Synthesis and Evaluation of AlgNa-g-Poly(QCL-co-HEMA) Hydrogels as Platform for Chondrocyte Proliferation and Controlled Release of Betamethasone
Abstract
1. Introduction
2. Results and Discussion
2.1. AlgNa-g-Poly(QCL-co-HEMA) Hydrogels
2.2. Hydrogel Characterizations
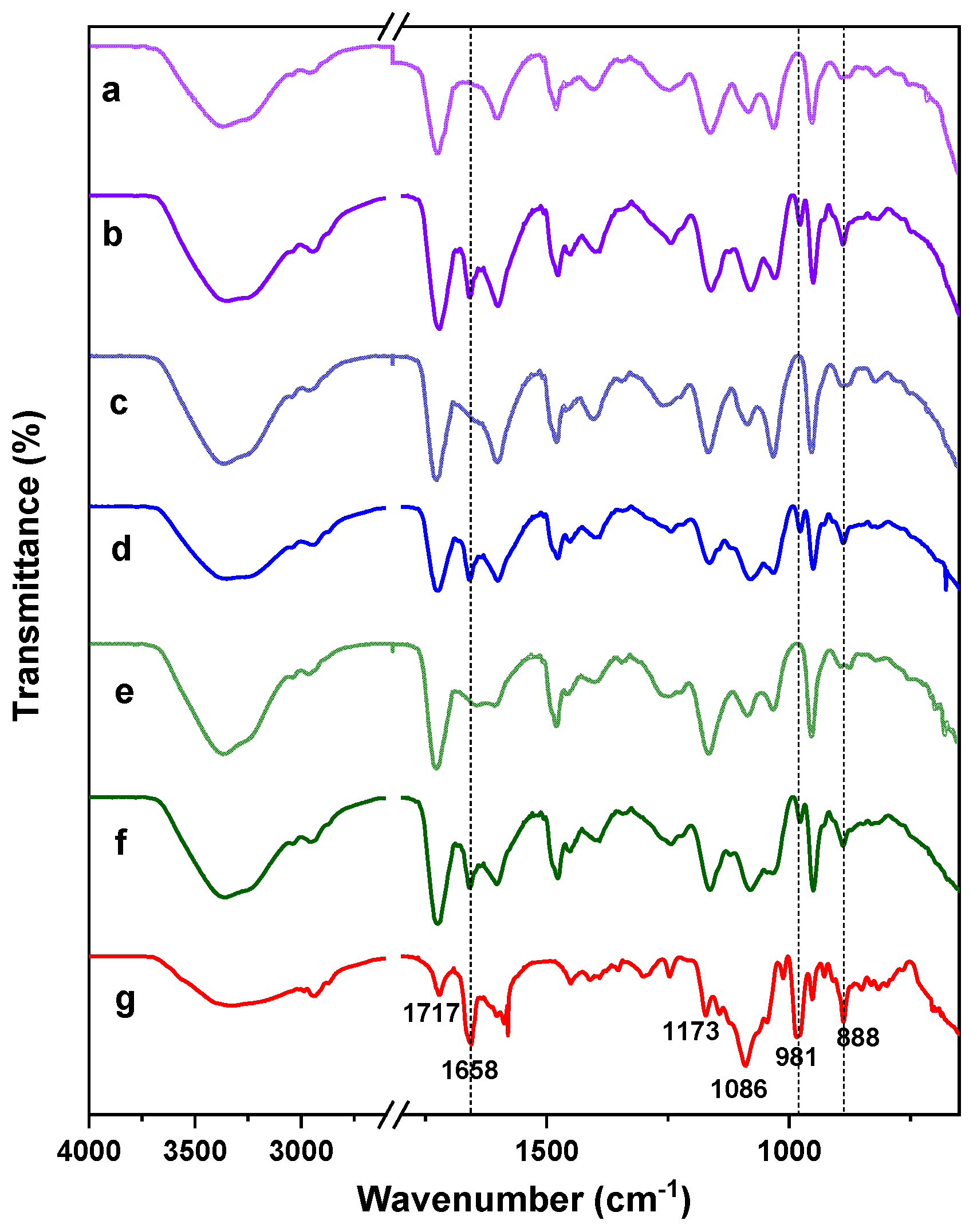
2.2.1. FTIR Spectroscopy Analysis
2.2.2. Morphology Characterization
2.2.3. Swelling Studies
2.2.4. Kinetic Swelling Study
2.3. Betamethasone Release Study
2.3.1. FTIR Characterization of BTM Loaded Hydrogels
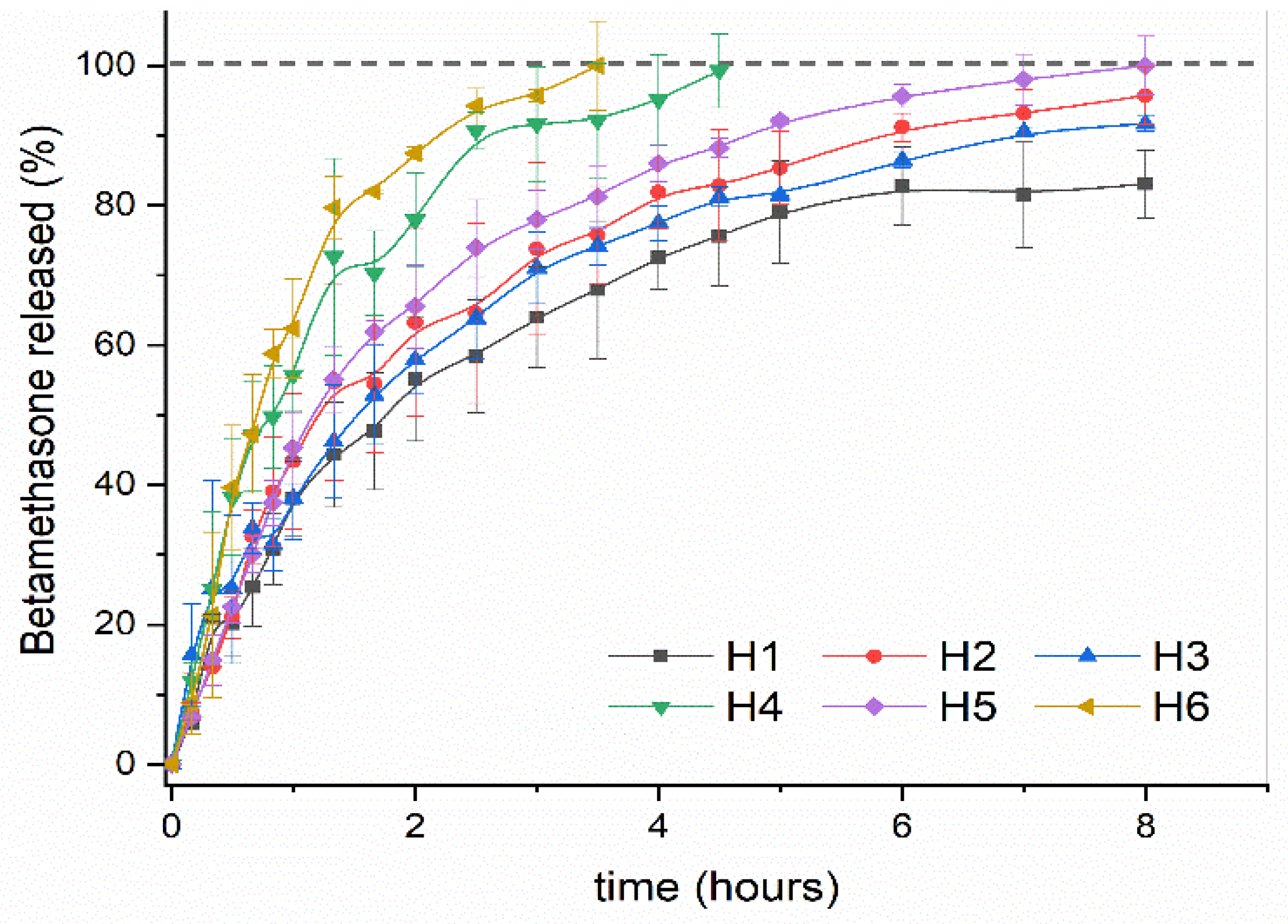
2.3.2. Drug Release Study
2.3.3. Kinetic Drug Release Study
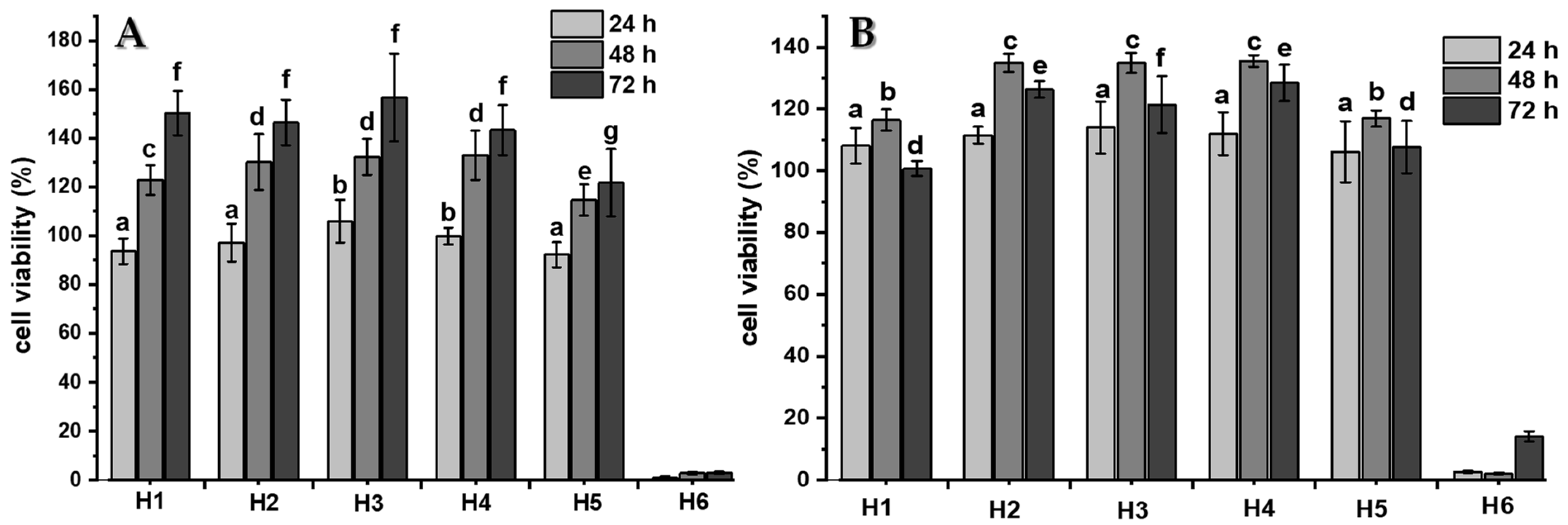
2.4. Cytocompatibility Studies
3. Materials and Methods
3.1. Materials
3.2. Preparation of AlgNa-g-Poly(QCL-co-HEMA) Hydrogels
3.3. Hydrogel Characterizations
3.3.1. Instrumental Analysis
3.3.2. Swelling Studies
3.4. Betamethasone In Vitro Release Study
3.5. Cell Culture and Cytotoxicity Assay
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kou, L.; Xiao, S.; Sun, R.; Bao, S.; Yao, Q.; Chen, R. Biomaterial-engineered intra-articular drug delivery systems for osteoarthritis therapy. Drug Deliv. 2019, 26, 870–885. [Google Scholar] [CrossRef]
- Samuel, S.; Ahmad, R.E.; Ramasamy, T.S.; Karunanithi, P.; Naveen, S.V.; Kamarul, T. Platelet-rich concentrate in serum-free medium enhances cartilage-specific extracellular matrix synthesis and reduces chondrocyte hypertrophy of human mesenchymal stromal cells encapsulated in alginate. Platelets 2019, 30, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Kaviani, A.; Zebarjad, S.M.; Javadpour, S.; Ayatollahi, M.; Bazargan-Lari, R. Fabrication and characterization of low-cost freeze-gelated chitosan/collagen/hydroxyapatite hydrogel nanocomposite scaffold. Int. J. Polym. Anal. Charact. 2019, 24, 191–203. [Google Scholar] [CrossRef]
- Silva, C.R.; Babo, P.S.; Gulino, M.; Costa, L.; Oliveira, J.M.; Silva-Correia, J.; Domingues, R.M.A.; Reis, R.L.; Gomes, M.E. Injectable and tunable hyaluronic acid hydrogels releasing chemotactic and angiogenic growth factors for endodontic regeneration. Acta Biomater. 