Novel Methods to Mobilize, Isolate, and Expand Mesenchymal Stem Cells
Abstract
:1. Introduction
2. Results
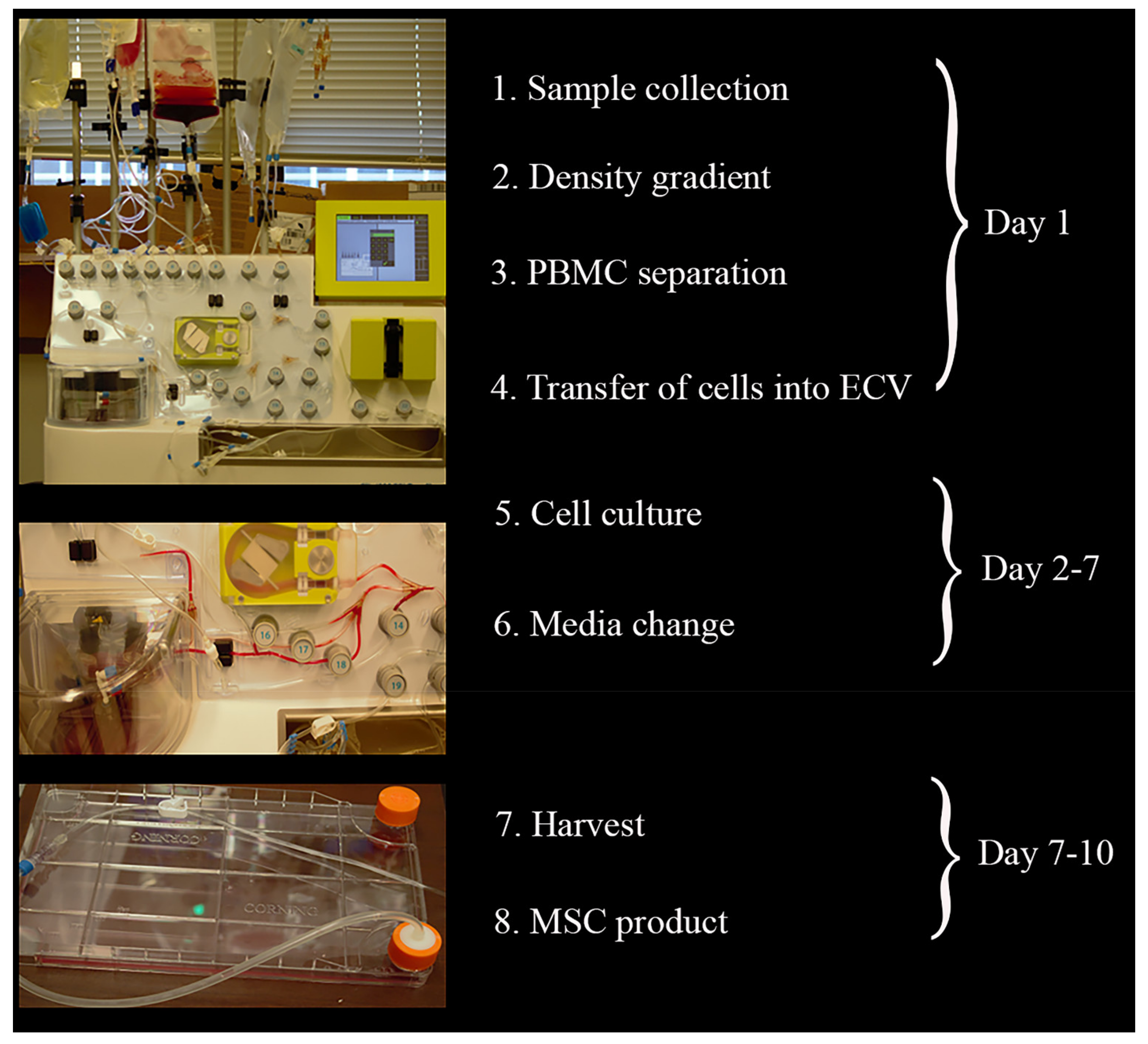
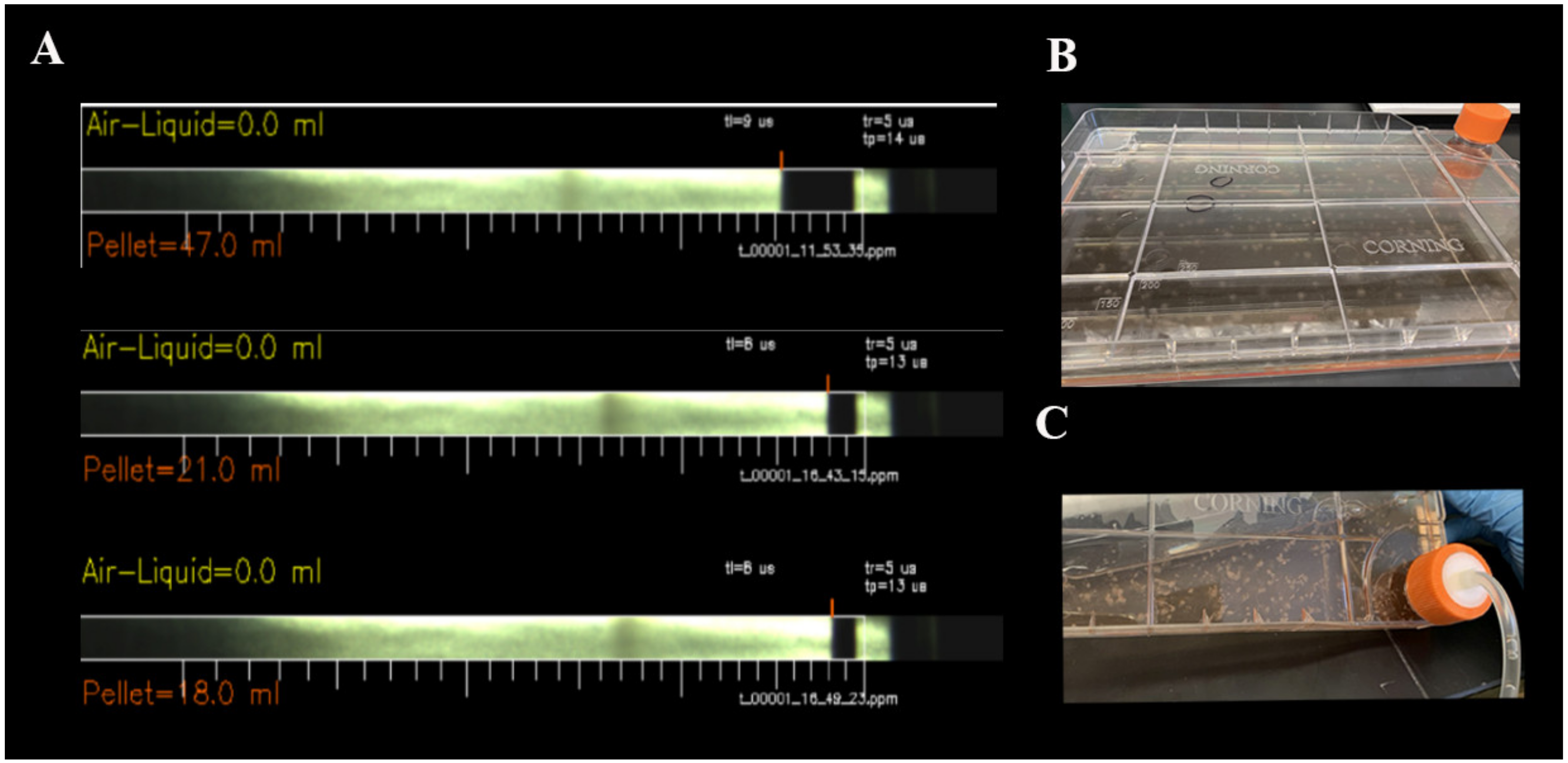
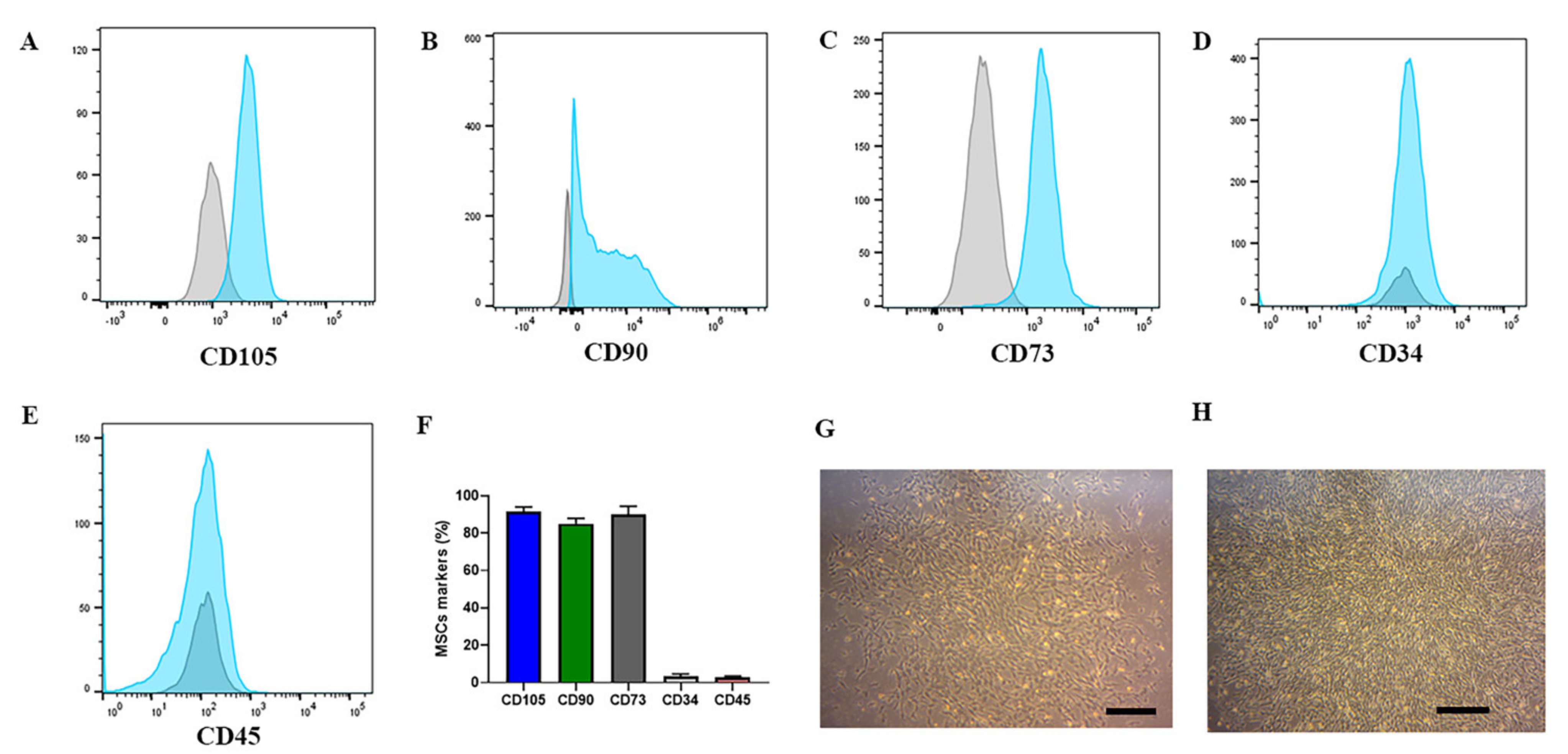
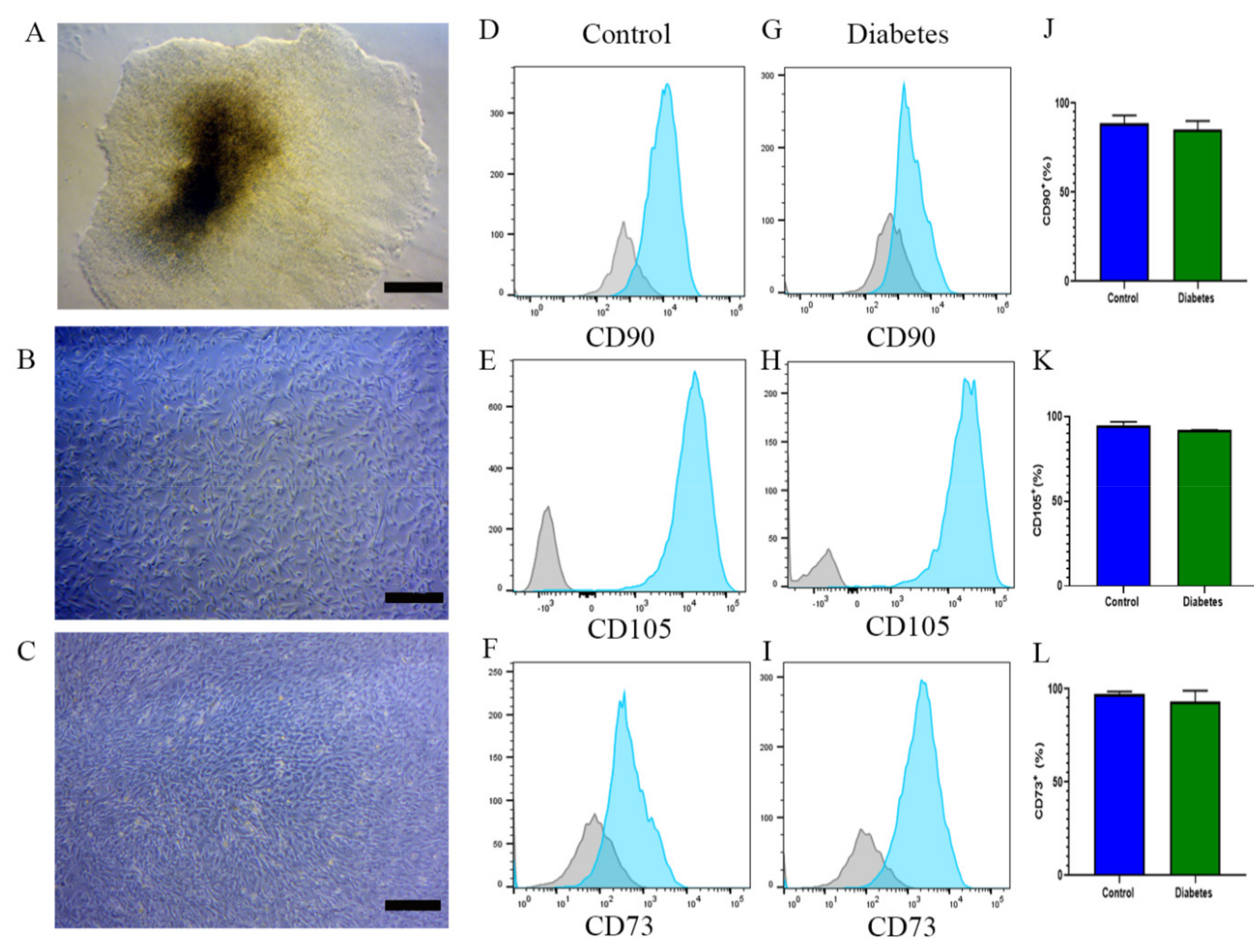
2.1. CliniMACS Prodigy® Isolates and Cultures Equine MSCs
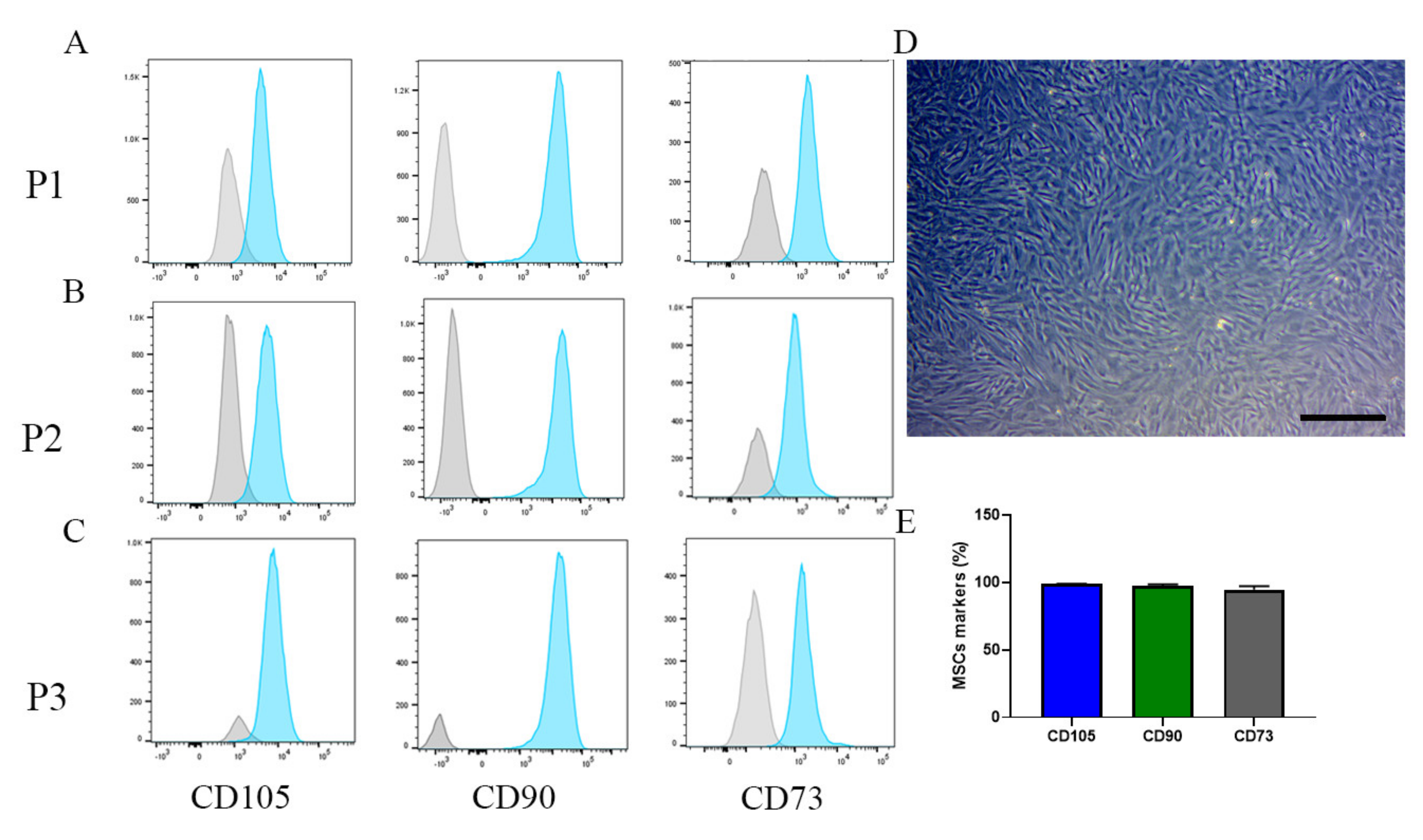
2.2. MSC Phenotype Remains the Same after Changing to a Manual System
2.3. EBs from iPSCs of Diabetic and Nondiabetic Subjects Can Differentiate into MSCs
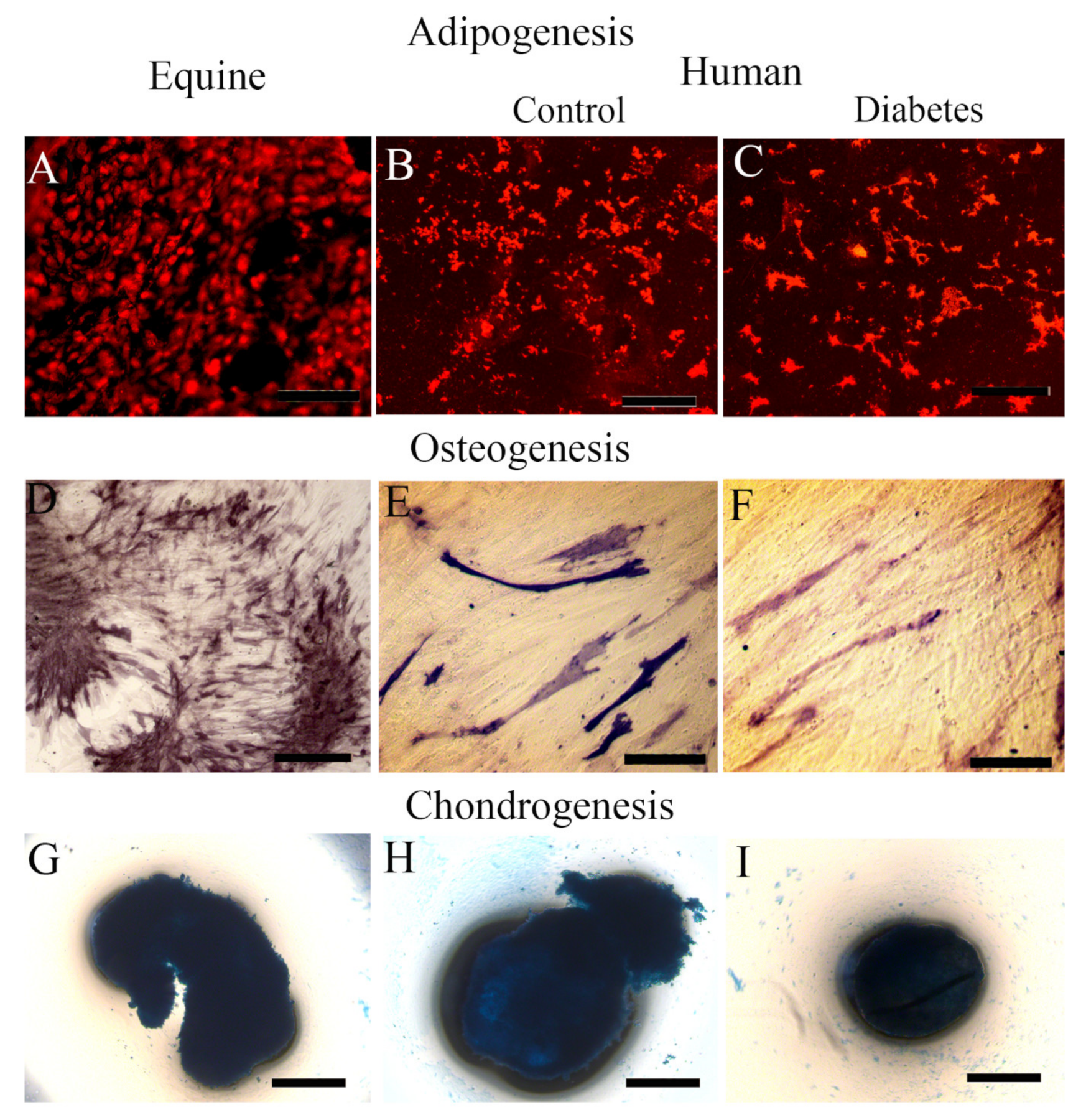
2.4. Equine MSCs and iPSC-Derived MSC Showed Their Lineage Capacity of Differentiation
3. Discussion
4. Material and Methods
4.1. Experimental Protocols
4.2. Electro-Acupuncture Procedure
4.3. Adherent Cell Culture Protocol Using CliniMACS Prodigy®
4.4. Cell Culture
4.5. hiPSC Generation and Culture
4.6. Phenotypic Characterization of Equine and Human MSCs
4.7. Adipogenic, Osteogenic, and Chondrogenic Differentiation of Equine and Human iPSC-Derived MSCs
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fitzsimmons, R.E.B.; Mazurek, M.S.; Soos, A.; Simmons, C.A. Mesenchymal Stromal/Stem Cells in Regenerative Medicine and Tissue Engineering. Stem Cells Int. 2018, 2018, 8031718. [Google Scholar] [CrossRef]
