Attachment of Cancer Urothelial Cells to the Bladder Epithelium Occurs on Uroplakin-Negative Cells and Is Mediated by Desmosomal and Not by Classical Cadherins
Abstract
1. Introduction
2. Results
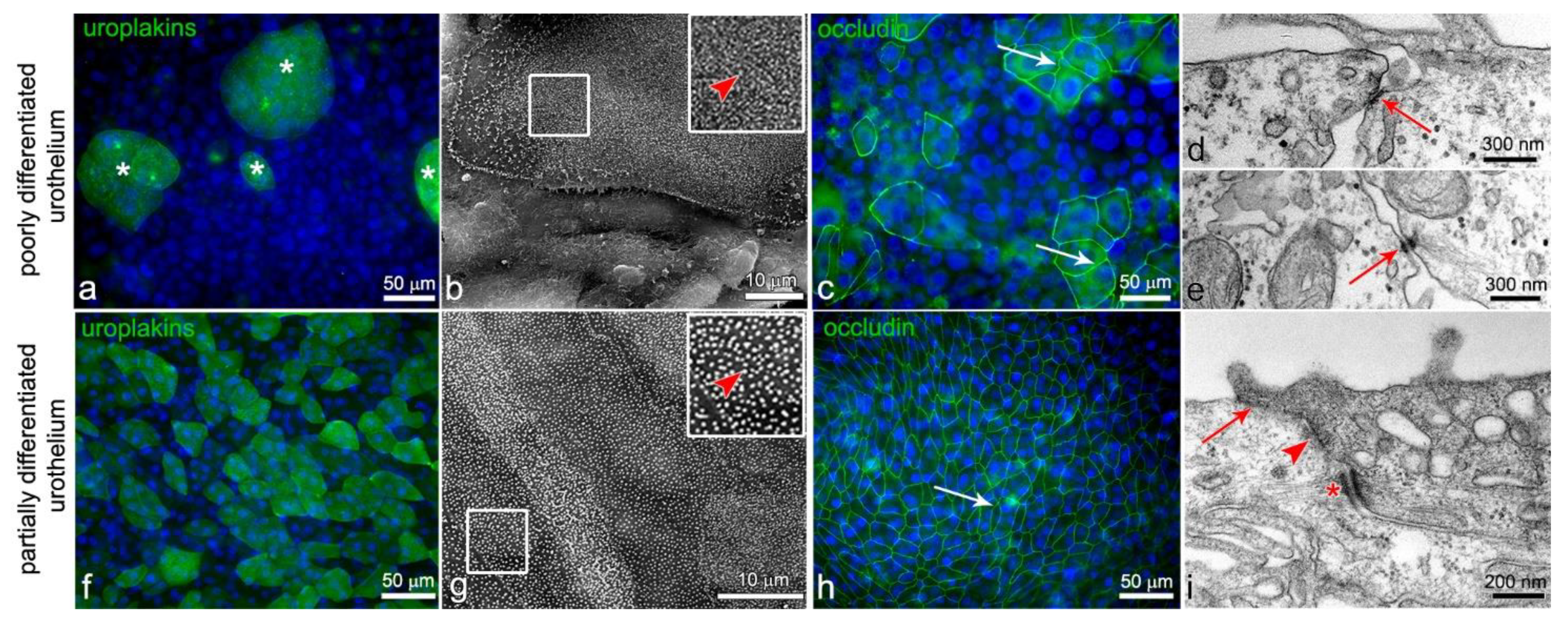
2.1. Urothelial In Vitro Models: Poorly Differentiated Urothelia from RT4 Cells and Partially Differentiated Urothelia Established from Normal Urothelial (NPU) Cells
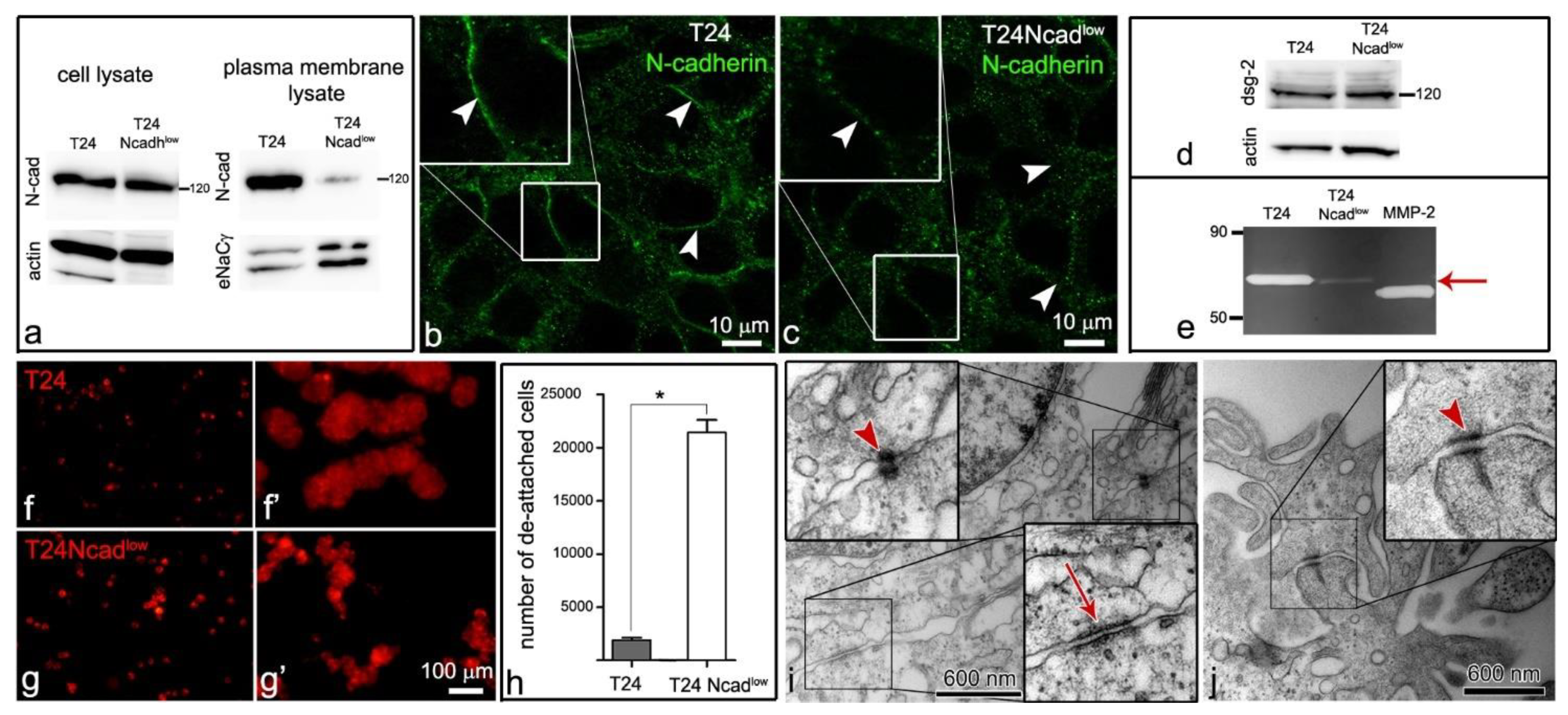
2.2. T24 Ncadlow Cells Are Characterized by Lower Expression of N-Cadherin in the Plasma Membrane Compared with T24 Cells
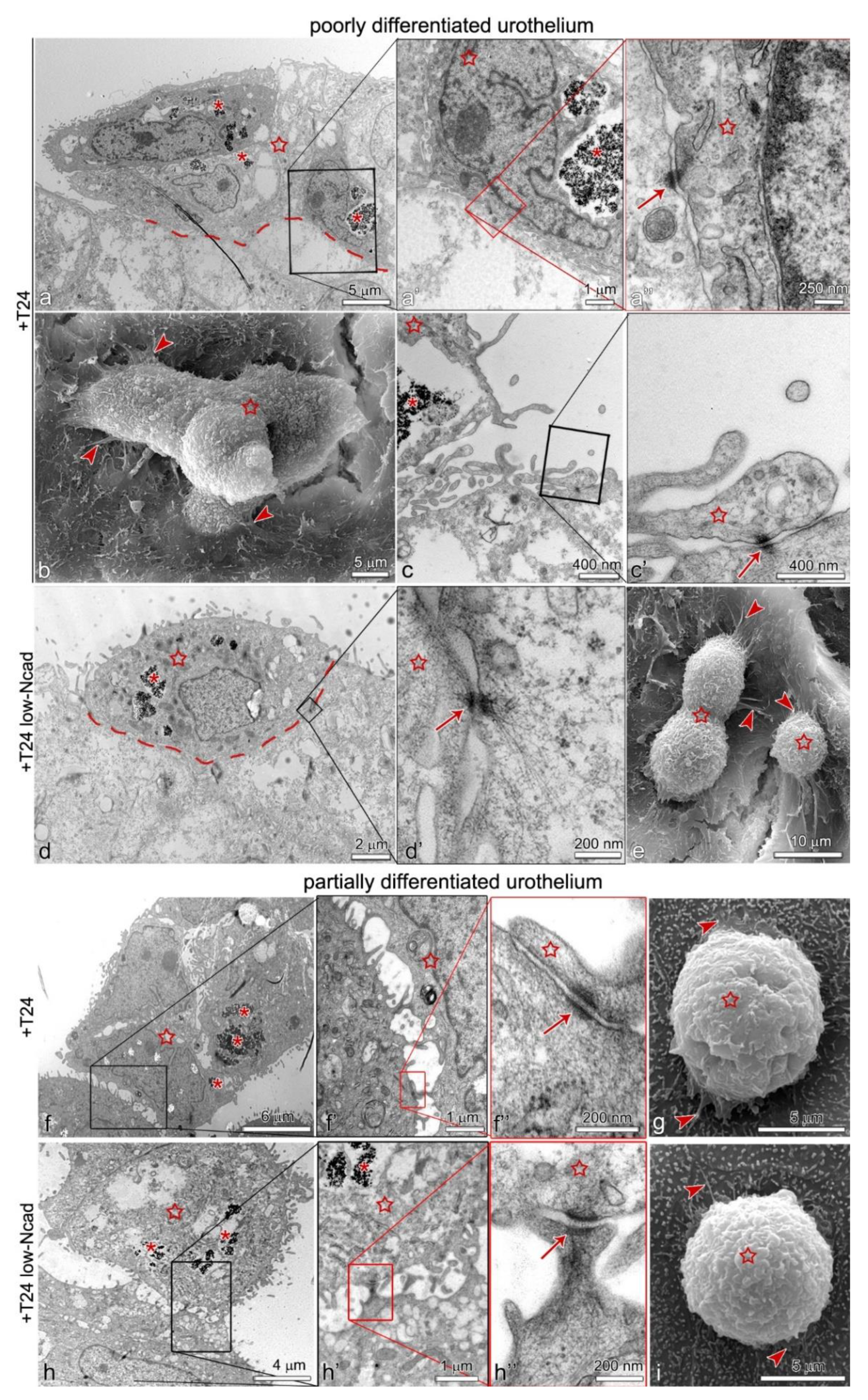
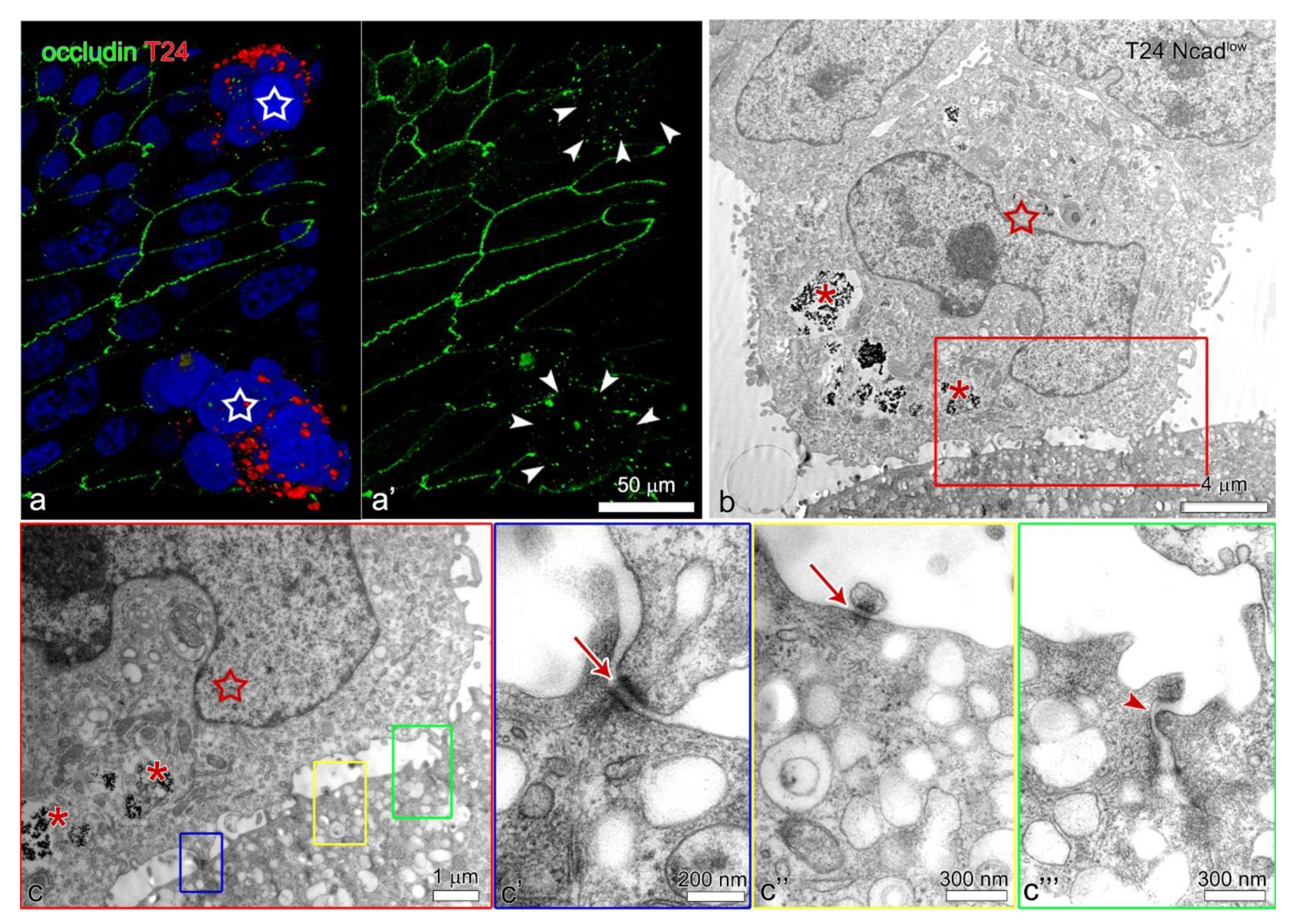
2.3. The Lower Expression of N-Cadherin in the Plasma Membrane of the T24 Ncadlow Cells Does Not Affect Their Attachment to the Poorly or Partially Differentiated Urothelium
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.2. Establishment of Cancer Urothelial Cell Line T24 with Low Expression of N-Cadherin
4.3. Seeding of Cancer Urothelial Cells on the Poorly or Partially Differentiated Urothelium
4.4. Quantitative Analysis of the Cancer Urothelial Cell Aggregates Adhered to the Poorly or Partially Differentiated Urothelium
4.5. Immunofluorescence Analysis
4.6. Transmission and Scanning Electron Microscopy
4.7. In Gel Gelatin Zymography
4.8. Cell Surface Biotinylation
4.9. Western Blot
4.10. Hanging Drop Assay
4.11. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antoni, S.; Ferlay, J.; Soerjomataram, I.; Znaor, A.; Jemal, A.; Bray, F. Bladder cancer incidence and mortality: A global overview and recent trends. Eur. Urol. 2017, 71, 96–108. [Google Scholar] [CrossRef]
- Millán-Rodríguez, F.; Chéchile-Toniolo, G.; Salvador-Bayarri, J.; Palou, J.; Vicente-Rodríguez, J. Multivariate analysis of the prognostic factors of primary superficial bladder cancer. J. Urol. 2000, 163, 73–78. [Google Scholar] [CrossRef]