2018, 77, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Urbanek, O.; Kołbuk, D.; Wróbel, M. Articular cartilage: New directions and barriers of scaffolds development—Review. Int. J. Polym. Mater. 2019, 68, 396–410. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Sun, X.; Liu, D.; Huang, C.; Wu, J.; Yang, C.; Zhang, Q. Biomimetic cartilage scaffold with orientated porous structure of two factors for cartilage repair of knee osteoarthritis. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1710–1721. [Google Scholar] [CrossRef]
- Passos, M.F.; Carvalho, N.M.S.; Rodrigues, A.A.; Bavaresco, V.P.; Jardini, A.L.; Maciel, M.R.W.; Maciel Filho, R. PHEMA Hydrogels Obtained by Infrared Radiation for Cartilage Tissue Engineering. Int. J. Chem. Eng. 2019, 2019, 10. [Google Scholar] [CrossRef]
- Chuang, E.-Y.; Chiang, C.-W.; Wong, P.-C.; Chen, C.-H. Hydrogels for the Application of Articular Cartilage Tissue Engineering: A Review of Hydrogels. Adv. Mater. Sci. Eng. 2018, 2018, 14. [Google Scholar] [CrossRef]
- Khalid, I.; Ahmad, M.; Minhas, M.U.; Barkat, K. Preparation and characterization of alginate-PVA-based semi-IPN: Controlled release pH-responsive composites. Polym. Bull. 2018, 75, 1075–1099. [Google Scholar] [CrossRef]
- Torres, M.L.; Oberti, T.G.; Fernández, J.M. HEMA and alginate-based chondrogenic semi-interpenetrated hydrogels: Synthesis and biological characterization. J. Biomater. Sci. Polym. Ed. 2020, 32, 504–523. [Google Scholar] [CrossRef]
- Hasnain, M.S.; Ray, P.; Nayak, A.K. Chapter 5—Alginate-based interpenetrating polymer networks for sustained drug release. In Alginates in Drug Delivery, 1st ed.; Nayak, A.K., Hasnain, M.S., Eds.; Academic Press: London, UK, 2020; pp. 101–128. [Google Scholar]
- Farokhi, M.; Jonidi Shariatzadeh, F.; Solouk, A.; Mirzadeh, H. Alginate Based Scaffolds for Cartilage Tissue Engineering: A Review. Int. J. Polym. Mater. 2020, 69, 230–247. [Google Scholar] [CrossRef]
- Yamaoka, H.; Asato, H.; Ogasawara, T.; Nishizawa, S.; Takahashi, T.; Nakatsuka, T.; Koshima, I.; Nakamura, K.; Kawaguchi, H.; Chung, U.I.; et al. Cartilage tissue engineering using human auricular chondrocytes embedded in different hydrogel materials. J. Biomed. Mater. Res. Part A 2006, 78, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Eslaminejad, M.B.; Taghiyar, L.; Falahi, F. Quantitative analysis of the proliferation and differentiation of rat articular chondrocytes in alginate 3D culture. Iran. Biomed. J. 2009, 13, 153–160. [Google Scholar]
