Visceral Adipose Tissue Displays Unique Metabolomic Fingerprints in Obesity, Pre-Diabetes and Type 2 Diabetes
Abstract
:1. Introduction
2. Results
2.1. Subjects’ Anthropometric and Clinical Features
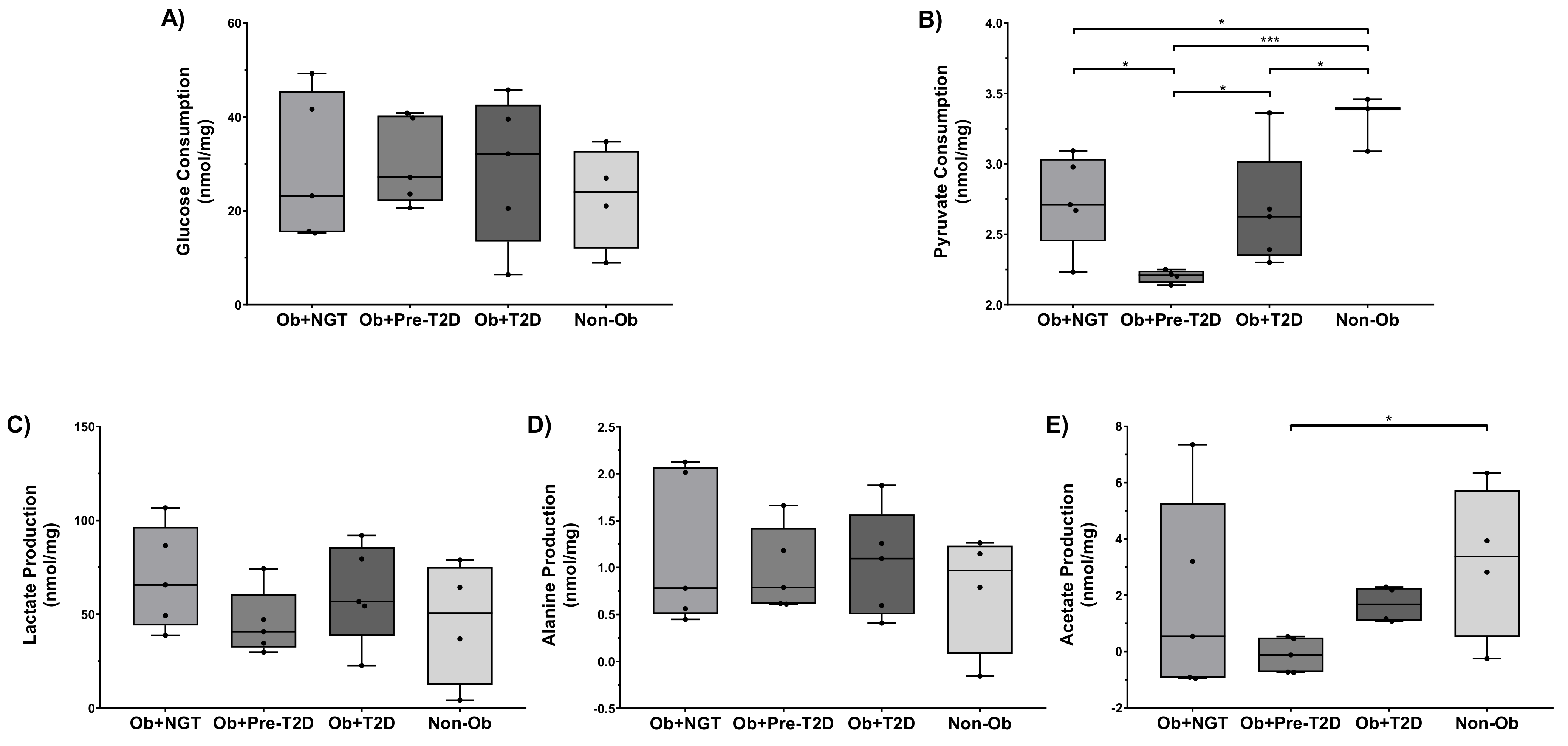
2.2. Metabolite Consumption and Production Rate
2.2.1. VAT Glucose Consumption Was Not Influenced, neither by Obesity nor by Dysglycemia, but Lower VAT Pyruvate Consumption Was Observed in Individuals with Obesity and Pre-Diabetes
2.2.2. VAT Lactate and Alanine Production Were Not Influenced, neither by Obesity nor by Dysglycemia, but Lower VAT Acetate Production Was Observed in Individuals with Obesity and Pre-Diabetes
2.2.3. A Higher Isoleucine Consumption Was Observed in the VAT of Individuals with Obesity and Pre-Diabetes, Whereas Leucine and Valine Consumption Were Not Influenced by Obesity or Dysglycemia
2.2.4. Lower Pyroglutamate Consumption Was Observed in the VAT of Individuals with Obesity, Particularly Those with Pre-Diabetes
2.2.5. Pyruvate Consumption Correlates with Several Metabolite Patterns in All Studied Groups, Whereas Glucose Consumption Only Correlates with Alanine Production and Pyroglutamate Consumption in Subjects with Obesity and Pre-Diabetes
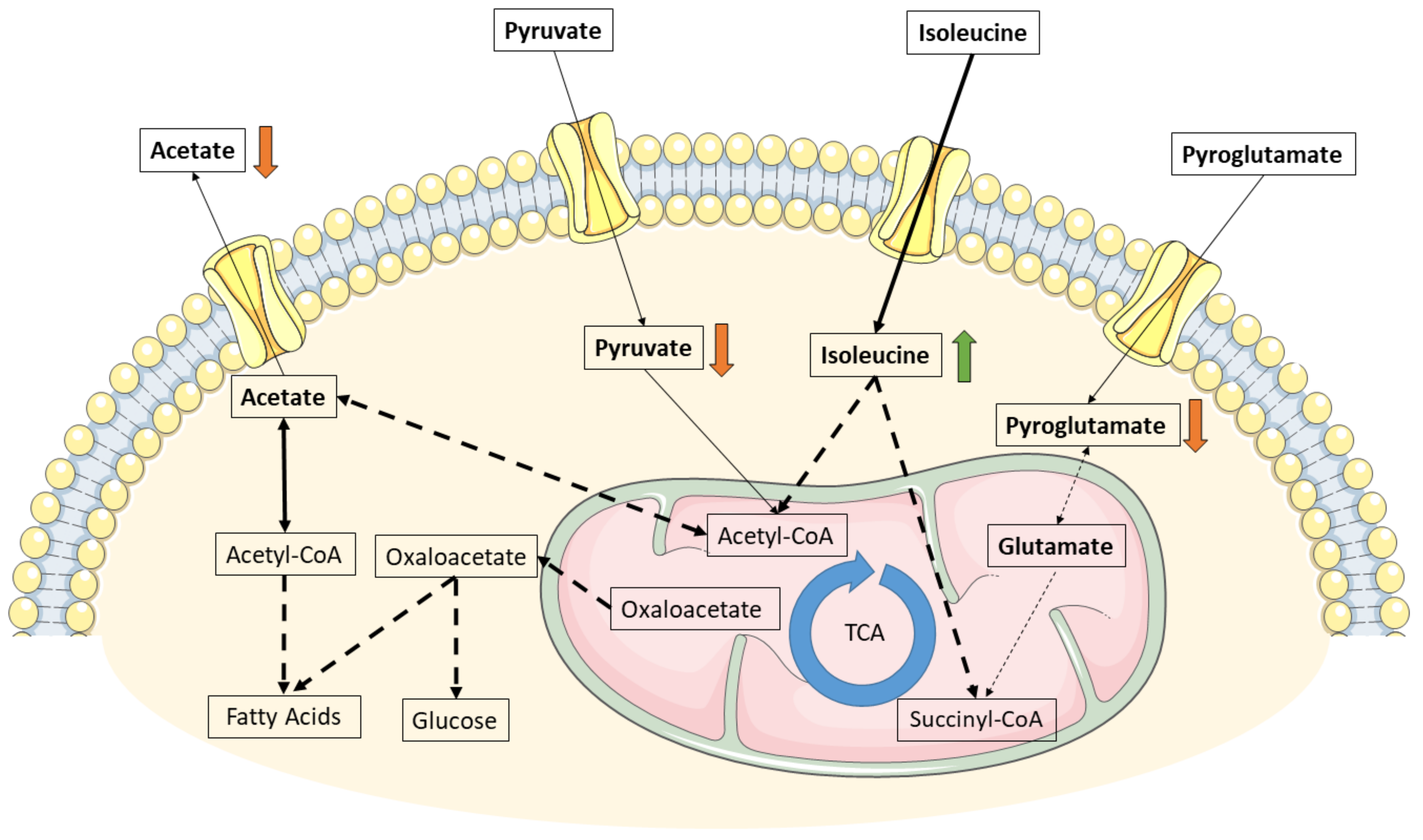
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Adipose Tissue Isolation and Explants Incubation
4.3. Proton Nuclear Magnetic Resonance (1H-NMR)
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdullah, A.; Peeters, A.; de Courten, M.; Stoelwinder, J. The magnitude of association between overweight and obesity and the risk of diabetes: A meta-analysis of prospective cohort studies. Diabetes Res. Clin. Pr. 2010, 89, 309–319. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Kajimura, S. Metabolic adaptation and maladaptation in adipose tissue. Nat. Metab. 2019, 1, 189–200. [Google Scholar] [CrossRef]
- Felber, J.-P.; Golay, A. Pathways from obesity to diabetes. Int. J. Obes. 2002, 26, S39–S45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muoio, D.M.; Newgard, C.B. Mechanisms of disease: Molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat. Rev. Mol. Cell Biol. 2008, 9, 193–205. [Google Scholar] [CrossRef]
- Guo, S.; Dunn, S.L.; White, M.F. The Reciprocal Stability of FOXO1 and IRS2 Creates a Regulatory Circuit that Controls Insulin Signaling. Mol. Endocrinol. 2006, 20, 3389–3399. [Google Scholar] [CrossRef] [Green Version]