- Höglund, M. Bladder cancer, a two phased disease? Semin. Cancer Biol. 2007, 17, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Simon, R.; Eltze, E.; Schäfer, K.L.; Bürger, H.; Semjonow, A.; Hertle, L.; Dockhorn-Dworniczak, B.; Terpe, H.J.; Böcker, W. Cytogenetic analysis of multifocal bladder cancer supports a monoclonal origin and intraepithelial spread of tumor cells. Cancer Res. 2001, 61, 355–362. [Google Scholar] [PubMed]
- Fadl-Elmula, I.; Gorunova, L.; Mandahl, N.; Elfving, P.; Lundgren, R.; Mitelman, F.; Heim, S. Cytogenetic monoclonality in multifocal uroepithelial carcinomas: Evidence of intraluminal tumour seeding. Br. J. Cancer 1999, 81, 6–12. [Google Scholar] [CrossRef]
- Acar, Ö.; Özkurt, E.; Demir, G.; Saraç, H.; Alkan, C.; Esen, T.; Somel, M.; Lack, N.A. Determining the origin of synchronous multifocal bladder cancer by exome sequencing. BMC Cancer 2015, 15, 871. [Google Scholar] [CrossRef]
- Hafner, C.; Knuechel, R.; Stoehr, R.; Hartmann, A. Clonality of multifocal urothelial carcinomas: 10 years of molecular genetic studies. Int. J. Cancer 2002, 101, 1–6. [Google Scholar] [CrossRef]
- Catto, J.W.; Hartmann, A.; Stoehr, R.; Bolderson, E.; Rehman, I.; Rosario, D.J.; Hamdy, F.C.; Meuth, M. Multifocal urothelial cancers with the mutator phenotype are of monoclonal origin and require panurothelial treatment for tumor clearance. J. Urol. 2006, 175, 2323–2330. [Google Scholar] [CrossRef]
- Conconi, D.; Panzeri, E.; Redaelli, S.; Bovo, G.; Volante, M.; Viganò, P.; Strada, G.; Dalprà, L.; Bentivegna, A. DNA copy number alterations and PPARG amplification in a patient with multifocal bladder urothelial carcinoma. BMC Res. Notes 2012, 5, 607. [Google Scholar] [CrossRef]