- Goel, N.K.; Kumar, V.; Bhardwaj, Y.K.; Chaudhari, C.V.; Dubey, K.A.; Sabharwal, S. Swelling Response of Radiation Synthesized 2-Hydroxyethylmethacrylate-co-[2-(methacryloyloxy)ethyl] Trimethylammonium Chloride Hydrogels Under Various In Vitro Conditions. J. Biomater. Sci. Polym. Ed. 2009, 20, 785–805. [Google Scholar] [CrossRef]
- Bölgen, N.; Yang, Y.; Korkusuz, P.; Güzel, E.; El Haj, A.J.; Pişkin, E. 3D ingrowth of bovine articular chondrocytes in biodegradable cryogel scaffolds for cartilage tissue engineering. J. Tissue Eng. Regen. Med. 2011, 5, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, C.; Verny, M.; Giraud, I.; Ollier, M.; Rapp, M.; Maurizis, J.-C.; Madelmont, J.-C. New Quaternary Ammonium Oxicam Derivatives Targeted toward Cartilage: Synthesis, Pharmacokinetic Studies, and Antiinflammatory Potency. J. Med. Chem. 1999, 42, 5235–5240. [Google Scholar] [CrossRef]
- Giraud, I.; Rapp, M.; Maurizis, J.-C.; Madelmont, J.-C. Application to a Cartilage Targeting Strategy: Synthesis and in Vivo Biodistribution of 14C-Labeled Quaternary Ammonium−Glucosamine Conjugates. Bioconjugate Chem. 2000, 11, 212–218. [Google Scholar] [CrossRef]
- Miot-Noirault, E.; Vidal, A.; Morlieras, J.; Bonazza, P.; Auzeloux, P.; Besse, S.; Dauplat, M.-M.; Peyrode, C.; Degoul, F.; Billotey, C.; et al. Small rigid platforms functionalization with quaternary ammonium: Targeting extracellular matrix of chondrosarcoma. Nanomed. NBM 2014, 10, 1887–1895. [Google Scholar] [CrossRef] [PubMed]
- Rivero, R.; Alustiza, F.; Capella, V.; Liaudat, C.; Rodriguez, N.; Bosch, P.; Barbero, C.; Rivarola, C. Physicochemical properties of ionic and non-ionic biocompatible hydrogels in water and cell culture conditions: Relation with type of morphologies of bovine fetal fibroblasts in contact with the surfaces. Colloids Surf. B 2017, 158, 488–497. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhu, Z. Adhesion of starch-g-poly(2-acryloyloxyethyl trimethyl ammonium chloride) to cotton and polyester fibers. Starch/Staerke 2014, 66, 566–575. [Google Scholar] [CrossRef]
