Abstract
E3 ubiquitin ligases, the most important part of the ubiquitination process, participate in various processes of plant immune response. RBR E3 ligase is one of the E3 family members, but its functions in plant immunity are still little known. NtRNF217 is a RBR E3 ligase in tobacco based on the sequence analysis. To assess roles of NtRNF217 in tobacco responding to Ralstonia solanacearum, overexpression experiments in Nicotiana tabacum (Yunyan 87, a susceptible cultivar) were performed. The results illuminated that NtRNF217-overexpressed tobacco significantly reduced multiplication of R. solanacearum and inhibited the development of disease symptoms compared with wild-type plants. The accumulation of H2O2 and O2− in NtRNF217-OE plants was significantly higher than that in WT-Yunyan87 plants after pathogen inoculation. The activities of CAT and SOD also increased rapidly in a short time after R. solanacearum inoculation in NtRNF217-OE plants. What is more, overexpression of NtRNF217 enhanced the transcript levels of defense-related marker genes, such as NtEFE26, NtACC Oxidase, NtHIN1, NtHSR201, and NtSOD1 in NtRNF217-OE plants after R. solanacearum inoculation. The results suggested that NtRNF217 played an important role in regulating the expression of defense-related genes and the antioxidant enzymes, which resulted in resistance to R. solanacearum infection.
1. Introduction
The ubiquitin-proteasome system (UPS) is widely found in eukaryotes and plays an important role in post-translational modification of proteins in cells [1]. This system is involved in regulation of biological growth and development, as well as the adaptability of organisms to the surrounding environment [2]. The ubiquitin modification of the target protein requires the sequential action of three enzymes: E1 ubiquitin-activating enzymes, E2 ubiquitin-conjugating enzymes, and E3 ubiquitin ligases [3]. In plants, there are a large number of E3 ubiquitin ligase gene families. For example, Arabidopsis has more than one thousand E3 ubiquitin ligase genes based on the genome analysis, while there are only a small number of E1 and E2 genes [1,4,5].
According to the structure and mechanism of action, E3 ubiquitin ligases can be divided into different categories including RING (Really Interesting New Gene), U-box, HECT (homologous to E6-associated protein C-terminus), and RBR (RING1-IBR-RING2) [5,6]. The RING and U-box type ligases can function as molecular adapters for binding both the E2~ubiquitin thioester and the substrate to accomplish ubiquitin transfer directly [7,8]. For HECT-type ligases, there is a common bilobal HECT domain, which contains a larger N-lobe that interacts with E2 ligases, and a smaller C-lobe that contains the active-site cysteine [9]. The HECT E3s form a covalent bond between ubiquitin and C-lobe to form a ubiquitin-E3 thiole-ester intermediate before transferring it to the target protein [5]. The RBR or TRIAD [two RING fingers and a DRIL (double RING finger linked motif)] E3 ubiquitin ligases have common features of the larger RING and HECT ubiquitin ligases [10,11]. However, unlike single subunit RING- or HECT-style E3 ligases, RBR E3 ubiquitin ligases are multidomain proteins, containing a N terminal RING finger (RING1), an IBR (in between rings) or DRIL, and a C terminal RING finger (RING2) [12].
Ubiquitination especially involving E3 ubiquitin ligases, plays an important role in modulation of plant immunity [13,14,15]. Living in complex environment conditions, plants face a lot of abiotic and biotic stress and develop a series of adaptation mechanisms. Plants can recognize microbial- or pathogen-associated molecular patterns (MAMP/PAMP) using transmembrane pattern recognition receptors (PRRs) to trigger the PAMP/pattern-triggered immunity (PTI), which is the first line of defense response. Pathogen could secrete effectors to suppress this line of plants. Then plants would activate the effector-triggered immunity (ETI) to contrast infection [16]. More and more reports demonstrate E3 ubiquitin ligases such as the RING, U-box, HECT, and CRL types are involved in various process of plant PTI, ETI, and signal pathways [2,3,13,15]. However, the functions of RBR E3 ligases in plant immunity are still little known.
Ralstonia solanacearum is a soil-borne plant pathogenic bacterium, which is considered as a complex species with extensive diversity, and is widely distributed in tropical, subtropical, and some temperate regions [17]. This pathogen could cause plant bacterial wilt in more than 50 plant families, especially many important economic crops, resulting in huge economic losses around the world [17]. There are many strategies that have been developed to control bacterial wilt, including cultural practices, biological control, integrated management, etc. [18]. However, bacterial wilt has remained a challenging problem in agricultural crop protection. Cultivating resistant varieties is one of the most environmentally friendly, economical, and effective methods, which is regarded as a key approach for integrated management of bacterial wilt [18]. A deep understanding of the role of resistance-related genes will help with breeding new resistant varieties.
In this study, we analyze the function of RBR E3 ligase NtRNF217 in Nicotiana tabacum responses to R. solanacearum. The results of this study provide evidence for the role of RBR E3 ligases in plant immunity, which may help with disease management of R. solanacearum.
2. Results
2.1. Amino Acids Sequence Analyses of NtRNF217
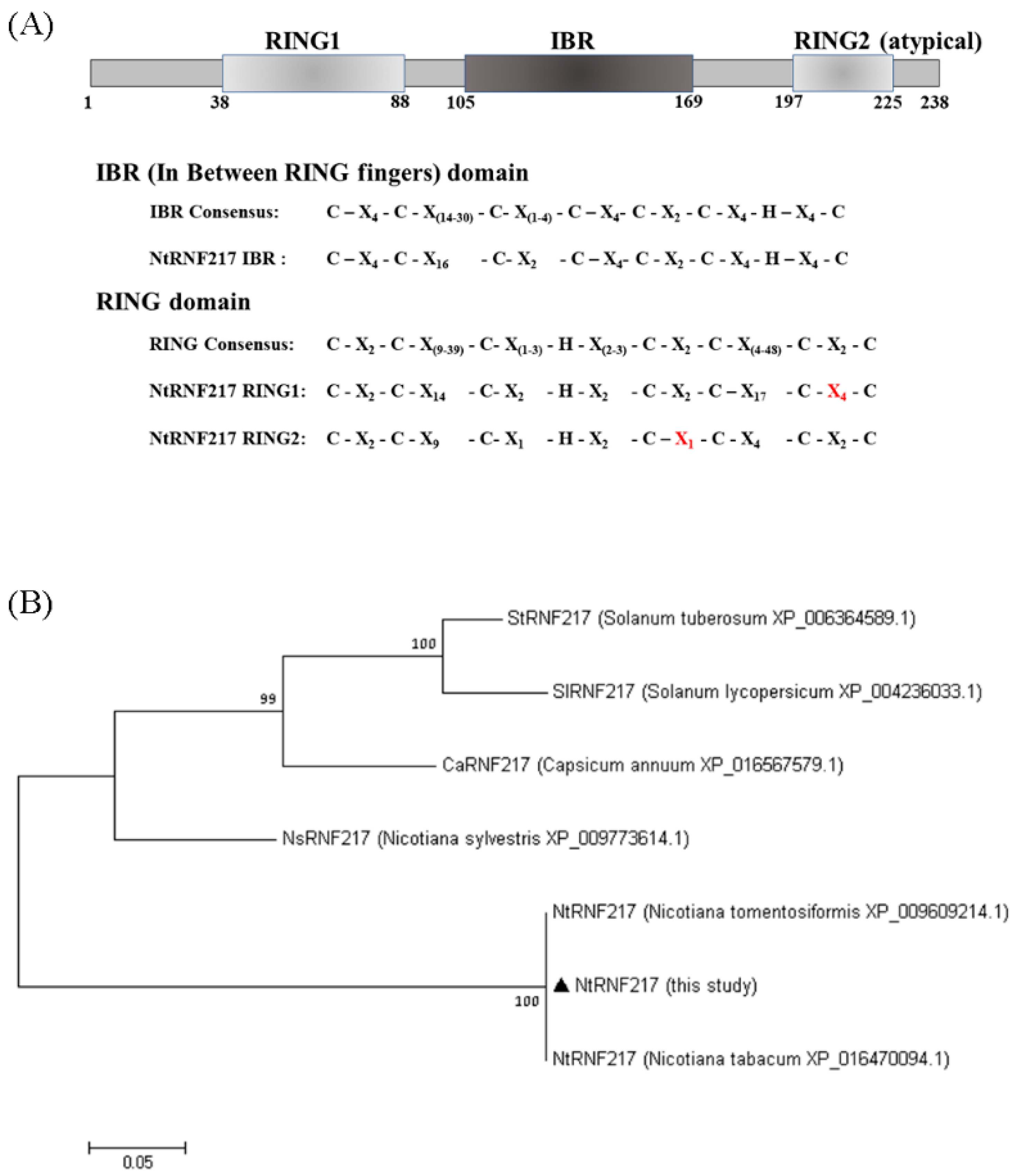
Using PROSITE search, three domains were identified in NtRNF217: a variant N terminal RING finger (RING1), an IBR, and an atypical RING finger (RING2) at the C terminal end (Figure 1A). This means that NtRNF217 belongs to the RBR E3 ligase protein. However, compared with the consensus RBR sequence, in the RING1 of NtRNF217, there were four amino acids between Cysteins 7 and 8 rather than two consensus amino acids, and in the RING2 of NtRNF217, there was only one amino acid between Cysteins 6 and 7 rather than two consensus amino acids. Aligning NtRNF217 with other RNF217 from Solanaceae, the protein sequence of N. tabacum was similar to that of N. tomentosiformis, and distant to that of Capsicum and Solanum (Figure 1B).
