Embryonic Trophectoderm Secretomics Reveals Chemotactic Migration and Intercellular Communication of Endometrial and Circulating MSCs in Embryonic Implantation
Abstract
1. Introduction
2. Results
2.1. The Proteomic Profiles of BBT-9 and BBT-18 Secretomes (EVs and Soluble Proteins) Are Compatible with Different EMT Stages
2.2. The BBT-Secretomes Contain Early Pregnancy-Related Proteins and Angiogenic Proteins Both in the Soluble Fraction and EV-Cargo
2.3. Endometrial and Blood MSCs Show Chemotactic Migration to the Secretome of the Embryonic Trophectoderm Cells
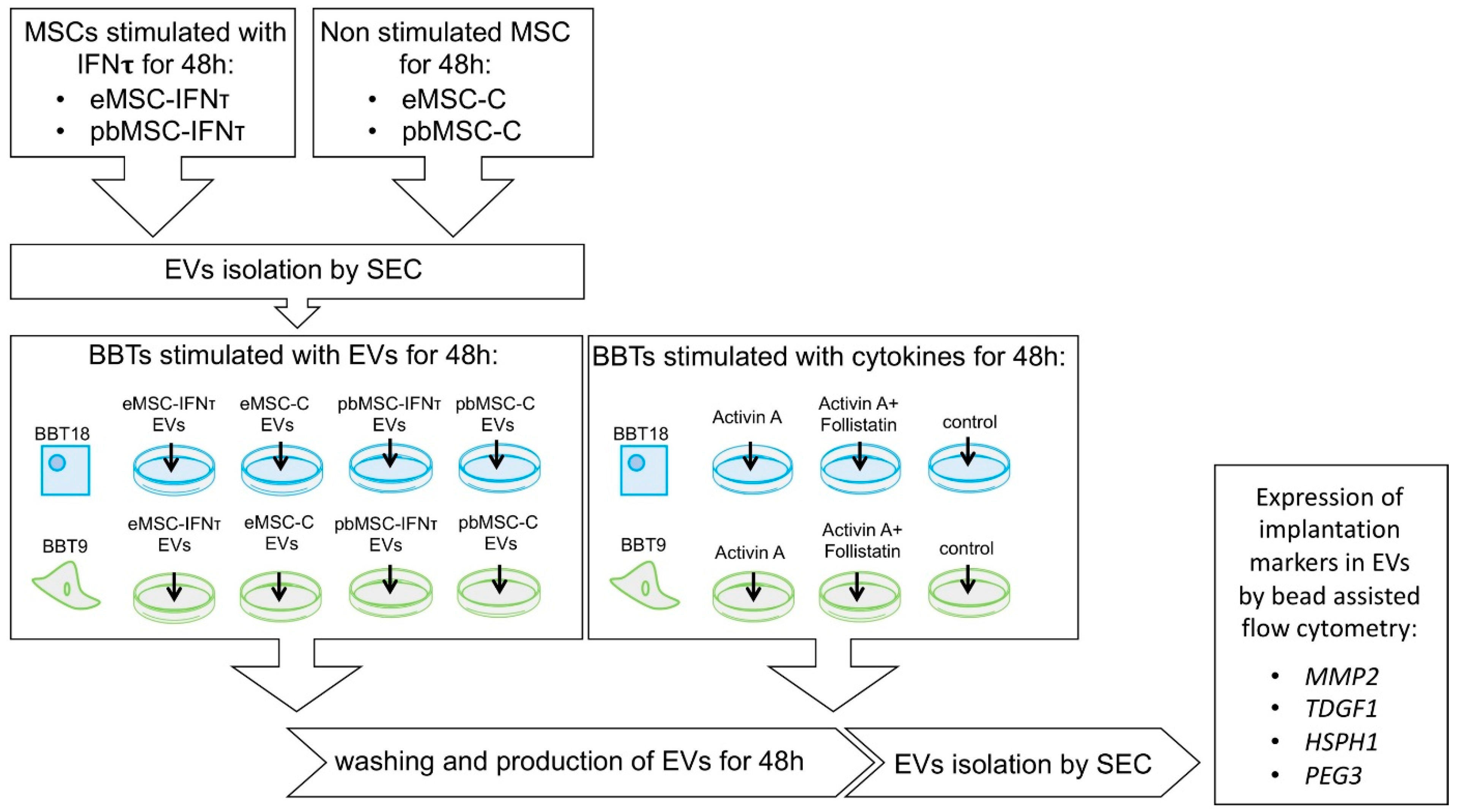
2.4. Embryonic Trophectoderm EVs Change Their Implantation Protein Profile after Uptake of Maternal MSC-EVs
3. Discussion
4. Materials and Methods
4.1. Cells
4.2. Production and Isolation of BBT Primary Cultures (BBT-9 and BBT-18) Secretome (EVs and Soluble Proteins)
4.3. Characterization of BBT Primary Cultures (BBT-9 and BBT-18) EVs by Bead-Assisted Flow Cytometry Assay
4.4. BCA Protein Analysis
4.5. Dot Blot Analysis
4.6. Transmission Electron Microscopy (TEM)
4.7. BBT-EV Binding to MSC
4.8. Identification and Quantification of Secretome (EVs and Soluble Fraction- Derived Proteins of BBT Cells by LC-MS/MS
4.9. Sample Preparation for LC-MS/MS
4.10. In Vitro Model of Chemotactic Migration of MSC by BBT Secretome
4.11. Analysis of Changes in BBT EVs Profile after Uterine Cytokines Stimuli or Communication with MSC EVs
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Yamakoshi, S.; Bai, R.; Chaen, T.; Ideta, A.; Aoyagi, Y.; Sakurai, T.; Konno, T.; Imakawa, K. Expression of mesenchymal-related genes by the bovine trophectoderm following conceptus attachment to the endometrial epithelium. Reproduction 2012, 143, 377–387. [Google Scholar] [CrossRef]
- Lee, B.; Villarreal-Ponce, A.; Fallahi, M.; Ovadia, J.; Sun, P.; Yu, Q.C.; Ito, S.; Sinha, S.; Nie, Q.; Dai, X. Transcriptional mechanisms link epithelial plasticity to adhesion and differentiation of epidermal progenitor cells. Dev. Cell. 2014, 29, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Pfarrer, C.D. Characterization of the bovine placenta by cytoskeleton, integrin receptors, and extracellular matrix. Methods Mol. Med. 2006, 121, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Acloque, H.; Adams, M.S.; Fishwick, K.; Bronner-Fraser, M.; Nieto, M.A. Epithelial-mesenchymal transitions: The importance of changing cell state in development and disease. J. Clin. Investig. 2009, 119, 1438–1449. [Google Scholar] [CrossRef]