- Cheng, S.; Rhee, E.P.; Larson, M.; Lewis, G.D.; McCabe, E.L.; Shen, D.; Palma, M.J.; Roberts, L.; Dejam, A.; Souza, A.L.; et al. Metabolite Profiling Identifies Pathways Associated With Metabolic Risk in Humans. Circulation 2012, 125, 2222–2231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2010, 11, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Lackey, D.E.; Lynch, C.J.; Olson, K.C.; Mostaedi, R.; Ali, M.; Smith, W.H.; Karpe, F.; Humphreys, S.; Bedinger, D.H.; Dunn, T.N.; et al. Regulation of adipose branched-chain amino acid catabolism enzyme expression and cross-adipose amino acid flux in human obesity. Am. J. Physiol. Metab. 2013, 304, E1175–E1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rietman, A.; Stanley, T.L.; Clish, C.; Mootha, V.; Mensink, M.; Grinspoon, S.K.; Makimura, H. Associations between plasma branched-chain amino acids, β-aminoisobutyric acid and body composition. J. Nutr. Sci. 2016, 5, e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basu, R.; Chandramouli, V.; Dicke, B.; Landau, B.; Rizza, R. Obesity and Type 2 Diabetes Impair Insulin-Induced Suppression of Glycogenolysis as Well as Gluconeogenesis. Diabetes 2005, 54, 1942–1948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, S.T.; Hsia, D.S.; Chacko, S.K.; Rodriguez, L.M.; Haymond, M.W. Increased gluconeogenesis in youth with newly diagnosed type 2 diabetes. Diabetologia 2015, 58, 596–603. [Google Scholar] [CrossRef] [Green Version]
- Boulet, M.M.; Chevrier, G.; Grenier-Larouche, T.; Pelletier, M.; Nadeau, M.; Scarpa, J.; Prehn, C.; Marette, A.; Adamski, J.; Tchernof, A. Alterations of plasma metabolite profiles related to adipose tissue distribution and cardiometabolic risk. Am. J. Physiol. Metab. 2015, 309, E736–E746. [Google Scholar] [CrossRef] [Green Version]
- Johnson, C.H.; Ivanisevic, J.; Siuzdak, G. Metabolomics: Beyond biomarkers and towards mechanisms. Nat. Rev. Mol. Cell Biol. 2016, 17, 451–459. [Google Scholar] [CrossRef] [Green Version]
