Bioactive Nanofiber-Based Conduits in a Peripheral Nerve Gap Management—An Animal Model Study
Abstract
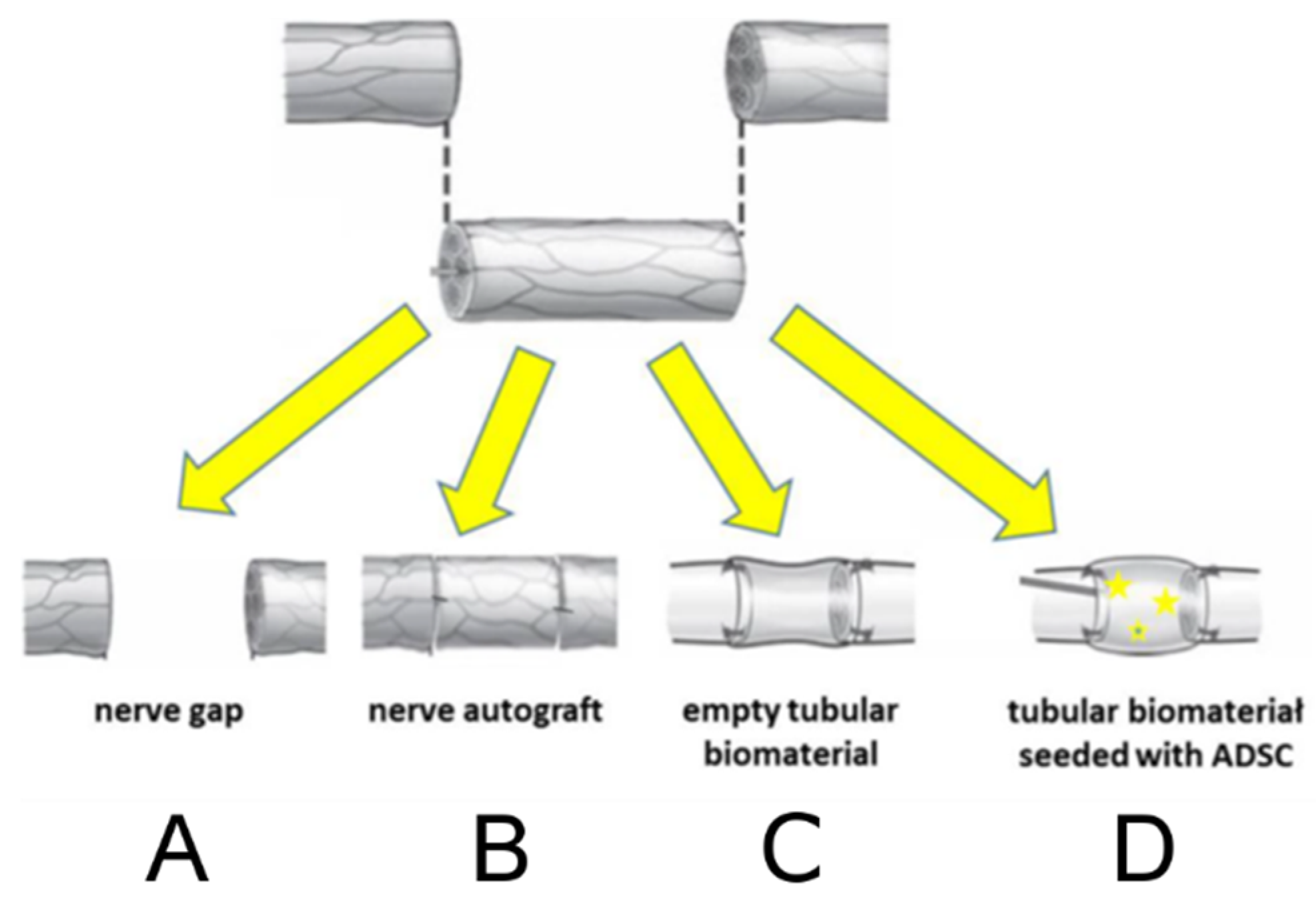
:1. Introduction
2. Results
2.1. Morphology of the Electrospun Tubes
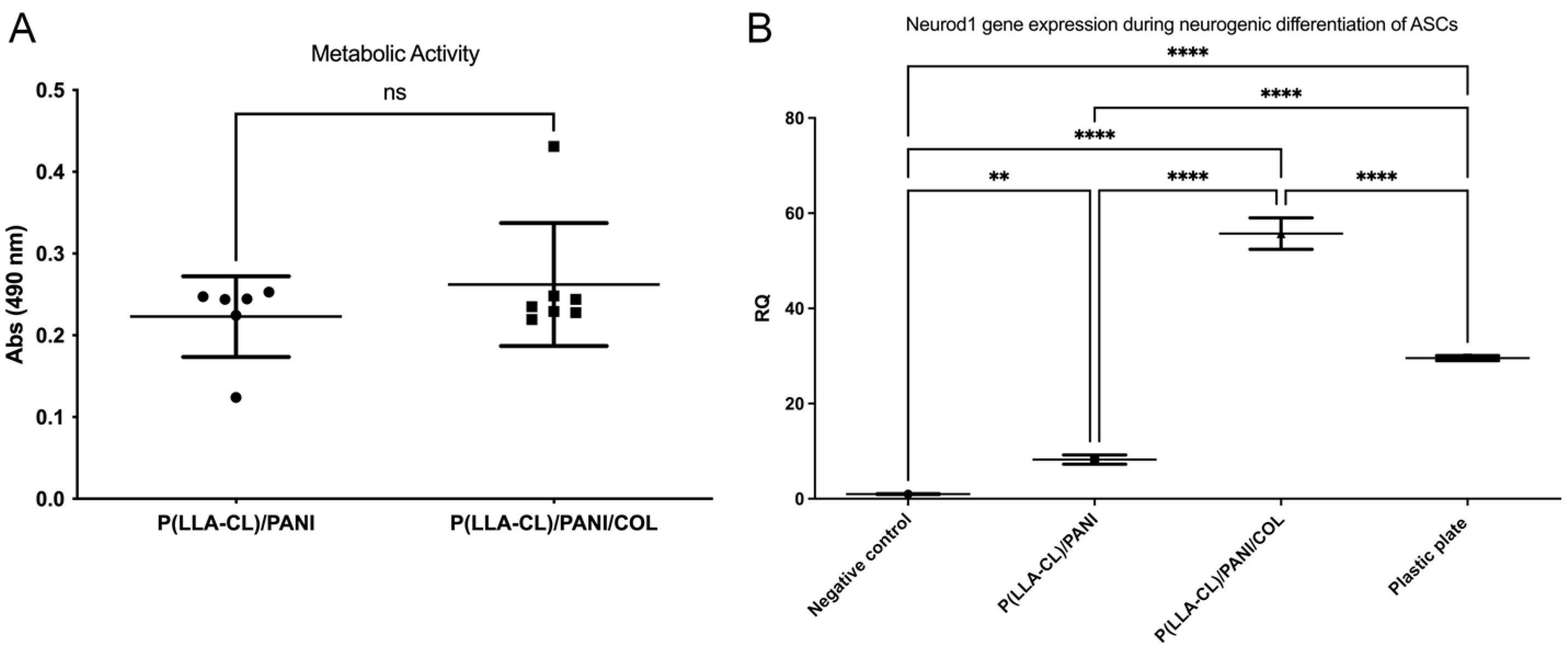
2.2. Biocompatibility Studies
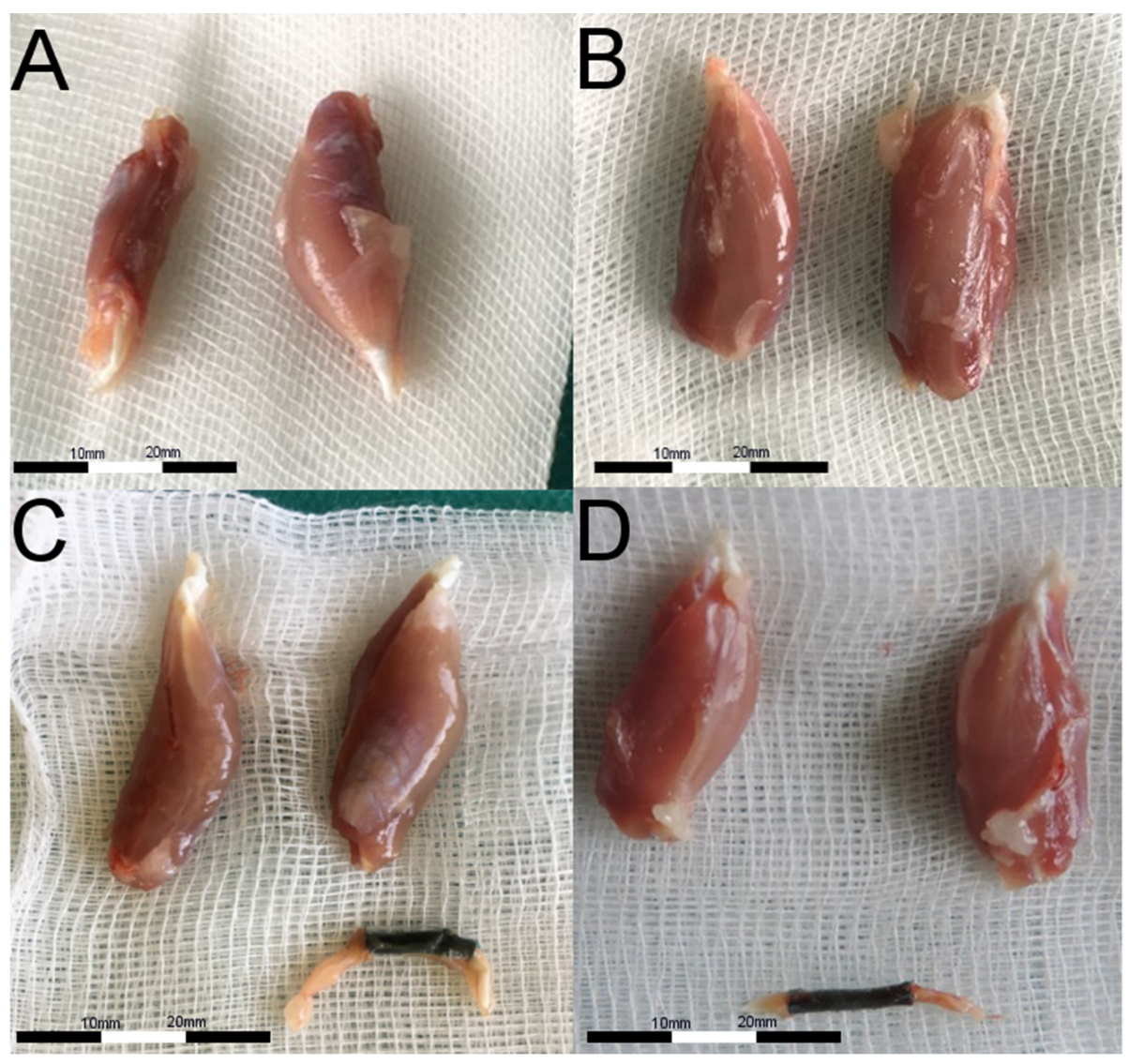
2.3. Animal Study and SFI
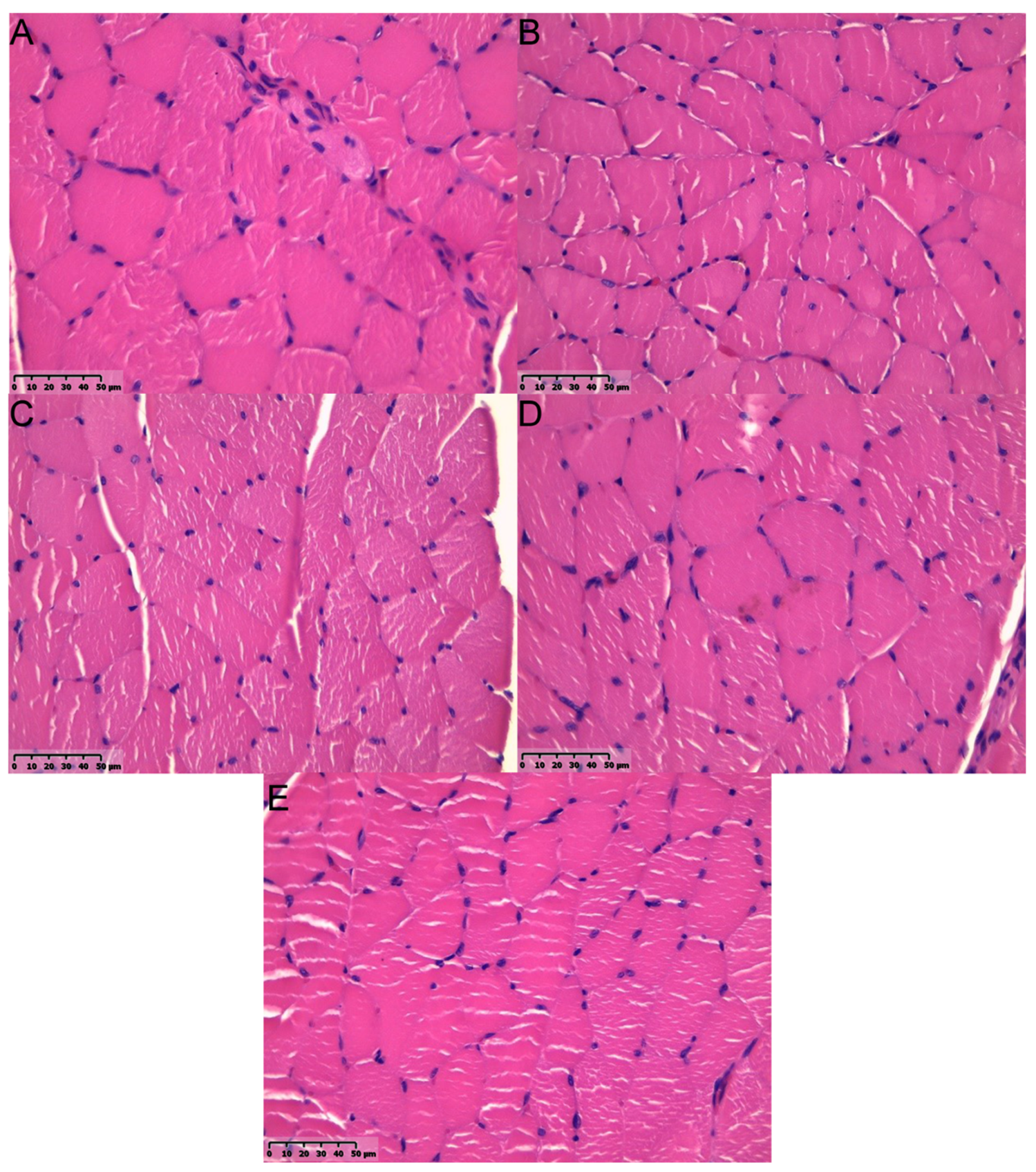
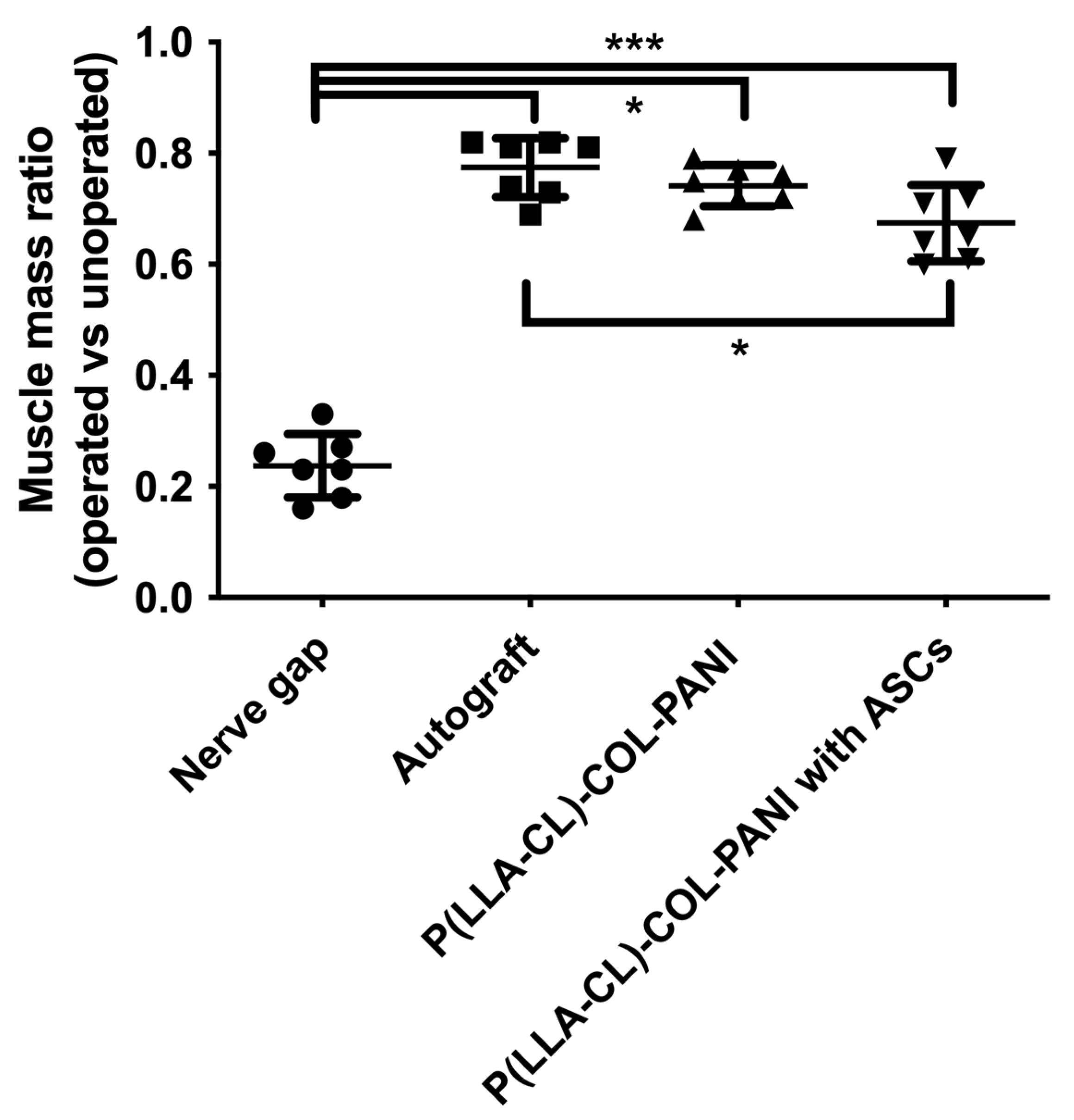
2.4. Muscular Tissue
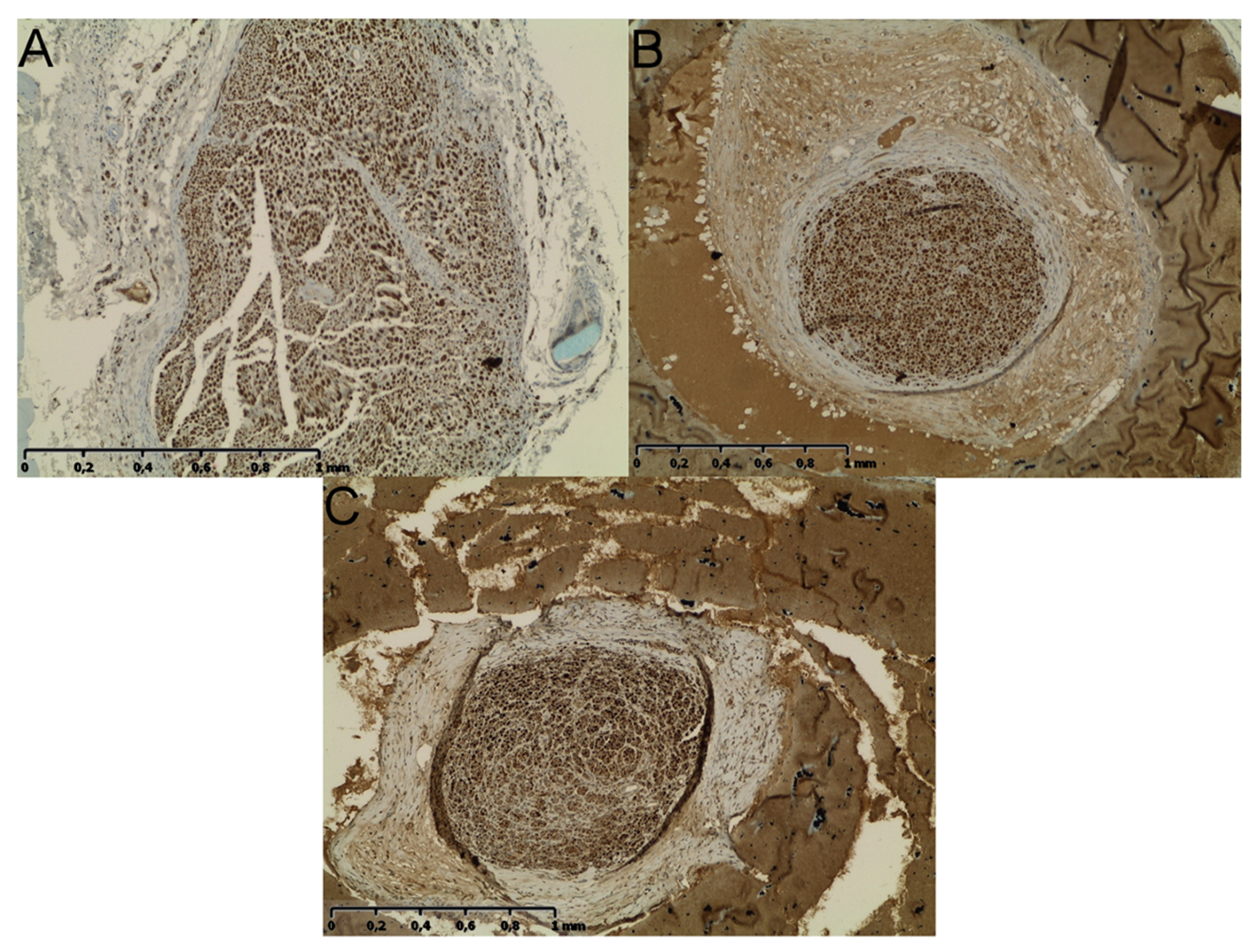
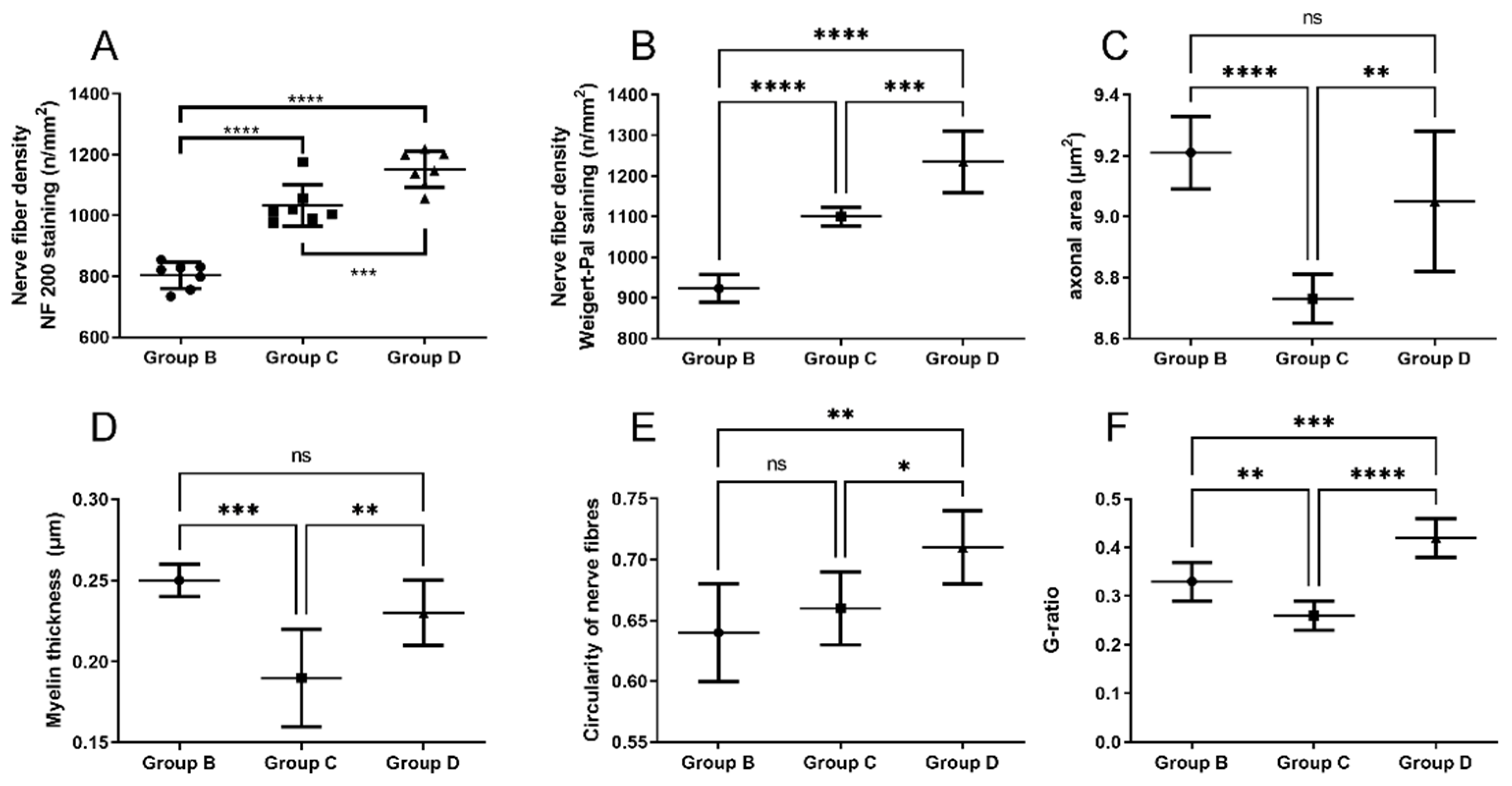
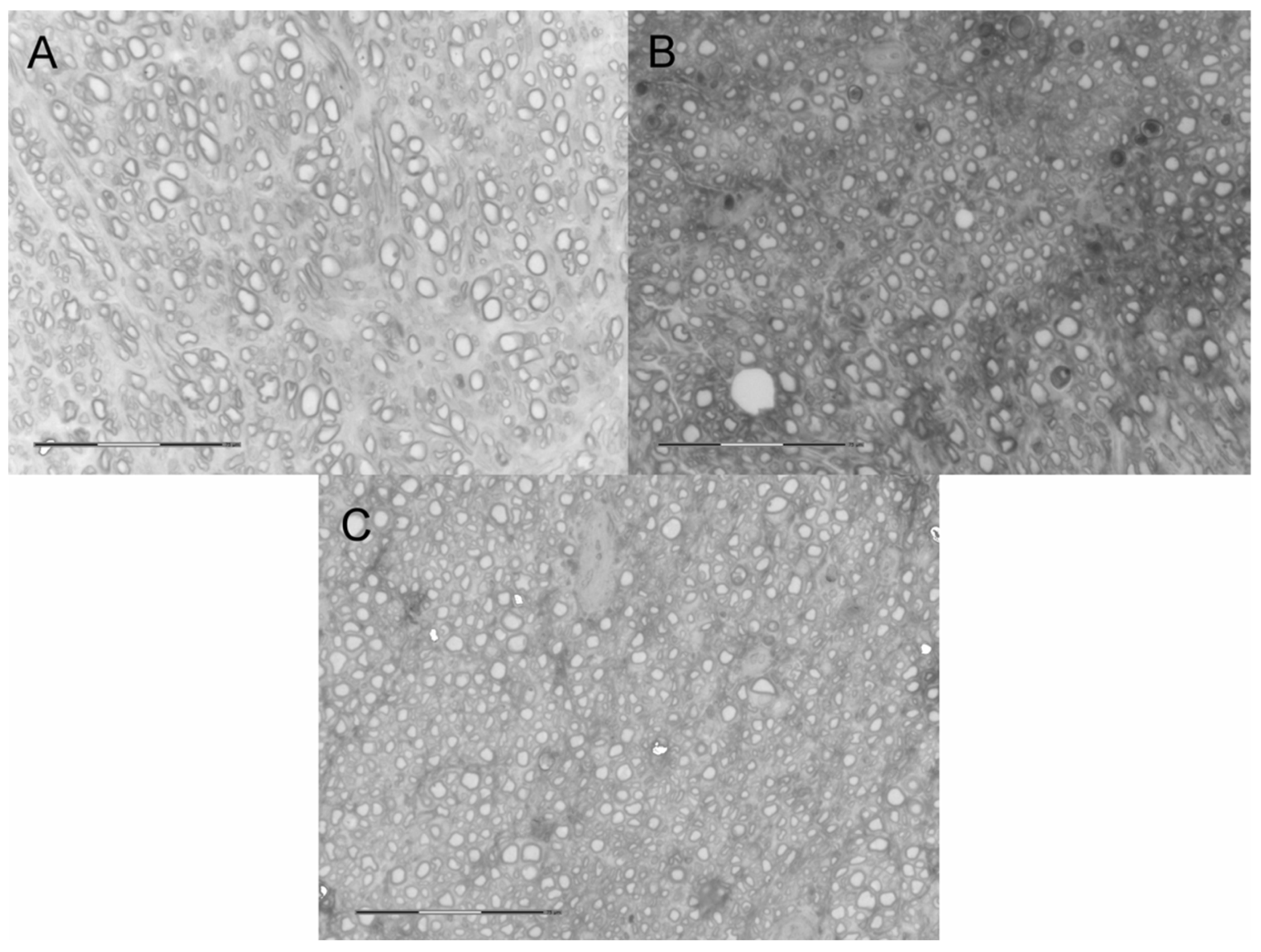
2.5. Neural Tissue
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Fabrication of Electrospun Tubular Fibrous Scaffolds
4.3. Characterization of the Electrospun Scaffolds
4.4. Biocompatibility of the Scaffolds and In Vitro Studies
4.4.1. Harvesting, Isolation, and Differentiation of ASCs
4.4.2. Metabolic Activity of ASCs Cultured on P(LLA-CL)-COL-PANI and P(LLA-CL)/PANI Meshes
4.4.3. ASCs Neural Differentiation on P(LLA-CL)-COL-PANI and P(LLA-CL)/PANI Meshes
4.4.4. Culture of ASCs on P(LLA-CL)-COL-PANI Tubular Scaffolds
4.4.5. Animal Study Design
4.5. Sciatic Function Index (SFI) Analysis
Samples’ Harvest and Histological Analyses
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dębski, T.; Noszczyk, B.H. Epidemiology of complex hand injuries treated in the Plastic Surgery Department of a tertiary referral hospital in Warsaw. Eur. J. Trauma Emerg. Surg. 2020, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Huckhagel, T.; Dgu, T.; Nüchtern, J.; Regelsberger, J.; Lefering, R. Nerve injury in severe trauma with upper extremity involvement: Evaluation of 49,382 patients from the TraumaRegister DGU® between 2002 and 2015. Scand. J. Trauma Resusc. Emerg. Med. 2018, 26, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hundepool, C.A.; Nijhuis, T.H.J.; Mohseny, B.; Selles, R.W.; Hovius, S.E.R. The effect of stem cells in bridging peripheral nerve defects: A meta-analysis. J. Neurosurg. 2014, 121, 195–209. [Google Scholar] [CrossRef] [Green Version]
- Clark, W.L.; Trumble, T.E.; Swiontkowski, M.F.; Tencer, A.F. Nerve tension and blood flow in a rat model of immediate and delayed repairs. J. Hand Surg. 1992, 17, 677–687. [Google Scholar] [CrossRef]