- Kusama, K.; Bai, R.; Ideta, A.; Aoyagi, Y.; Okuda, K.; Imakawa, K. Regulation of epithelial to mesenchymal transition in bovine conceptuses through the interaction between follistatin and activin A. Mol. Cell. Endocrinol. 2016, 434, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Kusama, K.; Nakamura, K.; Sakurai, T.; Kimura, K.; Ideta, A.; Aoyagi, Y.; Imakawa, K. Down-regulation of transcription factor OVOL2 contributes to epithelial-mesenchymal transition in a noninvasive type of trophoblast implantation to the maternal endometrium. FASEB J. 2018, 32, 3371–3384. [Google Scholar] [CrossRef] [PubMed]
- Talbot, N.C.; Caperna, T.J.; Edwards, J.L.; Garrett, W.; Wells, K.D.; Ealy, A.D. Bovine blastocyst-derived trophectoderm and endoderm cell cultures: Interferon tau and transferrin expression as respective in vitro markers. Biol. Reprod. 2000, 62, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Talbot, N.C.; Caperna, T.J.; Powell, A.M.; Garrett, W.M.; Ealy, A.D. Isolation and characterization of a bovine trophectoderm cell line derived from a parthenogenetic blastocyst. Mol. Reprod. Dev. 2004, 69, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Talbot, N.; Powell, A.; Camp, M.; Ealy, A. Establishment of a bovine blastocyst-derived cell line collection for the comparative analysis of embryos created in vivo and by in vitro fertilization, somatic cell nuclear transfer, or parthenogenetic activation. Vitr. Cell. Dev. Biol. Anim. 2007, 43, 59–71. [Google Scholar] [CrossRef]
- Ramos-Ibeas, P.; Calle, A.; Pericuesta, E.; Laguna-Barraza, R.; Moros-Mora, R.; Lopera-Vásquez, R.; Maillo, V.; Yáñez-Mó, M.; Gutiérrez-Adán, A.; Ramírez, M.Á.; et al. An Efficient System to Establish Biopsy-Derived Trophoblastic Cell Lines from Bovine Embryos. Biol. Reprod. 2014. [Google Scholar] [CrossRef]
- Calle, A.; López-Martín, S.; Monguió-Tortajada, M.; Borràs, F.E.; Yáñez-Mó, M.; Ramírez, M.Á. Bovine endometrial MSC: Mesenchymal to epithelial transition during luteolysis and tropism to implantation niche for immunomodulation. Stem Cell Res. Ther. 2019, 10, 239. [Google Scholar] [CrossRef] [PubMed]
- Calle, A.; Gutiérrez-Reinoso, M.Á.; Re, M.; Blanco, J.; De la Fuente, J.; Monguió-Tortajada, M.; Borràs, F.E.; Yáñez-Mó, M.; Ramírez, M.Á. Bovine peripheral blood MSCs chemotax towards inflammation and embryo implantation stimuli. J. Cell. Physiol. 2021, 236, 1054–1067. [Google Scholar] [CrossRef] [PubMed]
- Suárez, H.; Gámez-Valero, A.; Reyes, R.; López-Martín, S.; Rodríguez, M.J.; Carrascosa, J.L.; Cabañas, C.; Borràs, F.E.; Yáñez-Mó, M. A bead-assisted flow cytometry method for the semi-quantitative analysis of Extracellular Vesicles. Sci. Rep. 2017, 7, 11271. [Google Scholar] [CrossRef] [PubMed]
