Cell Biological Responses after Shiga Toxin-1 Exposure to Primary Human Glomerular Microvascular Endothelial Cells from Pediatric and Adult Origin
Abstract
1. Introduction
2. Results
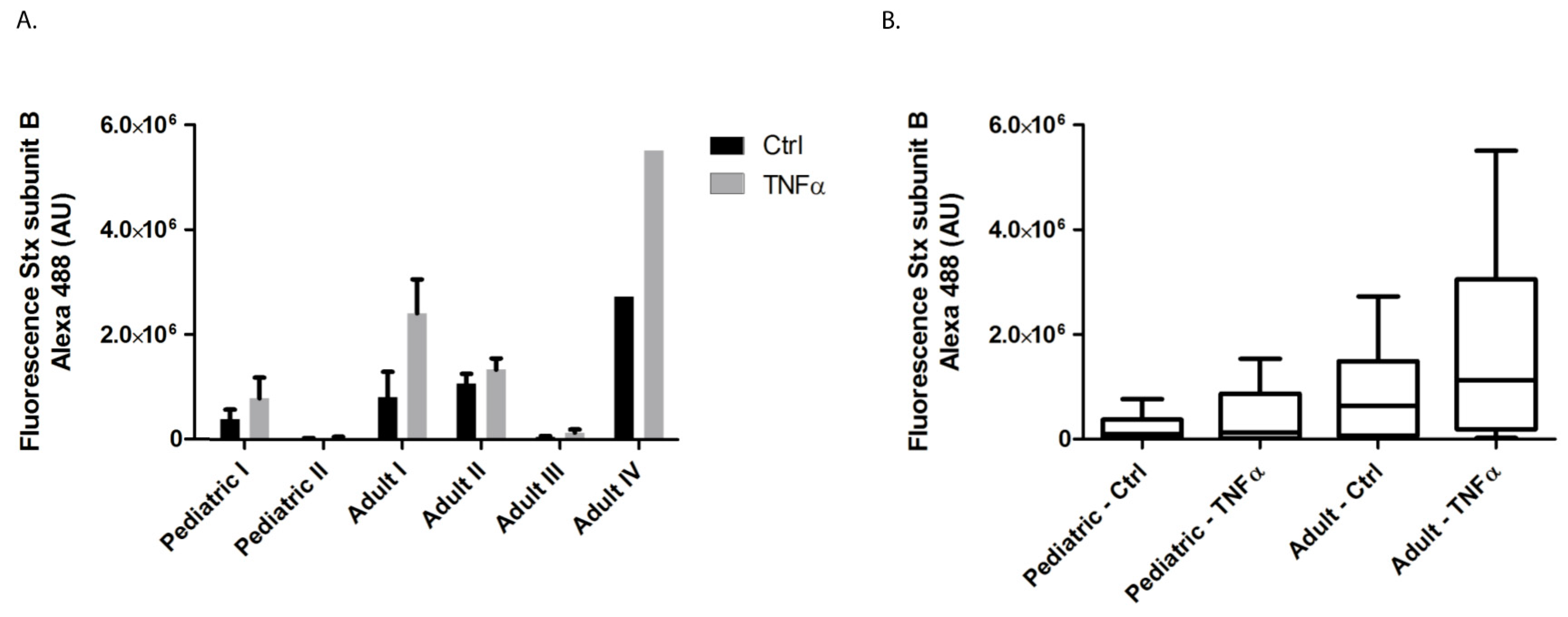
2.1. Stx-1 Binding to Pediatric and Adult Primary HGMVECs
2.2. Effect of Stx-1 on Protein Synthesis of Pediatric and Adult Primary HGMVECs
2.3. Effect of Stx-1 on the Proliferation of Pediatric and Adult HGMVECs
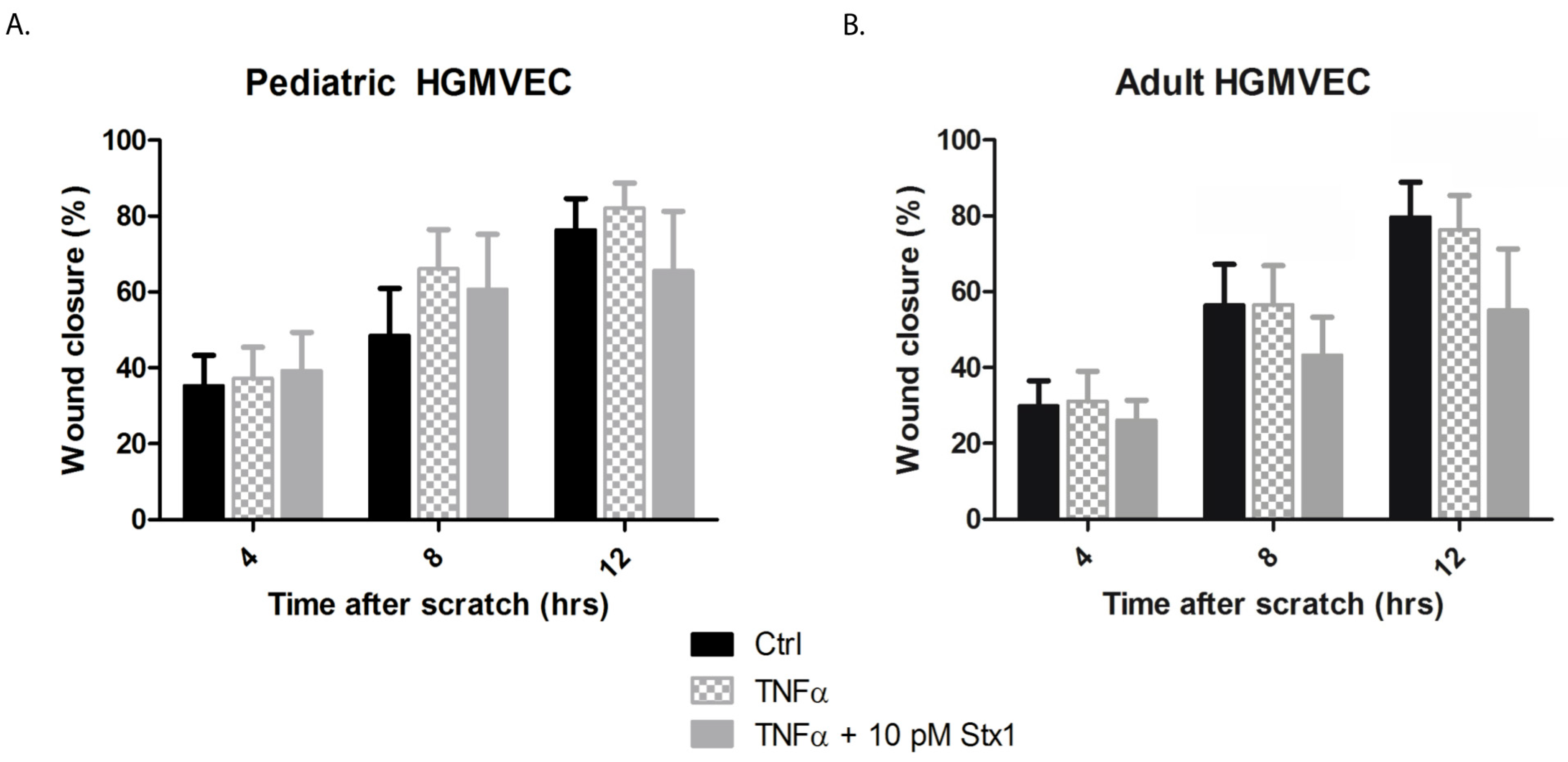
2.4. Effect of Stx-1 on the Migration of Pediatric and Adult HGMVECs
3. Discussion
4. Materials and Methods
4.1. Ethics
4.2. Isolation and Purification of Human Glomerular Microvascular Endothelial Cells (HGMVECs)
4.3. Reagents
4.4. Flow Cytometry
4.5. Protein Synthesis by Radiolabeled 3H-Leucine Incorporation Assay
4.6. Bromodeoxyuridine (BrdU) Proliferation Assay
4.7. Endothelial Wound Closure Assay
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mele, C.; Remuzzi, G.; Noris, M. Hemolytic uremic syndrome. Semin. Immunopathol. 2014, 36, 399–420. [Google Scholar] [CrossRef]
- Tarr, P.I.; Gordon, C.A.; Chandler, W.L. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet 2005, 365, 1073–1086. [Google Scholar] [CrossRef]
- Lingwood, C. Verotoxin Receptor-Based Pathology and Therapies. Front. Cell. Infect. Microbiol. 2020, 10, 123. [Google Scholar] [CrossRef] [PubMed]
- Karpman, D.; Loos, S.; Tati, R.; Arvidsson, I. Haemolytic uraemic syndrome. J. Intern. Med. 2016, 281, 123–148. [Google Scholar] [CrossRef] [PubMed]
- Pijpers, A.H.; Van Setten, P.; Heuvel, L.P.V.D.; Assmann, K.J.; Dijkman, H.B.; Pennings, A.H.; Monnens, L.; Van Hinsbergh, V.W. Verocytotoxin-induced apoptosis of human microvascular endothelial cells. J. Am. Soc. Nephrol. 2001, 12, 767–778. [Google Scholar] [CrossRef]
- Boyd, B.; Lingwood, C. Verotoxin Receptor Glycolipid in Human Renal Tissue. Nephron 1989, 51, 207–210. [Google Scholar] [CrossRef]