- Stolic, M.; Russell, A.; Hutley, L.; Fielding, G.; Hay, J.; MacDonald, G.; Whitehead, J.; Prins, J. Glucose uptake and insulin action in human adipose tissue—influence of BMI, anatomical depot and body fat distribution. Int. J. Obes. 2002, 26, 17–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westergren, H.; Danielsson, A.; Nystrom, F.H.; Strålfors, P. Glucose transport is equally sensitive to insulin stimulation, but basal and insulin-stimulated transport is higher, in human omental compared with subcutaneous adipocytes. Metabolism 2005, 54, 781–785. [Google Scholar] [CrossRef]
- Song, Z.; Xiaoli, A.M.; Yang, F. Regulation and Metabolic Significance of De Novo Lipogenesis in Adipose Tissues. Nutrients 2018, 10, 1383. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Cooper, D.E.; Cluntun, A.A.; Warmoes, M.O.; Zhao, S.; Reid, M.A.; Liu, J.; Lund, P.J.; Lopes, M.; Garcia, B.A.; et al. Abstract 792: Acetate production from glucose and coupling to mitochondrial metabolism in mammals. Mol. Cell. Biol. 2019, 175, 792. [Google Scholar] [CrossRef]
- Gray, L.R.; Tompkins, S.C.; Taylor, E.B. Regulation of pyruvate metabolism and human disease. Cell. Mol. Life Sci. 2014, 71, 2577–2604. [Google Scholar] [CrossRef] [Green Version]
- Yin, X.; Lanza, I.R.; Swain, J.M.; Sarr, M.G.; Nair, K.S.; Jensen, M.D. Adipocyte Mitochondrial Function Is Reduced in Human Obesity Independent of Fat Cell Size. J. Clin. Endocrinol. Metab. 2014, 99, E209–E216. [Google Scholar] [CrossRef] [Green Version]
- Alves, M.G.; Moreira, Â.; Guimarães, M.; Nora, M.; Sousa, M.; Oliveira, P.V.; Monteiro, M.P. Body mass index is associated with region-dependent metabolic reprogramming of adipose tissue. BBA Clin. 2017, 8, 1–6. [Google Scholar] [CrossRef]
- Hanzu, F.; Vinaixa, M.; Papageorgiou, A.; Parrizas, M.; Correig, X.; Delgado, S.; Carmona, F.; Samino, S.; Vidal, J.; Gomis, R. Obesity rather than regional fat depots marks the metabolomic pattern of adipose tissue: An untargeted metabolomic approach. Obesity 2013, 22, 698–704. [Google Scholar] [CrossRef]
- Doi, M.; Yamaoka, I.; Nakayama, M.; Sugahara, K.; Yoshizawa, F. Hypoglycemic effect of isoleucine involves increased muscle glucose uptake and whole body glucose oxidation and decreased hepatic gluconeogenesis. Am. J. Physiol. Metab. 2007, 292, E1683–E1693. [Google Scholar] [CrossRef]