- Nassimizadeh, M.; Nassimizadeh, A.K.; Power, D. Managing the nerve gap: New tools in the peripheral nerve repair toolbox. J. Musculoskelet. Surg. Res. 2019, 3, 4. [Google Scholar] [CrossRef]
- Huayllani, M.T.; Boczar, D.; Forte, A.J.; Rinker, B. Evidence-Based Approach to Nerve Gap Repair in the Upper Extremity: A Review of the Literature and Current Algorithm for Surgical Management. Ann. Plast Surg. 2020, 84, S369–S374. [Google Scholar] [CrossRef] [PubMed]
- Brooks, D.N.; Weber, R.V.; Chao, J.D.; Rinker, B.D.; Zoldos, J.; Robichaux, M.R.; Ruggeri, S.B.; Anderson, K.A.; Bonatz, E.E.; Wisotsky, S.M.; et al. Processed nerve allografts for peripheral nerve reconstruction: A multicenter study of utilization and outcomes in sensory, mixed, and motor nerve reconstructions. Microsurgery 2011, 32, 1–14. [Google Scholar] [CrossRef]
- Pi, H.-Y.; Gao, Y.; Wang, Y.-L.; Kong, D.; Qu, B.; Su, X.-J.; Li, H. Nerve autografts and tissue-engineered materials for the repair of peripheral nerve injuries: A 5-year bibliometric analysis. Neural Regen. Res. 2015, 10, 1003–1008. [Google Scholar] [CrossRef]
- Rebowe, R.; Rogers, A.; Yang, X.; Kundu, S.C.; Smith, T.L.; Li, Z. Nerve Repair with Nerve Conduits: Problems, Solutions, and Future Directions. J. Hand Microsurg. 2018, 10, 61–65. [Google Scholar] [CrossRef]
- Boecker, A.; Daeschler, S.C.; Kneser, U.; Harhaus, L. Relevance and Recent Developments of Chitosan in Peripheral Nerve Surgery. Front. Cell. Neurosci. 2019, 13, 104. [Google Scholar] [CrossRef] [Green Version]
- Kijeńska, E.; Prabhakaran, M.P.; Swieszkowski, W.; Kurzydlowski, K.J.; Ramakrishna, S. Electrospun bio-composite P(LLA-CL)/collagen I/collagen III scaffolds for nerve tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100B, 1093–1102. [Google Scholar] [CrossRef]
- Duda, S.; Dreyer, L.; Behrens, P.; Wienecke, S.; Chakradeo, T.; Glasmacher, B.; Haastert-Talini, K. Outer Electrospun Polycaprolactone Shell Induces Massive Foreign Body Reaction and Impairs Axonal Regeneration through 3D Multichannel Chitosan Nerve Guides. BioMed Res. Int. 2014, 2014, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Meek, M.F.; Dunnen, W.F.A.D. Porosity of the wall of a Neurolac® nerve conduit hampers nerve regeneration. Microsurgery 2009, 29, 473–478. [Google Scholar] [CrossRef]