- Shen, S.; Zhu, Z.; Liu, F. Introduction of poly[(2-acryloyloxyethyl trimethyl ammonium chloride)-co-(acrylic acid)] branches onto starch for cotton warp sizing. Carbohydr. Polym. 2016, 138, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Ozay, O.; Ilgin, P.; Ozay, H.; Gungor, Z.; Yilmaz, B.; Kıvanç, M.R. The preparation of various shapes and porosities of hydroxyethyl starch/p(HEMA-co-NVP) IPN hydrogels as programmable carrier for drug delivery. J. Macromol. Sci. Part A 2020, 57, 379–387. [Google Scholar] [CrossRef]
- Mandal, B.; Ray, S.K. Synthesis of interpenetrating network hydrogel from poly(acrylic acid-co-hydroxyethyl methacrylate) and sodium alginate: Modeling and kinetics study for removal of synthetic dyes from water. Carbohydr. Polym. 2013, 98, 257–269. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Y.; Zhang, L.; Xu, M.; Dong, W.; Zhang, J. Redox/pH dual stimuli-responsive degradable Salecan-g-SS-poly(IA-co-HEMA) hydrogel for release of doxorubicin. Carbohydr. Polym. 2017, 155, 242–251. [Google Scholar] [CrossRef]
- Clara, I.; Natchimuthu, N. Hydrogels of sodium alginate based copolymers grafted with sodium-2-acrylamido-2-methyl-1-propane sulfonate and methacrylic acid for controlled drug delivery applications. J. Macromol. Sci. Pure Appl. Chem. 2018, 55, 168–175. [Google Scholar] [CrossRef]
- Bardajee, G.R.; Hooshyar, Z.; Rastgo, F. Kappa carrageenan-g-poly (acrylic acid)/SPION nanocomposite as a novel stimuli-sensitive drug delivery system. Colloid Polym. Sci. 2013, 291, 2791–2803. [Google Scholar] [CrossRef]
- Jiang, H.; Yang, Y.; Lin, Z.; Zhao, B.; Wang, J.; Xie, J.; Zhang, A. Preparation of a novel bio-adsorbent of sodium alginate grafted polyacrylamide/graphene oxide hydrogel for the adsorption of heavy metal ion. Sci. Total Environ. 2020, 744, 10. [Google Scholar] [CrossRef]
- Rivas, B.L.; Aguirre, M.D.C. Water-soluble polymers: Optimization of arsenate species retention by ultrafiltration. J. Appl. Polym. Sci. 2009, 112, 2327–2333. [Google Scholar] [CrossRef]