- Zhou, Y.; Yamamoto, Y.; Xiao, Z.; Ochiya, T. The Immunomodulatory Functions of Mesenchymal Stromal/Stem Cells Mediated via Paracrine Activity. J. Clin. Med. 2019, 8, 1025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreira, A.; Kahlenberg, S.; Hornsby, P. Therapeutic potential of mesenchymal stem cells for diabetes. J. Mol. Endocrinol. 2017, 59, R109–R120. [Google Scholar] [CrossRef]
- Wang, M.; Song, L.; Strange, C.; Dong, X.; Wang, H. Therapeutic Effects of Adipose Stem Cells from Diabetic Mice for the Treatment of Type 2 Diabetes. Mol. Ther. 2018, 26, 1921–1930. [Google Scholar] [CrossRef] [Green Version]
- Päth, G.; Perakakis, N.; Mantzoros, C.S.; Seufert, J. Stem cells in the treatment of diabetes mellitus—Focus on mesenchymal stem cells. Metabolism 2019, 90, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Casiraghi, F.; Remuzzi, G. Mesenchymal stromal cells in kidney transplantation. Curr. Opin. Nephrol. Hypertens. 2019, 28, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Staff, N.P.; Jones, D.T.; Singer, W. Mesenchymal Stromal Cell Therapies for Neurodegenerative Diseases. Mayo Clin. Proc. 2019, 94, 892–905. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Ma, J.; Han, J.; Zhang, W.; Ma, J. Mesenchymal stem cell related therapies for cartilage lesions and osteoarthritis. Am. J. Transl. Res. 2019, 11, 6275–6289. [Google Scholar] [PubMed]
- Parekkadan, B.; Milwid, J. Mesenchymal Stem Cells as Therapeutics. Annu. Rev. Biomed. Eng. 2010, 12, 87–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Huang, B. Peripheral Blood Stem Cells: Phenotypic Diversity and Potential Clinical Applications. Stem Cell Rev. Rep. 2012, 8, 917–925. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Wan, C.; Li, G. Concise Review: Multipotent Mesenchymal Stromal Cells in Blood. Stem Cells 2006, 25, 69–77. [Google Scholar] [CrossRef]
