Recombinant Long-Acting Thioredoxin Ameliorates AKI to CKD Transition via Modulating Renal Oxidative Stress and Inflammation
Abstract
1. Introduction
2. Results
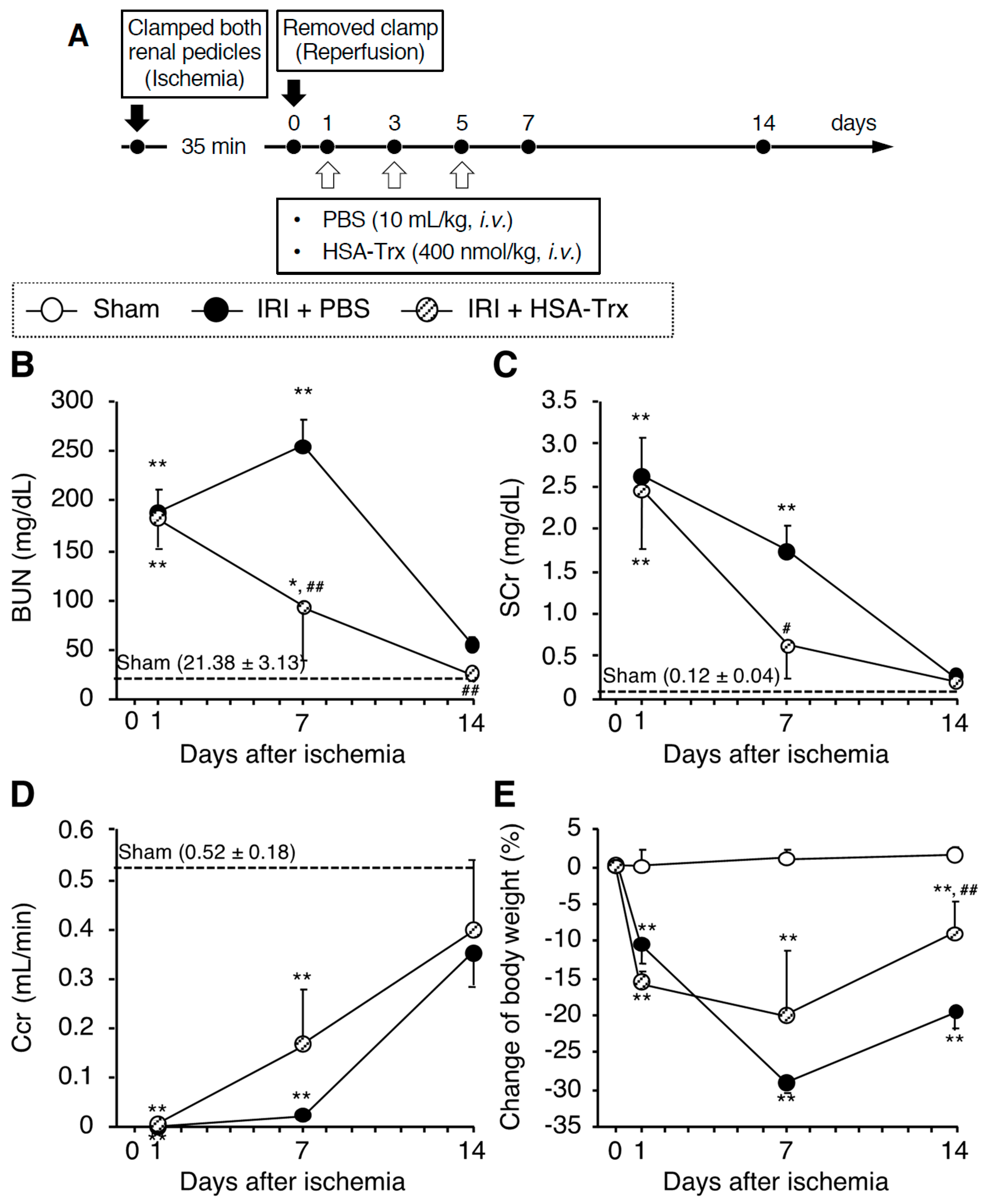
2.1. Evaluation of AKI to CKD Transition Model Mice
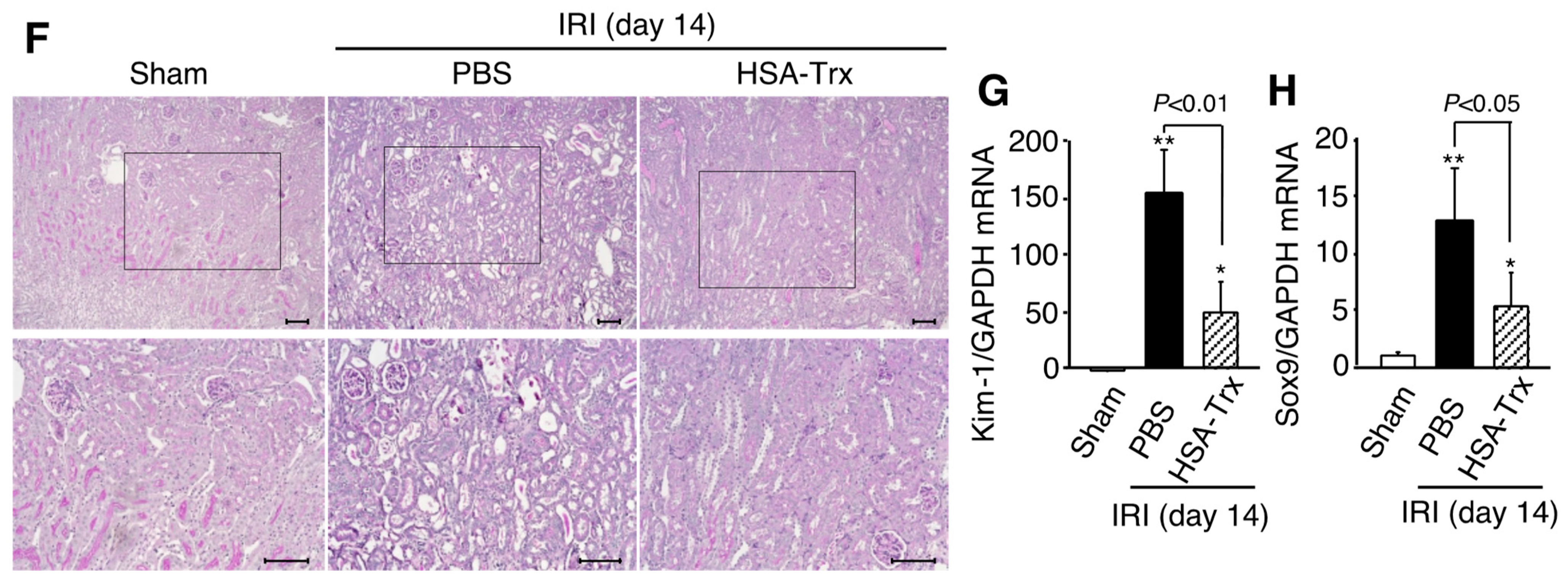
2.2. Effects of HSA-Trx on Renal Function and Renal Tissue Damage during the AKI to CKD Transition
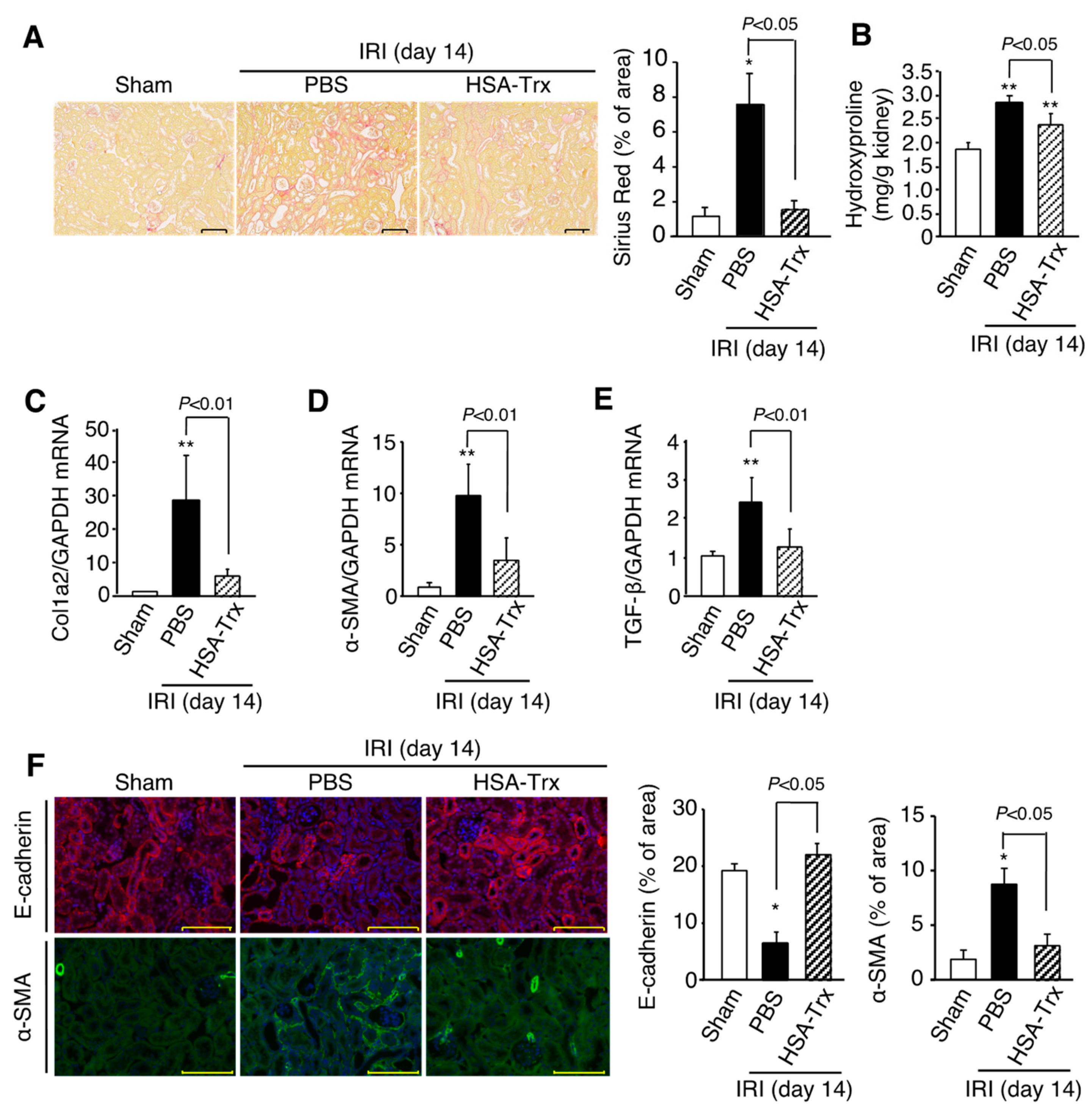
2.3. Effect of HSA-Trx on Renal Fibrosis
2.4. Effect of HSA-Trx on Oxidative Stress and Inflammation in the Kidney
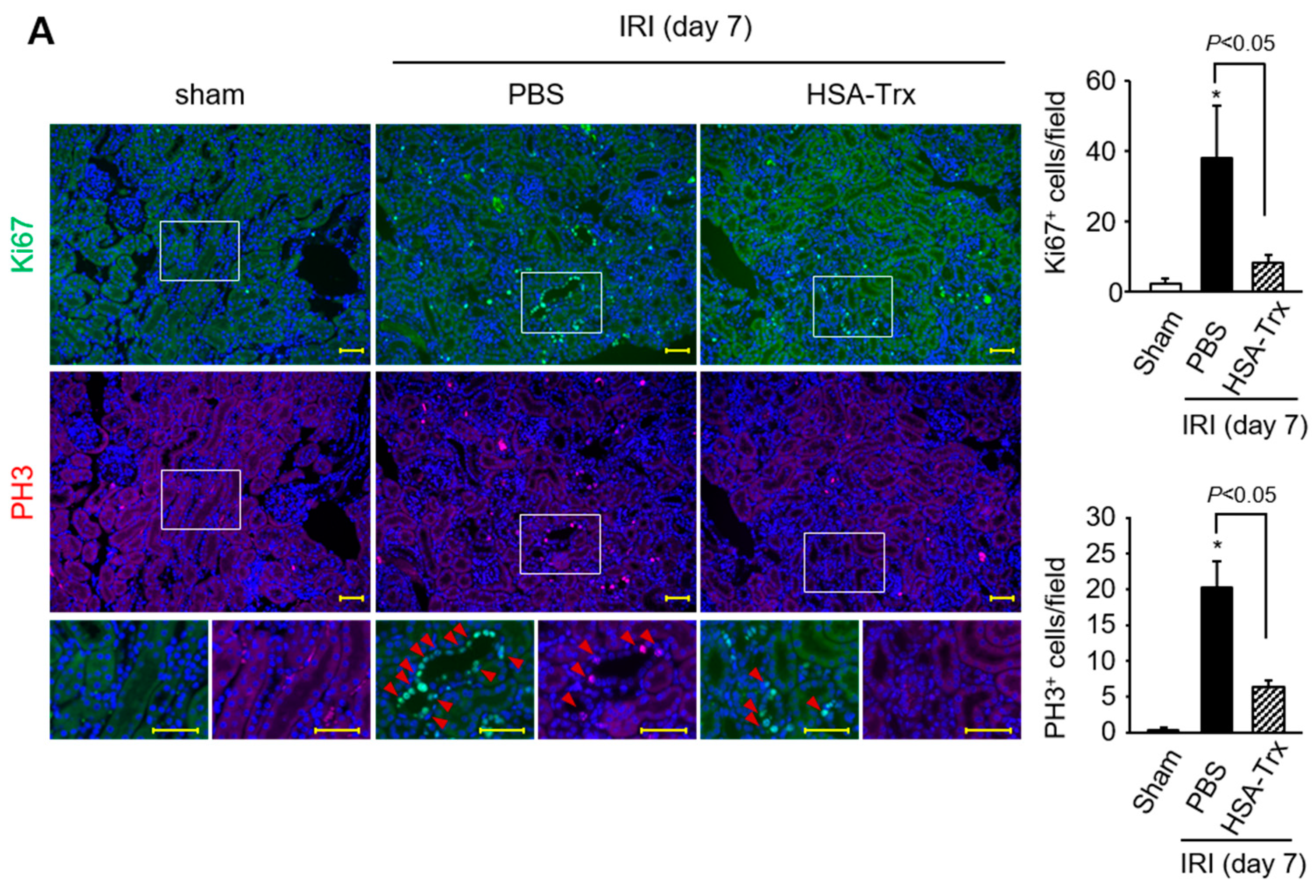
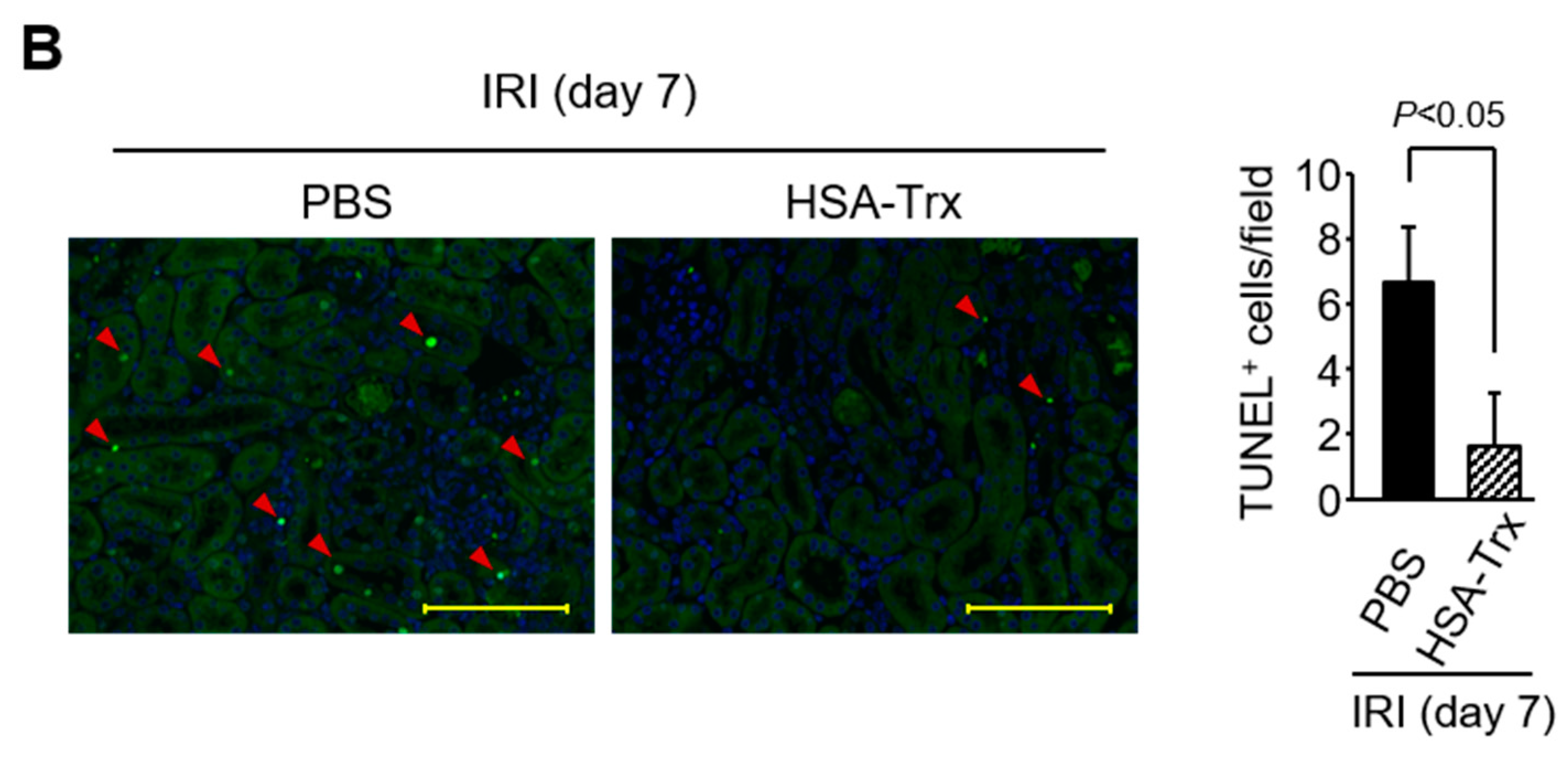
2.5. Effects of HSA-Trx on the Cell Cycle and Apoptosis of Tubular Cells
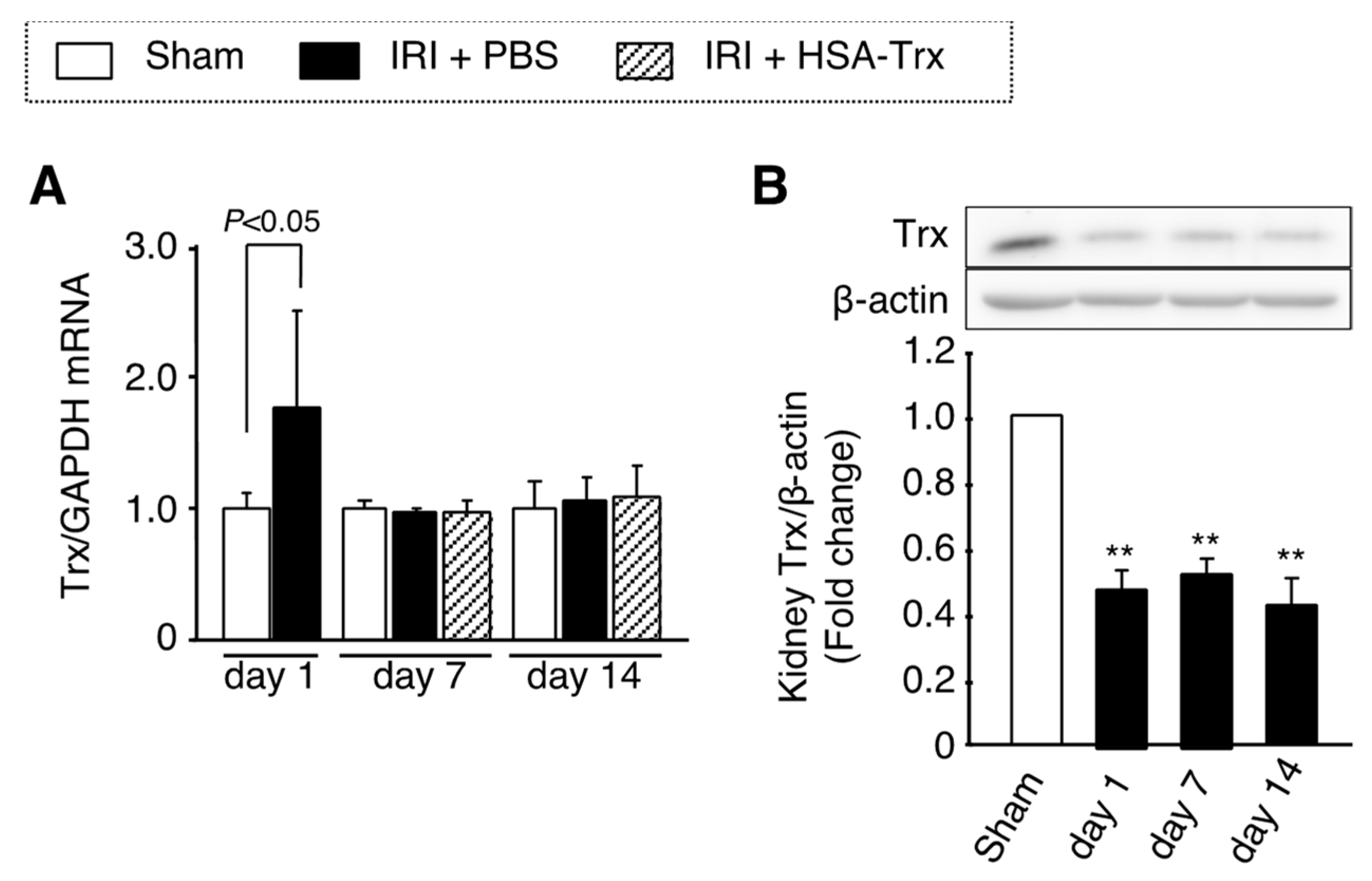
2.6. Endogenous Trx Expression in the AKI to CKD Transition
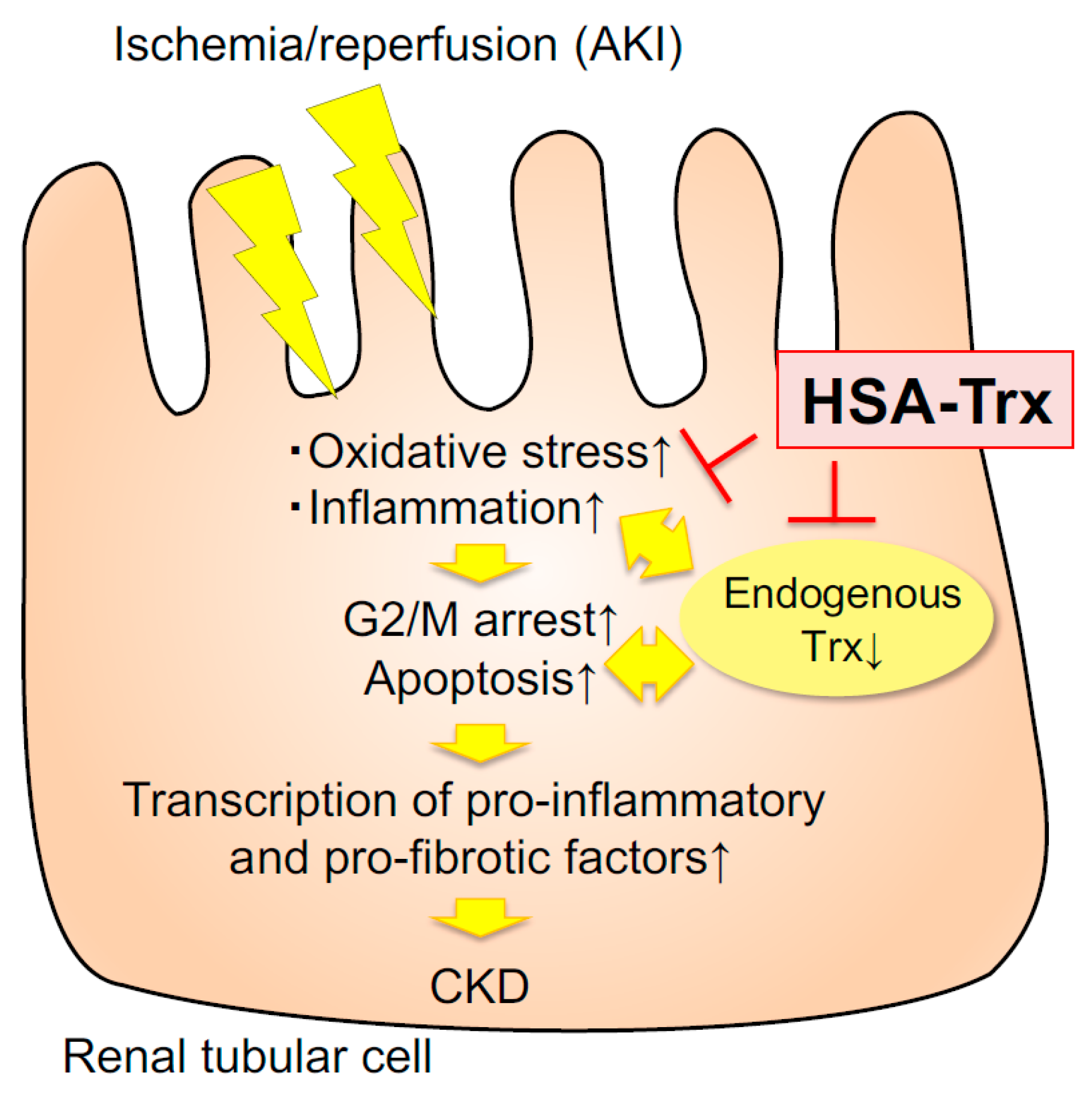
3. Discussion
4. Materials and Methods
4.1. Expression and Purification of HSA-Trx Fusion Protein
4.2. Mouse Model of AKI to CKD Transition
4.3. Biochemical Evaluation of Blood Samples
4.4. Histological Examination of Kidney Tissues
4.5. Measurement of Renal Hydroxyproline
4.6. mRNA Expression Analysis
4.7. Western Blot Analysis of Trx Expression in Kidney Tissue
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AKI | acute kidney injury |
| CKD | chronic kidney disease |
| HSA | human serum albumin |
| HSA-Trx | human serum albumin-thioredoxin fusion protein |
| Trx | thioredoxin |
References
- Coca, S.; Singanamala, S.; Parikh, C.R. Chronic kidney disease after acute kidney injury: A systematic review and meta-analysis. Kidney Int. 2012, 81, 442–448. [Google Scholar] [CrossRef]