- Courcoulas, A.P.; King, W.C.; Belle, S.H.; Berk, P.; Flum, D.R.; Garcia, L.; Gourash, W.; Horlick, M.; Mitchell, J.E.; Pomp, A.; et al. Seven-Year Weight Trajectories and Health Outcomes in the Longitudinal Assessment of Bariatric Surgery (LABS) Study. JAMA Surg. 2018, 153, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Gar, C.; Rottenkolber, M.; Prehn, C.; Adamski, J.; Seissler, J.; Lechner, A. Serum and plasma amino acids as markers of prediabetes, insulin resistance, and incident diabetes. Crit. Rev. Clin. Lab. Sci. 2017, 55, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Tseng, Y.; White, M.F. Insulin signaling meets mitochondria in metabolism. Trends Endocrinol. Metab. 2010, 21, 589–598. [Google Scholar] [CrossRef] [Green Version]
- Lynch, C.J.; Adams, S.H. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 2014, 10, 723–736. [Google Scholar] [CrossRef] [Green Version]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef] [Green Version]
- Yoo, H.C.; Yu, Y.C.; Sung, Y.; Han, J.M. Glutamine reliance in cell metabolism. Exp. Mol. Med. 2020, 52, 1496–1516. [Google Scholar] [CrossRef]
- Okekunle, A.P.; Li, Y.; Liu, L.; Du, S.; Wu, X.; Chen, Y.; Li, Y.; Qi, J.; Sun, C.; Feng, R. Abnormal circulating amino acid profiles in multiple metabolic disorders. Diabetes Res. Clin. Pract. 2017, 132, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, O.; Jacques-Silva, M.C.; Speier, S.; Yang, S.-N.; Köhler, M.; Fachado, A.; Vieira, E.; Zierath, J.R.; Kibbey, R.; Berman, D.M.; et al. Glutamate Is a Positive Autocrine Signal for Glucagon Release. Cell Metab. 2008, 7, 545–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nurjhan, N.; Bucci, A.; Perriello, G.; Stumvoll, M.; Dailey, G.; Bier, D.M.; Toft, I.; Jenssen, T.G.; Gerich, J.E. Glutamine: A major gluconeogenic precursor and vehicle for interorgan carbon transport in man. J. Clin. Investig. 1995, 95, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Snell, K. Alanine as a gluconeogenic carrier. Trends Biochem. Sci. 1979, 4, 124–128. [Google Scholar] [CrossRef]
- Lee, C.C.; Watkins, S.M.; Lorenzo, C.; Wagenknecht, L.E.; Il’Yasova, D.; Chen, Y.-D.I.; Haffner, S.M.; Hanley, A.J. Branched-Chain Amino Acids and Insulin Metabolism: The Insulin Resistance Atherosclerosis Study (IRAS). Diabetes Care 2016, 39, 582–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guasch-Ferré, M.; Hruby, A.; Toledo, E.; Clish, C.; Martínez-González, M.A.; Salas-Salvadó, J.; Hu, F.B. Metabolomics in Prediabetes and Diabetes: A Systematic Review and Meta-analysis. Diabetes Care 2016, 39, 833–846. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Holmes, M.V.; Smith, G.D.; Ala-Korpela, M. Genetic Support for a Causal Role of Insulin Resistance on Circulating Branched-Chain Amino Acids and Inflammation. Diabetes Care 2017, 40, 1779–1786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buse, J.B.; Wexler, D.J.; Tsapas, A.; Rossing, P.; Mingrone, G.; Mathieu, C.; D’Alessio, D.A.; Davies, M.J. 2019 update to: Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2020, 63, 221–228. [Google Scholar] [CrossRef] [Green Version]
- Hansen, M.; Lund, M.T.; Gregers, E.; Kraunsøe, R.; van Hall, G.; Helge, J.W.; Dela, F. Adipose tissue mitochondrial respiration and lipolysis before and after a weight loss by diet and RYGB. Obesity 2015, 23, 2022–2029. [Google Scholar] [CrossRef]
- Bertholdt, L.; Gudiksen, A.; Stankiewicz, T.; Villesen, I.; Tybirk, J.; van Hall, G.; Bangsbo, J.; Plomgaard, P.; Pilegaard, H. Impact of training state on fasting-induced regulation of adipose tissue metabolism in humans. J. Appl. Physiol. 2018, 124, 729–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madiraju, A.K.; Erion, D.M.; Rahimi, Y.; Zhang, X.-M.; Braddock, D.T.; Albright, R.A.; Prigaro, B.J.; Wood, J.L.; Bhanot, S.; Macdonald, M.J.; et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nat. Cell Biol. 2014, 510, 542–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tokubuchi, I.; Tajiri, Y.; Iwata, S.; Hara, K.; Wada, N.; Hashinaga, T.; Nakayama, H.; Mifune, H.; Yamada, K. Beneficial effects of metformin on energy metabolism and visceral fat volume through a possible mechanism of fatty acid oxidation in human subjects and rats. PLoS ONE 2017, 12, e0171293. [Google Scholar] [CrossRef]
- Breining, P.; Jensen, J.B.; Sundelin, E.I.; Gormsen, L.C.; Jakobsen, S.; Busk, M.; Rolighed, L.; Bross, P.; Fernandez-Guerra, P.; Markussen, L.K.; et al. Metformin targets brown adipose tissue in vivo and reduces oxygen consumption in vitro. Diabetes Obes. Metab. 2018, 20, 2264–2273. [Google Scholar] [CrossRef] [PubMed]