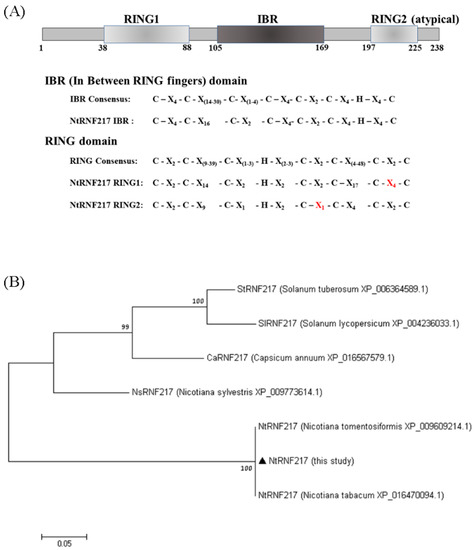
Figure 1.
Sequence analyses of NtRNF217. (A) Diagram of the NtRNF217 protein with the functional domains annotated, and comparison of the domains to the consensus sequences. RING means Really Interesting New Gene; IBR means in between rings. (B) Phylogenetic tree of RNF217 protein sequences from different species. Values at the branches indicate the percentage of bootstrap support for 1000 resamplings.
2.2. Response of NtRNF217 Transcript Levels to R. solanacearum and Exogenous Hormones
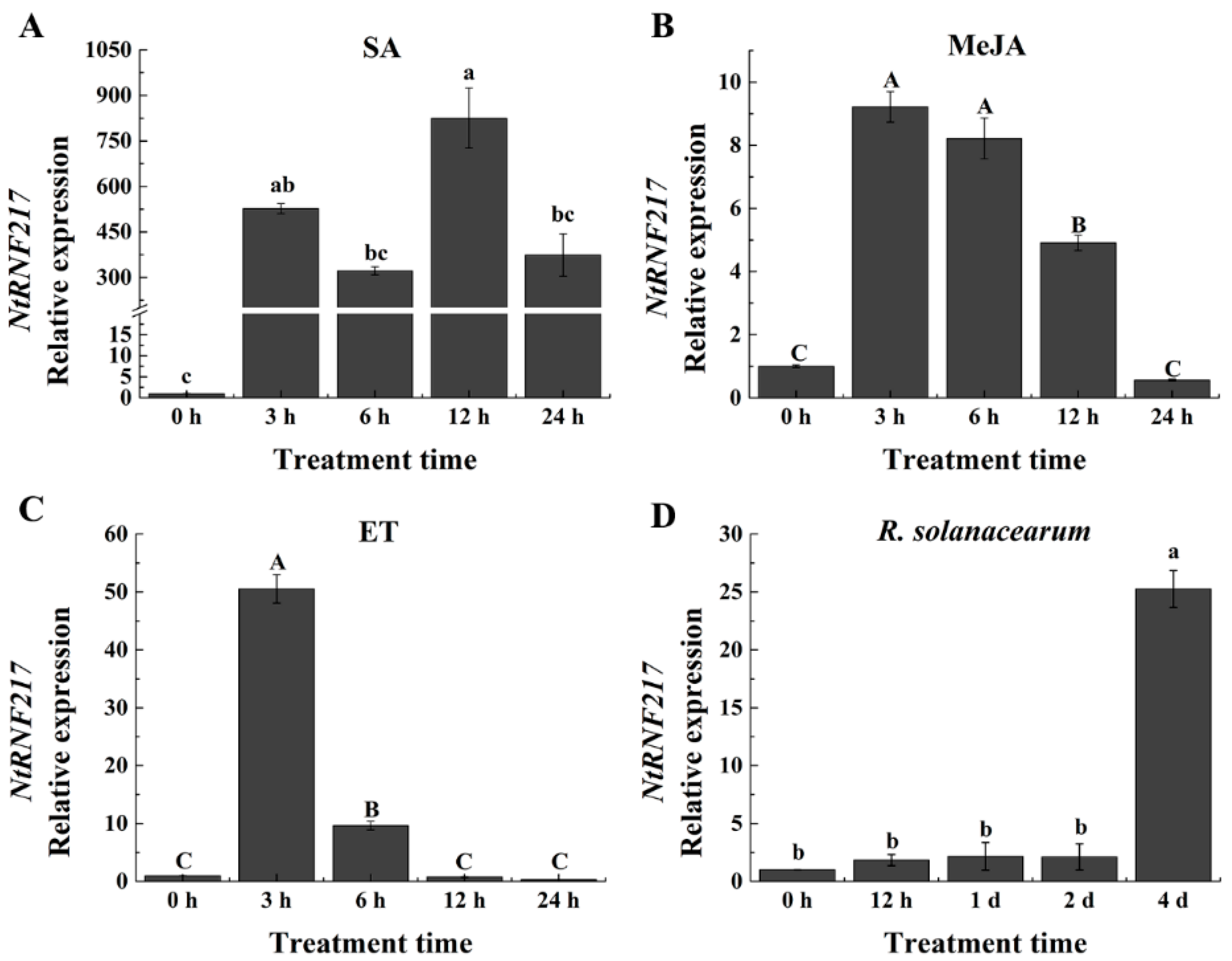
Salicylic acid (SA), jasmonic acid (JA), and ethylene (ET) are important resistance signal molecules in plants. To test whether NtRNF217 plays a role in plant resistance, qRT-PCR (quantitative real-time PCR) was used to analyze the relative expression of NtRNF217 after hormones treatment. As shown in Figure 2A–C, the expression of NtRNF217 in tobacco significantly fluctuated within 24 h post treatment (hpt) after ethephon, methyl jasmonate (MeJA), and SA treatment. We found transcript levels of NtRNF217 to be increased by 50.52-fold and 9.22-fold at 3 hpt with 7 mM ethephon and 0.1 mM MeJA application, individually, before slowly returning to the original state. In response to 2 mM SA treatment, the transcript levels of NtRNF217 were significantly enhanced, and reached the maximum at 12 hpt, almost 824.9-fold compared with the control.
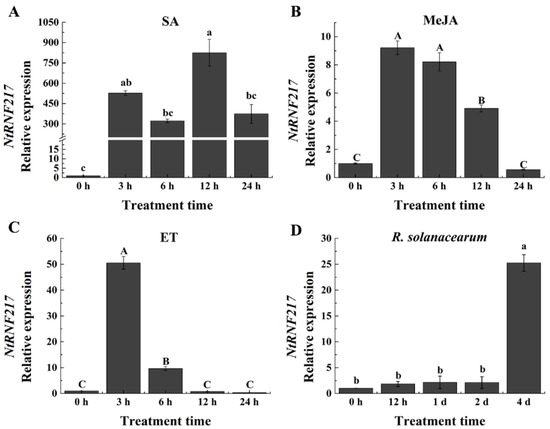
Figure 2.
qRT-PCR analysis of the relative NtRNF217 transcript levels in WT-Yunyan87 tobacco plants treated with exogenous hormones and pathogen. (A–C) NtRNF217 transcript levels measured at different time points in WT-Yunyan87 tobacco after treatment with salicylic acid (SA, 2 mM), methyl jasmonate (MeJA, 0.1 mM), and ethephon (ET, 7 mM). (D) NtRNF217 transcript levels measured in WT-Yunyan87 tobacco after R. solanacearum inoculation. Plants were inoculated by soil drenching with 10 mL cell suspension of Ralstonia solanacearum (108 CFU mL−1). Error bars indicate the standard error; values were based on three independent replicates. NtUBI3 used as the reference gene. Different letters indicated significant differences as determined by Tukey HSD (uppercase difference p-value < 0.01; lowercase difference p-value < 0.05).
