Demethoxycurcumin Suppresses Human Brain Glioblastoma Multiforme GBM 8401 Cell Xenograft Tumor in Nude Mice In Vivo
Abstract
:1. Introduction
2. Results
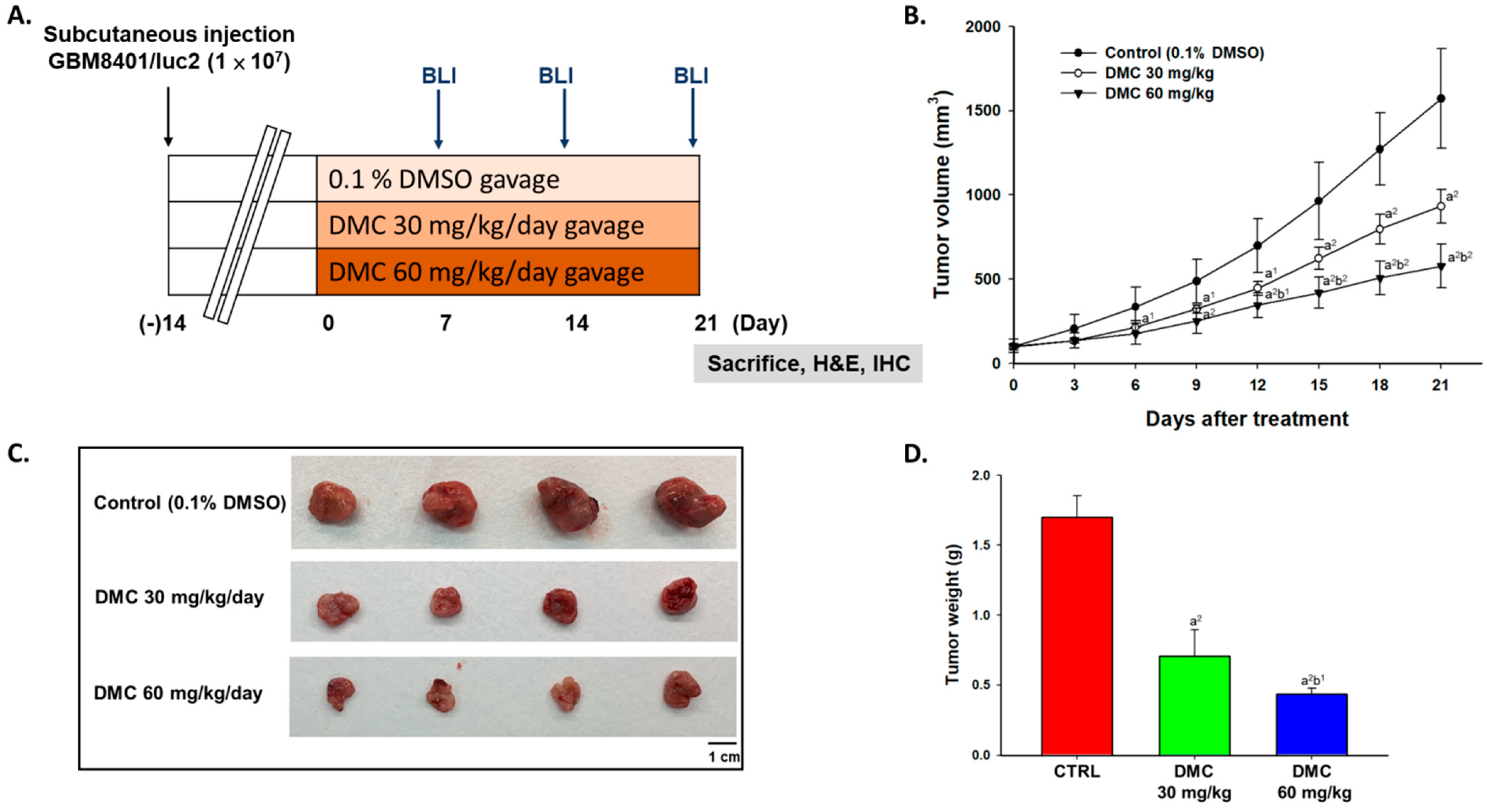
2.1. DMC Markedly Inhibited Glioblastoma Tumor Growth
2.2. DMC Markedly Reduced the Signal Intensity of Luc2 from Glioblastoma-Bearing Mice
2.3. DMC Suppressed Anti-Apoptosis and Induced Apoptosis Factors in Glioblastoma-Bearing Mice
2.4. DMC Treatment Did Not Induce Acute or Delayed Toxicity of Glioblastoma-Bearing Mice
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture of Human Glioblastoma GBM8401 Cells
4.3. Cell Transfection and Stable Clone Selection
4.4. Xenograft GBM 8401 Bearing Animal Model
4.5. Treatment and Physical Tumor Growth Validation
4.6. In Vivo Bioluminescent Imaging (BLI)
4.7. Liver Pathology and Tumor Immunohistochemistry Staining
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Kumar, V.; Aster, J.C. Robbins Basic Pathology E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Jiang, Y.; Uhrbom, L. On the origin of glioma. Upsala J. Med Sci. 2012, 117, 113–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Bao, Z.; Zhang, W.; Jiang, T. Progress on molecular biomarkers and classification of malignant gliomas. Front. Med. 2013, 7, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.W.; Huang, H.C. Effect of curcumin on cell cycle progression and apoptosis in vascular smooth muscle cells. Br. J. Pharmacol. 1998, 124, 1029–1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, J.; Lu, J.; Holmgren, A. Thioredoxin reductase is irreversibly modified by curcumin: A novel molecular mechanism for its anticancer activity. J. Biol. Chem. 2005, 280, 25284–25290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jurenka, J.S. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: A review of preclinical and clinical research. Altern. Med. Rev. A J. Clin. Ther. 2009, 14, 141–153. [Google Scholar]
- LoTempio, M.M.; Veena, M.S.; Steele, H.L.; Ramamurthy, B.; Ramalingam, T.S.; Cohen, A.N.; Chakrabarti, R.; Srivatsan, E.S.; Wang, M.B. Curcumin suppresses growth of head and neck squamous cell carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2005, 11, 6994–7002. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.C.; Patchva, S.; Koh, W.; Aggarwal, B.B. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin. Exp. Pharmacol. Physiol. 2012, 39, 283–299. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Hosseini, M.S.; Khalili, N.; Naimi, E.; Majeed, M.; Sahebkar, A. Antioxidant and anti-inflammatory effects of curcuminoid-piperine combination in subjects with metabolic syndrome: A randomized controlled trial and an updated meta-analysis. Clin. Nutr. 2015, 34, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, H.; Naseri, G.; Rezaee, R.; Mohammadi, M.; Banikazemi, Z.; Mirzaei, H.R.; Salehi, H.; Peyvandi, M.; Pawelek, J.M.; Sahebkar, A. Curcumin: A new candidate for melanoma therapy? Int. J. Cancer 2016, 139, 1683–1695. [Google Scholar] [CrossRef]