- Goldstein, S.L.; Jaber, B.L.; Faubel, S.; Chawla, L.S.; for the Acute Kidney Injury Advisory Group of the American Society of Nephrology. AKI Transition of Care: A Potential Opportunity to Detect and Prevent CKD. Clin. J. Am. Soc. Nephrol. 2013, 8, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Ishani, A.; Nelson, D.; Clothier, B.; Schult, T.; Nugent, S.; Greer, N.; Slinin, Y.; Ensrud, K. The Magnitude of Acute Serum Creatinine Increase After Cardiac Surgery and the Risk of Chronic Kidney Disease, Progression of Kidney Disease, and Death. Arch. Intern. Med. 2011, 171, 226–233. [Google Scholar] [CrossRef]
- Bonventre, J.V.; Yang, L. Cellular pathophysiology of ischemic acute kidney injury. J. Clin. Investig. 2011, 121, 4210–4221. [Google Scholar] [CrossRef]
- Ferenbach, D.A.; Bonventre, J.V. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat. Rev. Nephrol. 2015, 11, 264–276. [Google Scholar] [CrossRef]
- Takaori, K.; Nakamura, J.; Yamamoto, S.; Nakata, H.; Sato, Y.; Takase, M.; Nameta, M.; Yamamoto, T.; Economides, A.N.; Kohno, K.; et al. Severity and Frequency of Proximal Tubule Injury Determines Renal Prognosis. J. Am. Soc. Nephrol. 2015, 27, 2393–2406. [Google Scholar] [CrossRef]
- Liu, Y. Cellular and molecular mechanisms of renal fibrosis. Nat. Rev. Nephrol. 2011, 7, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Ma, N.; Fan, X.; Yu, Q.; Ci, X. The role of Nrf2 in acute kidney injury: Novel molecular mechanisms and therapeutic approaches. Free. Radic. Biol. Med. 2020, 158, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kasuno, K.; Shirakawa, K.; Yoshida, H.; Mori, K.; Kimura, H.; Takahashi, N.; Nobukawa, Y.; Shigemi, K.; Tanabe, S.; Yamada, N.; et al. Renal redox dysregulation in AKI: Application for oxidative stress marker of AKI. Am. J. Physiol. Physiol. 2014, 307, F1342–F1351. [Google Scholar] [CrossRef] [PubMed]
