Nanomechanical Hallmarks of Helicobacter pylori Infection in Pediatric Patients
Abstract
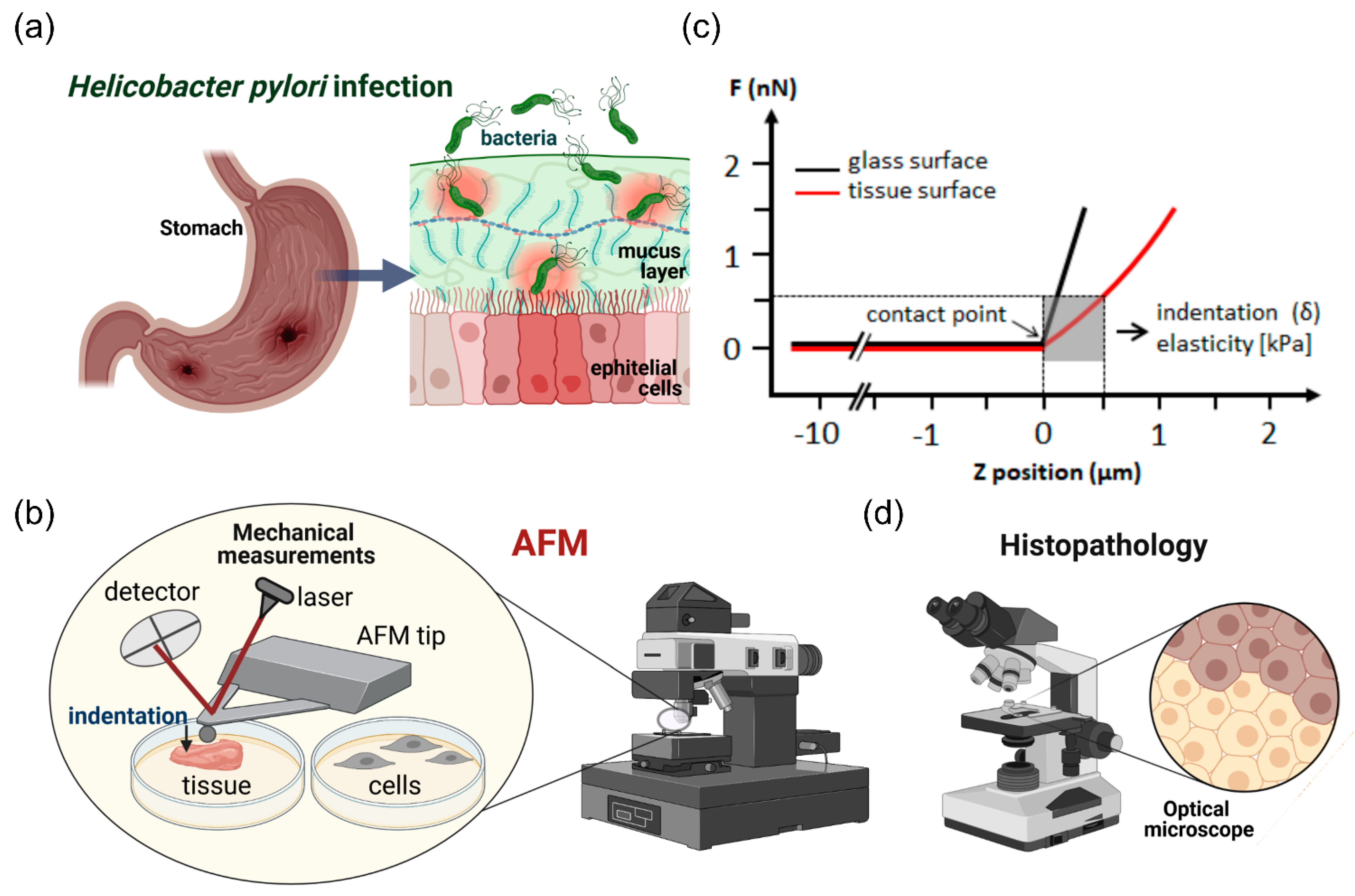
:1. Introduction
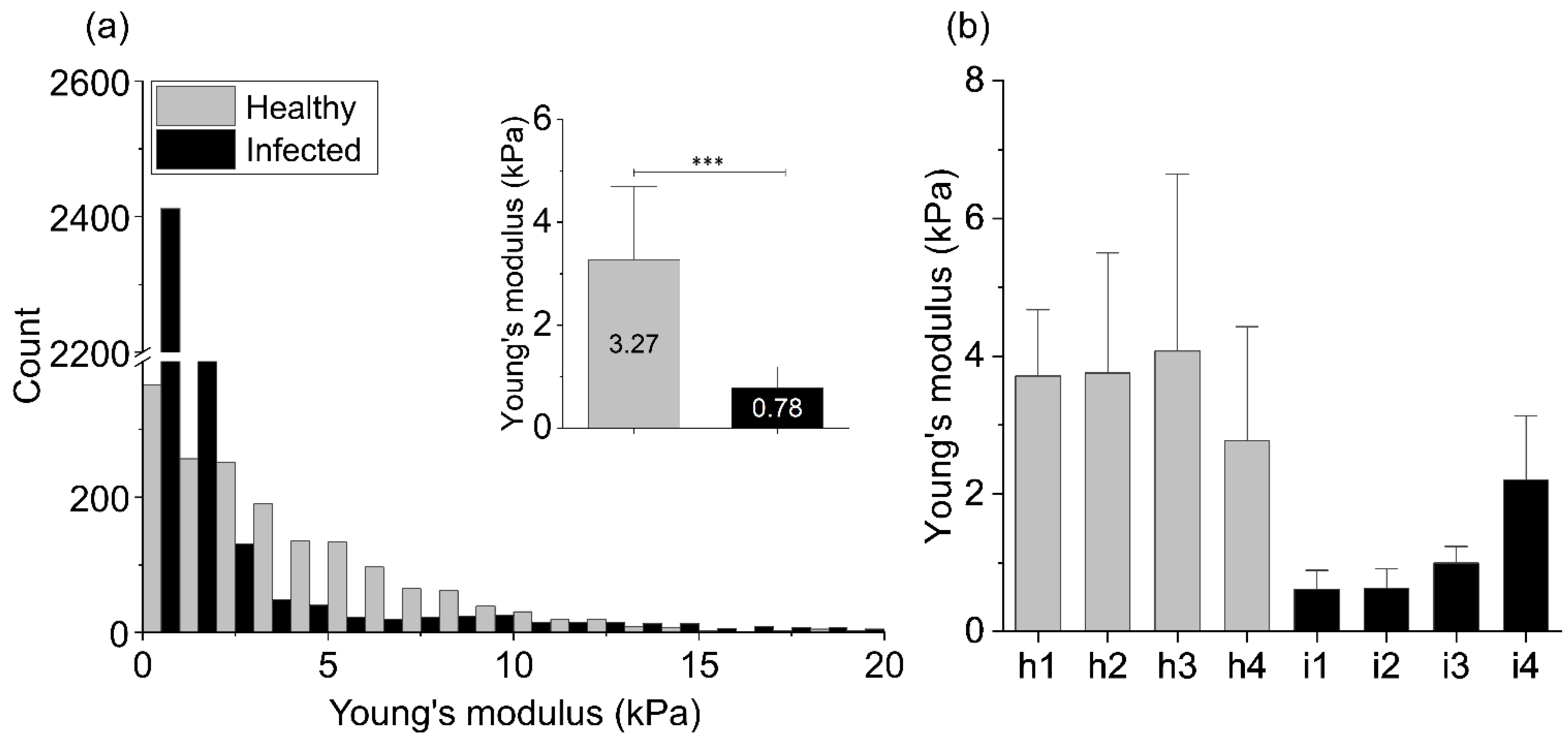
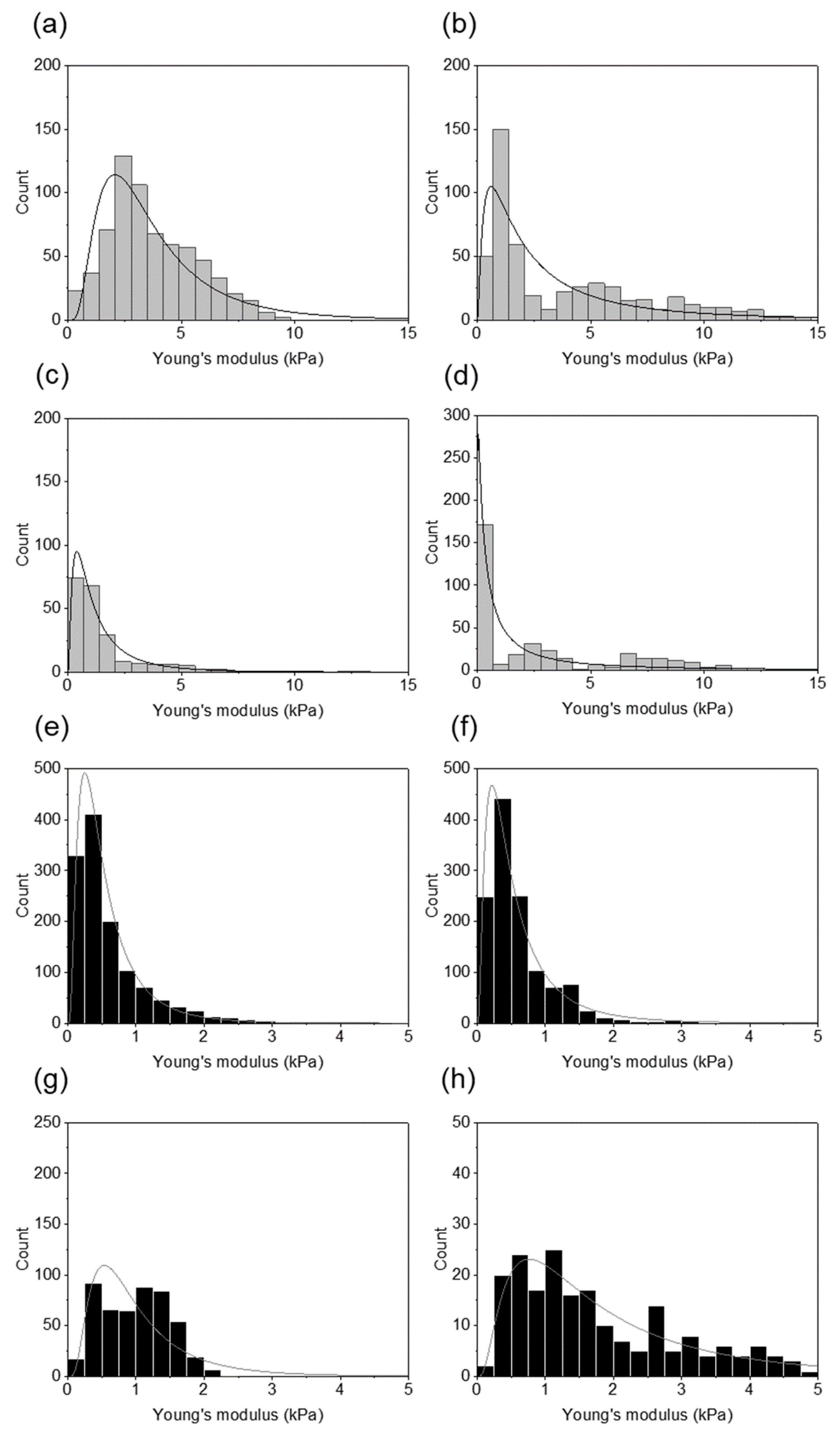
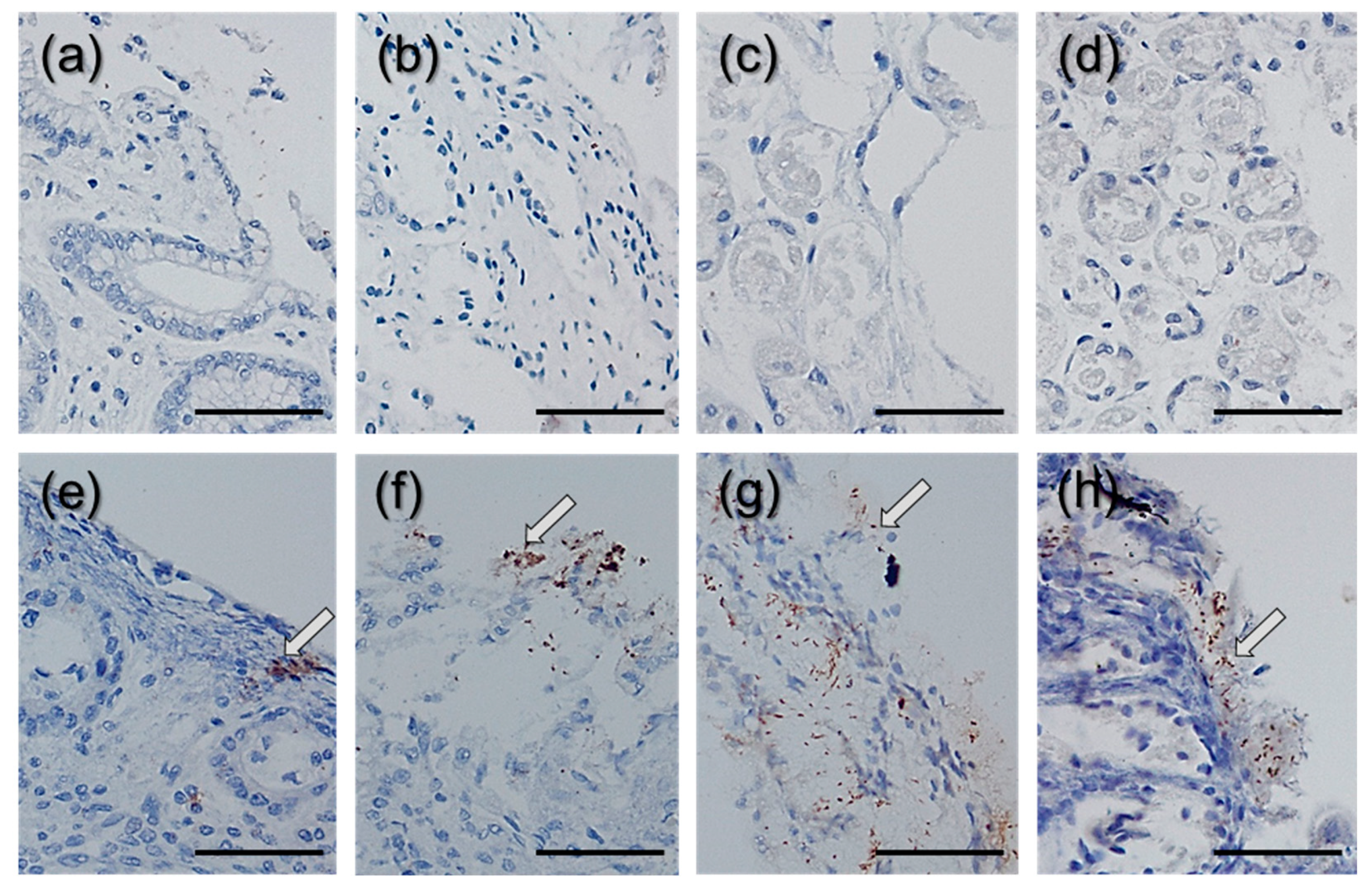
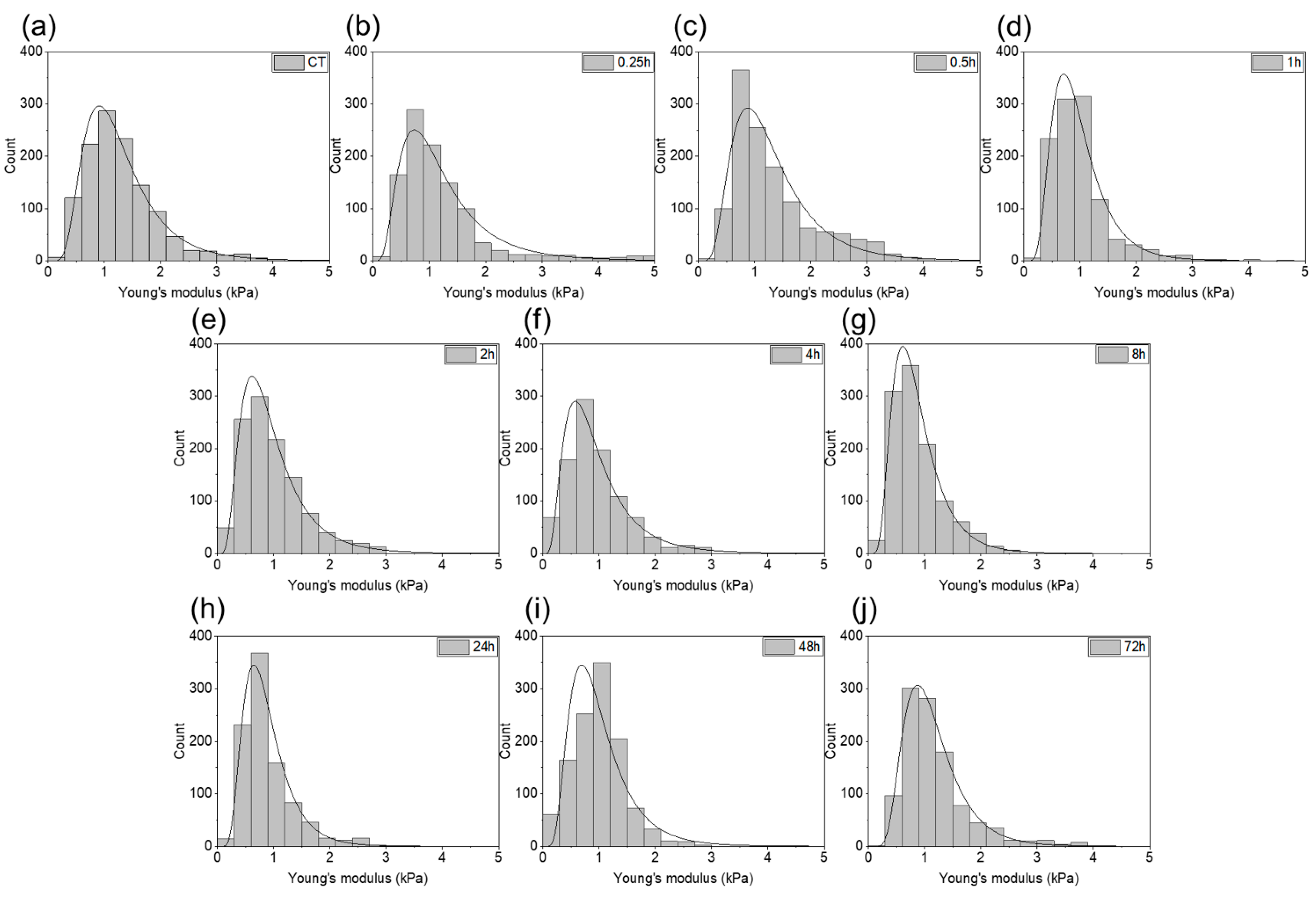
2. Results
3. Discussion
4. Materials and Methods
4.1. Tissue Samples
4.2. Bacterial Strain
4.3. Cell Culture
4.4. AFM Measurements
4.5. Fluorescent Visualization of the Cells
4.6. Immunohistochemistry of the Tissue Samples
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Soliman, C.; Eastwood, S.; Truong, V.K.; Ramsland, P.A.; Elbourne, A. The membrane effects of melittin on gastric and colorectal cancer. PLoS ONE 2019, 14, e0224028. [Google Scholar] [CrossRef] [Green Version]
- Alipour, M. Molecular Mechanism of Helicobacter pylori-Induced Gastric Cancer. J. Gastrointest. Cancer 2020, 1–8. [Google Scholar]
- Sabbagh, P.; Javanian, M.; Koppolu, V.; Vasigala, V.R.; Ebrahimpour, S. Helicobacter pylori infection in children: An overview of diagnostic methods. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 2019, 1–11. [Google Scholar] [CrossRef]
- Sokic-Milutinovic, A.; Alempijevic, T.; Milosavljevic, T. Role of Helicobacter pylori infection in gastric carcinogenesis: Current knowledge and future directions. World J. Gastroenterol. 2015, 21, 11654. [Google Scholar] [CrossRef]
- Necchi, V.; Candusso, M.E.; Tava, F.; Luinetti, O.; Ventura, U.; Fiocca, R.; Ricci, V.; Solcia, E. Intracellular, intercellular, and stromal invasion of gastric mucosa, preneoplastic lesions, and cancer by Helicobacter pylori. Gastroenterology 2007, 132, 1009–1023. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Hu, Y.; Ge, Z.-M.; Zou, Q.-M.; Lyu, N.-H. Diagnosis and treatment of Helicobacter pylori infections in children and elderly populations. Chronic Dis. Transl. Med. 2019, 5, 243–251. [Google Scholar] [CrossRef]