- Van Setten, P.A.; van Hinsbergh, V.W.; van der Velden, T.J.; van de Kar, N.C.; Vermeer, M.; Mahan, J.D.; Assmann, K.J.; van den Heuvel, L.P.; Monnens, L.A. Effects of TNF alpha on verocytotoxin cytotoxicity in purified human glomerular microvascular endothelial cells. Kidney Int. 1997, 51, 1245–1256. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Binnington, B.; Sakac, D.; Fernandes, K.R.; Shi, S.P.; Lingwood, C.A.; Branch, D.R. Comparison of detection methods for cell surface globotriaosylceramide. J. Immunol. Methods 2011, 371, 48–60. [Google Scholar] [CrossRef]
- Van de Kar, N.C.; Monnens, L.A.; Karmali, M.A.; van Hinsbergh, V.W. Tumor necrosis factor and interleukin-1 induce expression of the verocytotoxin receptor globotriaosylceramide on human endothelial cells: Implications for the pathogenesis of the hemolytic uremic syndrome. Blood 1992, 80, 2755–2764. [Google Scholar] [CrossRef] [PubMed]
- Exeni, R.A.; Fernandez-Brando, R.J.; Santiago, A.P.; Fiorentino, G.A.; Exeni, A.M.; Ramos, M.V.; Palermo, M.S. Pathogenic role of inflammatory response during Shiga toxin-associated hemolytic uremic syndrome (HUS). Pediatr. Nephrol. 2018, 33, 2057–2071. [Google Scholar] [CrossRef]
- Takeyoshi, M.; Yamasaki, K.; Yakabe, Y.; Takatsuki, M.; Kimber, I. Development of non-radio isotopic endpoint of murine local lymph node assay based on 5-bromo-2’-deoxyuridine (BrdU) incorporation. Toxicol. Lett. 2001, 119, 203–208. [Google Scholar] [CrossRef]
- Lingwood, C. Verotoxin-Binding in Human Renal Sections. Nephron 1994, 66, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Ergonul, Z.; Clayton, F.; Fogo, A.B.; Kohan, D.E. Shigatoxin-1 binding and receptor expression in human kidneys do not change with age. Pediatr. Nephrol. 2003, 18, 246–253. [Google Scholar] [CrossRef]
- Feitz, W.J.C.; Van De Kar, N.C.A.J.; Cheong, I.; Van Der Velden, T.J.A.M.; Ortiz-Sandoval, C.G.; Orth-Höller, D.; Heuvel, L.P.J.W.V.D.; Licht, C. Primary Human Derived Blood Outgrowth Endothelial Cells: An Appropriate In Vitro Model to Study Shiga Toxin Mediated Damage of Endothelial Cells. Toxins 2020, 12, 483. [Google Scholar] [CrossRef] [PubMed]