- Jankun, J.; Wyganowska-Swiatkowska, M.; Dettlaff, K.; Jelinska, A.; Surdacka, A.; Watrobska-Swietlikowska, D.; Skrzypczak-Jankun, E. Determining whether curcumin degradation/condensation is actually bioactivation (Review). Int. J. Mol. Med. 2016, 37, 1151–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamvakopoulos, C.; Dimas, K.; Sofianos, Z.D.; Hatziantoniou, S.; Han, Z.; Liu, Z.L.; Wyche, J.H.; Pantazis, P. Metabolism and anticancer activity of the curcumin analogue, dimethoxycurcumin. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2007, 13, 1269–1277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galluzzi, L.; Senovilla, L.; Vitale, I.; Michels, J.; Martins, I.; Kepp, O.; Castedo, M.; Kroemer, G. Molecular mechanisms of cisplatin resistance. Oncogene 2012, 31, 1869–1883. [Google Scholar] [CrossRef] [Green Version]
- Ni, X.; Zhang, A.; Zhao, Z.; Shen, Y.; Wang, S. Demethoxycurcumin inhibits cell proliferation, migration and invasion in prostate cancer cells. Oncol. Rep. 2012, 28, 85–90. [Google Scholar] [CrossRef]
- Huang, T.Y.; Hsu, C.W.; Chang, W.C.; Wang, M.Y.; Wu, J.F.; Hsu, Y.C. Demethoxycurcumin retards cell growth and induces apoptosis in human brain malignant glioma GBM 8401 cells. Evid.-Based Complementary Altern. Med. 2012, 2012, 396573. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Zhang, P.; Yang, H.; Ge, Y.; Xin, Y. Effects of demethoxycurcumin on the viability and apoptosis of skin cancer cells. Mol. Med. Rep. 2017, 16, 539–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, Y.C.; Lien, J.C.; Liu, H.C.; Hsu, S.C.; Lin, H.Y.; Chueh, F.S.; Ji, B.C.; Yang, M.D.; Hsu, W.H.; Chung, J.G. Demethoxycurcumin-induced DNA damage decreases DNA repair-associated protein expression levels in NCI-H460 human lung cancer cells. Anticancer Res. 2015, 35, 2691–2698. [Google Scholar]
- Ko, Y.C.; Lien, J.C.; Liu, H.C.; Hsu, S.C.; Ji, B.C.; Yang, M.D.; Hsu, W.H.; Chung, J.G. Demethoxycurcumin induces the apoptosis of human lung cancer NCI-H460 cells through the mitochondrial-dependent pathway. Oncol. Rep. 2015, 33, 2429–2437. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, Y.T.; Kuo, C.L.; Chueh, F.S.; Liu, K.C.; Bau, D.T.; Chung, J.G. Curcuminoids induce reactive oxygen species and autophagy to enhance apoptosis in human oral cancer cells. Am. J. Chin. Med. 2018, 46, 1145–1168. [Google Scholar] [CrossRef]
- Shi, L.; Fei, X.; Wang, Z. Demethoxycurcumin was prior to temozolomide on inhibiting proliferation and induced apoptosis of glioblastoma stem cells. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2015, 36, 7107–7119. [Google Scholar] [CrossRef] [PubMed]
- Leng, L.; Zhong, X.; Sun, G.; Qiu, W.; Shi, L. Demethoxycurcumin was superior to temozolomide in the inhibition of the growth of glioblastoma stem cells in vivo. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Darling, J.L. The In Vitro Biology of Human Brain Tumors; Johns Hopkins University Press: Baltimore, MD, USA, 1990; pp. 1–25. [Google Scholar]
- Slichenmyer, W.J.; Von Hoff, D.D. Taxol: A new and effective anti-cancer drug. Anti-Cancer Drugs 1991, 2, 519–530. [Google Scholar] [CrossRef]