- Debraekeleer, A.; Remaut, H. Future perspective for potential Helicobacter pylori eradication therapies. Future Microbiol. 2018, 13, 671–687. [Google Scholar] [CrossRef] [Green Version]
- Roszczenko-Jasińska, P.; Wojtyś, M.I.; Jagusztyn-Krynicka, E.K. Helicobacter pylori treatment in the post-antibiotics era—Searching for new drug targets. Appl. Microbiol. Biotechnol. 2020, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Magalhães Queiroz, D.M.; Luzza, F. Epidemiology of Helicobacter pylori infection. Helicobacter 2006, 11, 1–5. [Google Scholar] [CrossRef]
- Mentis, A.; Lehours, P.; Mégraud, F. Epidemiology and Diagnosis of Helicobacter pylori infection. Helicobacter 2015, 20, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, J.H.; Bortolin, K.; Jones, N.L. Helicobacter pylori infection in children. Helicobacter 2020, 25, e12742. [Google Scholar] [CrossRef] [PubMed]
- Thrift, A.P.; El-Serag, H.B. Burden of gastric cancer. Clin. Gastroenterol. Hepatol. 2020, 18, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Chenjing, X.; Djaleel, M.S.; Yao, W.; Shunfu, X. Virulence of Helicobacter Pylori Outer Membrane Proteins: An Updated Review. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1821–1830. [Google Scholar]
- Francescone, R.; Hou, V.; Grivennikov, S.I. Microbiome, inflammation and cancer. Cancer J. 2014, 20, 181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guevara, B.; Cogdill, A.G. Helicobacter pylori: A review of current diagnostic and management strategies. Dig. Dis. Sci. 2020, 65, 1917–1931. [Google Scholar] [CrossRef]
- Gravina, A.G.; Zagari, R.M.; de Musis, C.; Romano, L.; Loguercio, C.; Romano, M. Helicobacter pylori and extragastric diseases: A review. World J. Gastroenterol. 2018, 24, 3204. [Google Scholar] [CrossRef]