- Kijeńska-Gawrońska, E.; Bolek, T.; Bil, M.; Swieszkowski, W. Alignment and bioactive molecule enrichment of bio-composite scaffolds towards peripheral nerve tissue engineering. J. Mater. Chem. B 2019, 7, 4509–4519. [Google Scholar] [CrossRef]
- Anderson, M.; Shelke, N.B.; Manoukian, O.S.; Yu, X.; McCullough, L.D.; Kumbar, S.G. Peripheral Nerve Regeneration Strategies: Electrically Stimulating Polymer Based Nerve Growth Conduits. Crit. Rev. Biomed. Eng. 2015, 43, 131–159. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Dong, L.; Zhou, D.; Li, L.; Zhang, W.; Zhen, Y.; Wang, T.; Su, J.; Chen, D.; Mao, C.; et al. Extracellular vesicles from human umbilical cord mesenchymal stem cells improve nerve regeneration after sciatic nerve transection in rats. J. Cell. Mol. Med. 2019, 23, 2822–2835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, C.-D.; Wang, Y.; Wang, C.; Lu, C.-F.; Peng, J. Roles of neural stem cells in the repair of peripheral nerve injury. Neural Regen. Res. 2017, 12, 2106–2112. [Google Scholar] [CrossRef]
- Chen, J.; Ren, S.; Duscher, D.; Kang, Y.; Liu, Y.; Wang, C.; Yuan, M.; Guo, G.; Xiong, H.; Zhan, P.; et al. Exosomes from human adipose-derived stem cells promote sciatic nerve regeneration via optimizing Schwann cell function. J. Cell. Physiol. 2019, 234, 23097–23110. [Google Scholar] [CrossRef]
- Grimoldi, N.; Colleoni, F.; Tiberio, F.; Vetrano, I.G.; Cappellari, A.; Costa, A.; Belicchi, M.; Razini, P.; Giordano, R.; Spagnoli, D.; et al. Stem Cell Salvage of Injured Peripheral Nerve. Cell Transplant. 2015, 24, 213–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erba, P.; Mantovani, C.; Kalbermatten, D.F.; Pierer, G.; Terenghi, G.; Kingham, P. Regeneration potential and survival of transplanted undifferentiated adipose tissue-derived stem cells in peripheral nerve conduits. J. Plast. Reconstr. Aesthetic Surg. 2010, 63, e811–e817. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.M.; Vykoukal, J.; Li, D.P.; Pan, H.L.; Zeitler, K.; Alt, E.; Geis, S.; Felthaus, O.; Prantl, L. Peripheral Motor and Sensory Nerve Conduction following Transplantation of Undifferentiated Autologous Adipose Tissue-Derived Stem Cells in a Biodegradable U.S. Food and Drug Administration-Approved Nerve Conduit. Plast. Reconstr. Surg. 2016, 138, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Kappos, E.A.; Engels, P.E.; Tremp, M.; Zu Schwabedissen, M.M.; Di Summa, P.; Fischmann, A.; Von Felten, S.; Scherberich, A.; Schaefer, D.J.; Kalbermatten, D.F. Peripheral Nerve Repair: Multimodal Comparison of the Long-Term Regenerative Potential of Adipose Tissue-Derived Cells in a Biodegradable Conduit. Stem Cells Dev. 2015, 24, 2127–2141. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Y.; Zhang, K.-H.; Fan, C.-Y.; Mo, X.; Ruan, H.-J.; Li, F.-F. Aligned natural–synthetic polyblend nanofibers for peripheral nerve regeneration. Acta Biomater. 2011, 7, 634–643. [Google Scholar] [CrossRef]
- Panseri, S.; Cunha, C.; Lowery, J.; Del Carro, U.; Taraballi, F.; Amadio, S.; Vescovi, A.; Gelain, F. Electrospun micro- and nanofiber tubes for functional nervous regeneration in sciatic nerve transections. BMC Biotechnol. 2008, 8, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Bella, M.A.; Brucato, V.; Blanda, V.; Zummo, F.; Vitrano, I.; Di Liegro, C.M.; Ghersi, G.; Di Liegro, I.; Schiera, G. A 3D-scaffold of PLLA induces the morphological differentiation and migration of primary astrocytes and promotes the production of extracellular vesicles. Mol. Med. Rep. 2019, 20, 1288–1296. [Google Scholar] [CrossRef] [Green Version]
- Gelain, F.; Lomander, A.; Vescovi, A.L.; Zhang, S. Systematic Studies of a Self-Assembling Peptide Nanofiber Scaffold with Other Scaffolds. J. Nanosci. Nanotechnol. 2007, 7, 424–434. [Google Scholar] [CrossRef]
- Hsieh, S.-C.; Chang, C.-J.; Cheng, W.-T.; Tseng, T.-C.; Hsu, S.-H. Effect of an Epineurial-Like Biohybrid Nerve Conduit on Nerve Regeneration. Cell Transplant. 2016, 25, 559–574. [Google Scholar] [CrossRef] [Green Version]
