Nanotechnology Facilitated Cultured Neuronal Network and Its Applications
Abstract
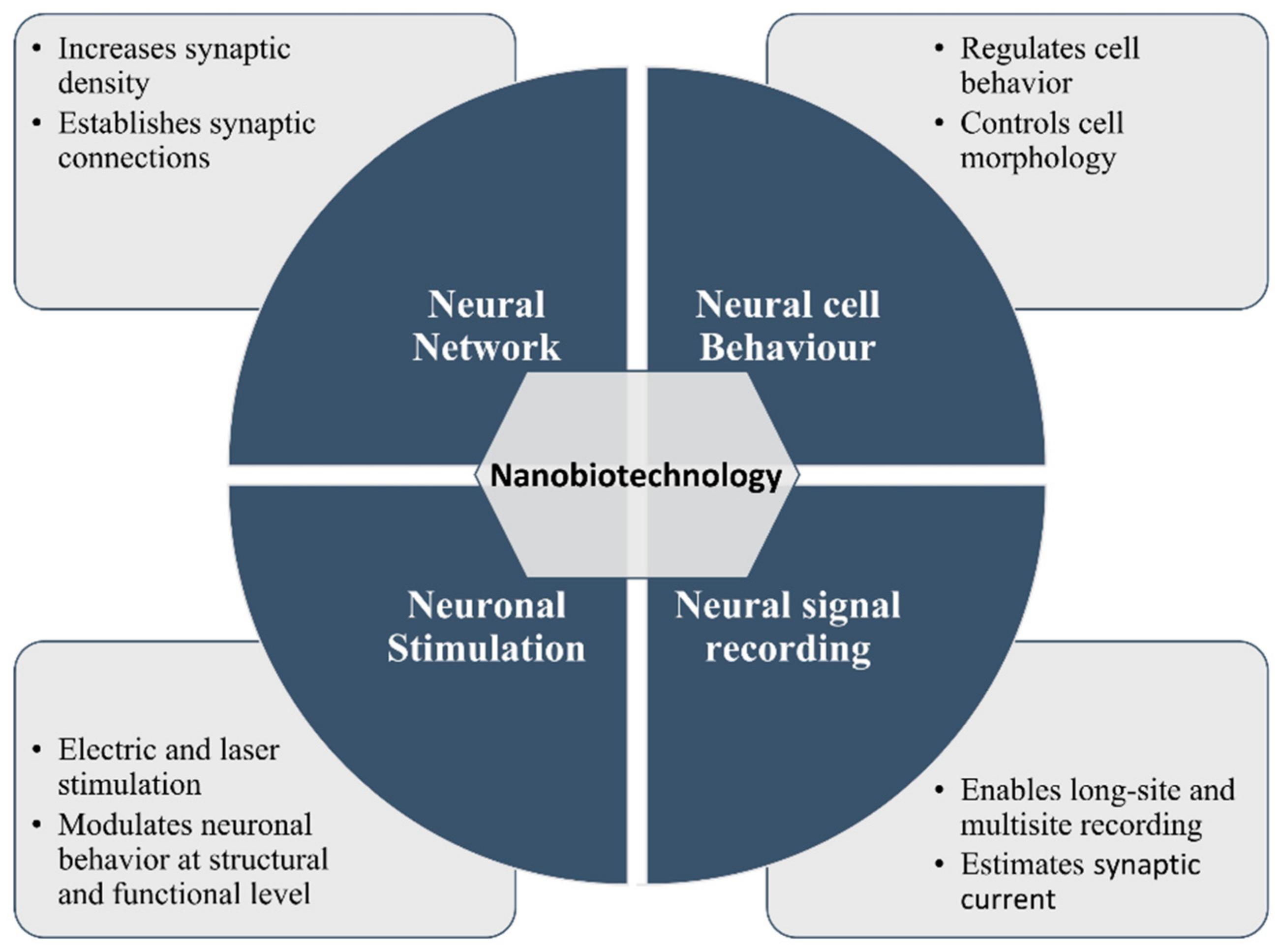
:1. Introduction
2. Nanotechnology for Neuronal Research
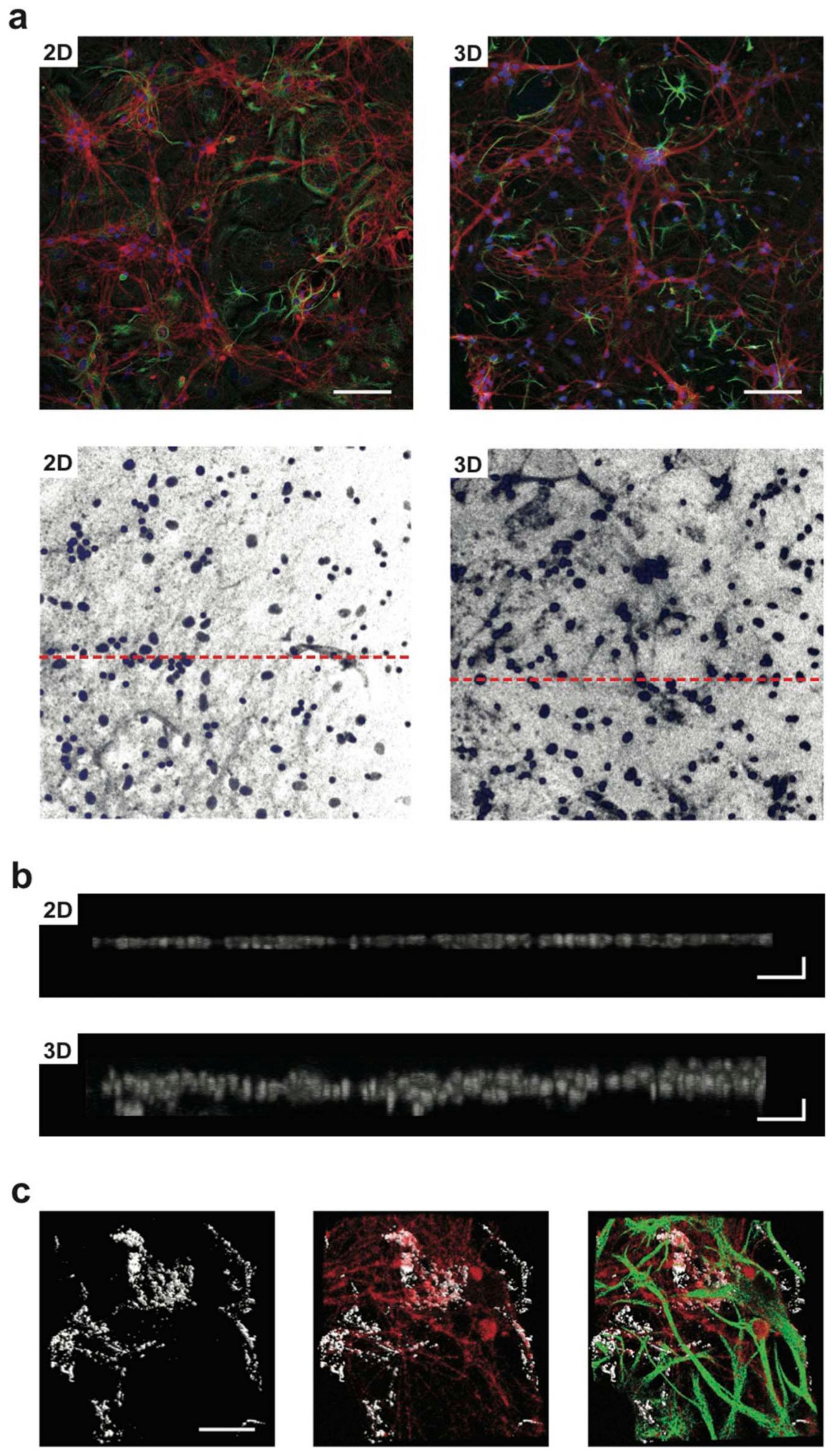
2.1. Nanomaterials for Neuronal Network Establishment
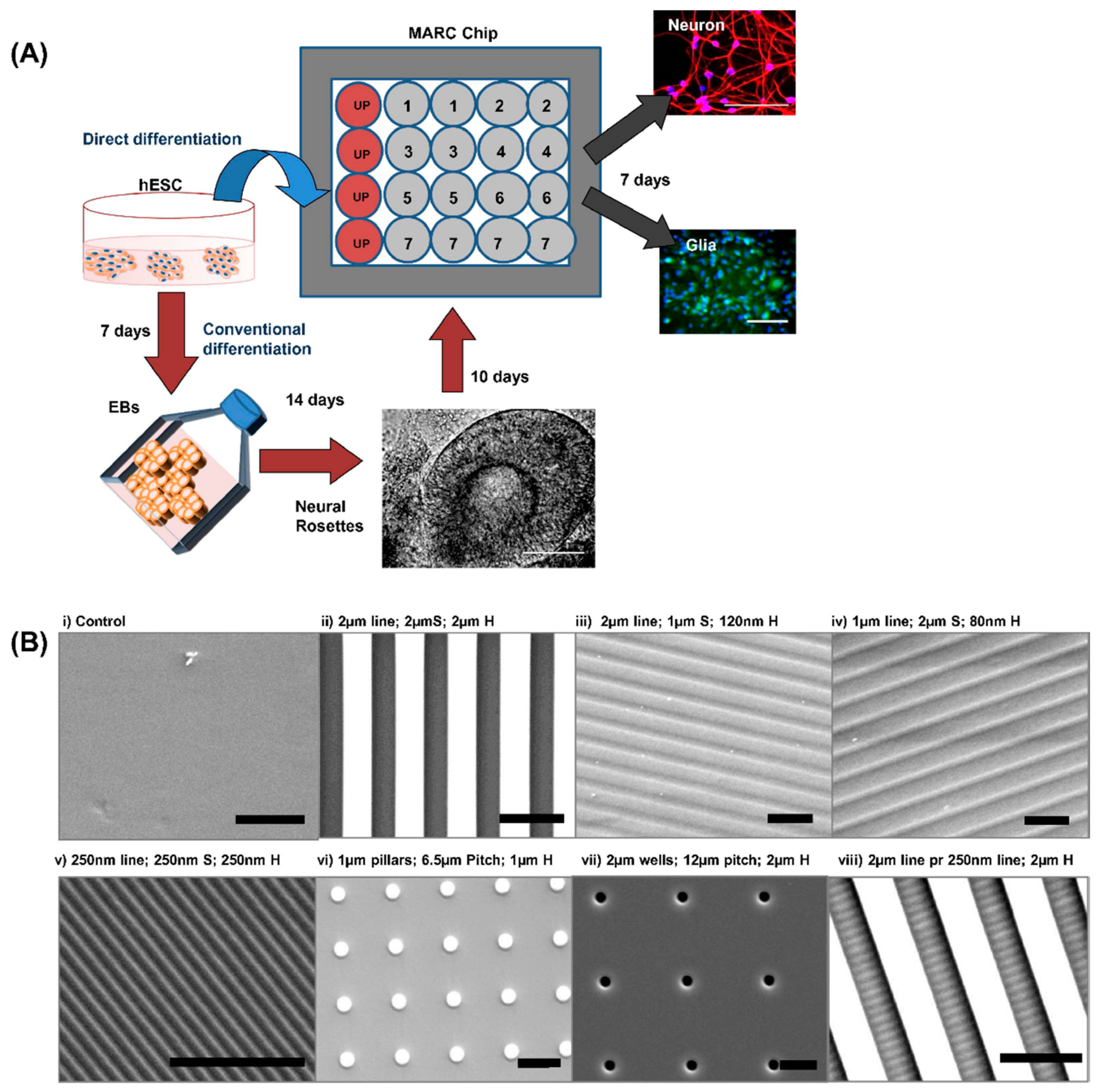
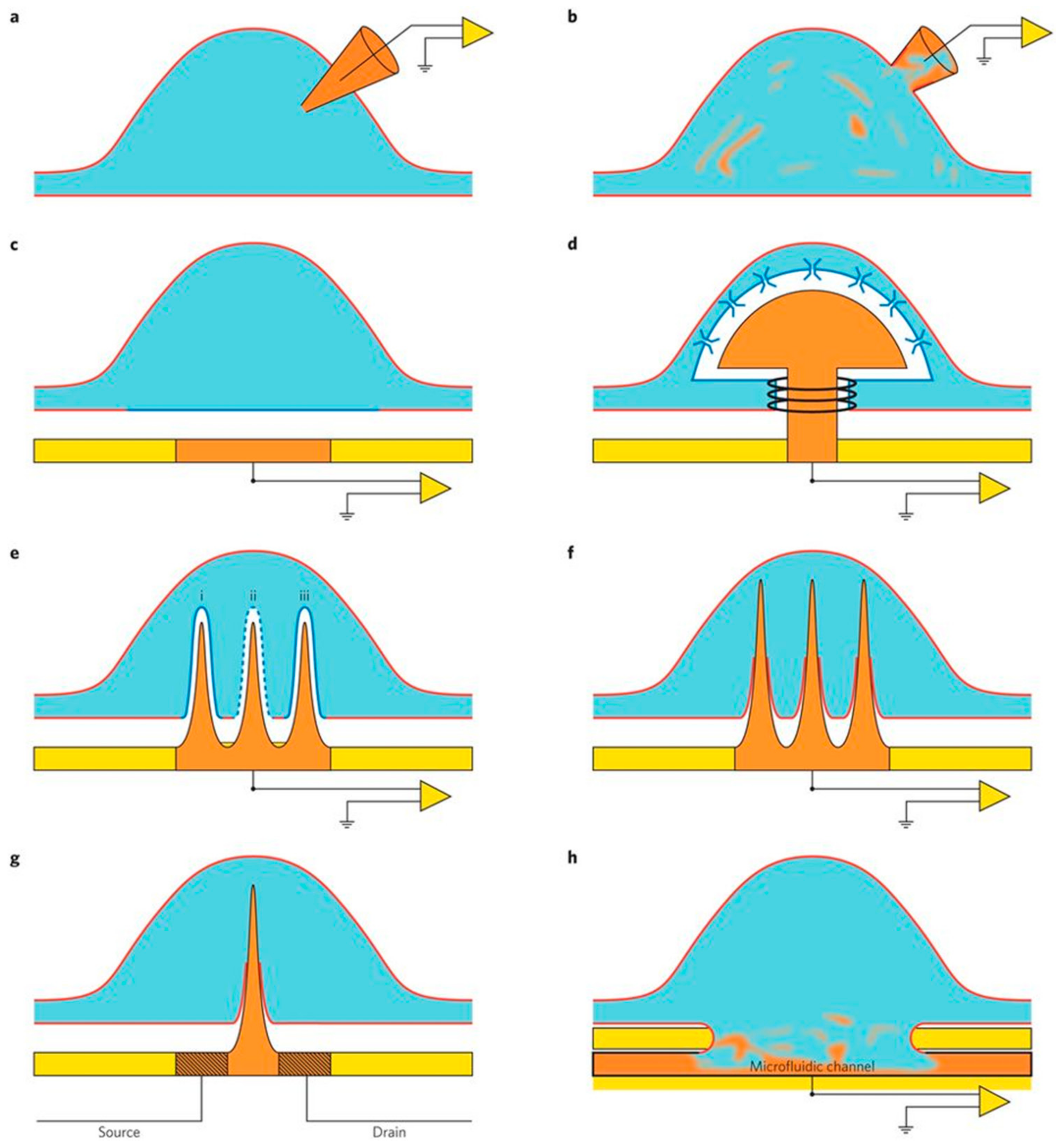
2.2. Nanostructural Design and Fabrication for Programming Cell Behavior
2.3. Nanotechnology-Assisted Neuronal Stimulation
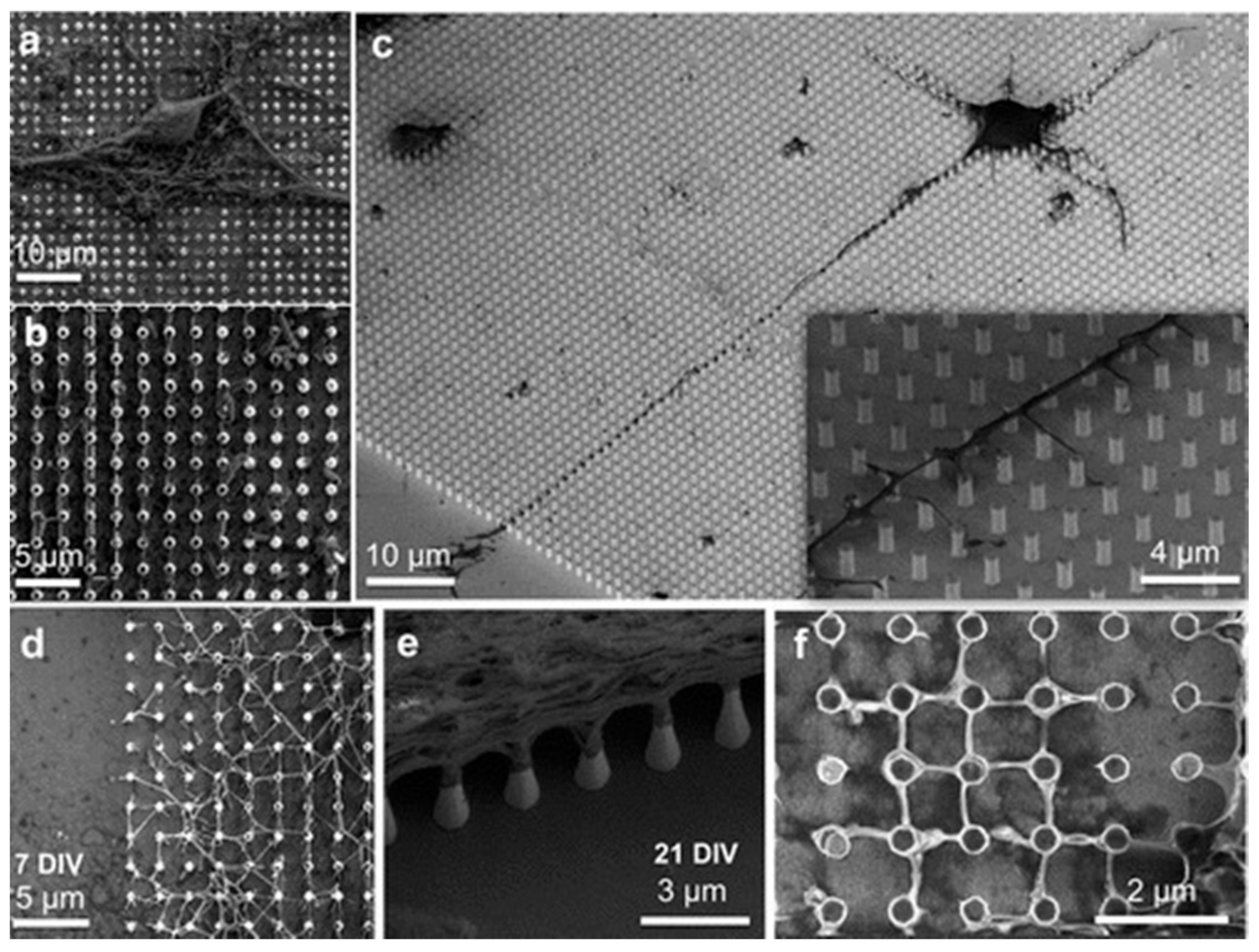
2.4. Nanodevices for Neuronal Signal Recording
3. Challenges and Future Perspectives
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Hua, J.Y.; Smith, S.J. Neural activity and the dynamics of central nervous system development. Nat. Neurosci. 2004, 7, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xu, Z.; Huang, J.; Lin, X.; Luo, R.; Chen, C.H.; Shi, P. NeuroArray: A universal interface for patterning and interrogating neural circuitry with single cell resolution. Sci. Rep. 2014, 4, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taupin, P. The therapeutic potential of adult neural stem cells. Curr. Opin. Mol. 2006, 8, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Kruskal, P.B.; Jiang, Z.; Gao, T.; Lieber, C.M. Beyond the Patch Clamp: Nanotechnologies for Intracellular Recording. Neuron 2015, 86, 21–24. [Google Scholar] [CrossRef] [Green Version]
- Bosi, S.; Rauti, R.; Laishram, J.; Turco, A.; Lonardoni, D.; Nieus, T.; Prato, M.; Scaini, D.; Ballerini, L. From 2D to 3D: Novel nanostructured scaffolds to investigate signalling in reconstructed neuronal networks. Sci. Rep. 2015, 5, 9562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pautot, S.; Wyart, C.; Isacoff, E.Y. Colloid-guided assembly of oriented 3D neuronal networks. Nat. Methods 2008, 5, 735–740. [Google Scholar] [CrossRef] [Green Version]
- Van den Ameele, J.; Tiberi, L.; Vanderhaeghen, P.; Espuny-Camacho, I. Thinking out of the dish: What to learn about cortical development using pluripotent stem cells. Trends Neurosci. 2014, 37, 334–342. [Google Scholar] [CrossRef] [Green Version]