- Kasuno, K.; Nakamura, H.; Ono, T.; Muso, E.; Yodoi, J. Protective roles of thioredoxin, a redox-regulating protein, in renal ischemia/reperfusion injury. Kidney Int. 2003, 64, 1273–1282. [Google Scholar] [CrossRef]
- Nakamura, H.; Herzenberg, L.A.; Bai, J.; Araya, S.; Kondo, N.; Nishinaka, Y.; Yodoi, J. Circulating thioredoxin suppresses lipopolysaccharide-induced neutrophil chemotaxis. Proc. Natl. Acad. Sci. USA 2001, 98, 15143–15148. [Google Scholar] [CrossRef]
- Furukawa, M.; Tanaka, R.; Chuang, V.T.G.; Ishima, Y.; Taguchi, K.; Watanabe, H.; Maruyama, T.; Otagiri, M. Human serum albumin–thioredoxin fusion protein with long blood retention property is effective in suppressing lung injury. J. Control. Release 2011, 154, 189–195. [Google Scholar] [CrossRef]
- Ikuta, S.; Chuang, V.T.G.; Ishima, Y.; Nakajou, K.; Furukawa, M.; Watanabe, H.; Maruyama, T.; Otagiri, M. Albumin fusion of thioredoxin — The production and evaluation of its biological activity for potential therapeutic applications. J. Control. Release 2010, 147, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Kodama, A.; Watanabe, H.; Tanaka, R.; Tanaka, H.; Chuang, V.T.G.; Miyamoto, Y.; Wu, Q.; Endo, M.; Hamasaki, K.; Ishima, Y.; et al. A human serum albumin–thioredoxin fusion protein prevents experimental contrast-induced nephropathy. Kidney Int. 2013, 83, 446–454. [Google Scholar] [CrossRef]
- Kodama, A.; Watanabe, H.; Tanaka, R.; Kondo, M.; Chuang, V.T.G.; Wu, Q.; Endo, M.; Ishima, Y.; Fukagawa, M.; Otagiri, M.; et al. Albumin fusion renders thioredoxin an effective anti-oxidative and anti-inflammatory agent for preventing cisplatin-induced nephrotoxicity. Biochim. Biophys. Acta (BBA) Gen. Subj. 2014, 1840, 1152–1162. [Google Scholar] [CrossRef]