- Howell, J.; Hellberg, K.; Turner, M.; Talbott, G.; Kolar, M.; Ross, D.S.; Hoxhaj, G.; Saghatelian, A.; Shaw, R.J.; Manning, B.D. Metformin Inhibits Hepatic mTORC1 Signaling via Dose-Dependent Mechanisms Involving AMPK and the TSC Complex. Cell Metab. 2017, 25, 463–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adam, J.; Brandmaier, S.; Leonhardt, J.; Scheerer, M.F.; Mohney, R.P.; Xu, T.; Bi, J.; Rotter, M.; Troll, M.; Chi, S.; et al. Metformin Effect on Nontargeted Metabolite Profiles in Patients with Type 2 Diabetes and in Multiple Murine Tissues. Diabetes 2016, 65, 3776–3785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Böhm, A.; Halama, A.; Meile, T.; Zdichavsky, M.; Lehmann, R.; Weigert, C.; Fritsche, A.; Stefan, N.; Königsrainer, A.; Häring, H.-U.; et al. Metabolic Signatures of Cultured Human Adipocytes from Metabolically Healthy versus Unhealthy Obese Individuals. PLoS ONE 2014, 9, e93148. [Google Scholar] [CrossRef]
- Kučera, J.; Spáčil, Z.; Friedecký, D.; Novák, J.; Pekař, M.; Bienertová-Vašků, J. Human White Adipose Tissue Metabolome: Current Perspective. Obesity 2018, 26, 1870–1878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinonen, S.; Buzkova, J.; Muniandy, M.; Kaksonen, R.; Ollikainen, M.; Ismail, K.; Hakkarainen, A.; Lundbom, J.; Lundbom, N.; Vuolteenaho, K.; et al. Impaired Mitochondrial Biogenesis in Adipose Tissue in Acquired Obesity. Diabetes 2015, 64, 3135–3145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chattopadhyay, M.; Guhathakurta, I.; Behera, P.; Ranjan, K.R.; Khanna, M.; Mukhopadhyay, S.; Chakrabarti, S. Mitochondrial bioenergetics is not impaired in nonobese subjects with type 2 diabetes mellitus. Metabolism 2011, 60, 1702–1710. [Google Scholar] [CrossRef]
- Bódis, K.; Jelenik, T.; Lundbom, J.; Markgraf, D.F.; Strom, A.; Zaharia, O.-P.; Karusheva, Y.; Burkart, V.; Müssig, K.; Kupriyanova, Y.; et al. Expansion and Impaired Mitochondrial Efficiency of Deep Subcutaneous Adipose Tissue in Recent-Onset Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2020, 105, e1331–e1343. [Google Scholar] [CrossRef] [Green Version]

| Ob+NGT (n = 5) | Ob+Pre-T2D (n = 5) | Ob+T2D (n = 5) | Non-Ob (n = 4) | |
|---|---|---|---|---|
| Age (years) | 44 ± 7 | 50 ± 3 | 56 ± 2 | 48 ± 7 |
| Sex (F:M) | 4:1 | 4:1 | 4:1 | 2:2 |
| BMI (kg/m2) | 41.4 ± 2.6 *** | 44.0 ± 2.8 *** | 41.5 ± 2.5 *** | 26.1 ± 1.0 |
| Fasting glucose (mg/dL) | 93.8 ± 0.8 | 109.2 ± 8.0 | 161.4 ± 26.8 *,† | 88.8 ± 3.2 |
| HbA1c (%) | 5.4 ± 0.2 | 6.2 ± 0.2 | 7.6 ± 1.3 † | Not available |
