An Overview on Dietary Polyphenols and Their Biopharmaceutical Classification System (BCS)
Abstract
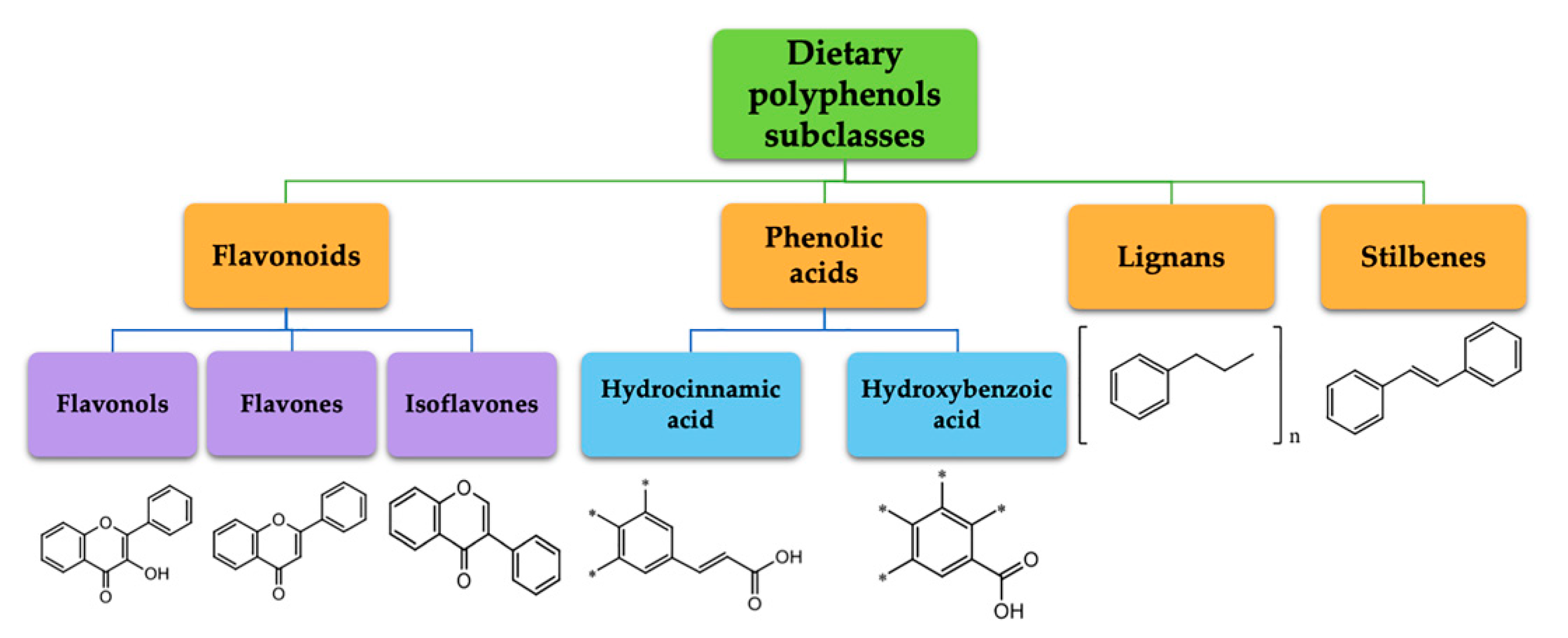
:1. Introduction
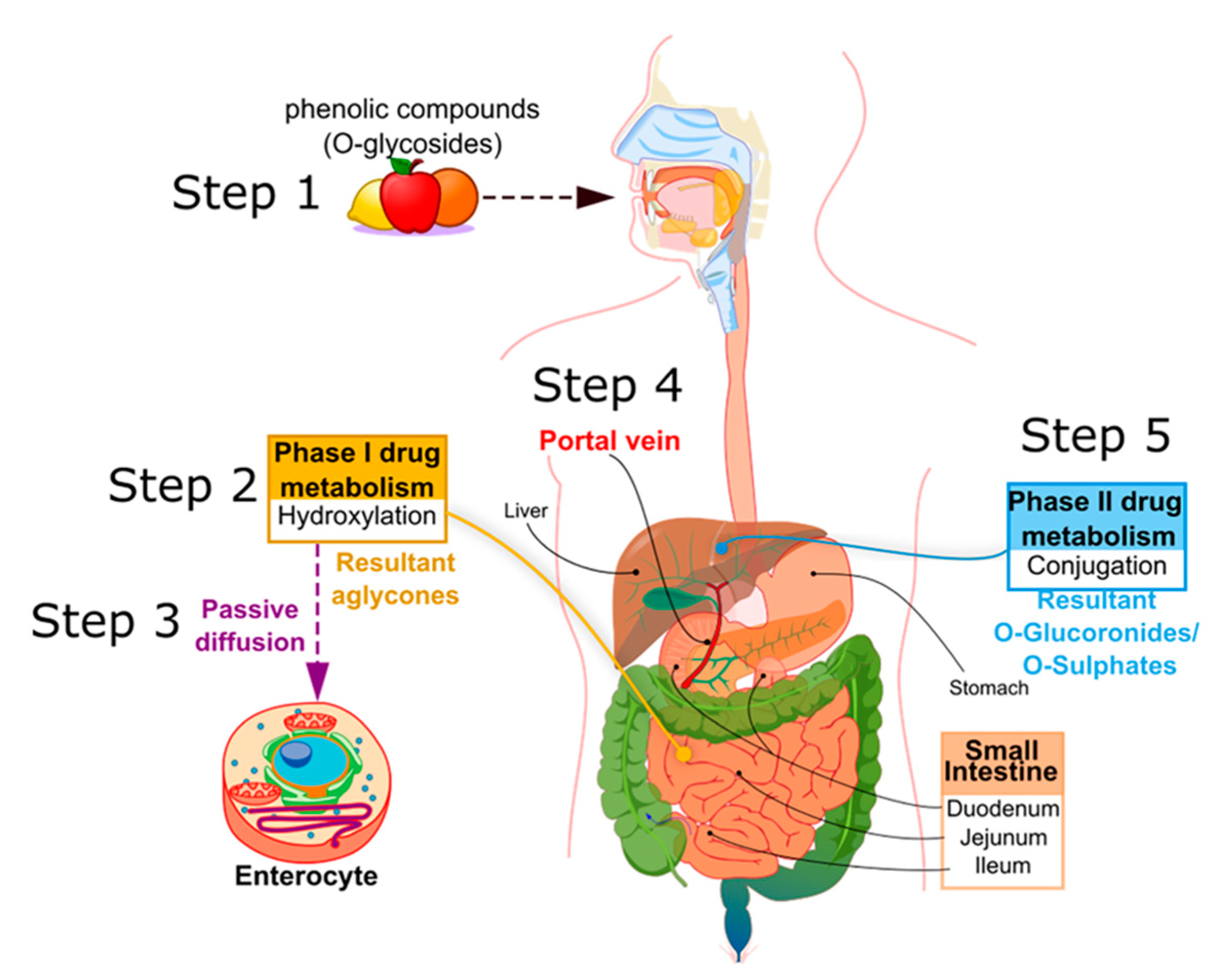
2. Polyphenol Metabolism
2.1. Metabolism Course
2.2. PC Metabolites Have Different Biological Activities
3. Polyphenol Bioavailability
3.1. Hydroxycinnamic Acids
3.2. Flavonols
3.3. Flavones
3.4. Isoflavones
3.5. Stilbenes
3.6. Tannins
3.7. Curcuminoids
4. Permeability and Solubility of Polyphenols
4.1. LogP
4.2. Solubility
5. BCS Classification of Polyphenols
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| BCS | Biopharmaceutical classification system |
| PC | Phenolic compound |
| P-gp | P-glycoprotein |
| SGLT1 | Sodium-glucose transporter |
| Nrf-2 | Nuclear factor (erythroid-derived 2)-like 2 |
| LogP | Partition coefficient logarithm P |
| RB | Rotatable bonds |
| HBA/HBD | Hydrogen-bonding acceptor/donor |
| FA | Ferulic acid |
| FDA | Food and Drug Administration |
| AUC | Area under the curve |
| LogD | Distribution coefficient logarithm D |
| QSAR | Quantitative structure-activity relationship |
| RP-HPLC | Reverse phase high-performance liquid chromatography |
| USFDA | United States Food and Drug Administration |
| WHO | World Health Organization |
| N/A | Not available |
| S | Solubility |
| API | Active pharmaceutical ingredient |
| D | Highest dose |
| D/S | Highest dose and solubility ratio |
| EMA | European Medicines Agency |
| IR | Immediate release |
| EML | List of Essential Medicines |
| SDAD | Single dietary abundant dose |
| ADI | Acceptable daily intake |
| NOAEL | No observed adverse effect level |
References
- Naczk, M.; Shahidi, F. Extraction and analysis of phenolics in food. J. Chromatogr. A 2004, 1054, 95–111. [Google Scholar] [CrossRef]
- Li, A.N.; Li, S.; Zhang, Y.J.; Xu, X.R.; Chen, Y.M.; Li, H. Bin Resources and biological activities of natural polyphenols. Nutrients. 2014, 6, 6020–6047. [Google Scholar] [CrossRef]
- González-Vallinas, M.; González-Castejón, M.; Rodríguez-Casado, A.; Ramírez de Molina, A. Dietary phytochemicals in cancer prevention and therapy: A complementary approach with promising perspectives. Nutr. Rev. 2013, 71, 585–599. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butterfield, D.A.; Castegna, A.; Pocernich, C.B.; Drake, J.; Scapagnini, G.; Calabrese, V. Nutritional approaches to combat oxidative stress in Alzheimer’s disease. J. Nutr. Biochem. 2002, 13, 444–461. [Google Scholar] [CrossRef]
- Han, X.; Shen, T.; Lou, H. Dietary polyphenols and their biological significance. Int. J. Mol. Sci. 2007, 8, 950–988. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, A.; Akoh, C.C.; Yi, W.; Fischer, J.; Krewer, G. Effect of storage conditions on the biological activity of phenolic compounds of blueberry extract packed in glass bottles. J. Agric. Food Chem. 2007, 55, 2705–2713. [Google Scholar] [CrossRef]
- Lacroix, S.; KlicicBadoux, J.; Scott-Boyer, M.P.; Parolo, S.; Matone, A.; Priami, C.; Morine, M.J.; Kaput, J.; Moco, S. A computationally driven analysis of the polyphenol-protein interactome. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of dietary polyphenols and gut microbiota metabolism: Antimicrobial properties. Biomed Res. Int. 2015, 2015, 905215. [Google Scholar] [CrossRef] [Green Version]
- Sevgi, K.; Tepe, B.; Sarikurkcu, C. Antioxidant and DNA damage protection potentials of selected phenolic acids. Food Chem. Toxicol. 2015, 77, 12–21. [Google Scholar] [CrossRef]
- Price, R.K.; Wallace, J.M.W.; Hamill, L.L.; Keaveney, E.M.; Strain, J.J.; Parker, M.J.; Welch, R.W. Evaluation of the effect of wheat aleurone-rich foods on markers of antioxidant status, inflammation and endothelial function in apparently healthy men and women. Br. J. Nutr. 2012, 108, 1644–1651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laddomada, B.; Durante, M.; Minervini, F.; Garbetta, A.; Cardinali, A.; D’Antuono, I.; Caretto, S.; Blanco, A.; Mita, G. Phytochemical composition and anti-inflammatory activity of extracts from the whole-meal flour of Italian durum wheat cultivars. Int. J. Mol. Sci. 2015, 16, 3512–3527. [Google Scholar] [CrossRef]
- Vitaglione, P.; Mennella, I.; Ferracane, R.; Rivellese, A.A.; Giacco, R.; Ercolini, D.; Gibbons, S.M.; La Storia, A.; Gilbert, J.A.; Jonnalagadda, S.; et al. Whole-grain wheat consumption reduces inflammation in a randomized controlled trial on overweight and obese subjects with unhealthy dietary and lifestyle behaviors: Role of polyphenols bound to cereal dietary fiber. Am. J. Clin. Nutr. 2015, 101, 251–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barera, A.; Buscemi, S.; Monastero, R.; Caruso, C.; Caldarella, R.; Ciaccio, M.; Vasto, S. β-glucans: Ex vivo inflammatory and oxidative stress resultsafter pasta intake. Immun. Ageing. 2016, 13, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Tighe, P.; Duthie, G.; Vaughan, N.; Brittenden, J.; Simpson, W.G.; Duthie, S.; Mutch, W.; Wahle, K.; Horgan, G.; Thies, F. Effect of increased consumption of whole-grain foods on blood pressure and other cardiovascular risk markers in healthy middle-aged persons: A randomized controlled trial. Am. J. Clin. Nutr. 2010, 92, 733–740. [Google Scholar] [CrossRef]
- Larsson, S.C.; Giovannucci, E.; Bergkvist, L.; Wolk, A. Whole grain consumption and risk of colorectal cancer: A population-based cohort of 60 000 women. Br. J. Cancer 2005, 92, 1803–1807. [Google Scholar] [CrossRef] [PubMed]
- Kyrø, C.; Skeie, G.; Loft, S.; Landberg, R.; Christensen, J.; Lund, E.; Nilsson, L.M.; Palmqvist, R.; Tjønneland, A.; Olsen, A. Intake of whole grains from different cereal and food sources and incidence of colorectal cancer in the Scandinavian HELGA cohort. Cancer Causes Control 2013, 24, 1363–1374. [Google Scholar] [CrossRef]
- Makarem, N.; Nicholson, J.M.; Bandera, E.V.; McKeown, N.M.; Parekh, N. Consumption of whole grains and cereal fiber in relation to cancer risk: A systematic review of longitudinal studies. Nutr. Rev. 2016, 74, 353–373. [Google Scholar] [CrossRef] [Green Version]