- Engstrand, L.; Graham, D.Y. Microbiome and gastric cancer. Dig. Dis. Sci. 2020, 65, 865–873. [Google Scholar] [CrossRef] [Green Version]
- Janmey, P.A.; Winer, J.P.; Murray, M.E.; Wen, Q. The hard life of soft cells. Cell Motil. Cytoskelet. 2009, 66, 597–605. [Google Scholar] [CrossRef]
- Pogoda, K.; Jaczewska, J.; Wiltowska-Zuber, J.; Klymenko, O.; Zuber, K.; Fornal, M.; Lekka, M. Depth-sensing analysis of cytoskeleton organization based on AFM data. Eur. Biophys. J. 2012, 41, 79–87. [Google Scholar] [CrossRef]
- Stylianou, A.; Lekka, M.; Stylianopoulos, T. AFM assessing of nanomechanical fingerprints for cancer early diagnosis and classification: From single cell to tissue level. Nanoscale 2018, 10, 20930–20945. [Google Scholar] [CrossRef]
- Zemła, J.; Danilkiewicz, J.; Orzechowska, B.; Pabijan, J.; Seweryn, S.; Lekka, M. Atomic force microscopy as a tool for assessing the cellular elasticity and adhesiveness to identify cancer cells and tissues. Semin. Cell Dev. Biol. 2018, 73, 115–124. [Google Scholar] [CrossRef]
- Hayashi, K.; Iwata, M. Stiffness of cancer cells measured with an AFM indentation method. J. Mech. Behav. Biomed. Mater. 2015, 49, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Wyss, H.M.; Henderson, J.M.; Byfield, F.J.; Bruggeman, L.A.; Ding, Y.; Huang, C.; Suh, J.H.; Franke, T.; Mele, E.; Pollak, M.R. Biophysical properties of normal and diseased renal glomeruli. Am. J. Physiol. Cell Physiol. 2011, 300, C397–C405. [Google Scholar] [CrossRef] [Green Version]
- Deptuła, P.; Łysik, D.; Pogoda, K.; Cieśluk, M.; Namiot, A.; Mystkowska, J.; Król, G.; Głuszek, S.; Janmey, P.A.; Bucki, R. Tissue Rheology as a Possible Complementary Procedure to Advance Histological Diagnosis of Colon Cancer. ACS Biomater. Sci. Eng. 2020, 6, 5620–5631. [Google Scholar] [CrossRef]