- Forde, N.; Bazer, F.W.; Spencer, T.E.; Lonergan, P. ‘Conceptualizing’ the Endometrium: Identification of Conceptus-Derived Proteins During Early Pregnancy in Cattle. Biol. Reprod. 2015, 92, 156. [Google Scholar] [CrossRef]
- Calle, A.; Barrajón-Masa, C.; Gómez-Fidalgo, E.; Martín-Lluch, M.; Cruz-Vigo, P.; Sánchez-Sánchez, R.; Ramírez, M.Á. Iberian pig mesenchymal stem/stromal cells from dermal skin, abdominal and subcutaneous adipose tissues, and peripheral blood: In vitro characterization and migratory properties in inflammation. Stem Cell Res. Ther. 2018, 9, 178. [Google Scholar] [CrossRef] [PubMed]
- Monguió-Tortajada, M.; Prat-Vidal, C.; Moron-Font, M.; Clos-Sansalvador, M.; Calle, A.; Gastelurrutia, P.; Cserkoova, A.; Morancho, A.; Ramírez, M.Á.; Roura, S.; et al. Local administration of porcine immunomodulatory, chemotactic and angiogenic extracellular vesicles using engineered cardiac scaffolds for myocardial infarction. Bioact. Mater. 2021, 6, 3314–3327. [Google Scholar] [CrossRef] [PubMed]
- Nakano, H.; Shimada, A.; Imai, K.; Takahashi, T.; Hashizume, K. The cytoplasmic expression of E-cadherin and beta-catenin in bovine trophoblasts during binucleate cell differentiation. Placenta 2005, 26, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Imakawa, K.; Bai, R.; Fujiwara, H.; Ideta, A.; Aoyagi, Y.; Kusama, K. Continuous model of conceptus implantation to the maternal endometrium. J. Endocrinol. 2017, 233, R53–R65. [Google Scholar] [CrossRef]
- Škovierová, H.; Okajčeková, T.; Strnádel, J.; Vidomanová, E.; Halašová, E. Molecular regulation of epithelial-to-mesenchymal transition in tumorigenesis. Int. J. Mol. Med. 2018, 41, 1187–1200. [Google Scholar] [CrossRef]
- Pang, M.F.; Georgoudaki, A.M.; Lambut, L.; Johansson, J.; Tabor, V.; Hagikura, K.; Jin, Y.; Jansson, M.; Alexander, J.S.; Fuxe, J.; et al. TGF-β1-induced EMT promotes targeted migration of breast cancer cells through the lymphatic system by the activation of CCR7/CCL21-mediated chemotaxis. Oncogene 2016, 35, 748–760. [Google Scholar] [CrossRef]
- Di Nicola, M.; Carlo-Stella, C.; Magni, M.; Milanesi, M.; Longoni, P.D.; Matteucci, P.; Grisanti, S.; Gianni, A.M. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002, 99, 3838–3843. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, D.; Rosato, B.; Nanni, M.; Magenta, A.; Belleudi, F.; Torrisi, M.R. Expression of the FGFR2 mesenchymal splicing variant in epithelial cells drives epithelial-mesenchymal transition. Oncotarget 2016, 7, 5440–5460. [Google Scholar] [CrossRef] [PubMed]
