 36, 529–535. [Google Scholar] [CrossRef]
- Rochwerg, B.; Neupane, B.; Zhang, Y.; Garcia, C.C.; Raghu, G.; Richeldi, L.; Brozek, J.; Beyene, J.; Schünemann, H. Treatment of idiopathic pulmonary fibrosis: A network meta-analysis. BMC Med. 2016, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Wilkinson, J.; Gatzoulis, M.A. Sildenafil in primary pulmonary hypertension. N. Engl. J. Med. 2000, 343, 1342. [Google Scholar] [CrossRef]
- Sansone, A.; Mollaioli, D.; Ciocca, G.; Limoncin, E.; Colonnello, E.; Vena, W.; Jannini, E. Addressing male sexual and reproductive health in the wake of COVID-19 outbreak. J. Endocrinol. Investig. 2021, 44, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Devillier, P.; Naline, E.; Grassin-Delyle, S. The pharmacology of bitter taste receptors and their role in human airways. Pharmacol. Ther. 2015, 155, 11–21. [Google Scholar] [CrossRef]
- Deshpande, D.A.; Wang, W.C.; McIlmoyle, E.L.; Robinett, K.S.; Schillinger, R.M.; An, S.S.; Sham, J.S.K.; Liggett, S. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat. Med. 2010, 16, 1299–1304. [Google Scholar] [CrossRef] [PubMed]
- Meyerhof, W.; Batram, C.; Kuhn, C.; Brockhoff, A.; Chudoba, E.; Bufe, B.; Appendino, G.; Behrens, M. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem. Senses 2010, 35, 157–170. [Google Scholar] [CrossRef]
- Grassin-Delyle, S.; Abrial, C.; Fayad-Kobeissi, S.; Brollo, M.; Faisy, C.; Alvarez, J.-C.; Naline, E.; DeVillier, P. The expression and relaxant effect of bitter taste receptors in human bronchi. Respir. Res. 2013, 14, 134. [Google Scholar] [CrossRef] [PubMed]
- Shaik, F.A.; Singh, N.; Arakawa, M.; Duan, K.; Bhullar, R.P.; Chelikani, P. Bitter taste receptors: Extraoral roles in pathophysiology. Int. J. Biochem. Cell Biol. 2016, 77, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Sanderson, M.J. Bitter tasting compounds dilate airways by inhibiting airway smooth muscle calcium oscillations and calcium sensitivity. Br. J. Pharmacol. 2014, 171, 646–662. [Google Scholar] [CrossRef]
- Monji, F.; Siddiquee, A.A.-M.; Hashemian, F. Can pentoxifylline and similar xanthine derivatives find a niche in COVID-19 therapeutic strategies? A ray of hope in the midst of the pandemic. Eur. J. Pharmacol. 2020, 887, 173561. [Google Scholar] [CrossRef]
- Bishop, N.C.; Fitzgerald, C.; Porter, P.J.; Scanlon, G.A.; Smith, A.C. Effect of caffeine ingestion on lymphocyte counts and subset activation in vivo following strenuous cycling. Graefe’s Arch. Clin. Exp. Ophthalmol. 2004, 93, 606–613. [Google Scholar] [CrossRef]
- Chen, L.; Bell, E.M.; Browne, M.L.; Druschel, C.M.; Romitti, P.A.; Schmidt, R.J.; Burns, T.L.; Moslehi, R.; Olney, R.S.; National Birth Defects Prevention Study. Maternal caffeine consumption and risk of congenital limb deficiencies. Birth Defects Res. Part A Clin. Mol. Teratol. 2012, 94, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Dulson, D.K.; Bishop, N.C. Effect of a high and low dose of caffeine on human lymphocyte activation in response to antigen stimulation. Appl. Physiol. Nutr. Metab. 2016, 41, 224–227. [Google Scholar] [CrossRef]