- Cieśluk, M.; Pogoda, K.; Deptuła, P.; Werel, P.; Kułakowska, A.; Kochanowicz, J.; Mariak, Z.; Łysoń, T.; Reszeć, J.; Bucki, R. Nanomechanics and Histopathology as Diagnostic Tools to Characterize Freshly Removed Human Brain Tumors. Int. J. Nanomed. 2020, 15, 7509. [Google Scholar] [CrossRef]
- Pogoda, K.; Janmey, P.A. Glial tissue mechanics and mechanosensing by glial cells. Front. Cell. Neurosci. 2018, 12, 25. [Google Scholar] [CrossRef] [PubMed]
- Chin, L.; Xia, Y.; Discher, D.E.; Janmey, P.A. Mechanotransduction in cancer. Curr. Opin. Chem. Eng. 2016, 11, 77–84. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, R.; Sherratt, M.J.; Cruickshank, J.K.; Derby, B. Characterizing the elastic properties of tissues. Mater. Today 2011, 14, 96–105. [Google Scholar] [CrossRef]
- Perepelyuk, M.; Chin, L.; Cao, X.; van Oosten, A.; Shenoy, V.B.; Janmey, P.A.; Wells, R.G. Normal and fibrotic rat livers demonstrate shear strain softening and compression stiffening: A model for soft tissue mechanics. PLoS ONE 2016, 11, e0146588. [Google Scholar] [CrossRef] [Green Version]
- Stylianou, A.; Stylianopoulos, T. Atomic force microscopy probing of cancer cells and tumor microenvironment components. BioNanoScience 2016, 6, 33–46. [Google Scholar] [CrossRef] [Green Version]
- Ivanov, A.I.; Parkos, C.A.; Nusrat, A. Cytoskeletal regulation of epithelial barrier function during inflammation. Am. J. Pathol. 2010, 177, 512–524. [Google Scholar] [CrossRef]
- Chakravortty, D.; Kumar, K.N. Bacterial lipopolysaccharide induces cytoskeletal rearrangement in small intestinal lamina propria fibroblasts: Actin assembly is essential for lipopolysaccharide signaling. Biochim. Biophys. Acta 2000, 1500, 125–136. [Google Scholar] [CrossRef] [Green Version]
- Haynes, J.; Srivastava, J.; Madson, N.; Wittmann, T.; Barber, D.L. Dynamic actin remodeling during epithelial–mesenchymal transition depends on increased moesin expression. Mol. Biol. Cell 2011, 22, 4750–4764. [Google Scholar] [CrossRef] [PubMed]