- Shin, K.; Lim, A.; Odegaard, J.I.; Honeycutt, J.D.; Kawano, S.; Hsieh, M.H.; Beachy, P.A. Cellular origin of bladder neoplasia and tissue dynamics of its progression to invasive carcinoma. Nat. Cell Biol. 2014, 16, 469–478. [Google Scholar] [CrossRef]
- Hoglund, M. On the origin of syn- and metachronous urothelial carcinomas. Eur. Urol. 2007, 51, 1185–1193. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Takahashi, T.; Kakehi, Y.; Mitsumori, K.; Akao, T.; Terachi, T.; Kato, T.; Ogawa, O.; Habuchi, T. Distinct microsatellite alterations in upper urinary tract tumors and subsequent bladder tumors. J. Urol. 2001, 165, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Fujiyama, C.; Tokuda, Y.; Sugihara, H.; Masaki, Z. Bladder cancer cell implantation in reconstructed bladder in vitro: A model of tumour recurrence. BJU Int. 2002, 89, 119–125. [Google Scholar] [CrossRef]
- Rebel, J.M.; Thijssen, C.D.; Vermey, M.; Delouvée, A.; Zwarthoff, E.C.; Van der Kwast, T.H. E-Cadherin expression determines the mode of replacement of normal urothelium by human bladder carcinoma cells. Cancer Res. 1994, 54, 5488–5492. [Google Scholar] [PubMed]
- Bindels, E.M.; Vermey, M.; Rebel, J.M.; Zwarthoff, E.C.; Van Der Kwast, T.H. In vitro modulation of implantation and intraepithelial expansion of bladder tumor cells by epidermal growth factor. Exp. Cell Res. 1997, 235, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Tucker, D.K.; Kohlhorst, D.; Niessen, C.M.; Kowalczyk, A.P. Classical and desmosomal cadherins at a glance. J. Cell Sci. 2012, 125, 2547–2552. [Google Scholar] [CrossRef]
- Wheelock, M.J.; Shintani, Y.; Maeda, M.; Fukumoto, Y.; Johnson, K.R. Cadherin switching. J. Cell Sci. 2008, 121, 727–735. [Google Scholar] [CrossRef]