- Nakada, M.; Itoh, S.; Tada, K.; Matsuta, M.; Murai, A.; Tsuchiya, H. Effects of hybridization of decellularized allogenic nerves with adipose-derive stem cell sheets to facilitate nerve regeneration. Brain Res. 2020, 1746, 147025. [Google Scholar] [CrossRef]
- Syu, W.-Z.; Hueng, D.-Y.; Chen, W.-L.; Chan, J.Y.-H.; Chen, S.-G.; Huang, S.-M. Adipose-Derived Neural Stem Cells Combined with Acellular Dermal Matrix as a Neural Conduit Enhances Peripheral Nerve Repair. Cell Transplant. 2019, 28, 1220–1230. [Google Scholar] [CrossRef] [Green Version]
- Volkmer, E.; Saller, M.M.; Huettl, R.-E.; Mayer, J.M.; Feuchtinger, A.; Krug, C.; Holzbach, T. Validation of a novel animal model for sciatic nerve repair with an adipose-derived stem cell loaded fibrin conduit. Neural Regen. Res. 2018, 13, 854–861. [Google Scholar] [CrossRef]
- Liu, G.-B.; Cheng, Y.-X.; Feng, Y.-K.; Pang, C.-J.; Li, Q.; Wang, Y.; Jia, H.; Tong, X.-J. Adipose-derived stem cells promote peripheral nerve repair. Arch. Med. Sci. 2011, 4, 592–596. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, R.; Zhang, C.; Yang, Y.; Xu, Y.; Wang, H.; Zhang, M.; Zhu, J.; Wang, Y.; Sun, Y.; et al. Therapeutic effects of nerve leachate-treated adipose-derived mesenchymal stem cells on rat sciatic nerve injury. Exp. Ther. Med. 2019, 19, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Gong, K.; Zheng, Z.; Liu, L.; Wang, A.; Zhang, L.; Ao, Q.; Gong, Y.; Zhang, X. Schwann-like cell differentiation of rat adipose-derived stem cells by indirect co-culture with Schwann cells in vitro. Cell Prolif. 2010, 43, 606–616. [Google Scholar] [CrossRef]
- Suganuma, S.; Tada, K.; Hayashi, K.; Takeuchi, A.; Tsuchiya, H.; Sugimoto, N.; Ikeda, K. Uncultured adipose-derived regenerative cells promote peripheral nerve regeneration. J. Orthop. Sci. 2013, 18, 145–151. [Google Scholar] [CrossRef]
- Dadaci, M.; Karagülle, N.; Sönmez, E.; Dadaci, Z.; Işci, E.T.; Ince, B.; Vargel, I.; Pişkin, E.; Erk, A.Y. Evaluation of the effectiveness of biodegradable electrospun caprolactoneand poly(lactic acid-ε-caprolactone) nerve conduits for peripheral nerveregenerations in a rat sciatic nerve defect model. Turk. J. Med. Sci. 2016, 46, 539–548. [Google Scholar] [CrossRef]