- Nishida, K.; Watanabe, H.; Ogaki, S.; Kodama, A.; Tanaka, R.; Imafuku, T.; Ishima, Y.; Chuang, V.T.G.; Toyoda, M.; Kondoh, M.; et al. Renoprotective effect of long acting thioredoxin by modulating oxidative stress and macrophage migration inhibitory factor against rhabdomyolysis-associated acute kidney injury. Sci. Rep. 2015, 5, 14471. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Watanabe, H.; Kodama, A.; Chuang, V.T.G.; Ishima, Y.; Hamasaki, K.; Tanaka, K.-I.; Mizushima, T.; Otagiri, M.; Maruyama, T. Long-Acting Human Serum Albumin-Thioredoxin Fusion Protein Suppresses Bleomycin-Induced Pulmonary Fibrosis Progression. J. Pharmacol. Exp. Ther. 2013, 345, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Ishima, Y.; Maeda, H.; Kodama, A.; Nagao, S.; Watanabe, H.; Chuang, V.T.G.; Otagiri, M.; Maruyama, T. Albumin Fusion Prolongs the Antioxidant and Anti-Inflammatory Activities of Thioredoxin in Mice with Acetaminophen-Induced Hepatitis. Mol. Pharm. 2014, 11, 1228–1238. [Google Scholar] [CrossRef]
- Tanaka, R.; Ishima, Y.; Enoki, Y.; Kimachi, K.; Shirai, T.; Watanabe, H.; Chuang, V.T.G.; Maruyama, T.; Otagiri, M. Therapeutic Impact of Human Serum Albumin–Thioredoxin Fusion Protein on Influenza Virus-Induced Lung Injury Mice. Front. Immunol. 2014, 5, 561. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.-I.; Shimoda, M.; Chuang, V.T.; Nishida, K.; Kawahara, M.; Ishida, T.; Otagiri, M.; Maruyama, T.; Ishima, Y. Thioredoxin-albumin fusion protein prevents copper enhanced zinc-induced neurotoxicity via its antioxidative activity. Int. J. Pharm. 