- Roufosse, C.; Direkze, N.; Otto, W.; Wright, N. Circulating mesenchymal stem cells. Int. J. Biochem. Cell Biol. 2004, 36, 585–597. [Google Scholar] [CrossRef]
- Xu, L.; Li, G. Circulating mesenchymal stem cells and their clinical implications. J. Orthop. Transl. 2014, 2, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Longhini, A.L.F.; Salazar, T.E.; Vieira, C.; Trinh, T.; Duan, Y.; Pay, L.M.; Calzi, S.L.; Losh, M.; Johnston, N.A.; Xie, H.; et al. Peripheral blood-derived mesenchymal stem cells demonstrate immunomodulatory potential for therapeutic use in horses. PLoS ONE 2019, 14, e0212642. [Google Scholar] [CrossRef] [PubMed]
- Salazar, T.E.; Richardson, M.R.; Beli, E.; Ripsch, M.S.; George, J.; Kim, Y.; Duan, Y.; Moldovan, L.; Yan, Y.; Bhatwadekar, A.; et al. Electroacupuncture Promotes Central Nervous System-Dependent Release of Mesenchymal Stem Cells. Stem Cells 2017, 35, 1303–1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chait, A.; Hartigh, L.J.D. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grundy, S.M. Metabolic Syndrome Pandemic. Arter. Thromb. Vasc. Biol. 2008, 28, 629–636. [Google Scholar] [CrossRef] [Green Version]
- Wu, K.K. Control of Mesenchymal Stromal Cell Senescence by Tryptophan Metabolites. Int. J. Mol. Sci. 2021, 22, 697. [Google Scholar] [CrossRef]