- Murphy, W.L.; McDevitt, T.C.; Engler, A.J. Materials as stem cell regulators. Nat. Mater. 2014, 13, 547–557. [Google Scholar] [CrossRef]
- Narayanan, K.; Mishra, S.; Singh, S.; Pei, M.; Gulyas, B.; Padmanabhan, P. Engineering Concepts in Stem Cell Research. Biotechnol. J. 2017, 12. [Google Scholar] [CrossRef]
- Frega, M.; Tedesco, M.; Massobrio, P.; Pesce, M.; Martinoia, S. Network dynamics of 3D engineered neuronal cultures: A new experimental model for in-vitro electrophysiology. Sci. Rep. 2014, 4, 5489. [Google Scholar] [CrossRef] [PubMed]
- Hanson Shepherd, J.N.; Parker, S.T.; Shepherd, R.F.; Gillette, M.U.; Lewis, J.A.; Nuzzo, R.G. 3D microperiodic hydrogel scaffolds for robust neuronal cultures. Adv. Funct. Mater. 2011, 21, 47–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monaco, A.M.; Giugliano, M. Carbon-based smart nanomaterials in biomedicine and neuroengineering. Beilstein J. Nanotechnol. 2014, 5, 1849–1863. [Google Scholar] [CrossRef] [PubMed]
- Limongi, T.; Cesca, F.; Gentile, F.; Marotta, R.; Ruffilli, R.; Barberis, A.; Dal Maschio, M.; Petrini, E.M.; Santoriello, S.; Benfenati, F.; et al. Nanostructured superhydrophobic substrates trigger the development of 3D neuronal networks. Small 2013, 9, 402–412. [Google Scholar] [CrossRef]
- Dowling, D.P.; Miller, I.S.; Ardhaoui, M.; Gallagher, W.M. Effect of surface wettability and topography on the adhesion of osteosarcoma cells on plasma-modified polystyrene. J. Biomater. Appl. 2011, 26, 327–347. [Google Scholar] [CrossRef]
- Li, N.; Folch, A. Integration of topographical and biochemical cues by axons during growth on microfabricated 3-D substrates. Exp. Cell Res. 2005, 311, 307–316. [Google Scholar] [CrossRef] [Green Version]
- Gautam, V.; Naureen, S.; Shahid, N.; Gao, Q.; Wang, Y.; Nisbet, D.; Jagadish, C.; Daria, V.R. Engineering Highly Interconnected Neuronal Networks on Nanowire Scaffolds. Nano Lett. 2017. [Google Scholar] [CrossRef]
- Abrams, G.A.; Schaus, S.S.; Goodman, S.L.; Nealey, P.F.; Murphy, C.J. Nanoscale topography of the corneal epithelial basement membrane and Descemet’s membrane of the human. Cornea 2000, 19, 57–64. [Google Scholar] [CrossRef]