- Billottet, C.; Tuefferd, M.; Gentien, D.; Rapinat, A.; Thiery, J.P.; Broët, P.; Jouanneau, J. Modulation of several waves of gene expression during FGF-1 induced epithelial-mesenchymal transition of carcinoma cells. J. Cell. Biochem. 2008, 104, 826–839. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.G.; Hosoe, M.; Fujii, S.; Kanahara, H.; Sakumoto, R. Temporal expression and localization of vascular endothelial growth factor family members in the bovine uterus during peri-implantation period. Theriogenology 2019, 133, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Hoffert-Goeres, K.A.; Batchelder, C.A.; Bertolini, M.; Moyer, A.L.; Famula, T.R.; Anderson, G.B. Angiogenesis in day-30 bovine pregnancies derived from nuclear transfer. Cloning Stem Cells 2007, 9, 595–607. [Google Scholar] [CrossRef]
- Pfarrer, C.; Hirsch, P.; Guillomot, M.; Leiser, R. Interaction of integrin receptors with extracellular matrix is involved in trophoblast giant cell migration in bovine placentomes. Placenta 2003, 24, 588–597. [Google Scholar] [CrossRef]
- Caltabiano, S.; Hum, W.T.; Attwell, G.J.; Gralnick, D.N.; Budman, L.J.; Cannistraci, A.M.; Bex, F.J. The integrin specificity of human recombinant osteopontin. Biochem. Pharmacol. 1999, 58, 1567–1578. [Google Scholar] [CrossRef]
- Bai, R.; Bai, H.; Kuse, M.; Ideta, A.; Aoyagi, Y.; Fujiwara, H.; Okuda, K.; Imakawa, K.; Sakurai, T. Involvement of VCAM-1 in the bovine conceptus adhesion to the uterine endometrium. Reproduction 2014. [Google Scholar] [CrossRef]
- Schnapp, L.M.; Hatch, N.; Ramos, D.M.; Klimanskaya, I.V.; Sheppard, D.; Pytela, R. The human integrin alpha 8 beta 1 functions as a receptor for tenascin, fibronectin, and vitronectin. J. Biol. Chem. 1995, 270, 23196–23202. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, G.; Fan, H.; Zhao, X.; Li, P.; Wang, Z.; Hu, Y.; Hou, Y. Mesenchymal stem cells ameliorate Th1-induced pre-eclampsia-like symptoms in mice via the suppression of TNF-α expression. PLoS ONE 2014, 9, e88036. [Google Scholar] [CrossRef]
- Vacca, P.; Montaldo, E.; Vitale, C.; Croxatto, D.; Moretta, L.; Mingari, M.C. MSC and innate immune cell interactions: A lesson from human decidua. Immunol. Lett. 2015, 168, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.H.; Zhu, X.H.; Yan, L.Y.; Zhang, Y.; Jin, H.Y.; Xia, X.; Li, R.; Qiao, J. Bone mesenchymal stem cells improve pregnancy outcome by inducing maternal tolerance to the allogeneic fetus in abortion-prone matings in mouse. Placenta 2016, 47, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, O.; Yanez-Mo, M.; Serrador, J.M.; Montoya, M.C.; Vicente-Manzanares, M.; Tejedor, R.; Furthmayr, H.; Sanchez-Madrid, F. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J. Cell Biol. 2002, 157, 1233–1245. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Huergo, S.P.; Hockl, P.F.; Stupirski, J.C.; Maller, S.M.; Morosi, L.G.; Pinto, N.A.; Berón, A.M.; Musuruana, J.L.; Nasswetter, G.G.; Rabinovich, G.A.; et al. Clinical Relevance of Galectin-1 and Galectin-3 in Rheumatoid Arthritis Patients: Differential Regulation and Correlation With Disease Activity. Front. Immunol. 2018, 9, 3057. [Google Scholar] [CrossRef]
- Colnot, C.; Fowlis, D.; Ripoche, M.A.; Bouchaert, I.; Poirier, F. Embryonic implantation in galectin 1/galectin 3 double mutant mice. Dev. Dyn. 1998, 211, 306–313. [Google Scholar] [CrossRef]
- Siegel, G.; Kluba, T.; Hermanutz-Klein, U.; Bieback, K.; Northoff, H.; Schäfer, R. Phenotype, donor age and gender affect function of human bone marrow-derived mesenchymal stromal cells. BMC Med. 2013, 11, 146. [Google Scholar] [CrossRef]
- Jones, R.L.; Salamonsen, L.A.; Findlay, J.K. Potential roles for endometrial inhibins, activins and follistatin during human embryo implantation and early pregnancy. Trends Endocrinol. Metab. 2002, 13, 144–150. [Google Scholar] [CrossRef]
- Fang, Z.F.; Gai, H.; Huang, Y.Z.; Li, S.G.; Chen, X.J.; Shi, J.J.; Wu, L.; Liu, A.; Xu, P.; Sheng, H.Z. Rabbit embryonic stem cell lines derived from fertilized, parthenogenetic or somatic cell nuclear transfer embryos. Exp. Cell Res. 2006, 312, 3669–3682. [Google Scholar] [CrossRef]
- Mitko, K.; Ulbrich, S.E.; Wenigerkind, H.; Sinowatz, F.; Blum, H.; Wolf, E.; Bauersachs, S. Dynamic changes in messenger RNA profiles of bovine endometrium during the oestrous cycle. Reproduction 2008, 135, 225–240. [Google Scholar] [CrossRef]
- Zhou, W.; Xiang, T.; Walker, S.; Abruzzese, R.V.; Hwang, E.; Farrar, V.; Farrar, V.; Findeisen, B.; Sadeghieh, S.; Polejaeva, I.; et al. Aggregation of bovine cloned embryos at the four-cell stage stimulated gene expression and in vitro embryo development. Mol. Reprod. Dev. 2008, 75, 1281–1289. [Google Scholar] [CrossRef]
- Clemente, M.; Lopez-Vidriero, I.; O’Gaora, P.; Mehta, J.P.; Forde, N.; Gutierrez-Adan, A.; Lonergan, P.; Rizos, D. Transcriptome changes at the initiation of elongation in the bovine conceptus. Biol. Reprod. 2011, 85, 285–295. [Google Scholar] [CrossRef]
- Hatayama, T.; Takigawa, T.; Takeuchi, S.; Shiota, K. Characteristic expression of high molecular mass heat shock protein HSP105 during mouse embryo development. Cell Struct. Funct. 1997, 22, 517–525. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yuan, J.X.; Xiao, L.J.; Lu, C.L.; Zhang, X.S.; Liu, T.; Chen, M.; Hu, Z.Y.; Gao, F.; Liu, Y.X. Increased expression of heat shock protein 105 in rat uterus of early pregnancy and its significance in embryo implantation. Reprod. Biol. Endocrinol. 