- Carcamo-Orive, I.; Hoffman, G.E.; Cundiff, P.; Beckmann, N.D.; D’Souza, S.L.; Knowles, J.W.; Patel, A.; Papatsenko, D.; Abbasi, F.; Reaven, G.M.; et al. Analysis of Transcriptional Variability in a Large Human iPSC Library Reveals Genetic and Non-genetic Determinants of Heterogeneity. Cell Stem Cell 2017, 20, 518–532.e9. [Google Scholar] [CrossRef] [Green Version]
- Carcamo-Orive, I.; Huang, N.F.; Quertermous, T.; Knowles, J.W. Induced Pluripotent Stem Cell-Derived Endothelial Cells in Insulin Resistance and Metabolic Syndrome. Arter. Thromb. Vasc. Biol. 2017, 37, 2038–2042. [Google Scholar] [CrossRef] [PubMed]
- Kudva, Y.C.; Ohmine, S.; Greder, L.V.; Dutton, J.R.; Armstrong, A.; De Lamo, J.G.; Khan, Y.K.; Thatava, T.; Hasegawa, M.; Fusaki, N.; et al. Transgene-Free Disease-Specific Induced Pluripotent Stem Cells from Patients with Type 1 and Type 2 Diabetes. Stem Cells Transl. Med. 2012, 1, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Karagiannis, P.; Takahashi, K.; Saito, M.; Yoshida, Y.; Okita, K.; Watanabe, A.; Inoue, H.; Yamashita, J.K.; Todani, M.; Nakagawa, M.; et al. Induced Pluripotent Stem Cells and Their Use in Human Models of Disease and Development. Physiol. Rev. 2019, 99, 79–114. [Google Scholar] [CrossRef] [PubMed]