2018, 535, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Furuichi, K.; Gao, J.-L.; Murphy, P.M. Chemokine Receptor CX3CR1 Regulates Renal Interstitial Fibrosis after Ischemia-Reperfusion Injury. Am. J. Pathol. 2006, 169, 372–387. [Google Scholar] [CrossRef]
- Shi, M.; Flores, B.; Gillings, N.; Bian, A.; Cho, H.J.; Yan, S.; Liu, Y.; Levine, B.; Moe, O.W.; Hu, M.C. αKlotho Mitigates Progression of AKI to CKD through Activation of Autophagy. J. Am. Soc. Nephrol. 2016, 27, 2331–2345. [Google Scholar] [CrossRef]
- Chang-Panesso, M.; Humphreys, B.D. Cellular plasticity in kidney injury and repair. Nat. Rev. Nephrol. 2016, 13, 39–46. [Google Scholar] [CrossRef]
- Kumar, S.; Liu, J.; Pang, P.; Krautzberger, A.M.; Reginensi, A.; Akiyama, H.; Schedl, A.; Humphreys, B.D.; McMahon, A.P. Sox9 Activation Highlights a Cellular Pathway of Renal Repair in the Acutely Injured Mammalian Kidney. Cell Rep. 2015, 12, 1325–1338. [Google Scholar] [CrossRef] [PubMed]
- Ratliff, B.B.; Abdulmahdi, W.; Pawar, R.; Wolin, M.S. Oxidant Mechanisms in Renal Injury and Disease. Antioxid. Redox Signal. 2016, 25, 119–146. [Google Scholar] [CrossRef]
- Lv, W.; Booz, G.W.; Fan, F.; Wang, Y.; Roman, R.J. Oxidative Stress and Renal Fibrosis: Recent Insights for the Development of Novel Therapeutic Strategies. Front. Physiol. 2018, 9, 105. [Google Scholar] [CrossRef] [PubMed]
- Okamura, D.M.; Pennathur, S. The balance of powers: Redox regulation of fibrogenic pathways in kidney injury. Redox Biol. 2015, 6, 495–504. [Google Scholar] [CrossRef]