2009, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Lopera-Vásquez, R.; Hamdi, M.; Fernandez-Fuertes, B.; Maillo, V.; Beltrán-Breña, P.; Calle, A.; Redruello, A.; López-Martín, S.; Gutierrez-Adán, A.; Rizos, D.; et al. Extracellular Vesicles from BOEC in In Vitro Embryo Development and Quality. PLoS ONE 2016, 11, e0148083. [Google Scholar] [CrossRef] [PubMed]
- Kotrbová, A.; Štěpka, K.; Maška, M.; Pálenik, J.J.; Ilkovics, L.; Klemová, D.; Kravec, M.; Hubatka, F.; Dave, Z.; Pospíchalová, V.; et al. TEM ExosomeAnalyzer: A computer-assisted software tool for quantitative evaluation of extracellular vesicles in transmission electron microscopy images. J. Extracell Vesicles 2019, 8, 1560808. [Google Scholar] [CrossRef] [PubMed]






| EV-Cargo Proteins | Mean Fold Change BBT-18:BBT-9 | Gene Name | Accession Number | Reproductive Event |
|---|---|---|---|---|
| Mucin-1 | 8.47 | MUC1 | Q8WML4 | Epithelial. Implantation |
| Ezrin | 7.69 | EZR | P31976 | Implantation |
| Macrophage-capping protein | 4.27 | CAPG | Q865V6 | Co. secretion d16 pregnant cow. |
| CD9 antigen | 4.27 | CD9 | P30932 | Implantation (in vitro) |
| 14-3-3 protein sigma | 4.17 | SFN | Q0VC36 | Trophoblast marker |
| Moesin | 4.11 | MSN | Q2HJ49 | Co. secretion d16 pregnant cow. |
| Thioredoxin | 3.94 | TXN | O97680 | Co. secretion d16 pregnant cow. |
| Cystatin-B | 3.73 | CSTB | A0A140T831 | Co. secretion d16 pregnant cow. |
| Keratin type II cytoskeletal 8 | 3.59 | KRT8 | P05786 | Implantation |
| PGM2 protein (Fragment) | 2.7 | PGM2 | A6QQ11 | Co. secretion d16 pregnant cow. |
| Actin-related protein 2/3 complex subunit 5-like protein | 2.19 | ARPC5L | Q5E963 | Co. secretion d16 pregnancy cow. |
| Heat shock protein 105 kDa | 2.1 | HSPH1 | Q0IIM3 | Co. secretion d16 pregnancy cow. |
| Galectin | −6.12 | LGALS3 | A6QLZ0 | Co. marker peri-elongation |
| Embryo-specific fibronectin 1 transcript variant | −5.56 | FN1 | B8Y9S9 | Co. secretion peri-gastrulation |
| Plastin-1 | −5.10 | PLS | A6H74 | Co. secretion d16 pregnant cow. |
| Vimentin | −5.00 | VIM | P48616 | Co. secretion and EMT |
| Embryo-specific fibronectin 1 transcript variant | −5.56 | FN1 | B8Y9S9 | Co. secretion peri-gastrulation |
| Alpha-fetoprotein | −2.86 | AFP | Q3SZ57 | Co. secretion peri-gastrulation |
| VCAM1 protein | −2.04 | VCAM1 | A7MBB0 | Implantation |
| Secreted Proteins | Mean Fold Change BBT-18:BBT-9 (≤1.5) | Gene Name | Accession Number |
|---|---|---|---|
| Plastin-3 | 1.8 | PLS3 * | A7E3Q8 |
| Heat shock cognate 71 kDa protein | 1.5 | HSPA8 * | P19120 |
| CD44 antigen | −1.36 | CD44 * | Q29423 |