- Eutamene, H.; Theodorou, V.; Schmidlin, F.; Tondereau, V.; Garcia-Villar, R.; Salvador-Cartier, C.; Chovet, M.; Bertrand, C.; Bueno, L. LPS-induced lung inflammation is linked to increased epithelial permeability: Role of MLCK. Eur. Respir. J. 2005, 25, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Ceponis, P.J.; Sherman, P.M. Cytoskeletal rearrangements in gastric epithelial cells in response to Helicobacter pylori infection. J. Med. Microbiol. 2003, 52, 861–867. [Google Scholar] [CrossRef] [Green Version]
- McKee, C.T.; Last, J.A.; Russell, P.; Murphy, C.J. Indentation versus tensile measurements of Young’s modulus for soft biological tissues. Tissue Eng. Part B Rev. 2011, 17, 155–164. [Google Scholar] [CrossRef] [Green Version]
- Baker, E.L.; Bonnecaze, R.T.; Zaman, M.H. Extracellular matrix stiffness and architecture govern intracellular rheology in cancer. Biophys. J. 2009, 97, 1013–1021. [Google Scholar] [CrossRef] [Green Version]
- Engstrom, T.; Pogoda, K.; Cruz, K.; Janmey, P.; Schwarz, J. Compression stiffening in biological tissues: On the possibility of classic elasticity origins. Phys. Rev. E 2019, 99, 052413. [Google Scholar] [CrossRef] [Green Version]
- Bilston, L.E. Soft tissue rheology and its implications for elastography: Challenges and opportunities. NMR Biomed. 2018, 31, e3832. [Google Scholar] [CrossRef]
- Malandrino, A.; Mak, M.; Kamm, R.D.; Moeendarbary, E. Complex mechanics of the heterogeneous extracellular matrix in cancer. Extrem. Mech. Lett. 2018, 21, 25–34. [Google Scholar] [CrossRef]
- Celli, J.P.; Turner, B.S.; Afdhal, N.H.; Keates, S.; Ghiran, I.; Kelly, C.P.; Ewoldt, R.H.; McKinley, G.H.; So, P.; Erramilli, S. Helicobacter pylori moves through mucus by reducing mucin viscoelasticity. Proc. Natl. Acad. Sci. USA 2009, 106, 14321–14326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bansil, R.; Celli, J.; Hardcastle, J.; Turner, B. The influence of mucus microstructure and rheology in Helicobacter pylori infection. Front. Immunol. 