| Metformin (%) | 0% | 0% | 100% | 0% |
| SBP (mmHg) | 137 ± 7 | 150 ± 6 | 146 ± 4 | 133 ± 7 |
| DBP (mmHg) | 79 ± 4 | 85 ± 3 | 73 ± 4 | 80 ± 4 |
| Total cholesterol (mg/dL) | 161 ± 7 | 220 ± 15 | 206 ± 24 | 177 ± 25 |
| HDL (mg/dL) | 52 ± 5 | 47 ± 7 | 48 ± 5 | 35 ± 11 |
| LDL (mg/dL) | 96.4 ± 10.3 | 142.4 ± 7.2 | 135.4 ± 23.2 | 100.9 ± 6.7 |
| Triglycerides (mg/dL) | 102 ± 8 | 111 ± 22 | 203 ± 49 | 206 ± 147 |
| Non-Ob (n = 4) | |||||||
|---|---|---|---|---|---|---|---|
| Lactate (P) | Acetate (P) | Alanine (P) | Isoleucine (C) | Leucine (C) | Valine (C) | Pyroglutamate (C) | |
| Glucose (C) | 0.839 | 0.457 | 0.788 | 0.742 | 0.660 | 0.681 | 0.511 |
| Pyruvate (C) | 0.869 | 0.367 | 0.998 * | −0.887 | −0.991 | −0.794 | −0.023 |
| Ob+NGT (n = 5) | |||||||
| Glucose (C) | 0.844 | 0.330 | 0.424 | 0.264 | −0.061 | 0.316 | 0.553 |
| Pyruvate (C) | 0.617 | 0.987 * | 0.958 * | −0.751 | −0.729 | −0.742 | 0.916 * |
| Ob+Pre−T2D (n = 5) | |||||||
| Glucose (C) | 0.860 | −0.909 | 0.988 ** | 0.660 | 0.868 | 0.394 | 0.911 * |
| Pyruvate (C) | 0.933 * | −0.469 | 0.734 | 0.064 | 0.825 | −0.354 | 0.904 * |
| Ob+T2D (n = 5) | |||||||
| Glucose (C) | −0.009 | −0.805 | 0.638 | 0.177 | 0.573 | 0.357 | 0.152 |
| Pyruvate (C) | 0.981 ** | 0.470 | 0.281 | −0.990 ** | −0.527 | −0.936 * | 0.761 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morais, T.; Seabra, A.L.; Patrício, B.G.; Guimarães, M.; Nora, M.; Oliveira, P.F.; Alves, M.G.; Monteiro, M.P. Visceral Adipose Tissue Displays Unique Metabolomic Fingerprints in Obesity, Pre-Diabetes and Type 2 Diabetes. Int. J. Mol. Sci. 2021, 22, 5695. https://doi.org/10.3390/ijms22115695
Morais T, Seabra AL, Patrício BG, Guimarães M, Nora M, Oliveira PF, Alves MG, Monteiro MP. Visceral Adipose Tissue Displays Unique Metabolomic Fingerprints in Obesity, Pre-Diabetes and Type 2 Diabetes. International Journal of Molecular Sciences. 2021; 22(11):5695. https://doi.org/10.3390/ijms22115695
Chicago/Turabian StyleMorais, Tiago, Alexandre L. Seabra, Bárbara G. Patrício, Marta Guimarães, Mário Nora, Pedro F. Oliveira, Marco G. Alves, and Mariana P. Monteiro. 2021. "Visceral Adipose Tissue Displays Unique Metabolomic Fingerprints in Obesity, Pre-Diabetes and Type 2 Diabetes" International Journal of Molecular Sciences 22, no. 11: 5695. https://doi.org/10.3390/ijms22115695