- Torres, C.C.; Urbano, B.F.; Campos, C.H.; Rivas, B.L.; Reyes, P. Composite hydrogel based on surface modified mesoporous silica and poly[(2-acryloyloxy)ethyl trimethylammonium chloride]. Mater. Chem. Phys. 2015, 152, 69–76. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, G.-J.; Guo, Y.-T.; Zhou, L.; Du, J.; He, H. Preparation of novel biodegradable pHEMA hydrogel for a tissue engineering scaffold by microwave-assisted polymerization. Asian Pac. J. Trop. Med. 2014, 7, 136–140. [Google Scholar] [CrossRef]
- Lee, B.-S.; Chen, Y.-J.; Wei, T.-C.; Ma, T.-L.; Chang, C.-C. Comparison of Antibacterial Adhesion When Salivary Pellicle Is Coated on Both Poly(2-hydroxyethyl-methacrylate)- and Polyethylene-glycol-methacrylate-grafted Poly(methyl methacrylate). Int. J. Mol. Sci. 2018, 19, 2764. [Google Scholar] [CrossRef]
- Yang, X.; Wang, B.; Qiao, C.; Li, Z.; Li, Y.; Xu, C.; Li, T. Molecular interactions in N-[(2-hydroxyl)-propyl-3-trimethyl ammonium] chitosan chloride-sodium alginate polyelectrolyte complexes. Food Hydrocoll. 2020, 100, 105400. [Google Scholar] [CrossRef]
- Onder, A.; Ilgin, P.; Ozay, H.; Ozay, O. Removal of dye from aqueous medium with pH-sensitive poly[(2-(acryloyloxy)ethyl]trimethylammonium chloride-co-1-vinyl-2-pyrrolidone] cationic hydrogel. J. Environ. Chem. Eng. 2020, 8, 104436. [Google Scholar] [CrossRef]
- Vargün, E.; Usanmaz, A. Degradation of Poly(2-hydroxyethyl methacrylate) Obtained by Radiation in Aqueous Solution. J. Macromol. Sci. Pure Appl. Chem. 2010, 47, 882–891. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M.; Abdel-Mogib, M.; Dawidar, A.-A.M.; Elsayed, A.; Smyth, H.D.C. Biodegradable pH-responsive alginate-poly (lactic-co-glycolic acid) nano/micro hydrogel matrices for oral delivery of silymarin. Carbohydr. Polym. 2011, 83, 1345–1354. [Google Scholar] [CrossRef]
- Das, D.; Pham, H.T.T.; Lee, S.; Noh, I. Fabrication of alginate-based stimuli-responsive, non-cytotoxic, terpolymric semi-IPN hydrogel as a carrier for controlled release of bovine albumin serum and 5-amino salicylic acid. Mater. Sci. Eng. C 2019, 98, 42–53. [Google Scholar] [CrossRef]
- Ghobashy, M.M.; Bassioni, G. pH stimuli-responsive poly(acrylamide-co-sodium alginate) hydrogels prepared by γ-radiation for improved compressive strength of concrete. Adv. Polym. Technol. 2018, 37, 2123–2133. [Google Scholar] [CrossRef]