- Vattem, D.A.; Shetty, K. Biological functionality of ellagic acid: A review. J. Food Biochem. 2005, 29, 234–266. [Google Scholar] [CrossRef]
- Del Bo, C.; Bernardi, S.; Marino, M.; Porrini, M.; Tucci, M.; Guglielmetti, S.; Cherubini, A.; Carrieri, B.; Kirkup, B.; Kroon, P.; et al. Systematic Review on Polyphenol Intake and Health Outcomes: Is there Sufficient Evidence to Define a Health-Promoting Polyphenol-Rich Dietary Pattern? Nutrients 2019, 11, 1355. [Google Scholar]
- D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the polyphenols: Status and controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef]
- De-Souza, J.E.; Casanova, L.M.; Costa, S.S. Bioavailability of phenolic compounds: A major challenge for drug development? Rev. Fitos 2015, 9, 55–67. [Google Scholar]
- Kamonpatana, K.; Giusti, M.M.; Chitchumroonchokchai, C.; Morenocruz, M.; Riedl, K.M.; Kumar, P.; Failla, M.L. Susceptibility of anthocyanins to ex vivo degradation in human saliva. Food Chem. 2012, 135, 738–747. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, S.; Shinohara, M.; Nagai, T.; Konishi, Y. Transport mechanisms for soy isoflavones and microbial metabolites dihydrogenistein and dihydrodaidzein across monolayers and membranes. Biosci. Biotechnol. Biochem. 2013, 77, 2210–2217. [Google Scholar] [CrossRef] [Green Version]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; da Silva Pinto, M. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Song, J.; Shi, X.; Miao, S.; Li, Y.; Wen, A. Absorption and metabolism characteristics of rutin in caco-2 cells. Sci. World J. 2013, 13, 382350. [Google Scholar] [CrossRef]
- Jaganath, I.B.; Mullen, W.; Edwards, C.A.; Crozier, A. The relative contribution of the small and large intestine to the absorption and metabolism of rutin in man. Free Radic. Res. 2006, 40, 1035–1046. [Google Scholar] [CrossRef]
- Stevens, J.F.; Maier, C.S. The chemistry of gut microbial metabolism of polyphenols. Phytochem. Rev. 2016, 15, 425–444. [Google Scholar] [CrossRef] [Green Version]
- Aura, A.M. Microbial metabolism of dietary phenolic compounds in the colon. Phytochem. Rev. 2008, 7, 407–429. [Google Scholar] [CrossRef]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [Green Version]
- Monagas, M.; Urpi-Sarda, M.; Sánchez-Patán, F.; Llorach, R.; Garrido, I.; Gómez-Cordovés, C.; Andres-Lacueva, C.; Bartolomé, B. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct. 2010, 1, 233–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crespy, V.; Morand, C.; Besson, C.; Manach, C.; Démigné, C.; Rémésy, C. Comparison of the Intestinal Absorption of Quercetin, Phloretin and Their Glucosides in Rats. J. Nutr. 2001, 131, 2109–2114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouzzine, M.; Barré, L.; Netter, P.; Magdalou, J.; Fournel-Gigleux, S. The Human UDP-Glucuronosyltransferases: Structural Aspects and Drug Glucuronidation. Drug Metab. Rev. 2003, 35, 287–303. [Google Scholar] [CrossRef] [PubMed]
- Bang, S.H.; Hyun, Y.J.; Shim, J.; Hong, S.W.; Kim, D.H. Metabolism of rutin and poncirin by human intestinal microbiota and cloning of their metabolizing α-L-rhamnosidase from Bifidobacterium dentium. J. Microbiol. Biotechnol. 2015, 25, 18–25. [Google Scholar] [CrossRef] [Green Version]
- Rechner, A.R.; Smith, M.A.; Kuhnle, G.; Gibson, G.R.; Debnam, E.S.; Srai, S.K.S.; Moore, K.P.; Rice-Evans, C.A. Colonic metabolism of dietary polyphenols: Influence of structure on microbial fermentation products. Free Radic. Biol. Med. 2004, 36, 212–225. [Google Scholar] [CrossRef]
- Tzounis, X.; Vulevic, J.; Kuhnle, G.G.C.; George, T.; Leonczak, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P.E. Flavanol monomer-induced changes to the human faecal microflora. Br. J. Nutr. 2008, 99, 782–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minamida, K.; Tanaka, M.; Abe, A.; Sone, T.; Tomita, F.; Hara, H.; Asano, K. Production of equol from daidzein by gram-positive rod-shaped bacterium isolated from rat intestine. J. Biosci. Bioeng. 2006, 102, 247–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Decroos, K.; Vanhemmens, S.; Cattoir, S.; Boon, N.; Verstraete, W. Isolation and characterisation of an equol-producing mixed microbial culture from a human faecal sample and its activity under gastrointestinal conditions. Arch. Microbiol. 2005, 183, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Hur, H.G.; Beger, R.D.; Heinze, T.M.; Lay, J.O.; Freeman, J.P.; Dore, J.; Rafii, F. Isolation of an anaerobic intestinal bacterium capable of cleaving the C-ring of the isoflavonoid daidzein. Arch. Microbiol. 2002, 178, 8–12. [Google Scholar] [CrossRef]
- Hanske, L.; Loh, G.; Sczesny, S.; Blaut, M.; Braune, A. The Bioavailability of Apigenin-7-Glucoside Is Influenced by Human Intestinal Microbiota in Rats. J. Nutr. 2009, 139, 1095–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Rio, D.; Costa, L.G.; Lean, M.E.J.; Crozier, A. Polyphenols and health: What compounds are involved? Nutr. Metab. Cardiovasc. Dis. 2010, 20, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lee-Hilz, Y.Y.; Boerboom, A.M.J.F.; Westphal, A.H.; Van Berkel, W.J.H.; Aarts, J.M.M.J.G.; Rietjens, I.M.C.M. Pro-oxidant activity of flavonoids induces EpRE-mediated gene expression. Chem. Res. Toxicol. 2006, 19, 1499–1505. [Google Scholar] [CrossRef]
- Kumar, H.; Kim, I.S.; More, S.V.; Kim, B.W.; Choi, D.K. Natural product-derived pharmacological modulators of Nrf2/ARE pathway for chronic diseases. Nat. Prod. Rep. 2014, 31, 109–139. [Google Scholar] [CrossRef] [PubMed]
- Varì, R.; D’Archivio, M.; Filesi, C.; Carotenuto, S.; Scazzocchio, B.; Santangelo, C.; Giovannini, C.; Masella, R. Protocatechuic acid induces antioxidant/detoxifying enzyme expression through JNK-mediated Nrf2 activation in murine macrophages. J. Nutr. Biochem. 2011, 22, 409–417. [Google Scholar] [CrossRef]