- Caputi, L.; Franke, J.; Farrow, S.C.; Chung, K.; Payne, R.M.E.; Nguyen, T.D.; Dang, T.T.; Soares Teto Carqueijeiro, I.; Koudounas, K.; Duge de Bernonville, T.; et al. Missing enzymes in the biosynthesis of the anticancer drug vinblastine in Madagascar periwinkle. Science 2018, 360, 1235–1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brahmer, J.R.; Ettinger, D.S. The role of topotecan in the treatment of small cell lung cancer. Oncologist 1998, 3, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.W.; Bode, A.M.; Dong, Z. Molecular targets of phytochemicals for cancer prevention. Nat. Rev. Cancer 2011, 11, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.V.; Kurkjian, C.D.; Yamada, H.Y. Mitosis-targeting natural products for cancer prevention and therapy. Curr. Drug Targets 2012, 13, 1820–1830. [Google Scholar] [CrossRef]
- Hao, F.; Kumar, S.; Yadav, N.; Chandra, D. Neem components as potential agents for cancer prevention and treatment. Biochim. Et Biophys. Acta 2014, 1846, 247–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, L.; Sun, G. DMC is not better than TMZ on intracranial anti-glioma effects. J. Cell Biochem. 2018, 119, 6057–6064. [Google Scholar] [CrossRef]
- Pore, S.K.; Hahm, E.R.; Latoche, J.D.; Anderson, C.J.; Shuai, Y.; Singh, S.V. Prevention of breast cancer-induced osteolytic bone resorption by benzyl isothiocyanate. Carcinogenesis 2018, 39, 134–145. [Google Scholar] [CrossRef]
- Dairam, A.; Limson, J.L.; Watkins, G.M.; Antunes, E.; Daya, S. Curcuminoids, curcumin, and demethoxycurcumin reduce lead-induced memory deficits in male Wistar rats. J. Agric. Food Chem. 2007, 55, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Lin, Y.J.; Huang, W.T.; Hung, C.C.; Lin, H.Y.; Tu, Y.C.; Liu, D.M.; Lan, S.J.; Sheu, M.J. Demethoxycurcumin-loaded chitosan nanoparticle downregulates DNA repair pathway to improve cisplatin-induced apoptosis in non-small cell lung cancer. Molecules 2018, 23, 3217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Florentin, A.; Arama, E. Caspase levels and execution efficiencies determine the apoptotic potential of the cell. J. Cell Biol. 2012, 196, 513–527. [Google Scholar] [CrossRef] [Green Version]
- Krajewska, M.; Zapata, J.M.; Meinhold-Heerlein, I.; Hedayat, H.; Monks, A.; Bettendorf, H.; Shabaik, A.; Bubendorf, L.; Kallioniemi, O.P.; Kim, H.; et al. Expression of Bcl-2 family member Bid in normal and malignant tissues. Neoplasia 2002, 4, 129–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vellanki, S.H.; Grabrucker, A.; Liebau, S.; Proepper, C.; Eramo, A.; Braun, V.; Boeckers, T.; Debatin, K.M.; Fulda, S. Small-molecule XIAP inhibitors enhance gamma-irradiation-induced apoptosis in glioblastoma. Neoplasia 2009, 11, 743–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.H.; Yeh, M.Y.; Tu, Y.C.; Han, S.H.; Wang, Y.C. Establishment and characterization of a malignant glioma cell line, GBM8401/TSGH, NDMC. J. Surg. Oncol. 1988, 38, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.; Yeh, C.C.; Lee, J.H.; Hung, C.F.; Chung, J.G. Berberine inhibited arylamine N-acetyltransferase activity and gene expression and DNA adduct formation in human malignant astrocytoma (G9T/VGH) and brain glioblastoma multiforms (GBM 8401) cells. Neurochem. Res. 2002, 27, 883–889. [Google Scholar] [CrossRef]
