Antibody-Based Therapeutics for Atherosclerosis and Cardiovascular Diseases
Abstract
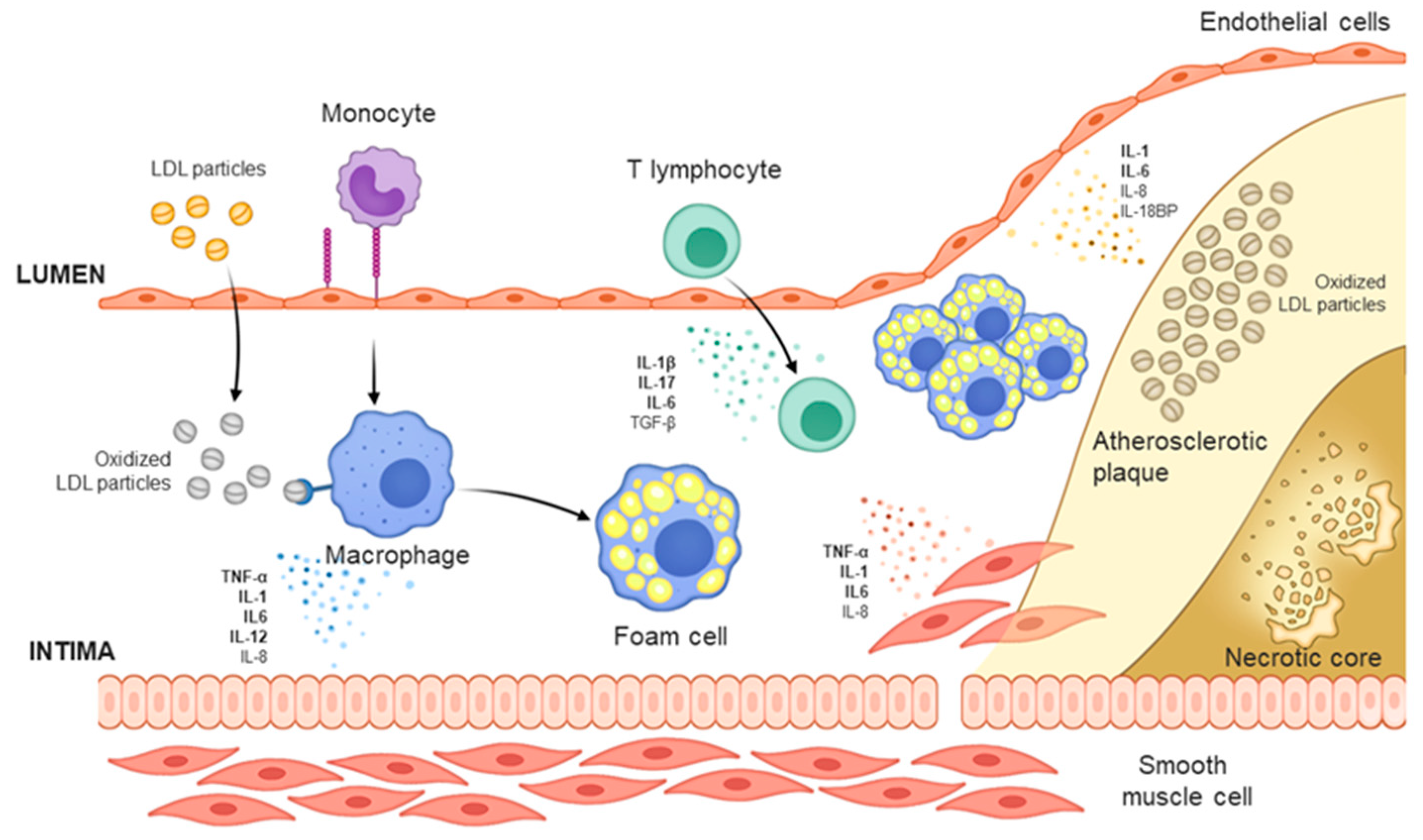
:1. Introduction
2. LDL- or oxLDL-Lowering Therapies
2.1. Apolipoprotein B Autoantibody
2.2. PCSK9 Inhibitor
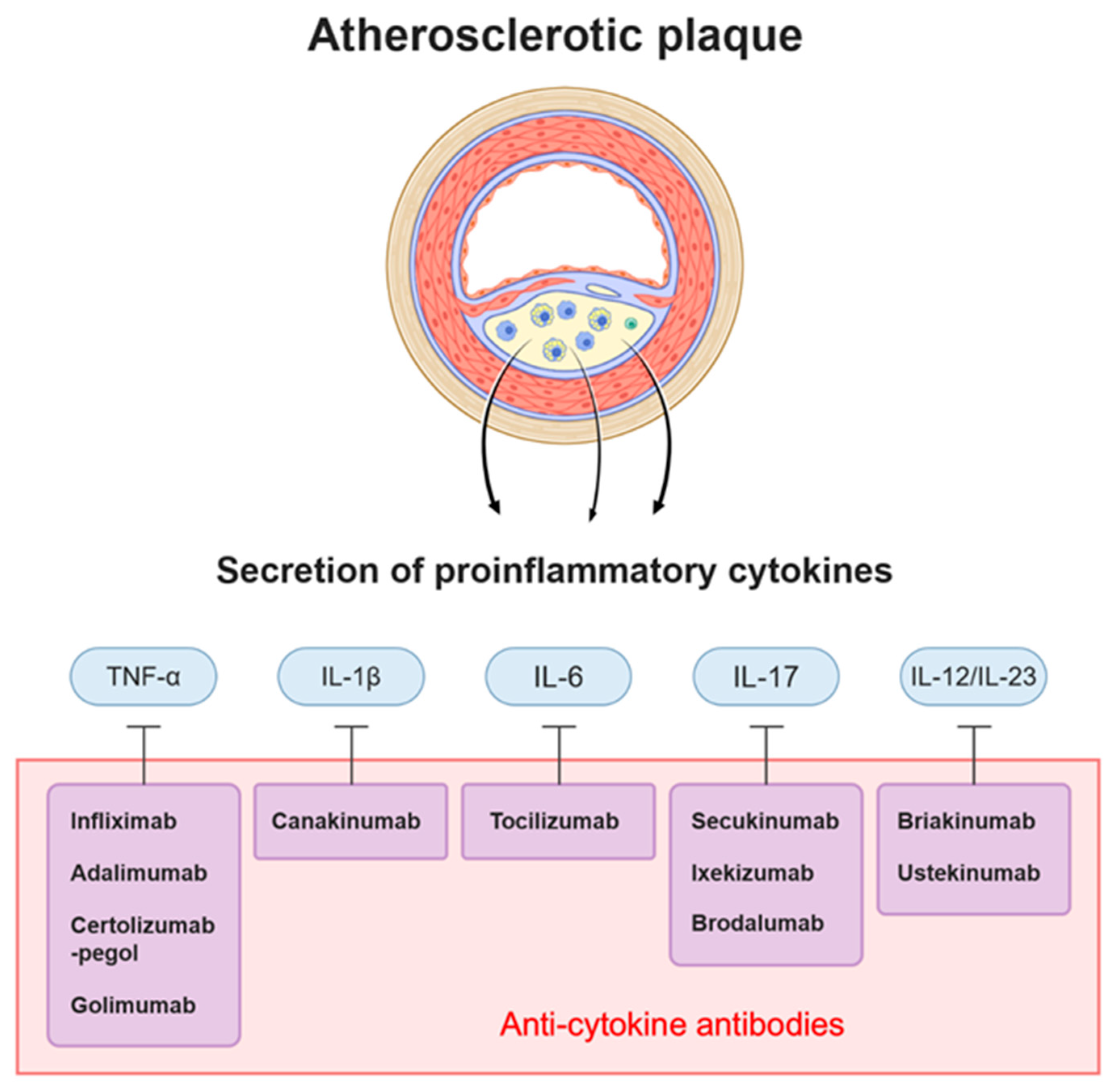
3. Cytokine-Targeting Therapy
3.1. Anti-TNF-α
3.2. Anti-IL-1β
3.3. Anti-IL-6
3.4. Anti-IL-17
3.5. Anti-IL-12/23
4. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Otreba, M.; Kosmider, L.; Rzepecka-Stojko, A. Polyphenols’ Cardioprotective Potential: Review of Rat Fibroblasts as Well as Rat and Human Cardiomyocyte Cell Lines Research. Molecules 2021, 26, 774. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, X.; Millican, R.; Sherwood, J.; Martin, S.; Jo, H.; Yoon, Y.S.; Brott, B.C.; Jun, H.W. Recent advances in nanomaterials for therapy and diagnosis for atherosclerosis. Adv. Drug Deliv. Rev. 2021, 170, 142–199. [Google Scholar] [CrossRef] [PubMed]
- Palasubramaniam, J.; Wang, X.W.; Peter, K. Myocardial Infarction-From Atherosclerosis to Thrombosis Uncovering New Diagnostic and Therapeutic Approaches. Arter. Throm. Vas. 2019, 39, E176–E185. [Google Scholar] [CrossRef]
- Adhyaru, B.B.; Jacobson, T.A. Safety and efficacy of statin therapy. Nat. Rev. Cardiol. 2018, 15, 757–769. [Google Scholar] [CrossRef] [PubMed]