- Den Braber, E.T.; de Ruijter, J.E.; Ginsel, L.A.; von Recum, A.F.; Jansen, J.A. Orientation of ECM protein deposition, fibroblast cytoskeleton, and attachment complex components on silicone microgrooved surfaces. J. Biomed. Mater. Res. 1998, 40, 291–300. [Google Scholar] [CrossRef]
- Cellot, G.; Toma, F.M.; Kasap Varley, Z.; Laishram, J.; Villari, A.; Quintana, M.; Cipollone, S.; Prato, M.; Ballerini, L. Carbon Nanotube Scaffolds Tune Synaptic Strength in Cultured Neural Circuits: Novel Frontiers in Nanomaterial-Tissue Interactions. J. Neurosci. 2011, 31, 12945–12953. [Google Scholar] [CrossRef] [Green Version]
- Mazzatenta, A.; Giugliano, M.; Campidelli, S.; Gambazzi, L.; Businaro, L.; Markram, H.; Prato, M.; Ballerini, L. Interfacing Neurons with Carbon Nanotubes: Electrical Signal Transfer and Synaptic Stimulation in Cultured Brain Circuits. J. Neurosci. 2007, 27, 6931–6936. [Google Scholar] [CrossRef] [PubMed]
- Fabbro, A.; Bosi, S.; Ballerini, L.; Prato, M. Carbon nanotubes: Artificial nanomaterials to engineer single neurons and neuronal networks. ACS Chem. Neurosci. 2012, 3, 611–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, X.; Huang, Y.; Cui, Y.; Wang, J.; Lieber, C.M. Indium phosphide nanowires as building blocks for nanoscale electronic and optoelectronic devices. Nature 2001, 409, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Piret, G.; Perez, M.T.; Prinz, C.N. Substrate porosity induces phenotypic alterations in retinal cells cultured on silicon nanowires. RSC Adv. 2014, 4, 27888–27897. [Google Scholar] [CrossRef] [Green Version]
- Onesto, V.; Cancedda, L.; Coluccio, M.L.; Nanni, M.; Pesce, M.; Malara, N.; Cesarelli, M.; Di Fabrizio, E.; Amato, F.; Gentile, F. Nano-topography Enhances Communication in Neural Cells Networks. Sci. Rep. 2017, 7, 9841. [Google Scholar] [CrossRef] [Green Version]
- Dawson, K.A.; Yan, Y. Current understanding of biological identity at the nanoscale and future prospects. Nat. Nanotechnol. 2021, 16, 229–242. [Google Scholar] [CrossRef]