- Gray, N.E.; Sampath, H.; Zweig, J.A.; Quinn, J.F.; Soumyanath, A. Centella asiatica Attenuates Amyloid-β-Induced Oxidative Stress and Mitochondrial Dysfunction. J. Alzheimer’s Dis. 2015, 45, 933–946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Z.-C.; Hong, Q.; Wang, Y.-G.; Tan, H.-L.; Xiao, C.-R.; Liang, Q.-D.; Zhang, B.-L.; Gao, Y. Ferulic acid protects human umbilical vein endothelial cells from radiation induced oxidative stress by phosphatidylinositol 3-kinase and extracellular signal-regulated kinase pathways. Biol. Pharm. Bull. 2010, 33, 29–34. [Google Scholar] [CrossRef] [Green Version]
- Williamson, G.; Clifford, M.N. Colonic metabolites of berry polyphenols: The missing link to biological activity? Br. J. Nutr. 2010, 104, S48–S66. [Google Scholar] [CrossRef] [Green Version]
- Shi, Z.H.; Li, N.G.; Wang, Z.J.; Tang, Y.P.; Dong, Z.X.; Zhang, W.; Zhang, P.X.; Gu, T.; Wu, W.Y.; Yang, J.P.; et al. Synthesis and biological evaluation of methylated scutellarein analogs based on metabolic mechanism of scutellarin in vivo. Eur. J. Med. Chem. 2015, 106, 95–105. [Google Scholar] [CrossRef]
- Domínguez-Avila, J.A.; Wall-Medrano, A.; Velderrain-Rodríguez, G.R.; Chen, C.Y.O.; Salazar-López, N.J.; Robles-Sánchez, M.; González-Aguilar, G.A. Gastrointestinal interactions, absorption, splanchnic metabolism and pharmacokinetics of orally ingested phenolic compounds. Food Funct. 2017, 8, 15–38. [Google Scholar] [CrossRef]
- Anderson, J.M. Molecular structure of tight junctions and their role in epithelial transport. News Physiol. Sci. 2001, 16, 126–130. [Google Scholar] [CrossRef]
- Matsui, T. Condensed catechins and their potential health-benefits. Eur. J. Pharmacol. 2015, 765, 495–502. [Google Scholar] [CrossRef]
- Box, K.; Comer, J. Using Measured pKa, LogP and Solubility to Investigate Supersaturation and Predict BCS Class. Curr. Drug Metab. 2008, 9, 869–878. [Google Scholar] [CrossRef]
- Iqbal, J.; Hussain, M.M. Intestinal lipid absorption. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1183–E1194. [Google Scholar] [CrossRef] [Green Version]
- Soler, A.; Romero, M.P.; Macià, A.; Saha, S.; Furniss, C.S.M.; Kroon, P.A.; Motilva, M.J. Digestion stability and evaluation of the metabolism and transport of olive oil phenols in the human small-intestinal epithelial Caco-2/TC7 cell line. Food Chem. 2010, 119, 703–714. [Google Scholar] [CrossRef]
- Rastogi, H.; Jana, S. Evaluation of physicochemical properties and intestinal permeability of six dietary polyphenols in human intestinal colon adenocarcinoma Caco-2 cells. Eur. J. Drug Metab. Pharmacokinet. 2016, 41, 33–43. [Google Scholar] [CrossRef]
- Poquet, L.; Clifford, M.N.; Williamson, G. Transport and metabolism of ferulic acid through the colonic epithelium. Drug Metab. Dispos. 2008, 36, 190–197. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Vázquez-Sánchez, K.; López-Barrera, D.; Loarca-Piña, G.; Mendoza-Díaz, S.; Oomah, B.D. Simulated gastrointestinal digestion and in vitro colonic fermentation of spent coffee (Coffea arabica L.): Bioaccessibility and intestinal permeability. Food Res. Int. 2015, 77, 156–161. [Google Scholar] [CrossRef]
- Piwowarski, J.P.; Granica, S.; Zwierzyńska, M.; Stefańska, J.; Schopohl, P.; Melzig, M.F.; Kiss, A.K. Role of human gut microbiota metabolism in the anti-inflammatory effect of traditionally used ellagitannin-rich plant materials. J. Ethnopharmacol. 2014, 155, 801–809. [Google Scholar] [CrossRef]
- D’Archivio, M.; Filesi, C.; Di Benedetto, R.; Gargiulo, R.; Giovannini, C.; Masella, R. Polyphenols, dietary sources and bioavailability. Ann. Ist. Super. Sanita 2007, 43, 348–361. [Google Scholar]
- Marrugat, J.; Covas, M.I.; Fitó, M.; Schröder, H.; Miró-Casas, E.; Gimeno, E.; López-Sabater, M.C.; De La Torre, R.; Farré, M. Effects of differing phenolic content in dietary olive oil on lipids and LDL oxidation: A randomized controlled trial. Eur. J. Nutr. 2004, 43, 140–147. [Google Scholar] [CrossRef]
- Fitó, M.; Guxens, M.; Corella, D.; Sáez, G.; Estruch, R.; de la Torre, R.; Francés, F.; Cabezas, C.; López-Sabater, M. del C.; Marrugat, J.; et al. Effect of a Traditional Mediterranean Diet on Lipoprotein Oxidation. Arch. Intern. Med. 2007, 167, 1195–1203. [Google Scholar] [CrossRef]
- Tian, Q.; Giusti, M.M.; Stoner, G.D.; Schwartz, S.J. Urinary excretion of black raspberry (Rubus occidentalis) anthocyanins and their metabolites. J. Agric. Food Chem. 2006, 54, 1467–1472. [Google Scholar] [CrossRef]
- Setchell, K.D.R.; Brown, N.M.; Zimmer-Nechemias, L.; Brashear, W.T.; Wolfe, B.E.; Kirschner, A.S.; Heubi, J.E. Evidence for lack of absorption of soy isoflavone glycosides in humans, supporting the crucial role of intestinal metabolism for bioavailability. Am. J. Clin. Nutr. 2002, 76, 447–453. [Google Scholar] [CrossRef] [Green Version]
- Regev-Shoshani, G.; Shoseyov, O.; Bilkis, I.; Kerem, Z. Glycosylation of resveratrol protects it from enzymic oxidation. Biochem. J. 2003, 374, 157–163. [Google Scholar] [CrossRef] [Green Version]
- CDER/FDA Guidances for industry: Waiver of in vivo bioavailability and bioequivalence studies for immediate-release solid oral dosage forms based on a Biopharmaceutics. In Guidance for Industry on Waiver of In Vivo Bioavailability and Bioequivalence Studies for Immediate Release Solid Oral Dosage Forms Based on a Biopharmaceutics Classification System; Center for Drug Evaluation and Research, Food and Drug Administration: Rockville, MD, USA, 2000.
- Chen, M.L.; Amidon, G.L.; Benet, L.Z.; Lennernas, H.; Yu, L.X. The BCS, BDDCS, and regulatory guidances. Pharm. Res. 2011, 28, 1774–1778. [Google Scholar] [CrossRef]
- Chen, M.L.; Yu, L. The use of drug metabolism for prediction of intestinal permeability. Mol. Pharm. 2009, 6, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Benet, L.Z.; Larregieu, C.A. The FDA should eliminate the ambiguities in the current BCS biowaiver guidance and make public the drugs for which bcs biowaivers have been granted. Clin. Pharmacol. Ther. 2010, 88, 405–407. [Google Scholar] [CrossRef] [Green Version]
- Scheff, J.D.; Almon, R.R.; Dubois, D.C.; Jusko, W.J.; Androulakis, I.P. Assessment of pharmacologic area under the curve when baselines are variable. Pharm. Res. 2011, 28, 1081–1089. [Google Scholar] [CrossRef]