2013, 4, 310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, C.; Padra, M.; Constantino, M.A.; Sharba, S.; Thorell, A.; Lindén, S.K.; Bansil, R. Influence of the viscosity of healthy and diseased human mucins on the motility of Helicobacter pylori. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Meng, W.; Wang, B.; Qiao, L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 2014, 345, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Dhiman, M. Inflammasome activation and regulation during Helicobacter pylori pathogenesis. Microb. Pathog. 2018, 125, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Shibata, W.; Sue, S.; Tsumura, S.; Ishii, Y.; Sato, T.; Kameta, E.; Sugimori, M.; Yamada, H.; Kaneko, H.; Sasaki, T. Helicobacter-induced gastric inflammation alters the properties of gastric tissue stem/progenitor cells. BMC Gastroenterol. 2017, 17, 145. [Google Scholar] [CrossRef] [Green Version]
- Plodinec, M.; Loparic, M.; Monnier, C.A.; Obermann, E.C.; Zanetti-Dallenbach, R.; Oertle, P.; Hyotyla, J.T.; Aebi, U.; Bentires-Alj, M.; Lim, R.Y.; et al. The nanomechanical signature of breast cancer. Nat. Nanotechnol. 2012, 7, 757–765. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [Green Version]
- Bessede, E.; Staedel, C.; Amador, L.A.; Nguyen, P.; Chambonnier, L.; Hatakeyama, M.; Belleannee, G.; Megraud, F.; Varon, C. Helicobacter pylori generates cells with cancer stem cell properties via epithelial–mesenchymal transition-like changes. Oncogene 2014, 33, 4123–4131. [Google Scholar] [CrossRef] [Green Version]
- Suprewicz, Ł.; Tokajuk, G.; Cieśluk, M.; Deptuła, P.; Sierpińska, T.; Wolak, P.; Wollny, T.; Tokajuk, J.; Głuszek, S.; Piktel, E. Bacteria Residing at Root Canals Can Induce Cell Proliferation and Alter the Mechanical Properties of Gingival and Cancer Cells. Int. J. Mol. Sci. 2020, 21, 7914. [Google Scholar] [CrossRef]