- Obrig, T.G.; Del Vecchio, P.J.; Brown, J.; Moran, T.P.; Rowland, B.M.; Judge, T.K.; Rothman, S.W. Direct cytotoxic action of Shiga toxin on human vascular endothelial cells. Infect. Immun. 1988, 56, 2373–2378. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, K.; Shimizu, T.; Matsumoto, A.; Hirai, S.; Yokoyama, E.; Takeuchi, H.; Yahiro, K.; Noda, M. Nitric oxide-enhanced Shiga toxin production was regulated by Fur and RecA in enterohemorrhagic Escherichia coli O157. Microbiology 2017, 6, e00461. [Google Scholar] [CrossRef] [PubMed]
- Psotka, M.A.; Obata, F.; Kolling, G.L.; Gross, L.K.; Saleem, M.A.; Satchell, S.C.; Mathieson, P.W.; Obrig, T.G. Shiga Toxin 2 Targets the Murine Renal Collecting Duct Epithelium. Infect. Immun. 2009, 77, 959–969. [Google Scholar] [CrossRef]
- Eremina, V.; Jefferson, J.A.; Kowalewska, J.; Hochster, H.; Haas, M.; Weisstuch, J.; Richardson, C.; Kopp, J.B.; Kabir, M.G.; Backx, P.H.; et al. VEGF Inhibition and Renal Thrombotic Microangiopathy. N. Engl. J. Med. 2008, 358, 1129–1136. [Google Scholar] [CrossRef]
- Conway, E.M. HUS and the case for complement. Blood 2015, 126, 2085–2090. [Google Scholar] [CrossRef] [PubMed]
- Orth, D.; Khan, A.B.; Naim, A.; Grif, K.; Brockmeyer, J.; Karch, H.; Joannidis, M.; Clark, S.J.; Day, A.J.; Fidanzi, S.; et al. Shiga Toxin Activates Complement and Binds Factor H: Evidence for an Active Role of Complement in Hemolytic Uremic Syndrome. J. Immunol. 2009, 182, 6394–6400. [Google Scholar] [CrossRef]
- Ehrlenbach, S.; Rosales, A.; Posch, W.; Wilflingseder, D.; Hermann, M.; Brockmeyer, J.; Karch, H.; Satchell, S.C.; Würzner, R.; Orth-Höller, D. Shiga Toxin 2 Reduces Complement Inhibitor CD59 Expression on Human Renal Tubular Epithelial and Glomerular Endothelial Cells. Infect. Immun. 2013, 81, 2678–2685. [Google Scholar] [CrossRef] [PubMed]
- Monnens, L.; Molenaar, J.; Lambert, P.H.; Proesmans, W.; Van Munster, P. The complement system in hemolytic-uremic syndrome in childhood. Clin. Nephrol. 1980, 13, 168–171. [Google Scholar] [PubMed]
- Thurman, J.M.; Marians, R.; Emlen, W.; Wood, S.; Smith, C.; Akana, H.; Holers, V.M.; Lesser, M.; Kline, M.; Hoffman, C.; et al. Alternative Pathway of Complement in Children with Diarrhea-Associated Hemolytic Uremic Syndrome. Clin. J. Am. Soc. Nephrol. 2009, 4, 1920–1924. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.-J.; Park, S.-K.; Yoon, S.-J.; Park, Y.J.; Lee, M.-S. Experimental In Vivo Models of Bacterial Shiga Toxin-Associated Hemolytic Uremic Syndrome. J. Microbiol. Biotechnol. 2018, 28, 1413–1425. [Google Scholar] [CrossRef]