- Higuchi, A.; Ling, Q.-D.; Chang, Y.; Hsu, S.-T.; Umezawa, A. Physical Cues of Biomaterials Guide Stem Cell Differentiation Fate. Chem. Rev. 2013, 113, 3297–3328. [Google Scholar] [CrossRef]
- Baptista, D.; Teixeira, L.; van Blitterswijk, C.; Giselbrecht, S.; Truckenmüller, R. Overlooked? Underestimated? Effects of Substrate Curvature on Cell Behavior. Trends Biotechnol. 2019, 37, 838–854. [Google Scholar] [CrossRef]
- Weaver, C.L.; Cui, X.T. Directed Neural Stem Cell Differentiation with a Functionalized Graphene Oxide Nanocomposite. Adv. Healthc. Mater. 2015, 4, 1408–1416. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Differentiation of human neural stem cells into neural networks on graphene nanogrids. J. Mater. Chem. B 2013, 1, 6291–6301. [Google Scholar] [CrossRef] [PubMed]
- Edgington, R.J.; Thalhammer, A.; Welch, J.O.; Bongrain, A.; Bergonzo, P.; Scorsone, E.; Jackman, R.B.; Schoepfer, R. Patterned neuronal networks using nanodiamonds and the effect of varying nanodiamond properties on neuronal adhesion and outgrowth. J. Neural Eng. 2013, 10, 56022. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.C.; González, C.H.; Miller, B.S.; Edgington, R.J.; Ferretti, P.; Jackman, R.B. Surface functionalisation of nanodiamonds for human neural stem cell adhesion and proliferation. Sci. Rep. 2017, 7, 7307. [Google Scholar] [CrossRef] [PubMed]
- Ankam, S.; Suryana, M.; Chan, L.Y.; Moe, A.A.K.; Teo, B.K.K.; Law, J.B.K.; Sheetz, M.P.; Low, H.Y.; Yim, E.K.F. Substrate topography and size determine the fate of human embryonic stem cells to neuronal or glial lineage. Acta Biomater. 2013, 9, 4535–4545. [Google Scholar] [CrossRef] [PubMed]
- Paviolo, C.; Haycock, J.W.; Yong, J.; Yu, A.; Stoddart, P.R.; McArthur, S.L. Laser exposure of gold nanorods can increase neuronal cell outgrowth. Biotechnol. Bioeng. 2013, 110, 2277–2291. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Lee, E.; Kim, H.Y.; Youn, D.H.; Jung, J.; Kim, H.; Chang, Y.; Lee, W.; Shin, J.; Baek, S.; et al. Electromagnetized gold nanoparticles mediate direct lineage reprogramming into induced dopamine neurons in vivo for Parkinson’s disease therapy. Nat. Nanotechnol. 2017, 12, 1006–1014. [Google Scholar] [CrossRef]