- Nair, G.G.; Tzanakakis, E.S.; Hebrok, M. Emerging routes to the generation of functional β-cells for diabetes mellitus cell therapy. Nat. Rev. Endocrinol. 2020, 16, 506–518. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, S.D.; Surampudi, V.; Rao, R.R. Analysis of Embryoid Bodies Derived from Human Induced Pluripotent Stem Cells as a Means to Assess Pluripotency. Stem Cells Int. 2012, 2012, 738910. [Google Scholar] [CrossRef] [Green Version]
- Guo, N.-N.; Liu, L.-P.; Zheng, Y.-W.; Li, Y.-M. Inducing human induced pluripotent stem cell differentiation through embryoid bodies: A practical and stable approach. World J. Stem Cells 2020, 12, 25–34. [Google Scholar] [CrossRef]
- Guo, N.-N.; Liu, L.-P.; Zhang, Y.-X.; Cai, Y.-T.; Guo, Y.; Zheng, Y.-W.; Li, Y.-M. Early prediction of the differentiation potential during the formation of human iPSC-derived embryoid bodies. Biochem. Biophys. Res. Commun. 2019, 516, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Ryujin, T.; Uno, M.; Wakayama, I. The Effect of Electroacupuncture with Different Frequencies on Muscle Oxygenation in Humans. Evid. Based Complement. Altern. Med. 2015, 2015, 620785. [Google Scholar] [CrossRef]
- Driscoll, J.; Patel, T. The mesenchymal stem cell secretome as an acellular regenerative therapy for liver disease. J. Gastroenterol. 2019, 54, 763–773. [Google Scholar] [CrossRef] [Green Version]
- Fernández, L.; Fernández, A.; Mirones, I.; Escudero, A.; Cardoso, L.; Vela, M.; Lanzarot, D.; De Paz, R.; Leivas, A.; Gallardo, M.; et al. GMP-Compliant Manufacturing of NKG2D CAR Memory T Cells Using CliniMACS Prodigy. Front. Immunol. 2019, 10, 2361. [Google Scholar] [CrossRef] [Green Version]