- Zhiyuan, Z.; Zhao, W.; Zhu, C.; Zhang, X.; Ye, D.; Zhang, W.; Zhou, Y.; Jiang, X.; Zhang, Z. Sciatic nerve regeneration in rats by a promising electrospun collagen/poly(ε-caprolactone) nerve conduit with tailored degradation rate. BMC Neurosci. 2011, 12, 68. [Google Scholar] [CrossRef] [Green Version]
- Lopez, J.; Xin, K.; Quan, A.; Xiang, S.; Barone, A.A.L.; Budihardjo, J.; Musavi, L.; Mulla, S.; Redett, R.; Martin, R.; et al. Poly(ε-Caprolactone) Nanofiber Wrap Improves Nerve Regeneration and Functional Outcomes after Delayed Nerve Repair. Plast. Reconstr. Surg. 2019, 144, 48e–57e. [Google Scholar] [CrossRef] [PubMed]
- Neal, R.A.; Tholpady, S.S.; Foley, P.L.; Swami, N.; Ogle, R.C.; Botchwey, E.A. Alignment and composition of laminin-polycaprolactone nanofiber blends enhance peripheral nerve regeneration. J. Biomed. Mater. Res. Part A 2011, 100, 406–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assaf, K.; Leal, C.V.; Derami, M.S.; Duek, E.A.D.R.; Ceragioli, H.; De Oliveira, A.L.R. Sciatic nerve repair using poly(ε-caprolactone) tubular prosthesis associated with nanoparticles of carbon and graphene. Brain Behav. 2017, 7, e00755. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Jiang, X.; Cai, M.; Zhao, W.; Ye, D.; Zhou, Y.; Zhu, C.; Zhang, X.; Lu, X.; Zhang, Z. A novel electrospun nerve conduit enhanced by carbon nanotubes for peripheral nerve regeneration. Nanotechnology 2014, 25, 165102. [Google Scholar] [CrossRef]
- Hong, S.; Kim, G. Electrospun micro/nanofibrous conduits composed of poly(epsilon-caprolactone) and small intestine submucosa powder for nerve tissue regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 94, 421–428. [Google Scholar]
- Fogli, B.; Corthout, N.; Kerstens, A.; Bosse, F.; Klimaschewski, L.; Munck, S.; Schweigreiter, R. Imaging axon regeneration within synthetic nerve conduits. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Klimaschewski, L.; Hausott, B.; Angelov, D.N. The Pros and Cons of Growth Factors and Cytokines in Peripheral Axon Regeneration. Int. Rev. Neurobiol. 2013, 108, 137–171. [Google Scholar] [CrossRef] [PubMed]