| Nuclear transport factor | −1.28 | NUTF2 * | Q32KP9 |
| Protein disulfide-isomerase | 1.15 | P4HB * | P05307 |
| Collagen type V alpha 1 chain | −1.29 | COL5A1 * | G3MZI7 |
| Alpha-actinin-1 OS=Bos taurus | 1.87 | ACTN1 * | Q3B7N2 |
| Creatine kinase U-type mitochondrial | 1.53 | CKMT1 * | Q9TTK8 |
| Elongation factor 2 OS=Bos taurus | 1.63 | EEF2 * | Q3SYU2 |
| Rab GDP dissociation inhibitor alpha | −1.42 | GDI1 * | P21856 |
| Phosphatidylinositol-glycan-specific phospholipase | −1.58 | GPLD1 * | P80109 |
| Heterogeneous nuclear ribonucleoprotein A1 | 1.23 | HNRNPA1 * | P09867| |
| Heterogeneous nuclear ribonucleoproteins A2/B1 | −1.21 | HNRNPA2B1 * | Q2HJ60 |
| Heterogeneous nuclear ribonucleoprotein F | −1.03 | HNRNPF * | Q5E9J1 |
| Proteasome subunit alpha type-4 | −1.04 | PSMA4 * | Q3ZCK9 |
| 26S proteasome regulatory subunit 6B | −1.63 | PSMC4 * | Q3T030 |
| Serpin A3-2 | −1.36 | SERPINA3 * | A2I7M9 |
| 14-3-3 protein gamma | −1.02 | YWHAG * | A7Z057 |
| Integrin alpha-V | ITGAV | P80746 | |
| Integrin beta-1 | ITGB1 | P53712 | |
| Integrin alpha-5 | ITGA5 | F1MK44 | |
| Integrin beta | ITGB3 | F1MTN1 |
| Description | Mean Fold Change BBT-18:BBT-9 | Gene Name | Accession Number | Reproductive Event |
|---|---|---|---|---|
| Pregnancy-associated glycoprotein 2 | 53.54 | PAG2 | Q28057 | co. marker peri-elongation |
| Secreted seminal-vesicle Ly-6 protein 1 | 16.24 | SSLP1 | P83107 | co. marker peri-gastrulation |
| 14-3-3 protein sigma | 15.76 | SFN | Q0VC36 | Trophoblast marker |
| Interferon tau-1 | 13.13 | IFNT1 | P15696 | Implantation/Co. secretion |
| Trophoblast Kunitz domain protein 1 | 10.96 | TKDP | Q28201 | Trophoblast marker/Co marker peri-elongation |
| Keratin type II cytoskeletal 8 | 9.67 | KRT8 | F1MU12 | Trophoblast marker |
| Ezrin | 7.52 | EZR | P31976 | Implantation |
| Galectin | 5.25 | LGALS3 | A6QLZ0 | co. marker peri-elongation |
| Interferon tau-3 | 4.99 | IFNT3 | P56831 | Implantation/Co. secretion |
| Furin | 3.53 | FURIN | Q28193 | co. marker peri-gastrulation |
| CD44 antigen | 2.91 | CD44 | Q29423 | co. marker peri-gastrulation |
| Retinoic acid receptor responder 1 | 2.32 | RARRES2 | Q29RS5 | Trophoblast marker |
| Macrophage-capping protein | 2.24 | CAPG, AFCP, MCP | Q865V6 | co. marker peri-elongation |
| Vimentin | −10 | VIM | P48616 | co. marker peri-gastrulation |
| 72 kDa type IV collagenase | −10 | MMP2 | F1MKH8 | Implantation/Co. secretion |
| Follistatin-related protein 1 | −5.3 | FSTL1 | Q58D84 | Implantation |
| Fibronectin | −5 | FN1 | G5E5A9 | co. marker peri-gastrulation |
| Heat shock cognate 71 kDa protein | −3 | HSPA8 | P19120 | Trophoblast marker/Co marker peri-elongation |