- Aleksandrova, K.; Leise, J.; Priesner, C.; Melk, A.; Kubaink, F.; Abken, H.; Hombach, A.; Aktas, M.; Essl, M.; Bürger, I.; et al. Functionality and Cell Senescence of CD4/CD8-Selected CD20 CAR T Cells Manufactured Using the Automated CliniMACS Prodigy® Platform. Transfus. Med. Hemother. 2019, 46, 47–54. [Google Scholar] [CrossRef]
- Zhu, F.; Shah, N.; Xu, H.; Schneider, D.; Orentas, R.; Dropulic, B.; Hari, P.; Keever-Taylor, C.A. Closed-system manufacturing of CD19 and dual-targeted CD20/19 chimeric antigen receptor T cells using the CliniMACS Prodigy device at an academic medical center. Cytotherapy 2018, 20, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, M.; Uslu, U.; Wiesinger, M.; Brüning, M.; Altmann, T.; Strasser, E.; Schuler, G.; Schuler-Thurner, B. Automated closed-system manufacturing of human monocyte-derived dendritic cells for cancer immunotherapy. J. Immunol. Methods 2018, 463, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Hynes, K.; Menicanin, D.; Gronthos, S.; Bartold, M.P. Differentiation of iPSC to Mesenchymal Stem-Like Cells and Their Characterization. Adv. Struct. Saf. Stud. 2014, 1357, 353–374. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Luo, Y.; Chen, M.; Wang, G.; Ding, M.; Petersen, C.C.; Kang, R.; Dagnaes-Hansen, F.; Zeng, Y.; Lv, N.; et al. A simple method for deriving functional MSCs and applied for osteogenesis in 3D scaffolds. Sci. Rep. 2013, 3, 2243. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Ma, J.; Li, S.; Liu, W. Applicability of adipose-derived mesenchymal stem cells in treatment of patients with type 2 diabetes. Stem Cell Res. Ther. 2019, 10, 274. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Cohrs, C.M.; Stertmann, J.; Bozsak, R.; Speier, S. Human beta cell mass and function in diabetes: Recent advances in knowledge and technologies to understand disease pathogenesis. Mol. Metab. 2017, 6, 943–957. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, X.; Wang, H.; Liu, X.; Zhang, T.; Wang, Y.; Hu, D. The challenges and promises of allogeneic mesenchymal stem cells for use as a cell-based therapy. Stem Cell Res. Ther. 2015, 6, 234. [Google Scholar] [CrossRef] [Green Version]
- Zang, L.; Hao, H.; Liu, J.; Li, Y.; Han, W.; Mu, Y. Mesenchymal stem cell therapy in type 2 diabetes mellitus. Diabetol. Metab. Syndr. 2017, 9, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakayama, C.; Fujita, Y.; Matsumura, W.; Ujiie, I.; Takashima, S.; Shinkuma, S.; Nomura, T.; Abe, R.; Shimizu, H. The development of induced pluripotent stem cell-derived mesenchymal stem/stromal cells from normal human and RDEB epidermal keratinocytes. J. Dermatol. Sci. 2018, 91, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Gorecka, J.; Kostiuk, V.; Fereydooni, A.; Gonzalez, L.; Luo, J.; Dash, B.; Isaji, T.; Ono, S.; Liu, S.; Lee, S.R.; et al. The potential and limitations of induced pluripotent stem cells to achieve wound healing. Stem Cell Res. Ther. 2019, 10, 87. [Google Scholar] [CrossRef] [Green Version]