- Heaney, R.P. Factors influencing the measurement of bioavailability, taking calcium as a model. J. Nutr. 2001, 131, 1344S–1348S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lempereur, I.; Rouau, X.; Abecassis, J. Genetic and agronomic variation in arabinoxylan and ferulic acid contents of durum wheat (Triticum durum l.) grain and its milling fractions. J. Cereal Sci. 1997, 25, 103–110. [Google Scholar] [CrossRef]
- Zhao, Z.; Egashira, Y.; Sanada, H. Ferulic Acid Is Quickly Absorbed from Rat Stomach as the Free Form and Then Conjugated Mainly in Liver. J. Nutr. 2004, 134, 3083–3088. [Google Scholar] [CrossRef] [PubMed]
- Adam, A.; Crespy, V.; Levrat-Verny, M.-A.; Leenhardt, F.; Leuillet, M.; Demigné, C.; Rémésy, C. The Bioavailability of Ferulic Acid Is Governed Primarily by the Food Matrix Rather than Its Metabolism in Intestine and Liver in Rats. J. Nutr. 2002, 132, 1962–1968. [Google Scholar] [CrossRef]
- Rondini, L.; Peyrat-Maillard, M.N.; Marsset-Baglieri, A.; Berset, C. Sulfated ferulic acid is the main in vivo metabolite found after short-term ingestion of free ferulic acid in rats. J. Agric. Food Chem. 2002, 50, 3037–3041. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P.E.; Chowrimootoo, G.; Choudhury, R.; Debnam, E.S.; Srai, S.K.; Rice-Evans, C. The small intestine can both absorb and glucuronidate luminal flavonoids. FEBS Lett. 1999, 458, 224–230. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Egashira, Y.; Sanada, H. Ferulic Acid Sugar Esters Are Recovered in Rat Plasma and Urine Mainly as the Sulfoglucuronide of Ferulic Acid. J. Nutr. 2003, 133, 1355–1361. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Zhang, Y.; Mi, S.; Wang, N. Pharmacokinetics of ferulic acid and potential interactions with Honghua and clopidogrel in rats. J. Ethnopharmacol. 2011, 137, 562–567. [Google Scholar] [CrossRef]
- Li, W.; Guo, J.; Tang, Y.; Wang, H.; Huang, M.; Qian, D.; Duan, J.A. Pharmacokinetic comparison of ferulic acid in normal and blood deficiency rats after oral administration of angelica sinensis, ligusticum chuanxiong and their combination. Int. J. Mol. Sci. 2012, 13, 3583–3597. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, Z.; Zhao, M.; Tang, J.; Pan, L. In vivo pharmacokinetic comparisons of ferulic acid and puerarin after oral administration of monomer, medicinal substance aqueous extract and Nao-De-Sheng to rats. Pharmacogn. Mag. 2012, 8, 256. [Google Scholar] [CrossRef] [Green Version]
- Yan, N.; Tang, Z.; Xu, Y.; Li, X.; Wang, Q. Pharmacokinetic Study of Ferulic Acid Following Transdermal or Intragastric Administration in Rats. AAPS PharmSciTech 2020, 21, 1–7. [Google Scholar] [CrossRef]
- Li, J.; Bai, Y.; Bai, Y.; Zhu, R.; Liu, W.; Cao, J.; An, M.; Tan, Z.; Chang, Y.X. Pharmacokinetics of Caffeic Acid, Ferulic Acid, Formononetin, Cryptotanshinone, and Tanshinone IIA after Oral Administration of Naoxintong Capsule in Rat by HPLC-MS/MS. Evidence-based Complement. Altern. Med. 2017, 2017, 9057238. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, T.; Pei, Q.; Liu, S.; Yuan, H. Pharmacokinetics and tissue distribution study of chlorogenic acid from loniceraejaponicaeflos following oral administrations in rats. Evidence-based Complement. Altern. Med. 2014, 2014, 979414. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.W.; Kim, J.M.; Jeong, J.S.; Son, M.; Lee, H.S.; Lee, M.G.; Kang, H.E. Pharmacokinetics of chlorogenic acid and corydaline in DA-9701, a new botanical gastroprokinetic agent, in rats. Xenobiotica 2014, 44, 635–643. [Google Scholar] [CrossRef]
- Nardini, M.; Cirillo, E.; Natella, F.; Scaccini, C. Absorption of phenolic acids in humans after coffee consumption. J. Agric. Food Chem. 2002, 50, 5735–5741. [Google Scholar] [CrossRef]
- Qi, W.; Zhao, T.; Yang, W.W.; Wang, G.H.; Yu, H.; Zhao, H.X.; Yang, C.; Sun, L.X. Comparative pharmacokinetics of chlorogenic acid after oral administration in rats. J. Pharm. Anal. 2011, 1, 270–274. [Google Scholar] [CrossRef] [Green Version]
- Atanassova, M.; Bagdassarian, V. Rutin Content in Plant Products. J. Univ. Chem. Technol. Metall. 2009, 44, 201–203. [Google Scholar]
- Justesen, U.; Knuthsen, P. Composition of flavonoids in fresh herbs and calculation of flavonoid intake by use of herbs in traditional Danish dishes. Food Chem. 2001, 73, 245–250. [Google Scholar] [CrossRef]
- Boyle, S.P.; Dobson, V.L.; Duthie, S.J.; Hinselwood, D.C.; Kyle, J.A.M.; Collins, A.R. Bioavailability and efficiency of rutin as an antioxidant: A human supplementation study. Eur. J. Clin. Nutr. 2000, 54, 774–782. [Google Scholar] [CrossRef]
- Yang, C.Y.; Hsiu, S.L.; Wen, K.C.; Lin, S.P.; Tsai, S.Y.; Hou, Y.C.; Chao, P.D.L. Bioavailability and metabolic pharmacokinetics of rutin and quercetin in rats. J. Food Drug Anal. 2005, 13, 244–250. [Google Scholar]
- Dong, X.; Lan, W.; Yin, X.; Yang, C.; Wang, W.; Ni, J. Simultaneous Determination and Pharmacokinetic Study of Quercetin, Luteolin, and Apigenin in Rat Plasma after Oral Administration of Matricaria chamomilla L. Extract by HPLC-UV. Evidence-based Complement. Altern. Med. 2017, 2017, 8370584. [Google Scholar] [CrossRef] [Green Version]
- Graefe, E.U.; Wittig, J.; Mueller, S.; Riethling, A.K.; Uehleke, B.; Drewelow, B.; Pforte, H.; Jacobasch, G.; Derendorf, H.; Veit, M. Pharmacokinetics and bioavailability of quercetin glycosides in humans. J. Clin. Pharmacol. 2001, 41, 492–499. [Google Scholar] [CrossRef]
- Ou-yang, Z.; Cao, X.; Wei, Y.; Zhang, W.-W.-Q.; Zhao, M.; Anh Tuan, L. Pharmacokinetic study of rutin and quercetin in rats after oral administration of total flavones of mulberry leaf extract. Rev. Bras. Farmacogn. 2013, 23, 776–782. [Google Scholar] [CrossRef]
- Kasikci, M.B.; Bagdatlioglu, N. Bioavailability of quercetin. Curr. Res. Nutr. Food Sci. 2016, 4, 146–151. [Google Scholar] [CrossRef]
- Shimoi, K.; Okada, H.; Furugori, M.; Goda, T.; Takase, S.; Suzuki, M.; Hara, Y.; Yamamoto, H.; Kinae, N. Intestinal absorption of luteolin and luteolin 7-O-β-glucoside in rats and humans. FEBS Lett. 1998, 438, 220–224. [Google Scholar] [CrossRef]