- Karmouty-Quintana, H.; Xia, Y.; Blackburn, M.R. Adenosine signaling during acute and chronic disease states. J. Mol. Med. 2013, 91, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Ter Horst, S.A.; Wagenaar, G.T.; De Boer, E.; Van Gastelen, M.A.; Meijers, J.C.; Biemond, B.J.; Ben, J.H.M.; Walther, F.J. Pentoxifylline reduces fibrin deposition and prolongs survival in neonatal hyperoxic lung injury. J. Appl. Physiol. 2004, 97, 2014–2019. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Karmouty-Quintana, H.; Chen, N.-Y.; Mills, T.; Molina, J.; Blackburn, M.R.; Davies, J. Loss of CD73-mediated extracellular adenosine production exacerbates inflammation and abnormal alveolar development in newborn mice exposed to prolonged hyperoxia. Pediatr. Res. 2017, 82, 1039–1047. [Google Scholar] [CrossRef]
- Chavez-Valdez, R.; Wills-Karp, M.; Ahlawat, R.; Cristofalo, E.A.; Nathan, A.; Gauda, E. Caffeine modulates TNF-alpha production by cord blood monocytes: The role of adenosine receptors. Pediatr. Res. 2009, 65, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Endesfelder, S.; Strauß, E.; Bendix, I.; Schmitz, T.; Bührer, C. Prevention of oxygen-induced inflammatory lung injury by caffeine in neonatal rats. Oxidative Med. Cell. Longev. 2020, 2020, 3840124. [Google Scholar] [CrossRef]
- Nayak, A.P.; Villalba, D.; Deshpande, D.A. Bitter taste receptors: An answer to comprehensive asthma control? Curr. Allergy Asthma Rep. 2019, 19, 48. [Google Scholar] [CrossRef]
- Dsamou, M.; Palicki, O.; Septier, C.; Chabanet, C.; Lucchi, G.; Ducoroy, P.; Chagnon, M.-C.; Morzel, M. Salivary protein profiles and sensitivity to the bitter taste of caffeine. Chem. Senses 2011, 37, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.J.; Kofonow, J.M.; Rosen, P.L.; Siebert, A.P.; Chen, B.; Doghramji, L.; Xiong, G.; Adappa, N.D.; Palmer, J.N.; Kennedy, D.W.; et al. Bitter and sweet taste receptors regulate human upper respiratory innate immunity. J. Clin. Investig. 2014, 124, 1393–1405. [Google Scholar] [CrossRef]
- Lee, R.J.; Xiong, G.; Kofonow, J.M.; Chen, B.; Lysenko, A.; Jiang, P.; Abraham, V.; Doghramji, L.; Adappa, N.D.; Palmer, J.N.; et al. T2R38 taste receptor polymorphisms underlie susceptibility to upper respiratory infection. J. Clin. Investig. 2012, 122, 4145–4159. [Google Scholar] [CrossRef]
- Gopallawa, I.; Freund, J.R.; Lee, R.J. Bitter taste receptors stimulate phagocytosis in human macrophages through calcium, nitric oxide, and cyclic-GMP signaling. Cell. Mol. Life Sci. 2021, 78, 271–286. [Google Scholar] [CrossRef]
- Orsmark-Pietras, C.; James, A.; Konradsen, J.R.; Nordlund, B.; Söderhäll, C.; Pulkkinen, V.; Pedroletti, C.; Daham, K.; Kupczyk, M.; Dahlén, B.; et al. Transcriptome analysis reveals upregulation of bitter taste receptors in severe asthmatics. Eur. Respir. J. 2013, 42, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Ekoff, M.; Choi, J.H.; James, A.; Dahlén, B.; Nilsson, G.; Dahlén, S.E. Bitter taste receptor (TAS2R) agonists inhibit IgE-dependent mast cell activation. J. Allergy Clin. Immunol. 2014, 134, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, G.; Hu, J.-L.; Fu, X.-H.; Zeng, Y.-J.; Zhou, Y.-G.; Xiong, G.; Yang, N.; Dai, S.-S.; He, F.-T. Chronic or high dose acute caffeine treatment protects mice against oleic acid-induced acute lung injury via an adenosine A2A receptor-independent mechanism. Eur. J. Pharmacol. 2011, 654, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Horrigan, L.A.; Kelly, J.P.; Connor, T.J. Caffeine suppresses TNF-α production via activation of the cyclic AMP/protein kinase A pathway. Int. Immunopharmacol. 2004, 4, 1409–1417. [Google Scholar] [CrossRef]