| Supply | Quantity | Catalog No. | Company |
|---|---|---|---|
| TS 730 | 1 unit | 170-076-603 | Miltenyi Biotec |
| Transfer bag | 3 units | T3108 | Chartermedical |
| Cell Stack | 1 unit | 3268 | Corning |
| 1 m tubing extension | 1 unit | 170-076-606 | Miltenyi Biotec |
| CliniMACS buffer | 3 L | 130-070-525 | Miltenyi Biotec |
| Ficoll-Paque | 145 mL | 17-1440-03 | GE Healthcare Bio Sciences |
| FBS heat inactivated | 300 ml | 10-082-147 | Gibco |
| Tryple Express | 100 mL | 1260013 | Gibco |
| EBM | 750 mL | CC3156 | Lonza Walkersville |
| MEM | 750 mL | 10009CV | Corning |
| Penicillin–streptomycin–glutamine | 20 mL | 10378016 | Gibco |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira, C.P.; McCarrel, T.M.; Grant, M.B. Novel Methods to Mobilize, Isolate, and Expand Mesenchymal Stem Cells. Int. J. Mol. Sci. 2021, 22, 5728. https://doi.org/10.3390/ijms22115728
Vieira CP, McCarrel TM, Grant MB. Novel Methods to Mobilize, Isolate, and Expand Mesenchymal Stem Cells. International Journal of Molecular Sciences. 2021; 22(11):5728. https://doi.org/10.3390/ijms22115728
Chicago/Turabian StyleVieira, Cristiano P., Taralyn M. McCarrel, and Maria B. Grant. 2021. "Novel Methods to Mobilize, Isolate, and Expand Mesenchymal Stem Cells" International Journal of Molecular Sciences 22, no. 11: 5728. https://doi.org/10.3390/ijms22115728