- Telange, D.R.; Patil, A.T.; Pethe, A.M.; Fegade, H.; Anand, S.; Dave, V.S. Formulation and characterization of an apigenin-phospholipid phytosome (APLC) for improved solubility, in vivo bioavailability, and antioxidant potential. Eur. J. Pharm. Sci. 2017, 108, 36–49. [Google Scholar] [CrossRef] [Green Version]
- Ding, S.M.; Zhang, Z.H.; Song, J.; Cheng, X.D.; Jiang, J.; Jia, X. Enhanced bioavailability of apigenin via preparation of a carbon nanopowder solid dispersion. Int. J. Nanomed. 2014, 9, 2327. [Google Scholar] [CrossRef] [Green Version]
- Meyer, H.; Bolarinwa, A.; Wolfram, G.; Linseisen, J. Bioavailability of apigenin from apiin-rich parsley in humans. Ann. Nutr. Metab. 2006, 50, 167–172. [Google Scholar] [CrossRef]
- Hasrat, J.A.; De Bruyne, T.; De Backer, J.P.; Vauquelin, G.; Vlietinck, A.J. Cirsimarin and cirsimaritin, flavonoids of Microtea debilis (Phytolaccaceae) with adenosine antagonistic properties in rats: Leads for new therapeutics in acute renal failure. J. Pharm. Pharmacol. 1997, 49, 1150–1156. [Google Scholar] [CrossRef]
- Eisen, B.; Ungar, Y.; Shimoni, E. Stability of isoflavones in soy milk stored at elevated and ambient temperatures. J. Agric. Food Chem. 2003, 51, 2212–2215. [Google Scholar] [CrossRef]
- Setchell, K.D.R.; Faughnan, M.S.; Avades, T.; Zimmer-Nechemias, L.; Brown, N.M.; Wolfe, B.E.; Brashear, W.T.; Desai, P.; Oldfield, M.F.; Botting, N.P.; et al. Comparing the pharmacokinetics of daidzein and genistein with the use of 13C-labeled tracers in premenopausal women. Am. J. Clin. Nutr. 2003, 77, 411–419. [Google Scholar] [CrossRef] [Green Version]
- Rüfer, C.E.; Bub, A.; Möseneder, J.; Winterhalter, P.; Stürtz, M.; Kulling, S.E. Pharmacokinetics of the soybean isoflavone daidzein in its aglycone and glucoside form: A randomized, double-blind, crossover study. Am. J. Clin. Nutr. 2008, 87, 1314–1323. [Google Scholar] [CrossRef] [Green Version]
- Kwiecień, A.; Ruda-Kucerova, J.; Kamiński, K.; Babinska, Z.; Popiołek, I.; Szczubiałka, K.; Nowakowska, M.; Walczak, M. Improved pharmacokinetics and tissue uptake of complexed daidzein in rats. Pharmaceutics 2020, 12, 162. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Liu, W.; Wang, J.; Liu, H.; Chen, Y. Preparation and Pharmacokinetic Study of Daidzein Long-Circulating Liposomes. Nanoscale Res. Lett. 2019, 14, 321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kano, M.; Takayanagi, T.; Harada, K.; Sawada, S.; Ishikawa, F. Bioavailability of Isoflavones after Ingestion of Soy Beverages in Healthy Adults. J. Nutr. 2006, 136, 2291–2296. [Google Scholar] [CrossRef] [PubMed]
- Bhullar, K.S.; Udenigwe, C.C. Clinical evidence of resveratrol bioactivity in cardiovascular disease. Curr. Opin. Food Sci. 2016. 8, 68–73. [CrossRef]
- Feng, M.; Zhong, L.X.; Zhan, Z.Y.; Huang, Z.H.; Xiong, J.P. Resveratrol treatment inhibits proliferation of and induces apoptosis in human colon cancer cells. Med. Sci. Monit. 2016, 22, 1101–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastianetto, S.; Ménard, C.; Quirion, R. Neuroprotective action of resveratrol. Biochim. Biophys. Acta Mol. Basis Dis. 2015. 1852, 1195–1201. [Google Scholar] [CrossRef] [Green Version]
- Summerlin, N.; Soo, E.; Thakur, S.; Qu, Z.; Jambhrunkar, S.; Popat, A. Resveratrol nanoformulations: Challenges and opportunities. Int. J. Pharm. 2015, 479, 282–290. [Google Scholar] [CrossRef]
- Seljak, K.B.; Berginc, K.; Trontelj, J.; Zvonar, A.; Kristl, A.; Gašperlin, M. A self-microemulsifying drug delivery system to overcome intestinal resveratrol toxicity and presystemic metabolism. J. Pharm. Sci. 2014, 103, 3491–3500. [Google Scholar] [CrossRef]
- Tang, P.C.T.; Ng, Y.F.; Ho, S.; Gyda, M.; Chan, S.W. Resveratrol and cardiovascular health—Promising therapeutic or hopeless illusion? Pharmacol. Res. 2014, 90, 88–115. [Google Scholar] [CrossRef]
- Pangeni, R.; Sahni, J.K.; Ali, J.; Sharma, S.; Baboota, S. Resveratrol: Review on therapeutic potential and recent advances in drug delivery. Expert Opin. Drug Deliv. 2014, 11, 1285–1298. [Google Scholar] [CrossRef]
- Bertelli, A.; Bertelli, A.A.E.; Gozzini, A.; Giovannini, L. Plasma and tissue resveratrol concentrations and pharmacological activity. Drugs Exp. Clin. Res. 1998, 24, 133–138. [Google Scholar]
- Gambini, J.; Inglés, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J.; et al. Properties of Resveratrol: In Vitro and In Vivo Studies about Metabolism, Bioavailability, and Biological Effects in Animal Models and Humans. Oxid. Med. Cell. Longev. 2015, 2015, 837042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldberg, D.M.; Yan, J.; Soleas, G.J. Absorption of three wine-related polyphenols in three different matrices by healthy subjects. Clin. Biochem. 2003, 36, 79–87. [Google Scholar] [CrossRef]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef] [Green Version]
- Kapetanovic, I.M.; Muzzio, M.; Huang, Z.; Thompson, T.N.; McCormick, D.L. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother. Pharmacol. 2011, 68, 593–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soleas, G.J.; Yan, J.; Goldberg, D.M. Measurement of trans-resveratrol, (+)-catechin, and quercetin in rat and human blood and urine by gas chromatography with mass selective detection. Methods Enzymol. 2001, 335, 130–145. [Google Scholar]
- Soleas, G.J.; Yan, J.; Goldberg, D.M. Ultrasensitive assay for three polyphenols (catechin, quercetin and resveratrol) and their conjugates in biological fluids utilizing gas chromatography with mass selective detection. J. Chromatogr. B Biomed. Sci. Appl. 2001, 757, 161–172. [Google Scholar] [CrossRef]
- Almeida, L.; Vaz-da-Silva, M.; Falcão, A.; Soares, E.; Costa, R.; Loureiro, A.I.; Fernandes-Lopes, C.; Rocha, J.F.; Nunes, T.; Wright, L.; et al. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol. Nutr. Food Res. 2009, 53 (Suppl. 1), S7–S15. [Google Scholar] [CrossRef]
- Rotches-Ribalta, M.; Andres-Lacueva, C.; Estruch, R.; Escribano, E.; Urpi-Sarda, M. Pharmacokinetics of resveratrol metabolic profile in healthy humans after moderate consumption of red wine and grape extract tablets. Pharmacol. Res. 2012, 66, 375–382. [Google Scholar] [CrossRef]