- Loh, C.Y.; Chai, J.Y.; Tang, T.F.; Wong, W.F.; Sethi, G.; Shanmugam, M.K.; Chong, P.P.; Looi, C.Y. The E-cadherin and N-cadherin switch in epithelial-to-mesenchymal transition: Signaling, therapeutic implications, and challenges. Cells 2019, 8, 1118. [Google Scholar] [CrossRef]
- Cao, Z.-Q.; Wang, Z.; Leng, P. Aberrant N-cadherin expression in cancer. Biomed. Pharmacother. 2019, 118, 109320. [Google Scholar] [CrossRef]
- Hazan, R.B.; Phillips, G.R.; Qiao, R.F.; Norton, L.; Aaronson, S.A. Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion, and metastasis. J. Cell Biol. 2000, 148, 779–790. [Google Scholar] [CrossRef]
- Suyama, K.; Shapiro, I.; Guttman, M.; Hazan, R.B. A signaling pathway leading to metastasis is controlled by N-cadherin and the FGF receptor. Cancer Cell 2002, 2, 301–314. [Google Scholar] [CrossRef]
- Labernadie, A.; Kato, T.; Brugués, A.; Serra-Picamal, X.; Derzsi, S.; Arwert, E.; Weston, A.; González-Tarragó, V.; Elosegui-Artola, A.; Albertazzi, L.; et al. A mechanically active heterotypic E-cadherin/N-cadherin adhesion enables fibroblasts to drive cancer cell invasion. Nat. Cell Biol. 2017, 19, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Straub, B.K.; Rickelt, S.; Zimbelmann, R.; Grund, C.; Kuhn, C.; Iken, M.; Ott, M.; Schirmacher, P.; Franke, W.W. E-N-cadherin heterodimers define novel adherens junctions connecting endoderm-derived cells. J. Cell Biol. 2011, 195, 873–887. [Google Scholar] [CrossRef] [PubMed]
- Shan, W.S.; Tanaka, H.; Phillips, G.R.; Arndt, K.; Yoshida, M.; Colman, D.R.; Shapiro, L. Functional cis-heterodimers of N- and R-cadherins. J. Cell Biol. 2000, 148, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Ounkomol, C.; Yamada, S.; Heinrich, V. Single-Cell adhesion tests against functionalized microspheres arrayed on AFM cantilevers confirm heterophilic E- and N-cadherin binding. Biophys. J. 2010, 99, L100–L102. [Google Scholar] [CrossRef]