The relative expression of NtRNF217 in WT-Yunyan87 also significantly changed after R. solanacearum infection, reaching the peak at the fourth day after inoculation (Figure 2D). In a word, the NtRNF217 gene is inducible by different hormones and R. solanacearum.
2.3. Overexpression of NtRNF217 Enhances Resistance of Tobacco to R. solanacearum
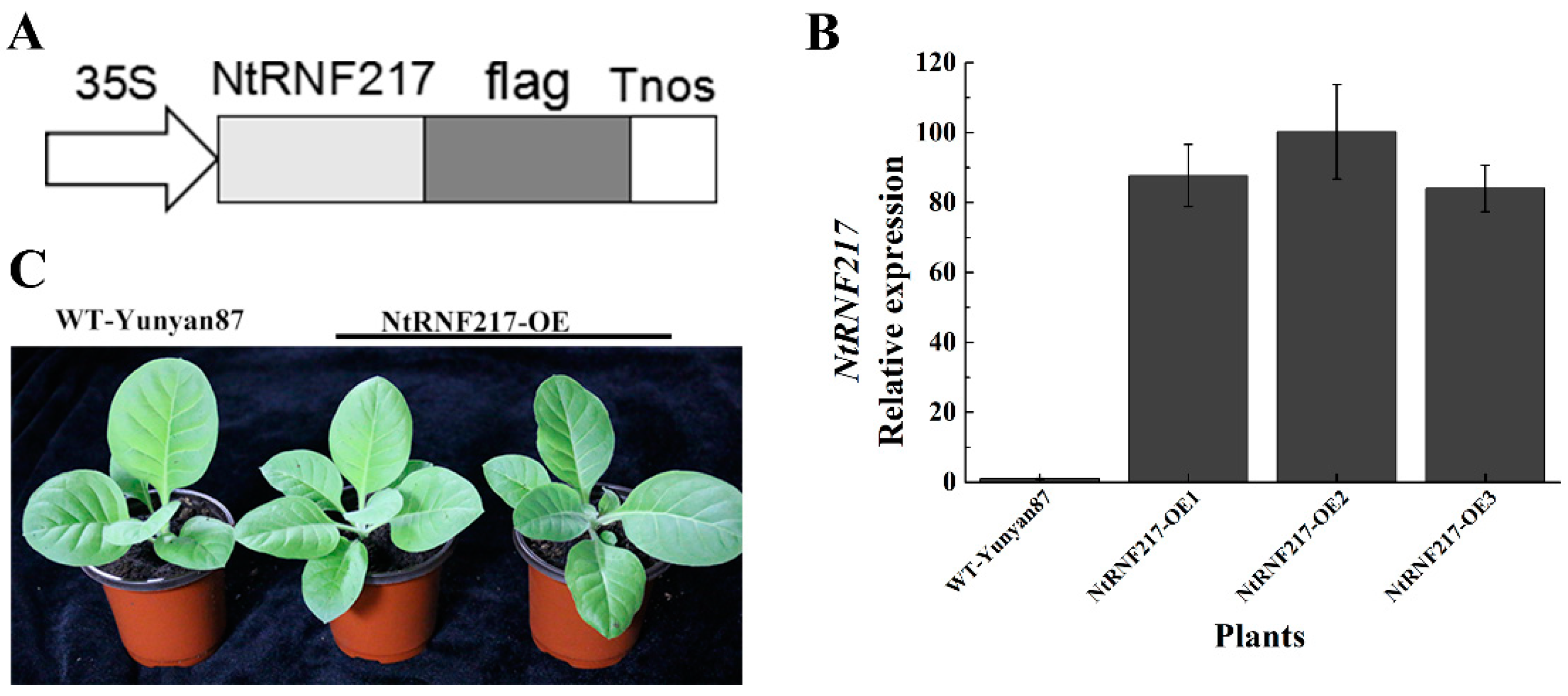
The high expression levels of NtRNF217 in response to R. solanacearum inoculation and hormone treatments had initially determined that the NtRNF217 gene played a role in plant resistance response. To clarify its role in tobacco resistance to R. solanacearum, we generated transgenic overexpression-NtRNF217 tobacco plants under the control of 35S promoter in the pBWAHS-ccdb vector (Figure 3A). Eventually, the cultivated overexpressed plants were analyzed focusing on the NtRNF217 transcript levels compared with WT-Yunyan87 plants by qRT-PCR. The result showed that the transcript levels of NtRNF217 in transgenic tobacco plants were significantly higher than for WT-Yunyan87 (Figure 3B). There were not any phenotypic differences between WT-Yunyan87 and NtRNF217-OE transgenic plants (Figure 3C), which were used in the next experiments.
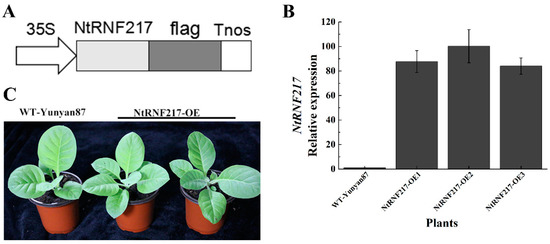
Figure 3.
Generation of NtRNF217-overexpressing tobacco plants. (A) Schematic diagram of the 35S::NtRNF217-flag fusion protein construct. (B) The relative transcripts of NtPRNF217 in transgenic plants and WT-Yunyan87 plants were tested by qRT-PCR. (C) Physiological phenotypes of NtRNF217-OE and WT-Yunyan87 plants.
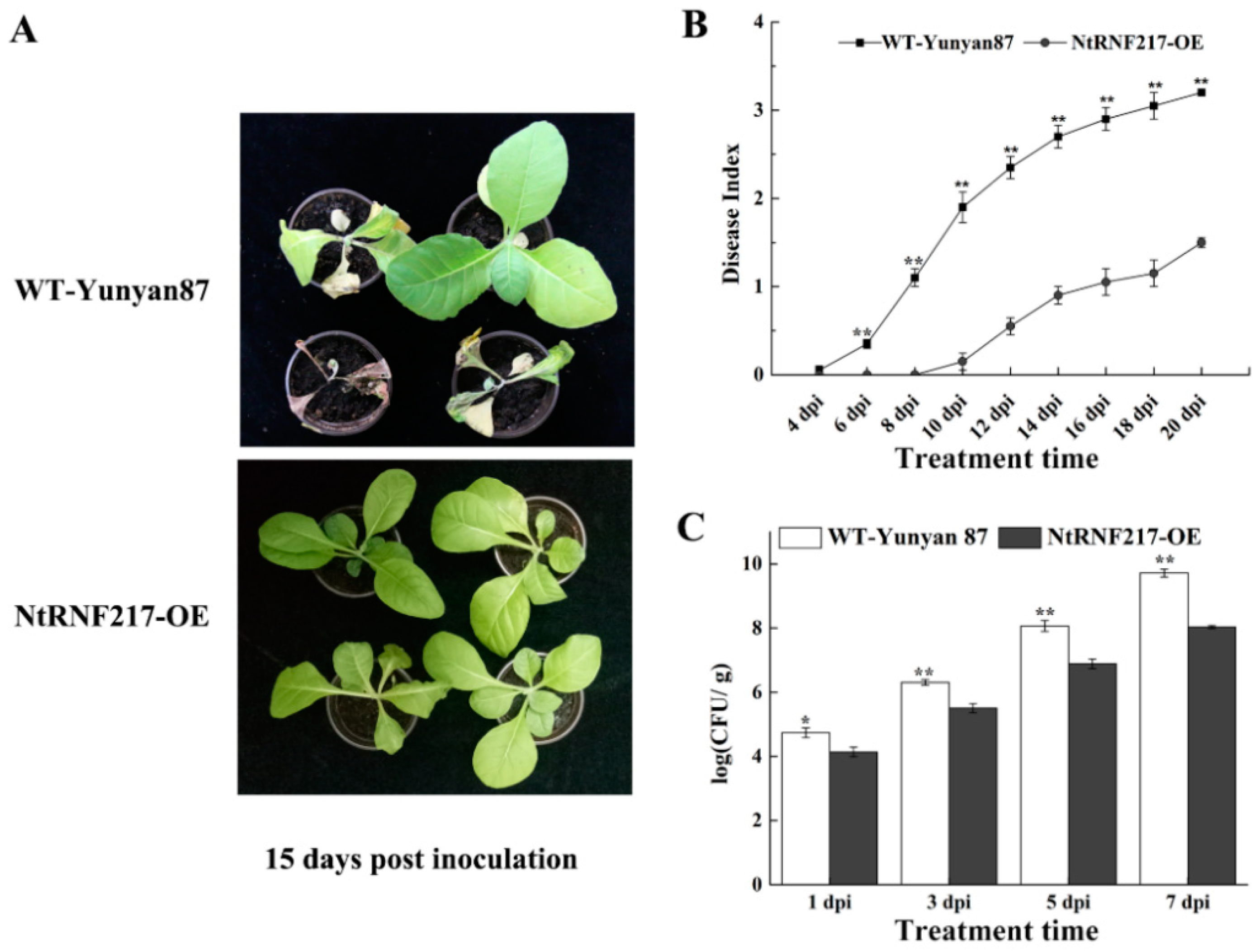
For exploring the ability of NtRNF217-OE plants to resist to bacterial wilt, plants were inoculated with the high virulent R. solanacearum strain CQPS-1 through root irrigation. At 15 days after inoculation (dpi), WT-Yunyan87 plants distinctly showed wilt symptoms, while NtRNF217-OE plants were in a healthy state (Figure 4A) and showed a significantly lower disease index (Figure 4B). Spread of R. solanacearum in the roots of NtRNF217-OE plants was also significantly lower than WT-Yunyan87 within 7 days after inoculation (Figure 4C). These results suggested that NtRNF217 can promote tobacco resistance to R. solanacearum.