- Boocock, D.J.; Faust, G.E.S.; Patel, K.R.; Schinas, A.M.; Brown, V.A.; Ducharme, M.P.; Booth, T.D.; Crowell, J.A.; Perloff, M.; Gescher, A.J.; et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol. Biomarkers Prev. 2007, 16, 1246–1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sergides, C.; Chirilă, M.; Silvestro, L.; Pitta, D.; Pittas, A. Bioavailability and safety study of resveratrol 500 mg tablets in healthy male and female volunteers. Exp. Ther. Med. 2016, 11, 164–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seeram, N.P.; Lee, R.; Heber, D. Bioavailability of ellagic acid in human plasma after consumption of ellagitannins from pomegranate (Punica granatum L.) juice. Clin. Chim. Acta. 2004, 348, 63–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mertens-Talcott, S.U.; Jilma-Stohlawetz, P.; Rios, J.; Hingorani, L.; Derendorf, H. Absorption, metabolism, and antioxidant effects of pomegranate (Punica granatum L.) polyphenols after ingestion of a standardized extract in healthy human volunteers. J. Agric. Food Chem. 2006, 54, 8956–8961. [Google Scholar] [CrossRef]
- Lei, F.; Xing, D.M.; Xiang, L.; Zhao, Y.N.; Wang, W.; Zhang, L.J.; Du, L.J. Pharmacokinetic study of ellagic acid in rat after oral administration of pomegranate leaf extract. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2003, 796, 189–194. [Google Scholar] [CrossRef]
- González-Sarrías, A.; García-Villalba, R.; Núñez-Sánchez, M.Á.; Tomé-Carneiro, J.; Zafrilla, P.; Mulero, J.; Tomás-Barberán, F.A.; Espín, J.C. Identifying the limits for ellagic acid bioavailability: A crossover pharmacokinetic study in healthy volunteers after consumption of pomegranate extracts. J. Funct. Foods 2015, 19, 225–235. [Google Scholar] [CrossRef]
- Hamad, A.W.R.; Al-Momani, W.M.; Janakat, S.; Oran, S.A. Bioavailability of Ellagic Acid after single dose administration using HPLC. Pakistan J. Nutr. 2009, 8, 1661–1664. [Google Scholar] [CrossRef] [Green Version]
- Vareed, S.K.; Kakarala, M.; Ruffin, M.T.; Crowell, J.A.; Normolle, D.P.; Djuric, Z.; Brenner, D.E. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol. Biomarkers Prev. 2008, 17, 1411–1417. [Google Scholar] [CrossRef] [Green Version]
- Balasubramanian, K. Molecular orbital basis for yellow curry spice curcumin’s prevention of Alzheimer’s disease. J. Agric. Food Chem. 2006, 54, 3512–3520. [Google Scholar] [CrossRef]
- Barry, J.; Fritz, M.; Brender, J.R.; Smith, P.E.S.; Lee, D.K.; Ramamoorthy, A. Determining the effects of lipophilic drugs on membrane structure by solid-state NMR spectroscopy: The case of the antioxidant curcumin. J. Am. Chem. Soc. 2009, 131, 4490–4498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Lara, A.; Ausili, A.; Aranda, F.J.; de Godos, A.; Torrecillas, A.; Corbalán-García, S.; Gómez-Fernández, J.C. Curcumin disorders 1,2-dipalmitoyl-sn-glycero-3-phosphocholine membranes and favors the formation of nonlamellar structures by 1,2-dielaidoyl-sn-glycero- 3-phosphoethanolamine. J. Phys. Chem. B 2010, 114, 9778–9786. [Google Scholar] [CrossRef]
- Heger, M.; van Golen, R.F.; Broekgaarden, M.; Michel, M.C. The molecular basis for the pharmacokinetics and pharmacodynamics of curcumin and its metabolites in relation to cancers. Pharmacol. Rev. 2014, 66, 222–307. [Google Scholar] [CrossRef] [PubMed]
- Berginc, K.; Trontelj, J.; Skalko Basnet, N.; Kristl, A. Physiological barriers to the oral delivery of curcumin. Pharmazie 2012, 67, 518–524. [Google Scholar] [PubMed]
- Dempe, J.S.; Scheerle, R.K.; Pfeiffer, E.; Metzler, M. Metabolism and permeability of curcumin in cultured Caco-2 cells. Mol. Nutr. Food Res. 2013, 57, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Huang, T.M.; Lin, J.K. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab. Dispos. 1999, 27, 486–494. [Google Scholar] [PubMed]
- Cheng, A.L.; Hsu, C.H.; Lin, J.K.; Hsu, M.M.; Ho, Y.F.; She, T.S.; Ko, J.Y.; Lin, J.T.; Lin, B.R.; Wu, M.S.; et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001, 21, 2895–2900. [Google Scholar] [PubMed]
- Lao, C.D.; Ruffin IV, M.T.; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 2006, 6, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Kanai, M.; Yoshimura, K.; Asada, M.; Imaizumi, A.; Suzuki, C.; Matsumoto, S.; Nishimura, T.; Mori, Y.; Masui, T.; Kawaguchi, Y.; et al. A phase I/II study of gemcitabine-based chemotherapy plus curcumin for patients with gemcitabine-resistant pancreatic cancer. Cancer Chemother. Pharmacol. 2011, 68, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Dhillon, N.; Aggarwal, B.B.; Newman, R.A.; Wolff, R.A.; Kunnumakkara, A.B.; Abbruzzese, J.L.; Ng, C.S.; Badmaev, V.; Kurzrock, R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin. Cancer Res. 2008, 14, 4491–4499. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.A.; Euden, S.A.; Platton, S.L.; Cooke, D.N.; Shafayat, A.; Hewitt, H.R.; Marczylo, T.H.; Morgan, B.; Hemingway, D.; Plummer, S.M.; et al. Phase I clinical trial of oral curcumin: Biomarkers of systemic activity and compliance. Clin. Cancer Res. 2004, 10, 6847–6854. [Google Scholar] [CrossRef] [Green Version]
- Ravindranath, V.; Chandrasekhara, N. Absorption and tissue distribution of curcumin in rats. Toxicology 1980, 16, 259–265. [Google Scholar] [CrossRef]
- Garcea, G.; Berry, D.P.; Jones, D.J.L.; Singh, R.; Dennison, A.R.; Farmer, P.B.; Sharma, R.A.; Steward, W.P.; Gescher, A.J. Consumption of the putative chemopreventive agent curcumin by cancer patients: Assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol. Biomarkers Prev. 2005, 14, 120–125. [Google Scholar] [PubMed]
- Mahale, J.; Singh, R.; Howells, L.M.; Britton, R.G.; Khan, S.M.; Brown, K. Detection of Plasma Curcuminoids from Dietary Intake of Turmeric-Containing Food in Human Volunteers. Mol. Nutr. Food Res. 2018, 62, 1800267. [Google Scholar] [CrossRef]