- Ma, Y.S.; Lin, J.J.; Lin, C.C.; Lien, J.C.; Peng, S.F.; Fan, M.J.; Hsu, F.T.; Chung, J.G. Benzyl isothiocyanate inhibits human brain glioblastoma multiforme GBM 8401 cell xenograft tumor in nude mice in vivo. Environ. Toxicol. 2018, 33, 1097–1104. [Google Scholar] [CrossRef]
- Weng, M.C.; Li, M.H.; Chung, J.G.; Liu, Y.C.; Wu, J.Y.; Hsu, F.T.; Wang, H.E. Apoptosis induction and AKT/NF-kappaB inactivation are associated with regroafenib-inhibited tumor progression in non-small cell lung cancer in vitro and in vivo. Biomed. Pharmacother. Biomed. Pharmacother. 2019, 116, 109032. [Google Scholar] [CrossRef]
- Chiu, W.C.; Yang, H.H.; Chiang, S.C.; Chou, Y.X.; Yang, H.T. Auricularia polytricha aqueous extract supplementation decreases hepatic lipid accumulation and improves antioxidative status in animal model of nonalcoholic fatty liver. BioMedicine 2014, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Weng, M.C.; Wang, M.H.; Tsai, J.J.; Kuo, Y.C.; Liu, Y.C.; Hsu, F.T.; Wang, H.E. Regorafenib inhibits tumor progression through suppression of ERK/NF-kappaB activation in hepatocellular carcinoma bearing mice. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef] [Green Version]
- Shu, J.; Dolman, G.E.; Duan, J.; Qiu, G.; Ilyas, M. Statistical colour models: An automated digital image analysis method for quantification of histological biomarkers. Biomed. Eng. Online 2016, 15, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.-P.; Ma, Y.-S.; Kuo, C.-L.; Liao, C.-L.; Chen, P.-Y.; Peng, S.-F.; Hsu, F.-T.; Lai, K.-C. Demethoxycurcumin Suppresses Human Brain Glioblastoma Multiforme GBM 8401 Cell Xenograft Tumor in Nude Mice In Vivo. Int. J. Mol. Sci. 2021, 22, 5503. https://doi.org/10.3390/ijms22115503
Huang Y-P, Ma Y-S, Kuo C-L, Liao C-L, Chen P-Y, Peng S-F, Hsu F-T, Lai K-C. Demethoxycurcumin Suppresses Human Brain Glioblastoma Multiforme GBM 8401 Cell Xenograft Tumor in Nude Mice In Vivo. International Journal of Molecular Sciences. 2021; 22(11):5503. https://doi.org/10.3390/ijms22115503
Chicago/Turabian StyleHuang, Yi-Ping, Yi-Shih Ma, Chao-Lin Kuo, Ching-Lung Liao, Po-Yuan Chen, Shu-Fen Peng, Fei-Ting Hsu, and Kuang-Chi Lai. 2021. "Demethoxycurcumin Suppresses Human Brain Glioblastoma Multiforme GBM 8401 Cell Xenograft Tumor in Nude Mice In Vivo" International Journal of Molecular Sciences 22, no. 11: 5503. https://doi.org/10.3390/ijms22115503
APA StyleHuang, Y.-P., Ma, Y.-S., Kuo, C.-L., Liao, C.-L., Chen, P.-Y., Peng, S.-F., Hsu, F.-T., & Lai, K.-C. (2021). Demethoxycurcumin Suppresses Human Brain Glioblastoma Multiforme GBM 8401 Cell Xenograft Tumor in Nude Mice In Vivo. International Journal of Molecular Sciences, 22(11), 5503. https://doi.org/10.3390/ijms22115503