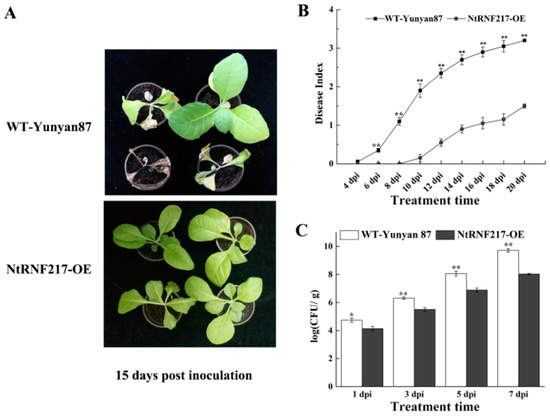
Figure 4.
Overexpression of NtRNF217 enhances tobacco resistance to Ralstonia solanacearum. (A) The phenotypes of representative NtRNF217-OE and wild-type (WT-Yunyan87) plants 15 days post inoculation with R. solanacearum. (B,C) Disease index of WT-Yunyan87 and NtRNF217-OE plants inoculated by R. solanacearum and the growth of R. solanacearum in roots. Plants were irrigated with a 10 mL suspension of the highly virulent R. solanacearum strain CQPS-1 (approximate 108 CFU mL−1). ‘dpi’ means day post inoculation. Error bars indicated the standard error of three independent biological replicates, Asterisks indicated a statistically significant (t-test, * p < 0.05 or ** p < 0.01).
2.4. Overexpression of NtRNF217 Increases the Accumulation of H2O2 and O2− Production
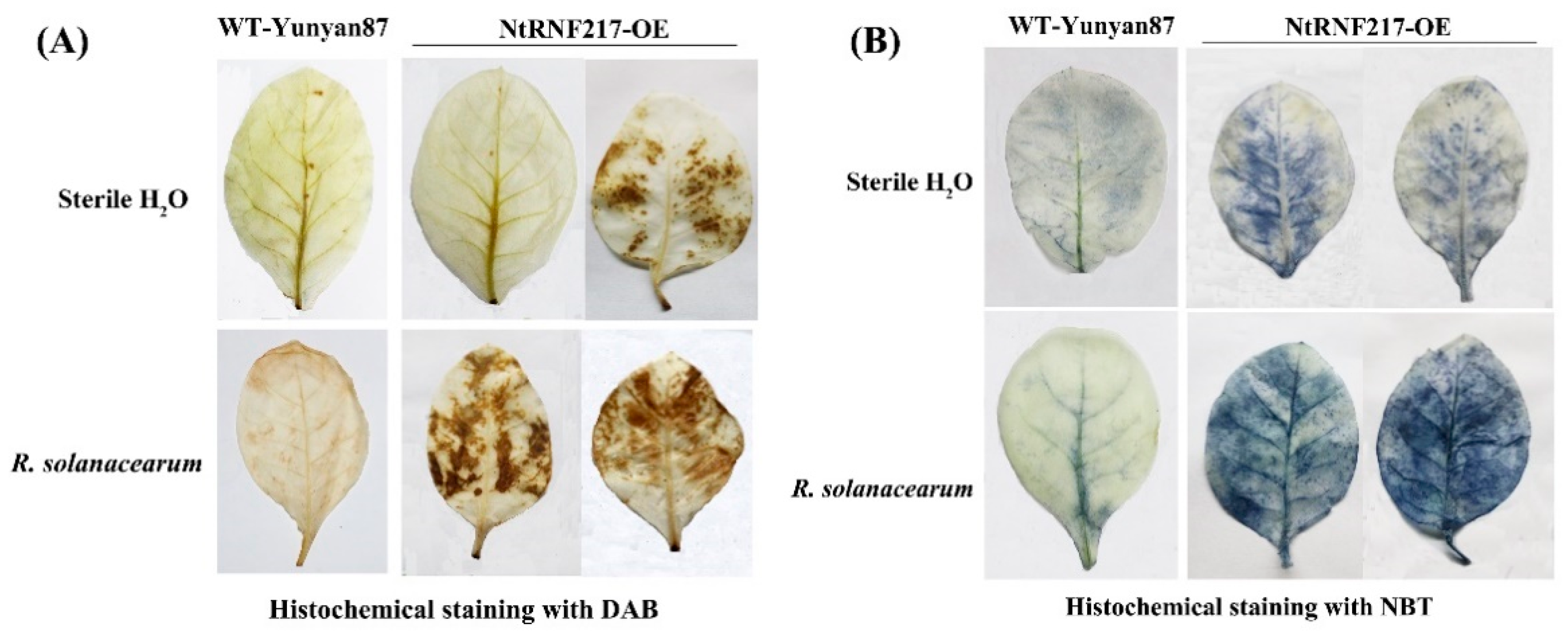
In order to observe the local defense responses of tobacco after R. solanacearum infection, H2O2 and O2− accumulation, a part of reactive oxygen species (ROS), were detected by histochemical staining. One day after inoculation by R. solanacearum, transgenic tobacco accumulated higher levels of H2O2 compared to WT-Yunyan87 tobacco, while there was no difference in the accumulation of H2O2 between NtRNF217-OE and WT-Yunyan87 plants treated with sterile water (Figure 5A). Similarly, when the bacteria were not inoculated, the production of O2− in NtRNF217-OE plants was not significantly different from WT-Yunyan87 plants. After R. solanacearum inoculation, the accumulation of O2− in NtRNF217-OE plants was significantly higher than in WT-Yunyan87 plants (Figure 5B).
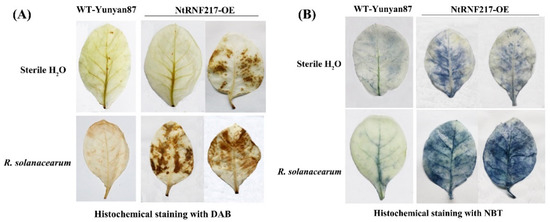
Figure 5.
Histochemical staining. DAB (A) and NBT (B) staining of WT-Yunyan87 and NtRNF217-OE plants before inoculation and after 24-h Ralstonia solanacearum inoculation. DAB: 3,3-diaminobenzidine, and NBT: nitro blue tetrazolium.