- Dou, W.-H.; Zhou, G.-M.; Kang, Q.-Q. Study of the Epimers of Dexamethasone Sodium Phosphate and Betamethasone Sodium Phosphate by FTIR, FT-Raman and SERS. Spectrosc. Spectr. Anal. 2012, 32, 2664–2668. [Google Scholar] [CrossRef]
- Monajjemzadeh, F.; Gholizadeh, N.; Yousefzadeh Mobaraki, N.; Jelvehgari, M. Physicochemical and in vitro mucoadhesive properties of microparticles/discs of betamethasone for the management of oral lichen planus. Pharm. Dev. Technol. 2016, 21, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Ghasemnejad, M.; Ahmadi, E.; Mohamadnia, Z.; Doustgani, A.; Hashemikia, S. Functionalized silica nanoparticles as a carrier for Betamethasone Sodium Phosphate: Drug release study and statistical optimization of drug loading by response surface method. Mater. Sci. Eng. C 2015, 56, 223–232. [Google Scholar] [CrossRef]
- Qi, X.; Li, J.; Wei, W.; Zuo, G.; Su, T.; Pan, X.; Zhang, J.; Dong, W. Cationic Salecan-based hydrogels for release of 5-fluorouracil. RSC Adv. 2017, 7, 14337–14347. [Google Scholar] [CrossRef]
- Rey-Rico, A.; Madry, H.; Cucchiarini, M. Hydrogel-Based Controlled Delivery Systems for Articular Cartilage Repair. Biomed. Res. Int. 2016, 2016, 1215263. [Google Scholar] [CrossRef]
- Wei, W.; Qi, X.; Li, J.; Zuo, G.; Sheng, W.; Zhang, J.; Dong, W. Smart Macroporous Salecan/Poly(N,N-diethylacrylamide) Semi-IPN Hydrogel for Anti-Inflammatory Drug Delivery. ACS Biomater. Sci. Eng. 2016, 2, 1386–1394. [Google Scholar] [CrossRef]
- Samanta, H.S.; Ray, S.K. Synthesis, characterization, swelling and drug release behavior of semi-interpenetrating network hydrogels of sodium alginate and polyacrylamide. Carbohydr. Polym. 2014, 99, 666–678. [Google Scholar] [CrossRef]
- Ilgin, P.; Ozay, H.; Ozay, O. A new dual stimuli responsive hydrogel: Modeling approaches for the prediction of drug loading and release profile. Eur. Polym. J. 2019, 113, 244–253. [Google Scholar] [CrossRef]
- Chen, J.; Liu, M.; Chen, W.; Zhang, N.; Zhu, S.; Zhang, S.; Xiong, Y. Synthesis, Swelling and Drug-Release Behaviour of a Poly(N,N-diethylacrylamide-co-(2-dimethylamino) ethyl methacrylate) Hydrogel. J. Biomater. Sci. Polym. Ed. 2011, 22, 1049–1068. [Google Scholar] [CrossRef]