- Eggers, R.; De Winter, F.; Hoyng, S.A.; Roet, K.C.D.; Ehlert, E.M.; Malessy, M.J.A.; Verhaagen, J.; Tannemaat, M.R. Lentiviral Vector-Mediated Gradients of GDNF in the Injured Peripheral Nerve: Effects on Nerve Coil Formation, Schwann Cell Maturation and Myelination. PLoS ONE 2013, 8, e71076. [Google Scholar] [CrossRef] [PubMed]
- Kijeńska, E.; Swieszkowski, W. 2—General requirements of electrospun materials for tissue engineering: Setups and strategy for successful electrospinning in laboratory and industry. In Electrospun Materials for Tissue Engineering and Biomedical Applications; Uyar, T., Kny, E., Eds.; Woodhead Publishing: UK, 2017; pp. 43–56. [Google Scholar]
- Dębski, T.; Kurzyk, A.; Ostrowska, B.; Wysocki, J.; Jaroszewicz, J.; Święszkowski, W.; Pojda, Z. Scaffold vascularization method using an adipose-derived stem cell (ASC)-seeded scaffold prefabricated with a flow-through pedicle. Stem Cell Res. Ther. 2020, 11, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.; Heydarkhan-Hagvall, S.; Hedrick, M.; Benhaim, P.; Zuk, P. Manual Isolation of Adipose-derived Stem Cells from Human Lipoaspirates. J. Vis. Exp. 2013, 79, e50585. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Bain, J.R.; Mackinnon, S.E.; Hunter, D.A. Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast. Reconstr. Surg. 1989, 83, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Sant’Anna, L.B.; Sant’Anna, N.; Parolini, O. Application of computer assisted image analysis for identifying and quantifying liver fibrosis in a experimental model. J. Comput. Interdiscip. Sci. 2011, 2, 139–148. [Google Scholar]
- Kiernan, J.A. Histochemistry of Staining Methods for Normal and Degenerating Myelin in the Central and Peripheral Nervous Systems. J. Histotechnol. 2007, 30, 87–106. [Google Scholar] [CrossRef]
- Paskal, A.M.; Paskal, W.; Pietruski, P.; Kusmierczyk, Z.; Jankowska-Steifer, E.; Andrychowski, J.; Wlodarski, P.K. Neuroregenerative effects of polyethylene glycol and FK-506 in a rat model of sciatic nerve injury. J. Plast. Reconstr. Aesthetic Surg. 2020, 73, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Bolleboom, A.; De Ruiter, G.C.W.; Coert, J.H.; Tuk, B.; Holstege, J.C.; Van Neck, J.W. Novel experimental surgical strategy to prevent traumatic neuroma formation by combining a 3D-printed Y-tube with an autograft. J. Neurosurg. 2018, 130, 184–196. [Google Scholar] [CrossRef] [PubMed]