| 10 kDa heat shock protein mitochondrial | −2.7 | HSPE1 | A0A3Q1N8Q5 | co. marker peri-elongation |
| Plastin-3 | −2.3 | PLS1 | A6H742 | Trophoblast marker |
| Fibroblast growth factor receptor | −1.8 | FGFR1 | F1MQI5 | co. marker peri-elongation |
| Angiogenesis Pathway | Mean Fold Change BBT-18:BBT-9 | Gene | Accession Number | Secretome |
|---|---|---|---|---|
| Catenin Beta-1 | co-expressed | CTNNB1 | Q0VCX4 | EV and soluble proteins |
| Mitogen-activated protein kinase 1 | co-expressed | MAPK1 | P46196 | EV and soluble proteins |
| Serine/threonine-protein kinase PAK-1 | co-expressed | PAK 1 | Q08E52 | Soluble proteins |
| Transforming protein RHOA | co-expressed | RHOA | P61585 | EV |
| MAP2K1 | co-expressed | MAP2K1 | Q0VD16 | EV |
| Serine/threonine-protein kinase A-RAF | 2.7 | ARAF | A0A452DI16 | Soluble proteins BBT-18 |
| Heat Shock Protein Beta-1 | 2.29 | HSPB1 | Q3T149 | EV BBT-18 |
| Fibroblast growth factor receptor 1 | −1.9 | FGFR1 | A0A3Q1LUE0 | Soluble proteins BBT-9 |
| RHO GTPase-activating protein 1 | −4 | ARHGAP1 | F6RWK1 | Soluble proteins BBT-9 |
| RHO-related GTP-binding protein RHOC | −3.57 | RHOC | Q1RMJ6 | Soluble proteins BBT-9 |
| Vascular cell-adhesion molecule | −2.04 | VCAM1 | Q9GKR2 | EV-BBT-9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calle, A.; Toribio, V.; Yáñez-Mó, M.; Ramírez, M.Á. Embryonic Trophectoderm Secretomics Reveals Chemotactic Migration and Intercellular Communication of Endometrial and Circulating MSCs in Embryonic Implantation. Int. J. Mol. Sci. 2021, 22, 5638. https://doi.org/10.3390/ijms22115638
Calle A, Toribio V, Yáñez-Mó M, Ramírez MÁ. Embryonic Trophectoderm Secretomics Reveals Chemotactic Migration and Intercellular Communication of Endometrial and Circulating MSCs in Embryonic Implantation. International Journal of Molecular Sciences. 2021; 22(11):5638. https://doi.org/10.3390/ijms22115638
Chicago/Turabian StyleCalle, Alexandra, Víctor Toribio, María Yáñez-Mó, and Miguel Ángel Ramírez. 2021. "Embryonic Trophectoderm Secretomics Reveals Chemotactic Migration and Intercellular Communication of Endometrial and Circulating MSCs in Embryonic Implantation" International Journal of Molecular Sciences 22, no. 11: 5638. https://doi.org/10.3390/ijms22115638
APA StyleCalle, A., Toribio, V., Yáñez-Mó, M., & Ramírez, M. Á. (2021). Embryonic Trophectoderm Secretomics Reveals Chemotactic Migration and Intercellular Communication of Endometrial and Circulating MSCs in Embryonic Implantation. International Journal of Molecular Sciences, 22(11), 5638. https://doi.org/10.3390/ijms22115638