- Watanabe, T.; Takahashi, A.; Suzuki, K.; Kurusu-Kanno, M.; Yamaguchi, K.; Fujiki, H.; Suganuma, M. Epithelial–mesenchymal transition in human gastric cancer cell lines induced by TNF-α-inducing protein of Helicobacter pylori. Int. J. Cancer 2014, 134, 2373–2382. [Google Scholar] [CrossRef]
- Bellows, C.F.; Wheatley, B.M.; Moroz, K.; Rosales, S.C.; Morici, L.A. The effect of bacterial infection on the biomechanical properties of biological mesh in a rat model. PLoS ONE 2011, 6, e21228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martino, F.; Perestrelo, A.R.; Vinarský, V.; Pagliari, S.; Forte, G. Cellular mechanotransduction: From tension to function. Front. Physiol. 2018, 9, 824. [Google Scholar] [CrossRef]
- Elosegui-Artola, A.; Oria, R.; Chen, Y.; Kosmalska, A.; Pérez-González, C.; Castro, N.; Zhu, C.; Trepat, X.; Roca-Cusachs, P. Mechanical regulation of a molecular clutch defines force transmission and transduction in response to matrix rigidity. Nat. Cell Biol. 2016, 18, 540–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Case, L.B.; Waterman, C.M. Integration of actin dynamics and cell adhesion by a three-dimensional, mechanosensitive molecular clutch. Nat. Cell Biol. 2015, 17, 955–963. [Google Scholar] [CrossRef]
- Levental, I.; Levental, K.R.; Klein, E.A.; Assoian, R.; Miller, R.T.; Wells, R.G.; Janmey, P.A. A simple indentation device for measuring micrometer-scale tissue stiffness. J. Phys. Condens. Matter 2010, 22, 194120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plodinec, M.; Lim, R.Y. Nanomechanical characterization of living mammary tissues by atomic force microscopy. In Mammary Stem Cells; Humana Press: New York, NY, USA, 2015; pp. 231–246. [Google Scholar]