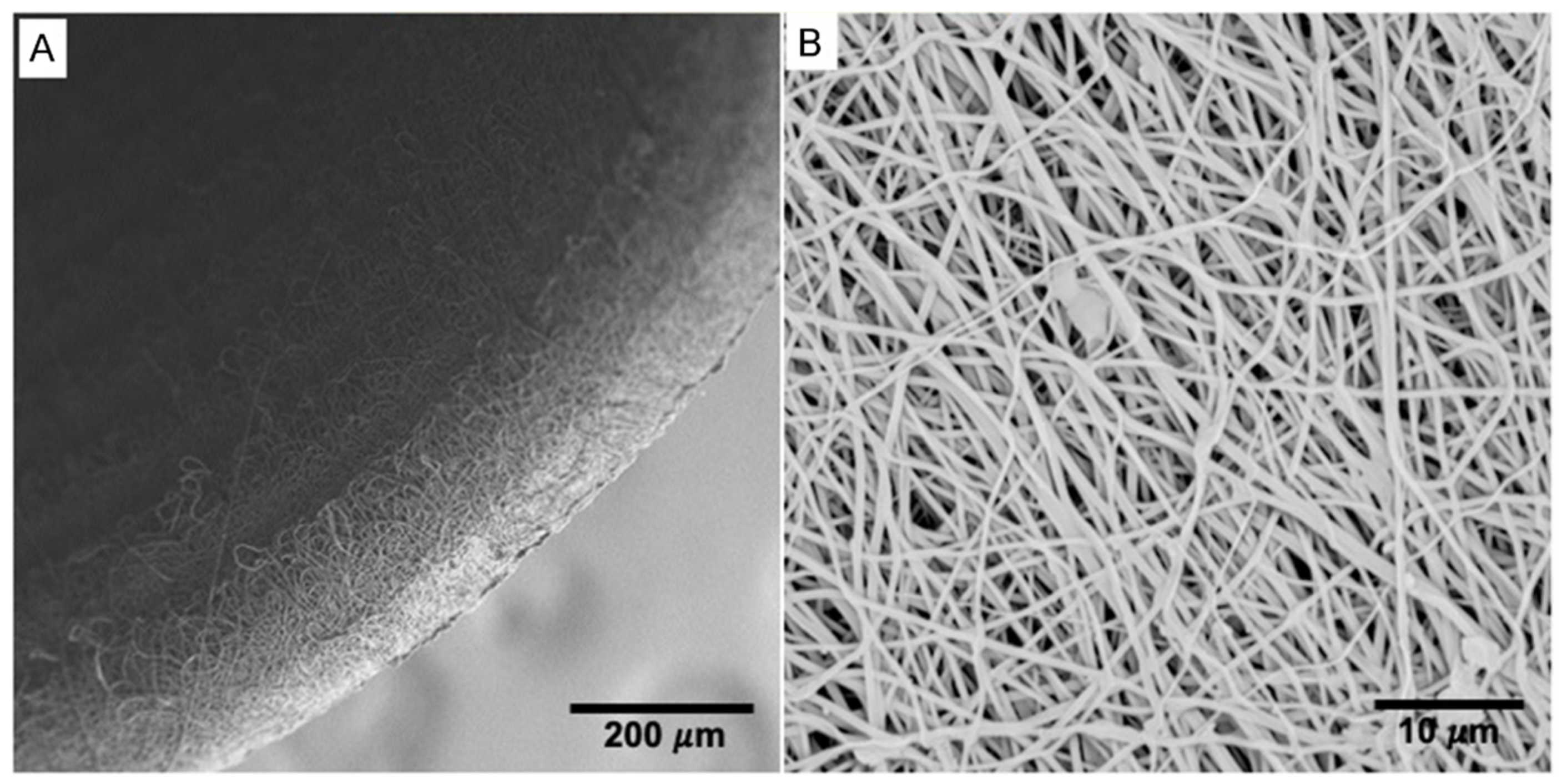




| Parameter | Value | Units |
|---|---|---|
| Wall thickness | 272 ± 12 | µm |
| External diameter of a conduit | 2.04 ± 0.04 | mm |
| Mean fiber thickness | 460 ± 143 | nm |
| Length of a conduit | 15 | mm |
| Tensile strength | 8.53 ± 1.43 | MPa |
| Elongation to break | 332.25 ± 72.11 | % |
| Contact angle | 81.23 ± 2.14 | ° |
| Group | Fibrosis |
|---|---|
| % of Positively Stained Pixels (Mean ± SD) | |
| A | 65.34 ± 12.3 |
| B | 13.61 ± 3.42 |
| C | 15.38 ± 2.81 |
| D | 12.11 ± 2.76 |
| Healthy muscle (n = 7) | 5.11 ± 2.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dębski, T.; Kijeńska-Gawrońska, E.; Zołocińska, A.; Siennicka, K.; Słysz, A.; Paskal, W.; Włodarski, P.K.; Święszkowski, W.; Pojda, Z. Bioactive Nanofiber-Based Conduits in a Peripheral Nerve Gap Management—An Animal Model Study. Int. J. Mol. Sci. 2021, 22, 5588. https://doi.org/10.3390/ijms22115588
Dębski T, Kijeńska-Gawrońska E, Zołocińska A, Siennicka K, Słysz A, Paskal W, Włodarski PK, Święszkowski W, Pojda Z. Bioactive Nanofiber-Based Conduits in a Peripheral Nerve Gap Management—An Animal Model Study. International Journal of Molecular Sciences. 2021; 22(11):5588. https://doi.org/10.3390/ijms22115588
Chicago/Turabian StyleDębski, Tomasz, Ewa Kijeńska-Gawrońska, Aleksandra Zołocińska, Katarzyna Siennicka, Anna Słysz, Wiktor Paskal, Paweł K. Włodarski, Wojciech Święszkowski, and Zygmunt Pojda. 2021. "Bioactive Nanofiber-Based Conduits in a Peripheral Nerve Gap Management—An Animal Model Study" International Journal of Molecular Sciences 22, no. 11: 5588. https://doi.org/10.3390/ijms22115588
APA StyleDębski, T., Kijeńska-Gawrońska, E., Zołocińska, A., Siennicka, K., Słysz, A., Paskal, W., Włodarski, P. K., Święszkowski, W., & Pojda, Z. (2021). Bioactive Nanofiber-Based Conduits in a Peripheral Nerve Gap Management—An Animal Model Study. International Journal of Molecular Sciences, 22(11), 5588. https://doi.org/10.3390/ijms22115588