- Cha, K.J.; Kong, S.Y.; Lee, J.S.; Kim, H.W.; Shin, J.Y.; La, M.; Han, B.W.; Kim, D.S.; Kim, H.J. Cell density-dependent differential proliferation of neural stem cells on omnidirectional nanopore-arrayed surface. Sci. Rep. 2017, 7, 13077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gliga, A.R.; Edoff, K.; Caputo, F.; Källman, T.; Blom, H.; Karlsson, H.L.; Ghibelli, L.; Traversa, E.; Ceccatelli, S.; Fadeel, B. Cerium oxide nanoparticles inhibit differentiation of neural stem cells. Sci. Rep. 2017, 7, 9284. [Google Scholar] [CrossRef]
- Ciofani, G.; Danti, S.; D’Alessandro, D.; Ricotti, L.; Moscato, S.; Bertoni, G.; Falqui, A.; Berrettini, S.; Petrini, M.; Mattoli, V.; et al. Enhancement of neurite outgrowth in neuronal-like cells following boron nitride nanotube-mediated stimulation. ACS Nano 2010, 4, 6267–6277. [Google Scholar] [CrossRef]
- Genchi, G.G.; Ceseracciu, L.; Marino, A.; Labardi, M.; Marras, S.; Pignatelli, F.; Bruschini, L.; Mattoli, V.; Ciofani, G. P(VDF-TrFE)/BaTiO3Nanoparticle Composite Films Mediate Piezoelectric Stimulation and Promote Differentiation of SH-SY5Y Neuroblastoma Cells. Adv. Healthc. Mater. 2016, 5, 1808–1820. [Google Scholar] [CrossRef]
- Marino, A.; Arai, S.; Hou, Y.; Sinibaldi, E.; Pellegrino, M.; Chang, Y.T.; Mazzolai, B.; Mattoli, V.; Suzuki, M.; Ciofani, G. Piezoelectric Nanoparticle-Assisted Wireless Neuronal Stimulation. ACS Nano 2015, 9, 7678–7689. [Google Scholar] [CrossRef]
- Rosenberg, S.S.; Spitzer, N.C. Calcium signaling in neuronal development. Cold Spring Harb. Perspect. Biol. 2011, 3, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johannsmeier, S.; Heeger, P.; Terakawa, M.; Kalies, S.; Heisterkamp, A.; Ripken, T.; Heinemann, D. Gold nanoparticle-mediated laser stimulation induces a complex stress response in neuronal cells. Sci. Rep. 2018, 8, 6533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kültz, D. Molecular and Evolutionary Basis of the Cellular Stress Response. Annu. Rev. Physiol. 2005, 67, 225–257. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S.; Gorman, A.M.; Hori, O.; Samali, A. Cellular stress responses: Cell survival and cell death. Int. J. Cell Biol. 2010, 2010, 214074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho-de-Souza, J.L.; Treger, J.S.; Dang, B.; Kent, S.B.H.; Pepperberg, D.R.; Bezanilla, F. Photosensitivity of neurons enabled by cell-targeted gold nanoparticles. Neuron 2015, 86, 207–217. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; O’Brien, C.; O’Brien, J.R.; Zhang, L.G. 3D nano/microfabrication techniques and nanobiomaterials for neural tissue regeneration. Nanomedicine 2014, 9, 859–875. [Google Scholar] [CrossRef]
- Aldinucci, A.; Turco, A.; Biagioli, T.; Toma, F.M.; Bani, D.; Guasti, D.; Manuelli, C.; Rizzetto, L.; Cavalieri, D.; Massacesi, L.; et al. Carbon nanotube scaffolds instruct human dendritic cells: Modulating immune responses by contacts at the nanoscale. Nano Lett. 2013, 13, 6098–6105. [Google Scholar] [CrossRef]
- Lovat, L.; Pantarotto, D.; Lagostena, L.; Cacciari, B.; Grandolfo, M.; Righi, M.; Spalluto, G.; Prato, M.; Ballerini, L. Carbon Nanotube Substrates Boost Neuronal Electrical Signaling. Nano Lett. 2005, 5, 1107–1110. [Google Scholar] [CrossRef]
- Matsumoto, K.; Shimizu, N. Activation of the phospholipase C signaling pathway in nerve growth factor-treated neurons by carbon nanotubes. Biomaterials 2013, 34, 5988–5994. [Google Scholar] [CrossRef]
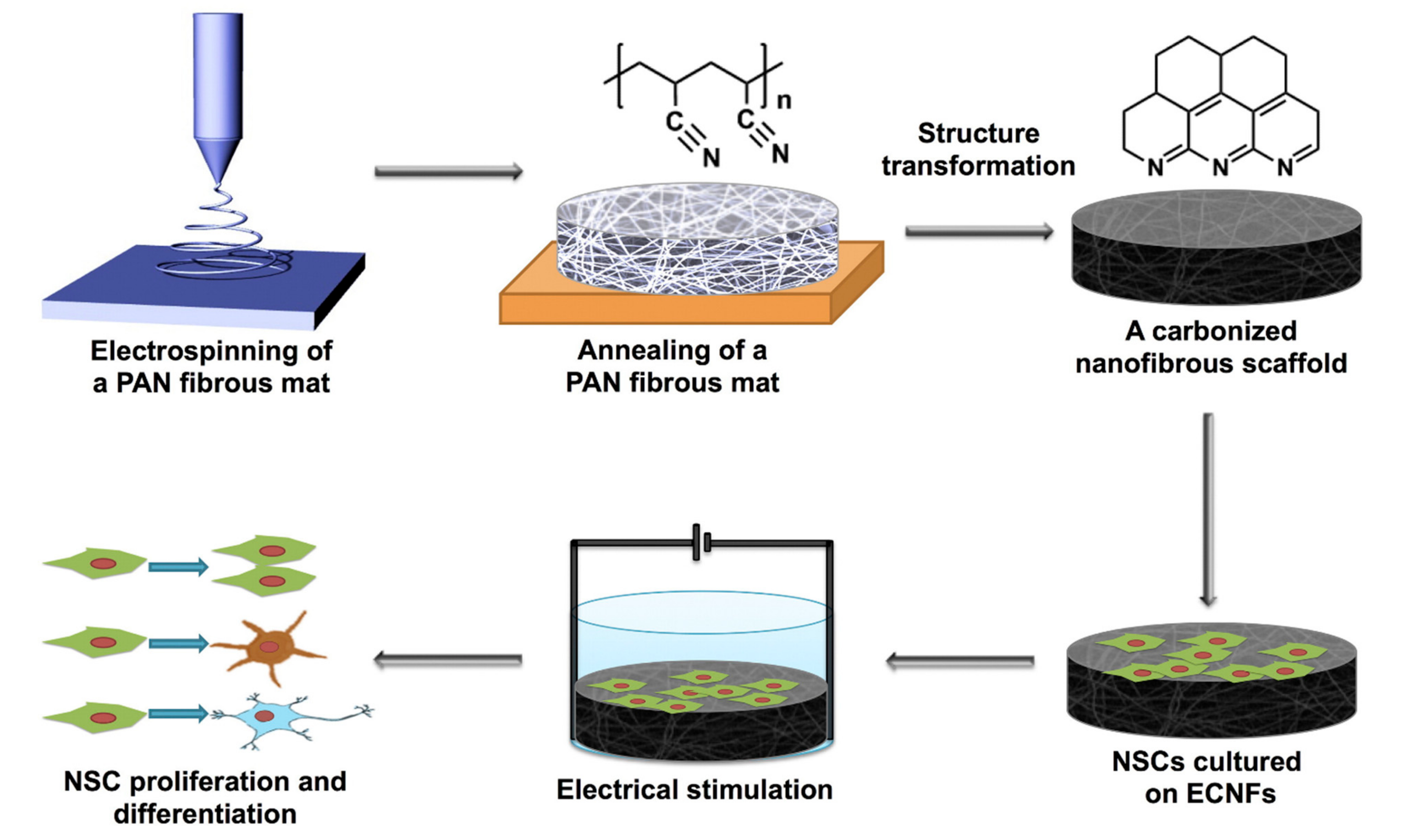
- Zhu, W.; Ye, T.; Lee, S.-J.; Cui, H.; Miao, S.; Zhou, X.; Shuai, D.; Zhang, L.G. Enhanced neural stem cell functions in conductive annealed carbon nanofibrous scaffolds with electrical stimulation. Nanomed. Nanotechnol. Biol. Med. 2017, 1–10. [Google Scholar] [CrossRef]
- Abbott, J.; Ye, T.; Qin, L.; Jorgolli, M.; Gertner, R.S.; Ham, D.; Park, H. CMOS Nanoelectrode Array for All-Electrical Intracellular Electrophysiological Imaging. Nat. Nanotechnol. 2017, 12, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Spira, M.E.; Hai, A. Multi-electrode array technologies for neuroscience and cardiology. Nat. Nanotechnol. 2013, 8, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.T.; Jorgolli, M.; Shalek, A.K.; Yoon, M.H.; Gertner, R.S.; Park, H. Vertical nanowire electrode arrays as a scalable platform for intracellular interfacing to neuronal circuits. Nat. Nanotechnol. 