- Okamura, D.M.; Bahrami, N.M.; Ren, S.; Pasichnyk, K.; Williams, J.M.; Gangoiti, J.A.; Lopez-Guisa, J.M.; Yamaguchi, I.; Barshop, B.A.; Duffield, J.S.; et al. Cysteamine Modulates Oxidative Stress and Blocks Myofibroblast Activity in CKD. J. Am. Soc. Nephrol. 2013, 25, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Mimura, I.; Nangaku, M.; Nishi, H.; Inagi, R.; Tanaka, T.; Fujita, T. Cytoglobin, a novel globin, plays an antifibrotic role in the kidney. Am. J. Physiol. Physiol. 2010, 299, F1120–F1133. [Google Scholar] [CrossRef]
- Nishi, H.; Inagi, R.; Kawada, N.; Yoshizato, K.; Mimura, I.; Fujita, T.; Nangaku, M. Cytoglobin, a Novel Member of the Globin Family, Protects Kidney Fibroblasts against Oxidative Stress under Ischemic Conditions. Am. J. Pathol. 2011, 178, 128–139. [Google Scholar] [CrossRef]
- Hawkes, H.-J.K.; Karlenius, T.C.; Tonissen, K.F. Regulation of the human thioredoxin gene promoter and its key substrates: A study of functional and putative regulatory elements. Biochim. Biophys. Acta (BBA) Gen. Subj. 2014, 1840, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014, 39, 199–218. [Google Scholar] [CrossRef]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef]
- Aggarwal, D.; Singh, G. Effects of single and dual RAAS blockade therapy on progressive kidney disease transition to CKD in rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019, 393, 615–627. [Google Scholar] [CrossRef]
- Rodríguez-Romo, R.; Benítez, K.; Barrera-Chimal, J.; Pérez-Villalva, R.; Gómez, A.; Aguilar-León, D.; Rangel-Santiago, J.F.; Huerta, S.; Gamba, G.; Uribe, N.; et al. AT1 receptor antagonism before ischemia prevents the transition of acute kidney injury to chronic kidney disease. Kidney Int. 2016, 89, 363–373. [Google Scholar] [CrossRef]