2.5. Overexpression of NtRNF217 Enhances the Antioxidant System of Tobacco
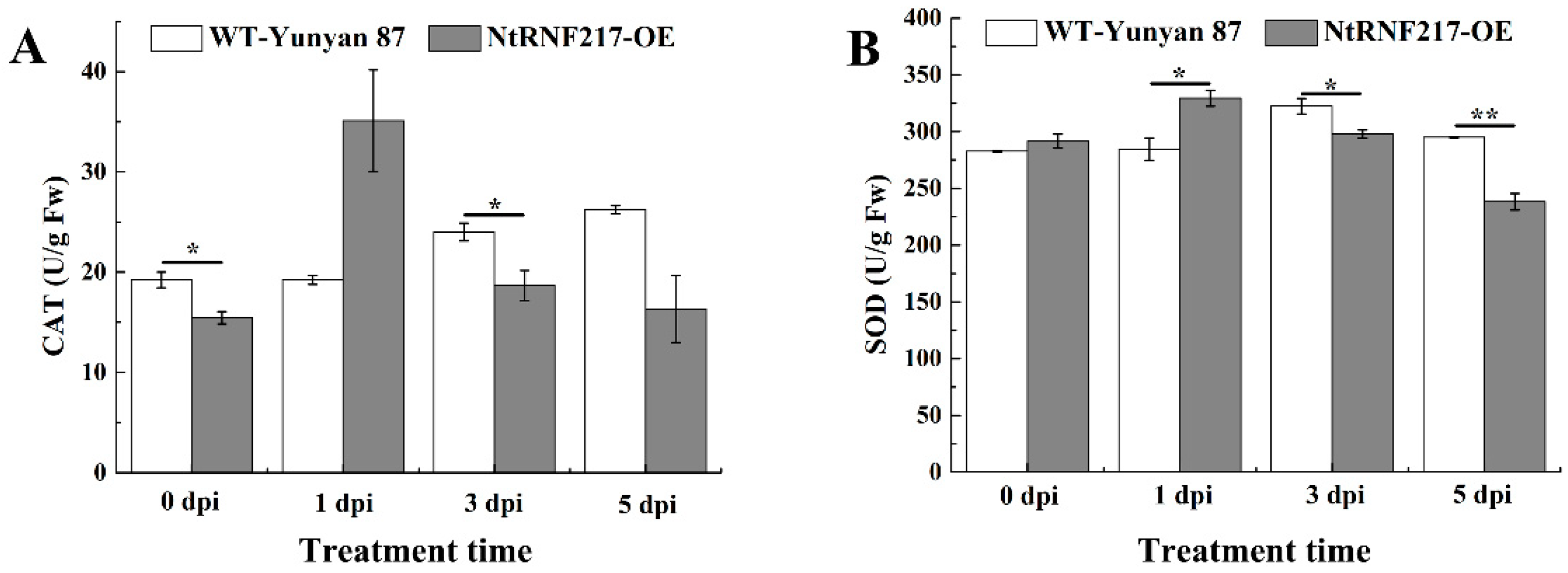
Previous experiments have shown that overexpression of NtRNF217 can enhance the accumulation of ROS. To further explore the effect of NtRNF217-overexpression on tobacco antioxidant system, the activity of catalase (CAT) and superoxide dismutase (SOD) in WT-Yunyan87 and NtRNF217-OE plants within 5 dpi after R. solanacearum inoculation were tested. As shown in Figure 6, the activities of CAT and SOD fluctuated and peaked at 1 day post inoculation in NtRNF217-OE plants, which is faster than in WT-Yunyan87 plants (Figure 6A,B). In general, the activities of the antioxidant enzymes in NtRNF217-OE plants were increased more quickly than that in wild type plants after R. solanacearum inoculation.
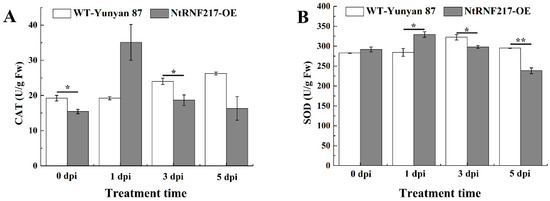
Figure 6.
The activities of CAT and SOD in WT-Yunyan87 and NtRNF217-OE plants after inoculation with 10 mL Ralstonia solanacearum suspension (approximate 108 CFU mL−1). (A) CAT activity. (B) SOD activity. dpi means day post inoculation. Error bars indicate the standard error of three overexpression lines. Asterisks indicate a significant difference determined by t-test (* p < 0.05 or ** p < 0.01).
2.6. Overexpression of NtRNF217 Activates the Expression of Defense-Related Genes
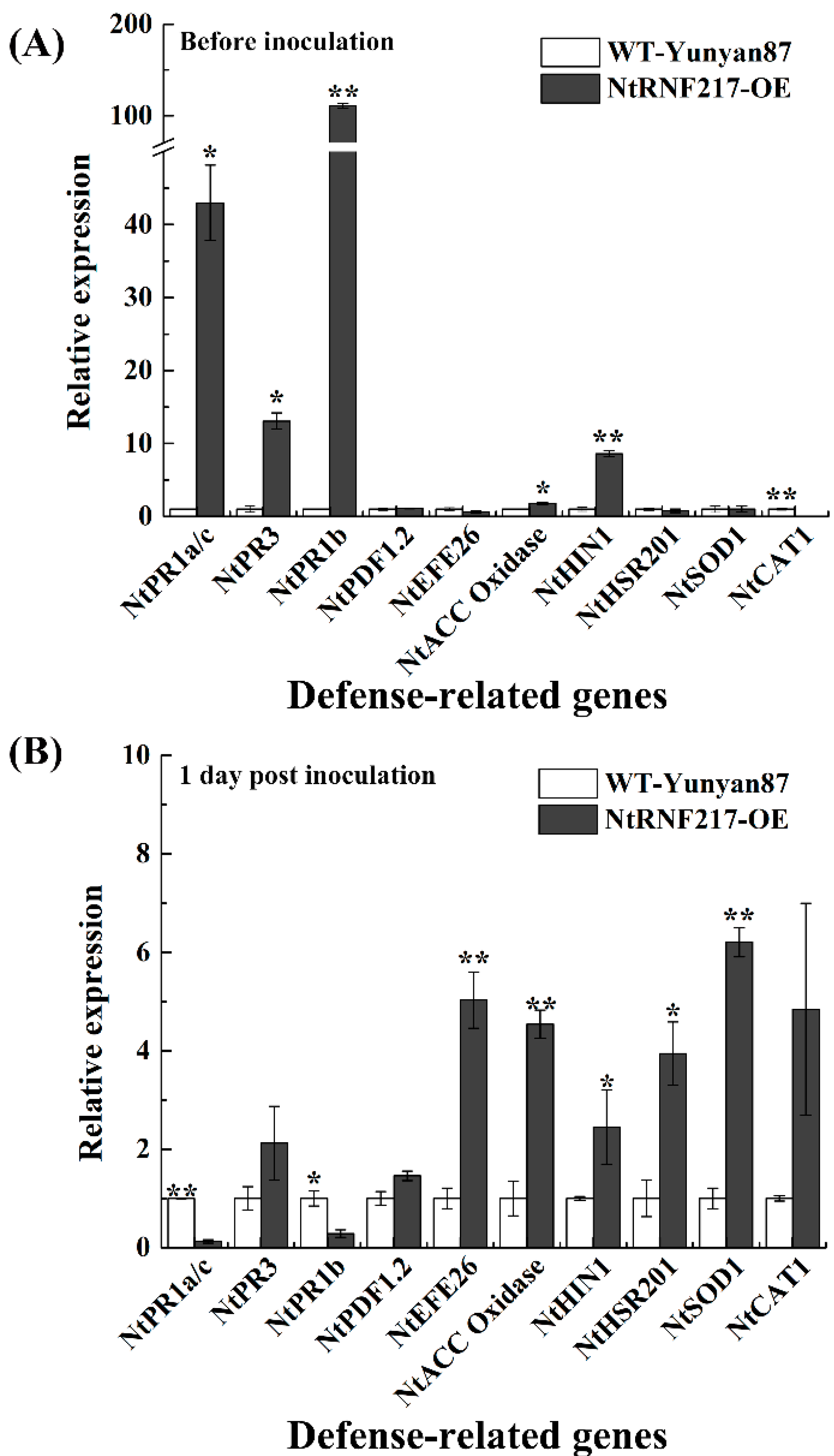
In order to further understand the resistance mechanism, the expression of defense-related genes in non-infected and infected tobacco plants were tested (Figure 7), including the SA-responsive genes NtPR1a/c and NtPR3, JA-responsive genes NtPR1b and NtPDF1.2, ET production-associated genes such as NtEFE26 and NtACC Oxidase, HR-associated genes NtHIN1 and NtHSR201, and ROS detoxification-associated genes NtCAT1 and NtSOD1 [19,20,21,22,23,24]. Before R. solanacearum infection, the transcript levels of NtPR1a/c, NtPR3, NtPR1b, NtACC Oxidase, and NtHIN1 were significantly higher in NtRNF217-OE plants compared with that in WT-Yunyan87 plants, increasing 42.97-fold, 13.06-fold, 110.81-fold, 1.77-fold, and 8.61-fold, respectively (Figure 7A). The expression levels of NtEFE26, NtACC Oxidase, NtHIN1, NtHSR201, and NtSOD1 were significantly higher in NtRNF217-OE than WT-Yunyan87 plants 24 h post inoculation, increasing 5.03-fold, 4.54-fold, 2.45-fold, 3.95-fold, and 6.21-fold, respectively (Figure 7B).