2012, 7, 180–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, C.; Lin, Z.; Hanson, L.; Cui, Y.; Cui, B. Intracellular recording of action potentials by nanopillar electroporation. Nat. Nanotechnol. 2012, 7, 185–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wijdenes, P.; Ali, H.; Armstrong, R.; Zaidi, W.; Dalton, C.; Syed, N.I. A novel bio-mimicking, planar nano-edge microelectrode enables enhanced long-term neural recording. Sci. Rep. 2016, 6, 34553. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.H.; Viventi, J.; Amsden, J.J.; Xiao, J.; Vigeland, L.; Kim, Y.S.; Blanco, J.A.; Panilaitis, B.; Frechette, E.S.; Contreras, D.; et al. Dissolvable films of silk fibroin for ultrathin conformal bio-integrated electronics. Nat. Mater. 2010, 9, 1–7. [Google Scholar] [CrossRef]
- Zhang, P.C.; Keleshian, A.M.; Sachs, F. Voltage-induced membrane movement. Nature 2001, 413, 428–432. [Google Scholar] [CrossRef]
- Matsuzaki, M.; Honkura, N.; Ellis-Davies, G.C.R.; Kasai, H. Structural basis of long-term potentiation in single dendritic spines. Nature 2004, 429, 761–766. [Google Scholar] [CrossRef]
- Lin, Y.; Freund, L.B. Forced detachment of a vesicle in adhesive contact with a substrate. Int. J. Solids Struct. 2007, 44, 1927–1938. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.D.; Deshmukh, N.; Nagarah, J.M.; Kramer, T.; Purohit, P.K.; Berry, M.J.; McAlpine, M.C. Piezoelectric nanoribbons for monitoring cellular deformations. Nat. Nanotechnol. 2012, 7, 587–593. [Google Scholar] [CrossRef]
- Zhao, Y.; You, S.S.; Zhang, A.; Lee, J.H.; Huang, J.; Lieber, C.M. Scalable ultrasmall three-dimensional nanowire transistor probes for intracellular recording. Nat. Nanotechnol. 2019, 14, 783–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Jimenez-Cruz, C.A.; Wang, J.; Zhou, B.; Yang, Z.; Zhou, R. Bio-mimicking of proline-rich motif applied to carbon nanotube reveals unexpected subtleties underlying nanoparticle functionalization. Sci. Rep. 2014, 4, 7229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rim, K.T.; Song, S.W.; Kim, H.Y. Oxidative