- Grynberg, K.; Ma, F.Y.; Nikolic-Paterson, D.J. The JNK Signaling Pathway in Renal Fibrosis. Front. Physiol. 2017, 8, 829. [Google Scholar] [CrossRef] [PubMed]
- Tesch, G.H.; Ma, F.Y.; Nikolic-Paterson, D.J. ASK1: A new therapeutic target for kidney disease. Am. J. Physiol. Physiol. 2016, 311, F373–F381. [Google Scholar] [CrossRef] [PubMed]
- Liles, J.T.; Corkey, B.K.; Notte, G.T.; Budas, G.R.; Lansdon, E.B.; Hinojosa-Kirschenbaum, F.; Badal, S.S.; Lee, M.; Schultz, B.E.; Wise, S.; et al. ASK1 contributes to fibrosis and dysfunction in models of kidney disease. J. Clin. Investig. 2018, 128, 4485–4500. [Google Scholar] [CrossRef]
- Hassoun, H.T.; Lie, M.L.; Grigoryev, D.N.; Liu, M.; Tuder, R.M.; Rabb, H. Kidney ischemia-reperfusion injury induces caspase-dependent pulmonary apoptosis. Am. J. Physiol. Physiol. 2009, 297, F125–F137. [Google Scholar] [CrossRef]
- Hassoun, H.T.; Grigoryev, D.N.; Lie, M.L.; Liu, M.; Cheadle, C.; Tuder, R.M.; Rabb, H. Ischemic acute kidney injury induces a distant organ functional and genomic response distinguishable from bilateral nephrectomy. Am. J. Physiol. Physiol. 2007, 293, F30–F40. [Google Scholar] [CrossRef] [PubMed]
- Oshiro, S.; Ishima, Y.; Maeda, H.; Honda, N.; Bi, J.; Kinoshita, R.; Ikeda, M.; Iwao, Y.; Imafuku, T.; Nishida, K.; et al. Dual Therapeutic Effects of an Albumin-Based Nitric Oxide Donor on 2 Experimental Models of Chronic Kidney Disease. J. Pharm. Sci. 2018, 107, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Enoki, Y.; Watanabe, H.; Arake, R.; Fujimura, R.; Ishiodori, K.; Imafuku, T.; Nishida, K.; Sugimoto, R.; Nagao, S.; Miyamura, S.; et al. Potential therapeutic interventions for chronic kidney disease-associated sarcopenia via indoxyl sulfate-induced mitochondrial dysfunction. J. Cachex Sarcopenia Muscle 2017, 8, 735–747. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishida, K.; Watanabe, H.; Murata, R.; Tokumaru, K.; Fujimura, R.; Oshiro, S.; Nagasaki, T.; Miyahisa, M.; Hiramoto, Y.; Nosaki, H.; et al. Recombinant Long-Acting Thioredoxin Ameliorates AKI to CKD Transition via Modulating Renal Oxidative Stress and Inflammation. Int. J. Mol. Sci. 2021, 22, 5600. https://doi.org/10.3390/ijms22115600
Nishida K, Watanabe H, Murata R, Tokumaru K, Fujimura R, Oshiro S, Nagasaki T, Miyahisa M, Hiramoto Y, Nosaki H, et al. Recombinant Long-Acting Thioredoxin Ameliorates AKI to CKD Transition via Modulating Renal Oxidative Stress and Inflammation. International Journal of Molecular Sciences. 2021; 22(11):5600. https://doi.org/10.3390/ijms22115600
Chicago/Turabian StyleNishida, Kento, Hiroshi Watanabe, Ryota Murata, Kai Tokumaru, Rui Fujimura, Shun Oshiro, Taisei Nagasaki, Masako Miyahisa, Yuto Hiramoto, Hiroto Nosaki, and et al. 2021. "Recombinant Long-Acting Thioredoxin Ameliorates AKI to CKD Transition via Modulating Renal Oxidative Stress and Inflammation" International Journal of Molecular Sciences 22, no. 11: 5600. https://doi.org/10.3390/ijms22115600
APA StyleNishida, K., Watanabe, H., Murata, R., Tokumaru, K., Fujimura, R., Oshiro, S., Nagasaki, T., Miyahisa, M., Hiramoto, Y., Nosaki, H., Imafuku, T., Maeda, H., Fukagawa, M., & Maruyama, T. (2021). Recombinant Long-Acting Thioredoxin Ameliorates AKI to CKD Transition via Modulating Renal Oxidative Stress and Inflammation. International Journal of Molecular Sciences, 22(11), 5600. https://doi.org/10.3390/ijms22115600