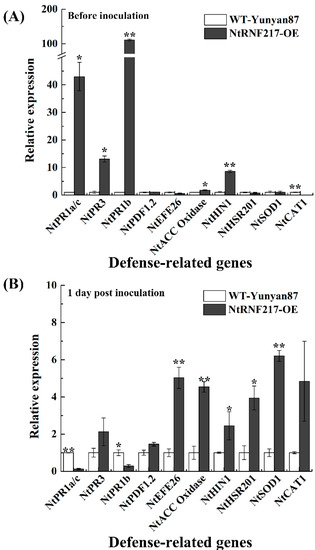
Figure 7.
The relative gene expressions before and after inoculation with Ralstonia solanacearum. qRT-PCR was performed before (A) and 1 day post inoculation (B). Plants were inoculated with 10 mL of R. solanacearum cells (approximate 108 CFU mL−1) by root irrigation. The NtUBI3 gene was used as a reference gene. The gene transcript levels in WT-Yunyan87 plants were converted to 1 and used as controls. Error bars indicate the standard error of three overexpression lines. Asterisks indicate a significant difference determined by t-test (* p < 0.05 or ** p < 0.01).
3. Discussion
E3 ubiquitin ligases have a large number of members, which mediate transportation of ubiquitin from an E2 Ub-conjugating enzyme to the target substrate, to confer specificity to ubiquitination [1]. Previous reports have shown that E3 ubiquitin ligases, such as maize RING-finger protein ZmRFP1 Gene and Pepper E3 Ubiquitin Ligase RING1 Gene CaRING1 are involved in plant immunity and regulate the adaptability of organisms to biotic and abiotic stress [25,26]. RBR E3 ligases are recently identified members of E3 ubiquitin ligases, containing two RINGs linked by an IBR domain [11]. They are important in the regulation of human and animal immune signaling, but there is still little known about their role in plant immunity [15,27,28]. In this study, we demonstrated that the RBR-type E3 ligase NtRNF217 in N. tabacum participated in immune regulation against R. solanacearum infection.
There is evidence that E3 ubiquitin ligases is important for control both of production of the HR and restriction of pathogen growth [29]. As a soil-borne plant pathogenic bacterium, R. solanacearum is able to enter root tissues from soil, and then invade the plant vascular system and cause the wilt of plants. The wilting symptoms causing by R. solanacearum are associated with strong bacterial multiplication in xylem vessels and abundant production of exopolysaccharides (EPS) [30]. The results of this study showed that overexpression of NtRNF217 in N. tabacum significantly reduced multiplication of R. solanacearum and inhibited the development of disease symptoms after irrigating pathogen compared with wild-type plants.
Histochemical staining demonstrated that NtRNF217-OE tobacco plants rapidly accumulated H2O2 and O2− after 24 h of R. solanacearum infection (Figure 5). H2O2 and O2− are part of ROS, which are usually considered as toxic by-products in the normal metabolism of plants and play important role in the defense response of plants [31,32,33]. On the one hand, ROS can trigger cell death at plant-infected sites, leading to hypersensitive response (HR) [34,35]. On the other hand, ROS may also act as a second messenger to regulate the expression of disease resistance-related genes and initiate transcription of plant antitoxin synthesis genes [36]. However, excessive accumulation of ROS would cause damage to the plant cells, so there is an antioxidant system in plants responsible for holding the steady state, which contains SOD, CAT, APX (ascorbate peroxidase), etc. [37,38]. Previous research has verified that E3 ubiquitin ligase can enhance the resistance of tobacco to abiotic stress by regulating the antioxidant system [26]. In the present study, the activities of SOD and CAT in NtRNF217-OE tobacco were increased at 1 day after inoculation (Figure 6). What is more, the relative expressions of NtCAT1 and NtSOD1 in NtRNF217-OE plants were significantly higher than in WT-Yunyan87 plants 24 h after inoculation with R. solanacearum (Figure 7). It could be speculated that NtRNF217-OE tobacco plants rapidly produce a large number of ROS when infected by R. solanacearum.
SA, JA, and ET are important signaling molecules in the plants, playing significant roles in regulating plant defense responses against different stress [39]. The expression levels of NtRNF217 were induced by exogenous SA, JA, and ET, indicating that NtRNF217 is involved in plant resistance (Figure 2). Overexpression of NtRNF217 in N. tabacum significantly improved the transcript levels of SA-responsive genes NtPR1a/c and NtPR3, JA-responsive genes NtPR1b without R. solanacearum infection (Figure 7), suggesting that NtRNF217 involved in regulating signal pathways. A previous study has reported that E3 ubiquitin ligase CUL3BPM regulated the JA signal pathway through targeted JA-pathway regulators MYC2, MYC3, and MYC4 proteins [40]. As an E3 ubiquitin ligase, the target of NtRNF217 is still unknown, which should be further studied. Solving this problem is the key to understanding how NtRNF217 gene participates in the regulation of signal pathways.
Localized HR and plant-wide systemic acquired resistance (SAR) are two well-characterized defense strategy in vascular plants [41]. SAR is a defense response that is activated at the infected site and triggered to the distal part to protect undamaged tissues [39,42]. It is known that SAR is a SA-dependent response, and is accompanied by the expression of various pathogenesis-related (PR) genes [42]. The qRT-PCR analysis showed that the NtRNF217 overexpression enhanced the expression of large range of defense-related genes, including the SA-responsive genes (Figure 7A). These results seem to support the conclusion that NtRNF217 is involved in plant SAR. Warding off diverse biotroph pathogens in plants, SAR confers broad-spectrum immunity [43]. It could be speculated that NtRNF217 may play an important role in resisting other biotic or abiotic stresses, which requires further verification.
Although a series of proteins related to R. solanacearum resistance have been reported in different plants, such as the WRKY family of pepper, PR family proteins of tobacco, etc. [44,45], there was no specific resistance gene found until now. This is also the biggest problem for breeding resistant varieties to control bacterial wilt. More research and methods should be used to analyze the functions of various resistance-related genes and regulatory networks, which would provide new ideas for future genetic breeding.
4. Materials and Methods
4.1. Plant Materials
A tobacco (N. tabacum) cultivar Yunyan 87, provided by Yuxi Zhong Yan Tobacco Seed CO., LTD, Yuxi, China, was used as a wild type (WT-Yunyan87) and for the transformation experiments in this study. The sterile plants to be used for obtaining transgenic plants were prepared following previous report [45].
4.2. Characterization of the NtRNF217 Gene and Construction of Over-Expressing Plants
Sequence of the NtRNF217 gene was assessed by BLAST and protein domains were identified using PROSITE pattern search. Alignment with reference sequences from NCBI was done using Clustal W [46]. Phylogenetic analysis was performed using neighbor-joining (NJ) and the algorithm of Poisson model with 1000 bootstrap resamplings in MEGA version 7 [47]. The GenBank accession number of NtRNF217 sequence is MK578824.
The full-length cDNA of NtRNF217 and flag sequences were amplified by RNF217 and flag primers (Table S1, see Supplementary Materials), respectively. The two sequences were inserted into Eco31I restriction sites of the binary vector pBWAHS-ccdb by digestion and ligation. The positive recombinant plasmid, pBWAHS-NtRNF217-flag, was transformed into Agrobacterium tumefaciens strain EHA 105, and then was transformed into WT-Yunyan87 using the leaf discs method [48]. Transgenic tobaccos were selected using hygromycin (25 mg L−1) and further confirmed by measuring the relative expression of NtRNF217. Three overexpression lines were screened for follow-up experiments. The overexpressed plants used for this study were obtained by asexual reproduction on MS medium [49], and then transferred to peat substrate. Greenhouse conditions were controlled at 25 ± 2 °C with 16/8 h light/dark cycle and a relative humidity of 80%.