DNA damage from nanoparticle exposure and its application to workers’ health: A literature review. Saf. Health Work 2013, 4, 177–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, Q.; Shao, D.; Ji, W.; Li, J.; Chen, L.; Song, J. The nanotoxicity investigation of optical nanoparticles to cultured cells in vitro. Toxicol. Rep. 2014, 1, 137–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, P.P.; Xia, Q.; Hwang, H.M.; Ray, P.C.; Yu, H. Mechanisms of nanotoxicity: Generation of reactive oxygen species. J. Food Drug Anal. 2014, 22, 64–75. [Google Scholar] [CrossRef] [Green Version]
- Kunzmann, A.; Andersson, B.; Thurnherr, T.; Krug, H.; Scheynius, A.; Fadeel, B. Toxicology of engineered nanomaterials: Focus on biocompatibility, biodistribution and biodegradation. Biochim. Biophys. Acta Gen. Subj. 2011, 1810, 361–373. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Chircov, C.; Grumezescu, A.M.; Teleanu, R.I. Neurotoxicity of nanomaterials: An up-to-date overview. Nanomaterials 2019, 9, 96. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, S.; Mishra, S.; Juha, S.; Pramanik, M.; Padmanabhan, P.; Gulyás, B. Nanotechnology Facilitated Cultured Neuronal Network and Its Applications. Int. J. Mol. Sci. 2021, 22, 5552. https://doi.org/10.3390/ijms22115552
Singh S, Mishra S, Juha S, Pramanik M, Padmanabhan P, Gulyás B. Nanotechnology Facilitated Cultured Neuronal Network and Its Applications. International Journal of Molecular Sciences. 2021; 22(11):5552. https://doi.org/10.3390/ijms22115552
Chicago/Turabian StyleSingh, Satnam, Sachin Mishra, Song Juha, Manojit Pramanik, Parasuraman Padmanabhan, and Balázs Gulyás. 2021. "Nanotechnology Facilitated Cultured Neuronal Network and Its Applications" International Journal of Molecular Sciences 22, no. 11: 5552. https://doi.org/10.3390/ijms22115552
APA StyleSingh, S., Mishra, S., Juha, S., Pramanik, M., Padmanabhan, P., & Gulyás, B. (2021). Nanotechnology Facilitated Cultured Neuronal Network and Its Applications. International Journal of Molecular Sciences, 22(11), 5552. https://doi.org/10.3390/ijms22115552