- Baum, L.; Lam, C.W.K.; Cheung, S.K.K.; Kwok, T.; Lui, V.; Tsoh, J.; Lam, L.; Leung, V.; Hui, E.; Ng, C.; et al. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J. Clin. Psychopharmacol. 2008, 28, 110–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ringman, J.M.; Frautschy, S.A.; Teng, E.; Begum, A.N.; Bardens, J.; Beigi, M.; Gylys, K.H.; Badmaev, V.; Heath, D.D.; Apostolova, L.G.; et al. Oral curcumin for Alzheimer’s disease: Tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimer’s Res. Ther. 2012, 4, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carroll, R.E.; Benya, R.V.; Turgeon, D.K.; Vareed, S.; Neuman, M.; Rodriguez, L.; Kakarala, M.; Carpenter, P.M.; McLaren, C.; Meyskens, F.L.; et al. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prev. Res. 2011, 4, 354–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sangster, J.M. Octanol-Water Partition Coefficients: Fundamentals and Physical Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 1997; Volume 2. [Google Scholar]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 23, 3–25. [Google Scholar] [CrossRef]
- Pérez-Sánchez, A.; Borrás-Linares, I.; Barrajón-Catalán, E.; Arráez-Román, D.; González-Álvarez, I.; Ibáñez, E.; Segura-Carretero, A.; Bermejo, M.; Micol, V. Evaluation of the intestinal permeability of rosemary (Rosmarinus officinalis L.) extract polyphenols and terpenoids in Caco-2 cell monolayers. PLoS ONE 2017, 12, e0172063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Liu, G.M.; Cao, M.J.; Chen, Q.; Sun, L.; Ji, B. Potential Retinal Benefits of Dietary Polyphenols Based on Their Permeability across the Blood-Retinal Barrier. J. Agric. Food Chem. 2017, 65, 3179–3189. [Google Scholar] [CrossRef]
- Fong, S.Y.K.; Liu, M.; Wei, H.; Löbenberg, R.; Kanfer, I.; Lee, V.H.L.; Amidon, G.L.; Zuo, Z. Establishing the pharmaceutical quality of Chinese herbal medicine: A provisional BCS classification. Mol. Pharm. 2013, 10, 1623–1643. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, J.A.; Day, A.J.; Morgan, M.R.A. Experimental determination of octanol-water partition coefficients of quercetin and related flavonoids. J. Agric. Food Chem. 2005, 53, 4355–4360. [Google Scholar] [CrossRef]
- Dima, C.; Assadpour, E.; Dima, S.; Jafari, S.M. Bioavailability and bioaccessibility of food bioactive compounds; overview and assessment by in vitro methods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2862–2884. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.M.; Curl, R.L.; Amidon, G.L. Estimating the Fraction Dose Absorbed from Suspensions of Poorly Soluble Compounds in Humans: A Mathematical Model. Pharm. Res. An. Off. J. Am. Assoc. Pharm. Sci. 1993, 10, 264–270. [Google Scholar]
- WHO expert committee on specifications for pharmaceutical preparations. Fortieth report. World Heal. Organ. Tech. Rep. Ser. 2006, 937, 1. [Google Scholar]
- Teng, Z.; Yuan, C.; Zhang, F.; Huan, M.; Cao, W.; Li, K.; Yang, J.; Cao, D.; Zhou, S.; Mei, Q. Intestinal absorption and first-pass metabolism of polyphenol compounds in rat and their transport dynamics in caco-2 cells. PLoS ONE 2012, 7, 1–9. [Google Scholar] [CrossRef]
- Hu, J.; Johnston, K.P.; Williams, R.O. Rapid dissolving high potency danazol powders produced by spray freezing into liquid process. Int. J. Pharm. 2004, 271, 145–154. [Google Scholar] [CrossRef]
- Kansara, H.; Panola, R.; Chauhan, C.S.; Mishra, A. Bioavailability Enhancement Techniques for BCS Class II Drugs: A Review. Int. J. Drug Dev. Res. 2015, 7, 250–261. [Google Scholar]
- Sediq, A.; Kubbinga, M.; Langguth, P.; Dressman, J. The impact of the EMA change in definition of “dose” on the BCS dose-solubility ratio: A review of the biowaiver monographs. J. Pharm. Sci. 2014, 103, 65–70. [Google Scholar] [CrossRef]
- Per capita fruit consumption. In Per Capita Fruit Consum; Economic Research Service, U.S. Department of Agriculture: Washington, DC, USA, 2013.
- Krewson, C.F.; Naghski, J. Some physical properties of rutin. J. Am. Pharm. Assoc. Am. Pharm. Assoc. (Baltim). 1952, 41, 582–587. [Google Scholar] [CrossRef]
- Kaur, H.; Kaur, G. A Critical Appraisal of Solubility Enhancement Techniques of Polyphenols. J. Pharm. 2014, 2014, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Bala, I.; Bhardwaj, V.; Hariharan, S.; Kumar, M.N.V.R. Analytical methods for assay of ellagic acid and its solubility studies. J. Pharm. Biomed. Anal. 2006, 40, 206–210. [Google Scholar] [CrossRef]
- Dahan, A.; Miller, J.M.; Amidon, G.L. Prediction of solubility and permeability class membership: Provisional BCS classification of the world’s top oral drugs. AAPS J. 2009, 11, 740–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dressman, J.; Butler, J.; Hempenstall, J.; Reppas, C. The BCS: Where do we go from here? Pharm. Technol. 2001, 25, 68–76. [Google Scholar]
- Kawabata, Y.; Wada, K.; Nakatani, M.; Yamada, S.; Onoue, S. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: Basic approaches and practical applications. Int. J. Pharm. 2011, 420, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Varma, M.V.; Gardner, I.; Steyn, S.J.; Nkansah, P.; Rotter, C.J.; Whitney-Pickett, C.; Zhang, H.; Di, L.; Cram, M.; Fenner, K.S.; et al. PH-dependent solubility and permeability criteria for provisional biopharmaceutics classification (BCS and BDDCS) in early drug discovery. Mol. Pharm. 2012, 9, 1199–1212. [Google Scholar] [CrossRef] [PubMed]
- Papich, M.G.; Martinez, M.N. Applying Biopharmaceutical Classification System (BCS) Criteria to Predict Oral Absorption of Drugs in Dogs: Challenges and Pitfalls. AAPS J. 2015, 17, 948–964. [Google Scholar] [CrossRef] [Green Version]
- Amidon, G.L.; Lennernäs, H.; Shah, V.P.; Crison, J.R. A Theoretical Basis for a Biopharmaceutic Drug Classification: The Correlation of in Vitro Drug Product Dissolution and in Vivo Bioavailability. Pharm. Res. An Off. J. Am. Assoc. Pharm. Sci. 1995, 12, 413–420. [Google Scholar]
- Verbeeck, R.K.; Junginger, H.E.; Midha, K.K.; Shah, V.P.; Barends, D.M. Biowaiver monographs for immediate release solid oral dosage forms based on Biopharmaceutics Classification System (BCS) literature data: Chloroquine phosphate, chloroquine sulfate, and chloroquine hydrochloride. J. Pharm. Sci. 2005, 94, 1389–1395. [Google Scholar] [CrossRef]