- Babjuk, M.; Böhle, A.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Hernández, V.; Kaasinen, E.; Palou, J.; Rouprêt, M.; et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: Update 2016. Eur. Urol. 2017, 71, 447–461. [Google Scholar] [CrossRef]
- Kakizoe, T. Development and progression of urothelial carcinoma. Cancer Sci. 2006, 97, 821–828. [Google Scholar] [CrossRef]
- Kim, B.; Choi, H.J.; Kim, M.H.; Cho, K.S. Recurrence patterns of bladder transitional cell carcinoma after radical cystectomy. Acta Radiol. 2012, 53, 943–949. [Google Scholar] [CrossRef]
- Herr, H.W. Tumor progression and survival of patients with high grade, noninvasive papillary (TaG3) bladder tumors: 15-year outcome. J. Urol. 2000, 163, 60–61. [Google Scholar] [CrossRef]
- Tang, D.H.; Chang, S.S. Management of carcinoma in situ of the bladder: Best practice and recent developments. Ther. Adv. Urol. 2015, 7, 351–364. [Google Scholar] [CrossRef]
- Abufaraj, M.; Shariat, S.F.; Haitel, A.; Moschini, M.; Foerster, B.; Chłosta, P.; Gust, K.; Babjuk, M.; Briganti, A.; Karakiewicz, P.I.; et al. Prognostic role of N-cadherin expression in patients with non–muscle-invasive bladder cancer. Urol. Oncol. Semin. Orig. Investig. 2017, 35, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Muramaki, M.; Miyake, H.; Terakawa, T.; Kumano, M.; Sakai, I.; Fujisawa, M. Expression profile of E-cadherin and N-cadherin in non-muscle-invasive bladder cancer as a novel predictor of intravesical recurrence following transurethral resection. Urol. Oncol. Semin. Orig. Investig. 2012, 30, 161–166. [Google Scholar] [CrossRef]