4.3. Application of Plant Hormones and Exogenous Inducers
WT-Yunyan87 plants at the four-leaf stage were sprayed with three plant hormones as previously described [45]: MeJA (0.1 mM), ethephon (7 mM), and SA (2 mM). The treated leaves were harvested at the indicated timepoints—0, 3, 6, 12, and 24 hpt, then were frozen and stored at −80 °C for the qRT-PCR analysis. The experiment was repeated three times.
4.4. Pathogens and Inoculation Procedures
R. solanacearum strain CQPS-1 (phylotype I/biovar 3), as highly virulent pathogen in this study [50], was cultured in B medium [51]. Soil drenching was used for testing pathogenicity, monitoring bacterial growth assay in roots, detecting the expression of resistance-related genes, and evaluating the activities of CAT and SOD: plants with four to five true leaves were inoculated with 10 mL inoculum (OD600 = 0.1, approximate 108 CFU mL−1 cell suspension). The pathogenicity tests were made with 10 plants and were repeated three times. The incidence and disease index were monitored every day for 20 days using a 0 to 4 scale as described by Dang et al. [52]. The leaves were harvested before or 24 h post inoculation for preparation of RNA. The activities of CAT and SOD were determined after 1 day, 3 days, and 5 days post inoculation by collecting the leaf samples. When collecting leaf samples, consisting of mixed leaves from two plants. At indicated time points 1, 3, 5, and 7 days post inoculation, the root samples were harvested for counting the number of colonies [45].
Infiltrated inoculation was used to study H2O2 and O2− accumulation in response to R. solanacearum infections. Plants were inoculated by bacterial suspension into the second leaves from the top using a syringe without a needle, and leaf samples were harvested at 0 h and 24 h.
4.5. Histochemical Staining and SOD, CAT Activities
H2O2 and O2− accumulation were detected by histochemical staining with 3,3-diaminobenzidine (DAB) and nitro blue tetrazolium (NBT), respectively [53,54]. After 24 h of infiltrating inoculum (5 × 107 CFU mL−1) into the second leaves from the bottom using a syringe without a needle, the leaves of WT-Yunyan87 and transgenic plants were dyed with 100 µg mL−1 DAB or NBT solution. Stained leaf samples were boiled in 97% (v/v) ethanol for removing chlorophyll, then photographed directly using a camera (Canon, EW-78E, Tokyo, Japan). SOD activity was determined by a NBT light reduction method described by Qu et al. [55]. CAT was assayed by following the methods described by Qin et al. [56].
4.6. RNA Extraction, cDNA Synthesis, and Quantitative Real-Time PCR
The mixed leaves samples of two plants from different treatment (approximate 0.1 g) were frozen in liquid nitrogen and ground into powder using mortars. Total RNA was extracted by TRNzol reagent (TIANGEN, Beijing, China). Then RNA samples were reverse transcribed with iScript™ cDNA Synthesis Kit (BIO-RAD, Hercules, CA, USA). qRT-PCR was used to analyze the relative transcript levels of various genes according to the previous description [45]; primers are shown in Table S1 (see Supplementary Materials).
5. Conclusions
In summary, our study provides evidence that NtRNF217 acts as a positive regulator in response to R. solanacearum infection, depending on production of a large number of ROS, regulating an antioxidant system and defense-related genes. As a member of RBR-type E3 ligases, NtRNF217 certainly participates in ubiquitination process; however, its target protein is still unknown. Further research should be carried out to elucidate this question.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijms22115507/s1.
Author Contributions
Data curation, Y.L. and Y.T.; Funding acquisition, W.D.; Investigation, Y.T. and X.T.; Methodology, Y.L. and Y.T.; Supervision, W.D.; Writing—original draft, Y.L. and Y.T.; Writing—review & editing, Y.L. and W.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Key Project from China National Tobacco Corporation, grant number 110202001024 (JY-07). The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Smalle, J.; Vierstra, R.D. The ubiquitin 26S proteasome proteolytic pathway. Annu. Rev. Plant Biol. 2004, 55, 555–590. [Google Scholar] [CrossRef]
- Sadanandom, A.; Bailey, M.; Ewan, R.; Lee, J.; Nelis, S. The ubiquitin-proteasome system: Central modifier of plant signalling. New Phytol. 2012, 196, 13–28. [Google Scholar] [CrossRef]
- Kelley, D.R.; Estelle, M. Ubiquitin-mediated control of plant hormone signaling. Plant Physiol. 2012, 160, 47–55. [Google Scholar] [CrossRef]
- Du, Z.; Zhou, X.; Li, L.; Su, Z. plantsUPS: A database of plants’ Ubiquitin Proteasome System. BMC Genom. 2009, 10, 227. [Google Scholar] [CrossRef]
- Mazzucotelli, E.; Belloni, S.; Marone, D.; De Leonardis, A.M.; Guerra, D.; Fonzo, N.; Cattivelli, L.; Mastrangelo, A.M. The E3 ubiquitin ligase gene family in plants: Regulation by degradation. Curr. Genom. 2006, 7, 509–522. [Google Scholar] [CrossRef]
- Berndsen, C.E.; Wolberger, C. New insights into ubiquitin E3 ligase mechanism. Nat. Struct. Mol. Biol. 2014, 21, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Deshaies, R.J.; Joazeiro, C.A.P. RING domain E3 ubiquitin ligases. Annu. Rev. Biochem. 2009, 78, 399–434. [Google Scholar] [CrossRef] [PubMed]
- Metzger, M.B.; Hristova, V.A.; Weissman, A.M. HECT and RING finger families of E3 ubiquitin ligases at a glance. J. Cell Sci. 2012, 125, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Kee, Y.; Huibregtse, J.M. Regulation of catalytic activities of HECT ubiquitin ligases. Biochem. Biophys. Res. Commun. 2007, 354, 329–333. [Google Scholar] [CrossRef]
- Eisenhaber, B.; Chumak, N.; Eisenhaber, F.; Hauser, M.T. The ring between ring fingers (RBR) protein family. Genome Biol. 2007, 8, 209. [Google Scholar] [CrossRef] [PubMed]
- Spratt, D.E.; Walden, H.; Shaw, G.S. RBR E3 ubiquitin ligases: New structures, new insights, new questions. Biochem. J. 2014, 458, 421–437. [Google Scholar] [CrossRef]
- Marin, I.; Ferrus, A. Comparative Genomics of the RBR family, including the Parkinson’s disease-related gene Parkin and the genes of the Ariadne subfamily. Mol. Biol. Evol. 2002, 19, 2039–2050. [Google Scholar] [CrossRef] [PubMed]
- Marino, D.; Peeters, N.; Rivas, S. Ubiquitination during plant immune signaling. Plant Physiol. 2012, 160, 15–27. [Google Scholar] [CrossRef]
- Serrano, I.; Campos, L.; Rivas, S. Roles of E3 ubiquitin-ligases in nuclear protein homeostasis during plant stress responses. Front. Plant Sci. 2018, 9, 139. [Google Scholar] [CrossRef]
- Zhou, B.J.; Zeng, L.R. Conventional and unconventional ubiquitination in plant immunity. Mol. Plant Pathol. 2017, 18, 1313–1330. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Genin, S.; Denny, T.P. Pathogenomics of the Ralstonia solanacearum species complex. Annu. Rev. Phytopathol. 2012, 50, 67–89. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.F.; Wei, Z.; Xu, J.; Chen, H.L.; Zhang, Y.; She, X.M.; Macho, A.P.; Ding, W.; Liao, B.S. Bacterial wilt in China: History, current Status, and future perspectives. Front. Plant. Sci. 2017, 8, 1549. [Google Scholar] [CrossRef]