- Dalapati, S.; Alam, M.A.; Jana, S.; Guchhait, N. Reduced Schiff-base assisted novel dihydrogenphosphate–water polymer. CrystEngComm 2012, 14, 6029–6034. [Google Scholar] [CrossRef]
- Basaran, I.; Emami Khansari, M.; Pramanik, A.; Wong, B.M.; Hossain, M.A. Binding and selectivity of dihydrogen phosphate by H-bond donors and acceptors in a tripodal-based thiourea receptor. Tetrahedron Lett. 2015, 56, 115–118. [Google Scholar] [CrossRef]
- Kim, A.R.; Lee, S.L.; Park, S.N. Properties and in vitro drug release of pH- and temperature-sensitive double cross-linked interpenetrating polymer network hydrogels based on hyaluronic acid/poly (N-isopropylacrylamide) for transdermal delivery of luteolin. Int. J. Biol. Macromol. 2018, 118, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Zhang, X.; Meng, Y.; Chen, L.; Zhang, Q. Development of a dual drug-loaded hydrogel delivery system for enhanced cancer therapy: In situ formation, degradation and synergistic antitumor efficiency. J. Mater. Chem. B 2017, 5, 8487–8497. [Google Scholar] [CrossRef] [PubMed]
- Roointan, A.; Farzanfar, J.; Mohammadi-Samani, S.; Behzad-Behbahani, A.; Farjadian, F. Smart pH responsive drug delivery system based on poly(HEMA-co-DMAEMA) nanohydrogel. Int. J. Pharm. 2018, 552, 301–311. [Google Scholar] [CrossRef] [PubMed]






| Samples | pH = 2.0 | PBS, pH = 7.4 | ||||
|---|---|---|---|---|---|---|
| k | n | R2 | k | n | R2 | |
| H1 | 38 ± 3 | 0.55 ± 0.02 | 99.67 | 33 ± 2 | 0.74 ± 0.02 | 99.90 |
| H2 | 40 ± 4 | 0.60 ± 0.03 | 99.56 | 41 ± 3 | 0.69 ± 0.03 | 99.79 |
| H3 | 52 ± 4 | 0.60 ± 0.03 | 99.55 | 65 ± 3 | 0.69 ± 0.02 | 99.93 |
| H4 | 35 ± 1 | 0.59 ± 0.01 | 99.97 | 47 ± 2 | 0.64 ± 0.01 | 99.95 |
| H5 | 45 ± 4 | 0.71 ± 0.03 | 99.80 | 68 ± 6 | 0.72 ± 0.03 | 99.84 |
| H6 | 49 ± 3 | 0.59 ± 0.02 | 99.72 | 53 ± 2 | 0.69 ± 0.02 | 99.95 |
| Samples | Composition (%) | Drug Load (µg of Drug/mg of Hydrogel) | Loading Efficiency (%) | BTM Released (%) | ||
|---|---|---|---|---|---|---|
| HEMA | QCL | AlgNa | ||||
| H6 | 10 | 80 | 10 | 58.0 | 31.8 | 100 * |
| H4 | 10 | 70 | 20 | 57.8 | 27.9 | 100 ** |
| H5 | 20 | 70 | 10 | 74.1 | 52.7 | 99.7 |