- Lascombe, I.; Clairotte, A.; Fauconnet, S.; Bernardini, S.; Wallerand, H.; Kantelip, B.; Bittard, H. N-Cadherin as a novel prognostic marker of progression in superficial urothelial tumors. Clin. Cancer Res. 2006, 12, 2780–2787. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Erman, A.; Kapun, G.; Novak, S.; Pavlin, M.; Drazic, G.; Drobne, D.; Veranic, P. How cancer cells attach to urinary bladder epithelium in vivo: Study of the early stages of tumorigenesis in an orthotopic mouse bladder tumor model. Histochem. Cell Biol. 2019, 151, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Alroy, J.; Pauli, B.U.; Weinstein, R.S. Correlation between numbers of desmosomes and the aggressiveness of transitional cell carcinoma in human urinary bladder. Cancer 1981, 47, 104–112. [Google Scholar] [CrossRef]
- Yang, Y.; Estrada, E.Y.; Thompson, J.F.; Liu, W.; Rosenberg, G.A. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J. Cereb. Blood Flow Metab. 2007, 27, 697–709. [Google Scholar] [CrossRef]
- Xin, H.; Liang, W.; Mang, J.; Lin, L.; Guo, N.; Zhang, F.; Xu, Z. Relationship of gelatinases-tight junction proteins and blood-brain barrier permeability in the early stage of cerebral ischemia and reperfusion. Neural Regen. Res. 2012, 7, 2405–2412. [Google Scholar] [CrossRef]
- Lischper, M.; Beuck, S.; Thanabalasundaram, G.; Pieper, C.; Galla, H.J. Metalloproteinase mediated occludin cleavage in the cerebral microcapillary endothelium under pathological conditions. Brain Res. 2010, 1326, 114–127. [Google Scholar] [CrossRef]
- Delva, E.; Tucker, D.K.; Kowalczyk, A.P. The desmosome. Cold Spring Harb. Perspect. Biol. 2009, 1, a002543. [Google Scholar] [CrossRef]
- Roberts, B.J.; Pashaj, A.; Johnson, K.R.; Wahl, J.K., 3rd. Desmosome dynamics in migrating epithelial cells requires the actin cytoskeleton. Exp. Cell Res. 2011, 317, 2814–2822. [Google Scholar] [CrossRef]