- Im, H.; Ammit, A.J. The NLRP3 inflammasome: Role in airway inflammation. Clin. Exp. Allergy 2014, 44, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Callaway, J.B.; Ting, J.P.Y. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Ma, L.; Cai, C.; Gong, X. Caffeine inhibits NLRP3 Iinflammasome activation by suppressing MAPK/NF-κB and A2aR signaling in LPS-induced THP-1 macrophages. Int. J. Biol. Sci. 2019, 15, 1571–1581. [Google Scholar] [CrossRef] [PubMed]
- Dahl, J.; You, J.; Benjamin, T.L. Induction and utilization of an ATM signaling pathway by polyomavirus. J. Virol. 2005, 79, 13007–13017. [Google Scholar] [CrossRef] [PubMed]
- Daniel, R.; Marusich, E.; Argyris, E.; Zhao, R.Y.; Skalka, A.M.; Pomerantz, R.J. Caffeine inhibits human immunodeficiency virus Type 1 transduction of nondividing cells. J. Virol. 2005, 79, 2058–2065. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Heidarizadeh, M.; Entesari, M.; Esmailpour, A.; Esmailpour, M.; Moradi, R.; Sakhaee, N.; Doustkhah, E. In silico investigation on the inhibiting role of Nicotine/Caffeine by Blocking the S Protein of SARS-CoV-2 Versus ACE2 Receptor. Microorganisms 2020, 8, 1600. [Google Scholar] [CrossRef]
- Tong, S.; Su, Y.; Yu, Y.; Wu, C.; Chen, J.; Wang, S.; Jiang, J. Ribavirin therapy for severe COVID-19: A retrospective cohort study. Int. J. Antimicrob. Agents 2020, 56, 106114. [Google Scholar] [CrossRef] [PubMed]
- Elzupir, A.O. Caffeine and caffeine-containing pharmaceuticals as promising inhibitors for 3-chymotrypsin-like protease of SARS-CoV-2. J. Biomol. Struct. Dyn. 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Elmezayen, A.D.; Al-Obaidi, A.; Şahin, A.T.; Yelekçi, K. Potential inhibitors of coronavirus 3-chymotrypsin-like protease (3CL(pro)): An in silico screening of alkaloids and terpenoids from African medicinal plants. J. Biomol. Struct. Dyn. 2020, 39, 2980–2992. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero-Martínez, B.S.; Montaño, L.M.; Solís-Chagoyán, H.; Sommer, B.; Ramírez-Salinas, G.L.; Pérez-Figueroa, G.E.; Flores-Soto, E. Possible Beneficial Actions of Caffeine in SARS-CoV-2. Int. J. Mol. Sci. 2021, 22, 5460. https://doi.org/10.3390/ijms22115460
Romero-Martínez BS, Montaño LM, Solís-Chagoyán H, Sommer B, Ramírez-Salinas GL, Pérez-Figueroa GE, Flores-Soto E. Possible Beneficial Actions of Caffeine in SARS-CoV-2. International Journal of Molecular Sciences. 2021; 22(11):5460. https://doi.org/10.3390/ijms22115460
Chicago/Turabian StyleRomero-Martínez, Bianca S., Luis M. Montaño, Héctor Solís-Chagoyán, Bettina Sommer, Gemma Lizbeth Ramírez-Salinas, Gloria E. Pérez-Figueroa, and Edgar Flores-Soto. 2021. "Possible Beneficial Actions of Caffeine in SARS-CoV-2" International Journal of Molecular Sciences 22, no. 11: 5460. https://doi.org/10.3390/ijms22115460
APA StyleRomero-Martínez, B. S., Montaño, L. M., Solís-Chagoyán, H., Sommer, B., Ramírez-Salinas, G. L., Pérez-Figueroa, G. E., & Flores-Soto, E. (2021). Possible Beneficial Actions of Caffeine in SARS-CoV-2. International Journal of Molecular Sciences, 22(11), 5460. https://doi.org/10.3390/ijms22115460