| H2 | 20 | 60 | 20 | 99.8 | 37.6 | 95.5 |
| H3 | 30 | 60 | 10 | 113.7 | 58.8 | 91.4 |
| H1 | 30 | 50 | 20 | 137.4 | 59.6 | 83.2 |
| Samples | Korsmeyer-Peppas | ||
|---|---|---|---|
| k | n | R2 | |
| H1 | 2.7 ± 0.6 | 0.63 ± 0.05 | 97.53% |
| H2 | 1.8 ± 0.6 | 0.76 ± 0.08 | 96.24% |
| H3 | 4.4 ± 0.7 | 0.54 ± 0.04 | 98.28% |
| H4 | 2.9 ± 0.9 | 0.73 ± 0.08 | 97.82% |
| H5 | 1.0 ± 0.1 | 0.93 ± 0.04 | 99.55% |
| H6 | 1.0 ± 0.4 | 1.0 ± 0.1 | 98.57% |
| Sample | QCL (% wt) | HEMA (% wt) | AlgNa (% wt) |
|---|---|---|---|
| H1 | 50 | 30 | 20 |
| H2 | 60 | 20 | 20 |
| H3 | 60 | 30 | 10 |
| H4 | 70 | 10 | 20 |
| H5 | 70 | 20 | 10 |
| H6 | 80 | 10 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Couce, J.; Vernhes, M.; Bada, N.; Agüero, L.; Valdés, O.; Alvarez-Barreto, J.; Fuentes, G.; Almirall, A.; Cruz, L.J. Synthesis and Evaluation of AlgNa-g-Poly(QCL-co-HEMA) Hydrogels as Platform for Chondrocyte Proliferation and Controlled Release of Betamethasone. Int. J. Mol. Sci. 2021, 22, 5730. https://doi.org/10.3390/ijms22115730
García-Couce J, Vernhes M, Bada N, Agüero L, Valdés O, Alvarez-Barreto J, Fuentes G, Almirall A, Cruz LJ. Synthesis and Evaluation of AlgNa-g-Poly(QCL-co-HEMA) Hydrogels as Platform for Chondrocyte Proliferation and Controlled Release of Betamethasone. International Journal of Molecular Sciences. 2021; 22(11):5730. https://doi.org/10.3390/ijms22115730
Chicago/Turabian StyleGarcía-Couce, Jomarien, Marioly Vernhes, Nancy Bada, Lissette Agüero, Oscar Valdés, José Alvarez-Barreto, Gastón Fuentes, Amisel Almirall, and Luis J. Cruz. 2021. "Synthesis and Evaluation of AlgNa-g-Poly(QCL-co-HEMA) Hydrogels as Platform for Chondrocyte Proliferation and Controlled Release of Betamethasone" International Journal of Molecular Sciences 22, no. 11: 5730. https://doi.org/10.3390/ijms22115730
APA StyleGarcía-Couce, J., Vernhes, M., Bada, N., Agüero, L., Valdés, O., Alvarez-Barreto, J., Fuentes, G., Almirall, A., & Cruz, L. J. (2021). Synthesis and Evaluation of AlgNa-g-Poly(QCL-co-HEMA) Hydrogels as Platform for Chondrocyte Proliferation and Controlled Release of Betamethasone. International Journal of Molecular Sciences, 22(11), 5730. https://doi.org/10.3390/ijms22115730