- Michels, C.; Buchta, T.; Bloch, W.; Krieg, T.; Niessen, C.M. Classical cadherins regulate desmosome formation. J. Investig. Dermatol. 2009, 129, 2072–2075. [Google Scholar] [CrossRef] [PubMed]
- Shafraz, O.; Rübsam, M.; Stahley, S.N.; Caldara, A.L.; Kowalczyk, A.P.; Niessen, C.M.; Sivasankar, S. E-Cadherin binds to desmoglein to facilitate desmosome assembly. eLife 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Resnik, N.; Repnik, U.; Kreft, M.E.; Sepčić, K.; Maček, P.; Turk, B.; Veranič, P. Highly selective anti-cancer activity of cholesterol-interacting agents methyl-β-cyclodextrin and ostreolysin A/pleurotolysin B protein complex on urothelial cancer cells. PLoS ONE 2015, 10, e0137878. [Google Scholar] [CrossRef] [PubMed]
- Lojk, J.; Bregar, V.B.; Strojan, K.; Hudoklin, S.; Veranič, P.; Pavlin, M.; Kreft, M.E. Increased endocytosis of magnetic nanoparticles into cancerous urothelial cells versus normal urothelial cells. Histochem. Cell Biol. 2018, 149, 45–59. [Google Scholar] [CrossRef]
- Zupančič, D.; Mrak Poljšak, K.; Kreft, M.E. Co-Culturing porcine normal urothelial cells, urinary bladder fibroblasts and smooth muscle cells for tissue engineering research. Cell Biol. Int. 2018, 42, 411–424. [Google Scholar] [CrossRef]
- Jerman, U.D.; Kolenc, M.; Steyer, A.; Veranic, P.; Prijatelj, M.P.; Kreft, M.E. A novel strain of porcine adenovirus detected in urinary bladder urothelial cell culture. Viruses 2014, 6, 2505–2518. [Google Scholar] [CrossRef]
- Bregar, V.B.; Lojk, J.; Suštar, V.; Veranič, P.; Pavlin, M. Visualization of internalization of functionalized cobalt ferrite nanoparticles and their intracellular fate. Int. J. Nanomed. 2013, 8, 919–931. [Google Scholar] [CrossRef]
- Granelli-Piperno, A.; Reich, E. A study of proteases and protease-inhibitor complexes in biological fluids. J. Exp. Med. 1978, 148, 223–234. [Google Scholar] [CrossRef]