- Pepin, K.M.; McGee, K.P.; Arani, A.; Lake, D.S.; Glaser, K.J.; Manduca, A.; Parney, I.F.; Ehman, R.L.; Huston, J., III. MR Elastography Analysis of Glioma Stiffness and IDH1-Mutation Status. Am. J. Neuroradiol. 2018, 39, 31–36. [Google Scholar] [CrossRef] [Green Version]
- Yin, Z.; Romano, A.J.; Manduca, A.; Ehman, R.L.; Huston, J., III. Stiffness and Beyond: What MR Elastography Can Tell Us About Brain Structure and Function Under Physiologic and Pathologic Conditions. Top. Magn. Reason. Imaging 2018, 27, 305–318. [Google Scholar] [CrossRef]
- Kennedy, E.A.; Tordonado, D.S.; Duma, S.M. Effects of freezing on the mechanical properties of articular cartilage. Biomed. Sci. Instrum. 2007, 43, 342–347. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deptuła, P.; Suprewicz, Ł.; Daniluk, T.; Namiot, A.; Chmielewska, S.J.; Daniluk, U.; Lebensztejn, D.; Bucki, R. Nanomechanical Hallmarks of Helicobacter pylori Infection in Pediatric Patients. Int. J. Mol. Sci. 2021, 22, 5624. https://doi.org/10.3390/ijms22115624
Deptuła P, Suprewicz Ł, Daniluk T, Namiot A, Chmielewska SJ, Daniluk U, Lebensztejn D, Bucki R. Nanomechanical Hallmarks of Helicobacter pylori Infection in Pediatric Patients. International Journal of Molecular Sciences. 2021; 22(11):5624. https://doi.org/10.3390/ijms22115624
Chicago/Turabian StyleDeptuła, Piotr, Łukasz Suprewicz, Tamara Daniluk, Andrzej Namiot, Sylwia Joanna Chmielewska, Urszula Daniluk, Dariusz Lebensztejn, and Robert Bucki. 2021. "Nanomechanical Hallmarks of Helicobacter pylori Infection in Pediatric Patients" International Journal of Molecular Sciences 22, no. 11: 5624. https://doi.org/10.3390/ijms22115624
APA StyleDeptuła, P., Suprewicz, Ł., Daniluk, T., Namiot, A., Chmielewska, S. J., Daniluk, U., Lebensztejn, D., & Bucki, R. (2021). Nanomechanical Hallmarks of Helicobacter pylori Infection in Pediatric Patients. International Journal of Molecular Sciences, 22(11), 5624. https://doi.org/10.3390/ijms22115624