- Ward, E.R.; Uknes, S.J.; Williams, S.C.; Dincher, S.S.; Wiederhold, D.L.; Alexander, D.C.; Ahlgoy, P.; Metraux, J.P.; Ryals, J.A. Coordinate gene activity in response to agents that induce systemic acquired resistance. Plant Cell 1991, 3, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.-W.; Li, G.-L.; Cheng, Z.-P.; Hu, M.; Liu, W.-Q. Introns identification and transient expression analysis of NtNAC-R1 gene in tobacco (Nicotiana tabacum L.). J. Henan Agric. Sci. 2013, 42, 27–31. (In Chinese) [Google Scholar]
- Sohn, S.-I.; Kim, Y.-H.; Kim, B.-R.; Lee, S.-Y.; Lim, C.K.; Hur, J.H.; Lee, J.-Y. Transgenic tobacco expressing the hrpNEP gene from Erwinia pyrifoliae triggers defense responses against Botrytis cinerea. Mol. Cells 2007, 24, 232–239. [Google Scholar] [PubMed]
- Chen, N.; Goodwin, P.H.; Hsiang, T. The role of ethylene during the infection of Nicotiana tabacum by Colletotrichum destructivum. J. Exp. Bot. 2003, 54, 2449–2456. [Google Scholar] [CrossRef] [PubMed]
- Ghannam, A.; Jacques, A.; de Ruffray, P.; Kauffmann, S. NtRING1, putative RING-finger E3 ligase protein, is a positive regulator of the early stages of elicitin-induced HR in tobacco. Plant Cell Rep. 2016, 35, 415–428. [Google Scholar] [CrossRef]
- Takahashi, H.; Chen, Z.X.; Du, H.; Liu, Y.D.; Klessig, D.F. Development of necrosis and activation of disease resistance in transgenic tobacco plants with severely reduced catalase levels. Plant J. 1997, 11, 993–1005. [Google Scholar] [CrossRef]
- Lee, D.H.; Choi, H.W.; Hwang, B.K. The pepper E3 ubiquitin ligase RING1 Gene, CaRING1, is required for cell death and the salicylic acid-dependent defense response. Plant Physiol. 2011, 156, 2011–2025. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Xia, Z.L.; Wang, M.P.; Zhang, X.Q.; Yang, T.Z.; Wu, J.Y. Overexpression of a maize E3 ubiquitin ligase gene enhances drought tolerance through regulating stomatal aperture and antioxidant system in transgenic tobacco. Plant Physiol. Biochem. 2013, 73, 114–120. [Google Scholar] [CrossRef]
- Uchida, C.; Kitagawa, M. RING-, HECT-, and RBR-type E3 ubiquitin ligases: Involvement in human cancer. Curr. Cancer Drug Targets 2016, 16, 157–174. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Dai, X.M.; Jiang, W.X.; Li, Y.Y.; Wei, W.Y. RBR E3 ubiquitin ligases in tumorigenesis. Semin. Cancer Biol. 2020, 67, 131–144. [Google Scholar] [CrossRef]
- Devoto, A.; Muskett, P.R.; Shirasu, K. Role of ubiquitination in the regulation of plant defence against pathogens. Curr. Opin. Plant Biol. 2003, 6, 307–311. [Google Scholar] [CrossRef]
- Genin, S. Molecular traits controlling host range and adaptation to plants in Ralstonia solanacearum. New Phytol. 2010, 187, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Camejo, D.; Guzman-Cedeno, A.; Moreno, A. Reactive oxygen species, essential molecules, during plant-pathogen interactions. Plant Physiol. Biochem. 2016, 103, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Singh, S.; Parihar, P.; Mishra, R.K.; Tripathi, D.K.; Singh, V.P.; Chauhan, D.K.; Prasad, S.M. Reactive oxygen species (ROS): Beneficial companions of plants’ developmental processes. Front. Plant Sci. 2016, 7, 1299. [Google Scholar] [CrossRef]
- Van Breusegem, F.; Dat, J.F. Reactive oxygen species in plant cell death. Plant Physiol. 2006, 141, 384–390. [Google Scholar] [CrossRef]
- Levine, A.; Pennell, R.I.; Alvarez, M.E.; Palmer, R.; Lamb, C. Calcium-mediated apoptosis in a plant hypersensitive disease resistance response. Curr. Biol. 1996, 6, 427–437. [Google Scholar] [CrossRef]
- Brisson, L.F.; Tenhaken, R.; Lamb, C. Function of oxidative cross-linking of cell wall structural proteins in plant disease resistance. Plant Cell 1994, 6, 1703–1712. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Lopez-Delgado, H.; Dat, J.F.; Scott, I.M. Hydrogen peroxide- and glutathione-associated mechanisms of acclimatory stress tolerance and signalling. Physiol. Plant. 1997, 100, 241–254. [Google Scholar] [CrossRef]
- Lamb, C.; Dixon, R.A. The oxidative burst in plant disease resistance. Annu. Rev. Plant Phys. 1997, 48, 251–275. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Chico, J.M.; Lechner, E.; Fernandez-Barbero, G.; Canibano, E.; Garcia-Casado, G.; Franco-Zorrilla, J.M.; Hammann, P.; Zamarreno, A.M.; Garcia-Mina, J.M.; Rubioa, V.; et al. CUL3(BPM) E3 ubiquitin ligases regulate MYC2, MYC3, and MYC4 stability and JA responses. Proc. Natl. Acad. Sci. USA 2020, 117, 6205–6215. [Google Scholar] [CrossRef]
- Fu, Z.Q.; Dong, X.N. Systemic acquired resistance: Turning local infection into global defense. Annu. Rev. Plant Biol. 2013, 64, 839–863. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.M.; Zhu, S.F.; Kachroo, P.; Kachroo, A. Signal regulators of systemic acquired resistance. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef]
- Reimer-Michalski, E.M.; Conrath, U. Innate immune memory in plants. Semin. Immunol. 2016, 28, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Dang, F.; Wang, Y.; She, J.; Lei, Y.; Liu, Z.; Eulgem, T.; Lai, Y.; Lin, J.; Yu, L.; Lei, D.; et al. Overexpression of CaWRKY27, a subgroup IIe WRKY transcription factor of Capsicum annuum, positively regulates tobacco resistance to Ralstonia solanacearum infection. Physiol. Plant. 2014, 150, 397–411. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, Q.; Liu, Y.; Zhang, L.; Ding, W. Overexpression of NtPR-Q up-regulates multiple defense-related genes in Nicotiana tabacum and enhances plant resistance to Ralstonia solanacearum. Front. Plant. Sci. 2017, 8, 1963. [Google Scholar] [CrossRef] [PubMed]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Oh, S.K.; Park, J.M.; Joung, Y.H.; Lee, S.; Chung, E.; Kim, S.Y.; Yu, S.H.; Choi, D. A plant EPF-type zinc-finger protein, CaPIF1, involved in defence against pathogens. Mol. Plant Pathol. 2005, 6, 269–285. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, D.; Liu, Q.; Zhang, S.; Tang, Y.; Jiang, G.; Li, S.; Ding, W. The sequevar distribution of Ralstonia solanacearum in tobacco-growing zones of China is structured by elevation. Eur. J. Plant Pathol. 2017, 147, 541–551. [Google Scholar] [CrossRef]
- Boucher, C.A.; Barberis, P.A.; Demery, D.A. Transposon mutagenesis of Pseudomonas solanacearum: Isolation of Tn5-induced avirulent mutants. Microbiology 1985, 131, 2449–2457. [Google Scholar] [CrossRef]
- Dang, F.F.; Wang, Y.N.; Yu, L.; Eulgem, T.; Lai, Y.; Liu, Z.Q.; Wang, X.; Qiu, A.L.; Zhang, T.X.; Lin, J.; et al. CaWRKY40, a WRKY protein of pepper, plays an important role in the regulation of tolerance to heat stress and resistance to Ralstonia solanacearum infection. Plant Cell Environ. 2013, 36, 757–774. [Google Scholar] [CrossRef] [PubMed]
- Doke, N. Generation of superoxide anion by potato tuber protoplasts during the hypersensitive response to hyphal wall components of Phytophthora infestans and specific inhibition of the reaction by suppressors of hypersensitivity. Physiol. Plant Pathol. 1983, 23, 359–367. [Google Scholar] [CrossRef]
- Thordal-Christensen, H.; Zhang, Z.G.; Wei, Y.D.; Collinge, D.B. Subcellular localization of H2O2 in plants: H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 1997, 11, 1187–1194. [Google Scholar] [CrossRef]
- Qu, M.; Qin, L.N.; Liu, Y.J.; Fan, H.C.; Zhu, S.; Wang, J.F. The comparison of two methods of testing superoxide dismutase activity. J. Food Saf. Qual. 2014, 5, 3318–3323. (In Chinese) [Google Scholar]
- Qin, H.X.; Liu, J.M.; Song, Y.X. Detection of APX and CAT activity in AtDREB1A transgenic Yinxin poplar. Acta Agric. Jiangxi 2007, 19, 89–91. (In Chinese) [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).