- Bala, I.; Bhardwaj, V.; Hariharan, S.; Sitterberg, J.; Bakowsky, U.; Ravi Kumar, M.N.V. Design of biodegradable nanoparticles: A novel approach to encapsulating poorly soluble phytochemical ellagic acid. Nanotechnology 2005, 16, 2819. [Google Scholar] [CrossRef]
- Madaan, K.; Lather, V.; Pandita, D. Evaluation of polyamidoamine dendrimers as potential carriers for quercetin, a versatile flavonoid. Drug Deliv. 2016, 23, 254–262. [Google Scholar] [CrossRef]
- Waldmann, S.; Almukainzi, M.; Bou-Chacra, N.A.; Amidon, G.L.; Lee, B.J.; Feng, J.; Kanfer, I.; Zuo, J.Z.; Wei, H.; Bolger, M.B.; et al. Provisional biopharmaceutical classification of some common herbs used in western medicine. Mol. Pharm. 2012, 9, 815–822. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, D.; Huang, Y.; Gao, Y.; Qian, S. Biopharmaceutics classification and intestinal absorption study of apigenin. Int. J. Pharm. 2012, 436, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Li, W.; Sun, W.J.; Lu, T.; Tong, H.H.Y.; Sun, C.C.; Zheng, Y. Resveratrol cocrystals with enhanced solubility and tabletability. Int. J. Pharm. 2016, 509, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Nyamba, I.; Lechanteur, A.; Semdé, R.; Evrard, B. Physical formulation approaches for improving aqueous solubility and bioavailability of ellagic acid: A review. Eur. J. Pharm. Biopharm. 2021, 159, 198–210. [Google Scholar] [CrossRef] [PubMed]
- John, M.K.; Huan, X.I.E.; Bell, E.C.; Liang, D. Development and pharmacokinetic evaluation of a curcumin co-solvent formulation. Anticancer Res. 2013, 33, 4285–4291. [Google Scholar] [PubMed]
- Franciosoa, A.; Mastromarino, P.; Masci, A.; d’Erme, M.; Mosca, L. Chemistry, Stability and Bioavailability of Resveratrol. Med. Chem. (Los. Angeles). 2014, 10, 237–245. [Google Scholar] [CrossRef]
- Mattarei, A.; Azzolini, M.; Carraro, M.; Sassi, N.; Zoratti, M.; Paradisi, C.; Biasutto, L. Acetalderivativesasprodrugs of resveratrol. Mol. Pharm. 2013, 10, 2781–2792. [Google Scholar] [CrossRef] [PubMed]
- Mateos, R.; Goya, L.; Bravo, L. Uptake and metabolism of hydroxycinnamic acids (chlorogenic, caffeic, and ferulic acids) by HepG2 cells as a model of the human liver. J. Agric. Food Chem. 2006, 54, 8724–8732. [Google Scholar] [CrossRef] [Green Version]
- Panizzon, G.P.; Bueno, F.G.; Ueda-Nakamura, T.; Nakamura, C.V.; Dias Filho, B.P. Manufacturing different types of solid dispersions of BCS class iv polyphenol (daidzein) by spray drying: Formulation and bioavailability. Pharmaceutics 2019, 11, 492. [Google Scholar] [CrossRef] [Green Version]
| Compound | Structure | Drugbank LogP | Other Sources LogP |
|---|---|---|---|
| Ferulic acid |  | 1.58 | 1.42 [150] |
| 0.96 [151] | |||
| Chlorogenic acid |  | 0.17 | 0.37 [151] |
| −0.3 [153] | |||
| Rutin |  | 0.15 | 0.47 ± 0.01 [55] |
| −2.28 [150] | |||
| −0.9 [151] | |||
| Quercetin |  | 1.81 | 1.59 ± 0.06 [55] |
| 0.35 [150] | |||
| 1.82 ± 0.32 [152] | |||
| 1.48 [153] | |||
| Apigenin |  | 3.07 | 1.51 [150] |
| 1.9 [149] | |||
| 2.92 ± 0.06 [152] | |||
| Cirsimaritin |  | N/A | 2.04 [149] |
| Daidzein |  | 3.3 | 2.13 [150] |
| 2.51 ± 0.06 [152] | |||
| Resveratrol |  | 2.57 | 2.99 ± 0.13 [55] |
| 3.06 [150] | |||
| 3.1 [153] | |||
| Ellagic acid |  | 1.59 | 1.366 http://www.chemspider.com (accessed on 28th April 2021) |
| Curcumin |  | 3.62 | 3.29 [153] |
| Compound | Experimental Water Solubility | Richest Source | SDAD | D/S |
|---|---|---|---|---|
| Ferulic acid | 0.78 mg mL−1 http://www.chemspider.com (accessed on 28th April 2021) | Dark chocolate (240 mg kg−1) | 7.56 mg | 9.69 mL |
| Chlorogenic acid | 40 mg mL−1 [153] | Plums (758.8 mg kg−1) | 259.9 mg | 6.49 mL |
| Rutin | 0.13 mg mL−1 [161] | Capers (3323 mg kg−1) | 13.45 mg | 103.46 mL |
| Quercetin | 0.0003 mg mL−1 [162] | Dark chocolate (250 mg kg−1) | 7.87 mg | 26.23 mL |
| Apigenin | 0.00216 mg mL−1 [162] | Origanum majorana (44 mg kg−1) | 178.2 µg | 82.5 mL |
| Cirsimaritin | 2.57 × 10−4 mg mL−1 [149] | Origanum vulgare (684.3 mg kg−1) | 2.77 mg | 10.78 mL |
| Daidzein | 0.008215 mg mL−1 [162] | Tempeh (processed soy) 136 mg kg−1 | 20.4 mg | 2483.2 mL |
| Resveratrol | 0.03 mg mL−1 [162] | Cranberry, red (30 mg kg−1) | 10.27 mg | 342.3 mL |
| Ellagic acid | 0.0093 mg mL−1 [162] 0.0097 mg mL−1 [163] | Chestnuts (7354 mg kg−1) | 2.518 g | 270,752 mL |
| Curcumin | 11 × 10−6 mg mL−1 [162] | Curry powder (2853 mg kg−1) | 11.55 mg | 10,500,000 mL |
| Family | Compounds | BCS Classification | |
|---|---|---|---|
| Reviewed | Literature | ||
| Hydroxycinnamic acid | Ferulic acid | III | III [151] |
| Chlorogenic acid | III | III [151] | |
| Flavonols | Rutin | III | III [151] |
| Quercetin | I | I [151] or II [172] or IV [173] | |
| Flavones | Apigenin | I | II [174] |
| Cirsimaritin | I | III [149] | |
| Isoflavones | Daidzein | II | IV [175] |
| Stilbenes | Resveratrol | II | II [176] |
| Tannins | Ellagic acid | IV | IV [177] |
| Curcuminoids | Curcumin | II | IV [178] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Truzzi, F.; Tibaldi, C.; Zhang, Y.; Dinelli, G.; D′Amen, E. An Overview on Dietary Polyphenols and Their Biopharmaceutical Classification System (BCS). Int. J. Mol. Sci. 2021, 22, 5514. https://doi.org/10.3390/ijms22115514
Truzzi F, Tibaldi C, Zhang Y, Dinelli G, D′Amen E. An Overview on Dietary Polyphenols and Their Biopharmaceutical Classification System (BCS). International Journal of Molecular Sciences. 2021; 22(11):5514. https://doi.org/10.3390/ijms22115514
Chicago/Turabian StyleTruzzi, Francesca, Camilla Tibaldi, Yanxin Zhang, Giovanni Dinelli, and Eros D′Amen. 2021. "An Overview on Dietary Polyphenols and Their Biopharmaceutical Classification System (BCS)" International Journal of Molecular Sciences 22, no. 11: 5514. https://doi.org/10.3390/ijms22115514
APA StyleTruzzi, F., Tibaldi, C., Zhang, Y., Dinelli, G., & D′Amen, E. (2021). An Overview on Dietary Polyphenols and Their Biopharmaceutical Classification System (BCS). International Journal of Molecular Sciences, 22(11), 5514. https://doi.org/10.3390/ijms22115514