- Obrig, T.G. Escherichia coli Shiga Toxin Mechanisms of Action in Renal Disease. Toxins 2010, 2, 2769–2794. [Google Scholar] [CrossRef]
- Maciag, T.; Cerundolo, J.; Ilsley, S.; Kelly, P.R.; Forand, R. An endothelial cell growth factor from bovine hypothalamus: Identification and partial characterization. Proc. Natl. Acad. Sci. USA 1979, 76, 5674–5678. [Google Scholar] [CrossRef]
- Liang, C.-C.; Park, A.Y.; Guan, J.-L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feitz, W.J.C.; van Setten, P.A.; van der Velden, T.J.A.M.; Licht, C.; van den Heuvel, L.P.J.W.; van de Kar, N.C.A.J. Cell Biological Responses after Shiga Toxin-1 Exposure to Primary Human Glomerular Microvascular Endothelial Cells from Pediatric and Adult Origin. Int. J. Mol. Sci. 2021, 22, 5615. https://doi.org/10.3390/ijms22115615
Feitz WJC, van Setten PA, van der Velden TJAM, Licht C, van den Heuvel LPJW, van de Kar NCAJ. Cell Biological Responses after Shiga Toxin-1 Exposure to Primary Human Glomerular Microvascular Endothelial Cells from Pediatric and Adult Origin. International Journal of Molecular Sciences. 2021; 22(11):5615. https://doi.org/10.3390/ijms22115615
Chicago/Turabian StyleFeitz, Wouter J. C., Petra A. van Setten, Thea J. A. M. van der Velden, Christoph Licht, Lambert P. J. W. van den Heuvel, and Nicole C. A. J. van de Kar. 2021. "Cell Biological Responses after Shiga Toxin-1 Exposure to Primary Human Glomerular Microvascular Endothelial Cells from Pediatric and Adult Origin" International Journal of Molecular Sciences 22, no. 11: 5615. https://doi.org/10.3390/ijms22115615
APA StyleFeitz, W. J. C., van Setten, P. A., van der Velden, T. J. A. M., Licht, C., van den Heuvel, L. P. J. W., & van de Kar, N. C. A. J. (2021). Cell Biological Responses after Shiga Toxin-1 Exposure to Primary Human Glomerular Microvascular Endothelial Cells from Pediatric and Adult Origin. International Journal of Molecular Sciences, 22(11), 5615. https://doi.org/10.3390/ijms22115615