- Busch, G.; Hoder, D.; Reutter, W.; Tauber, R. Selective isolation of individual cell surface proteins from tissue culture cells by a cleavable biotin label. Eur. J. Cell Biol. 1989, 50, 257–262. [Google Scholar]
- Teng, Y. Hanging drop aggregation assay of breast cancer cells. Bio Protoc. 2015, 5. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jerman, U.D.; Višnjar, T.; Bratkovič, I.H.; Resnik, N.; Pavlin, M.; Veranič, P.; Kreft, M.E. Attachment of Cancer Urothelial Cells to the Bladder Epithelium Occurs on Uroplakin-Negative Cells and Is Mediated by Desmosomal and Not by Classical Cadherins. Int. J. Mol. Sci. 2021, 22, 5565. https://doi.org/10.3390/ijms22115565
Jerman UD, Višnjar T, Bratkovič IH, Resnik N, Pavlin M, Veranič P, Kreft ME. Attachment of Cancer Urothelial Cells to the Bladder Epithelium Occurs on Uroplakin-Negative Cells and Is Mediated by Desmosomal and Not by Classical Cadherins. International Journal of Molecular Sciences. 2021; 22(11):5565. https://doi.org/10.3390/ijms22115565
Chicago/Turabian StyleJerman, Urška Dragin, Tanja Višnjar, Iva Hafner Bratkovič, Nataša Resnik, Mojca Pavlin, Peter Veranič, and Mateja Erdani Kreft. 2021. "Attachment of Cancer Urothelial Cells to the Bladder Epithelium Occurs on Uroplakin-Negative Cells and Is Mediated by Desmosomal and Not by Classical Cadherins" International Journal of Molecular Sciences 22, no. 11: 5565. https://doi.org/10.3390/ijms22115565
APA StyleJerman, U. D., Višnjar, T., Bratkovič, I. H., Resnik, N., Pavlin, M., Veranič, P., & Kreft, M. E. (2021). Attachment of Cancer Urothelial Cells to the Bladder Epithelium Occurs on Uroplakin-Negative Cells and Is Mediated by Desmosomal and Not by Classical Cadherins. International Journal of Molecular Sciences, 22(11), 5565. https://doi.org/10.3390/ijms22115565