- Cholesterol Treatment Trialists Collaborators; Mihaylova, B.; Emberson, J.; Blackwell, L.; Keech, A.; Simes, J.; Barnes, E.H.; Voysey, M.; Gray, A.; Collins, R.; et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: Meta-analysis of individual data from 27 randomised trials. Lancet 2012, 380, 581–590. [Google Scholar] [CrossRef]
- Wu, M.-Y.; Li, C.-J.; Hou, M.-F.; Chu, P.-Y. New Insights into the Role of Inflammation in the Pathogenesis of Atherosclerosis. Int. J. Mol. Sci. 2017, 18, 2034. [Google Scholar] [CrossRef]
- Shah, P.K.; Lecis, D. Inflammation in atherosclerotic cardiovascular disease. F1000Research 2019, 8, 1402. [Google Scholar] [CrossRef] [Green Version]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef]
- Valanti, E.-K.; Dalakoura-Karagkouni, K.; Siasos, G.; Kardassis, D.; Eliopoulos, A.G.; Sanoudou, D. Advances in biological therapies for dyslipidemias and atherosclerosis. Metabolism 2021, 116, 154461. [Google Scholar] [CrossRef]
- Gistera, A.; Hansson, G.K. The immunology of atherosclerosis. Nat. Rev. Nephrol. 2017, 13, 368–380. [Google Scholar] [CrossRef]
- Goldstein, J.L.; Ho, Y.K.; Basu, S.K.; Brown, M.S. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc. Natl. Acad. Sci. USA 1979, 76, 333–337. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.M.; Febbraio, M.; Silverstein, R.L. CD36 modulates migration of mouse and human macrophages in response to oxidized LDL and may contribute to macrophage trapping in the arterial intima. J. Clin. Investig. 2009, 119, 136–145. [Google Scholar] [CrossRef] [Green Version]
- Robbins, C.S.; Hilgendorf, I.; Weber, G.F.; Theurl, I.; Iwamoto, Y.; Figueiredo, J.-L.; Gorbatov, R.; Sukhova, G.K.; Gerhardt, L.M.S.; Smyth, D.; et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat. Med. 2013, 19, 1166–1172. [Google Scholar] [CrossRef]
- Libby, P. Targeting Inflammatory Pathways in Cardiovascular Disease: The Inflammasome, Interleukin-1, Interleukin-6 and Beyond. Cells 2021, 10, 951. [Google Scholar] [CrossRef] [PubMed]
- Cybulsky, M.I.; Gimbrone, M.A., Jr. Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science 1991, 251, 788–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ait-Oufella, H.; Taleb, S.; Mallat, Z.; Tedgui, A. Recent advances on the role of cytokines in atherosclerosis. Arter. Thromb. Vasc. Biol. 2011, 31, 969–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, N.; Xu, S.; Xu, Y.; Little, P.J.; Jin, Z.G. Targeting Mechanosensitive Transcription Factors in Atherosclerosis. Trends Pharm. Sci. 2019, 40, 253–266. [Google Scholar] [CrossRef]
- Tiller, K.E.; Tessier, P.M. Advances in Antibody Design. Annu. Rev. Biomed. Eng. 2015, 17, 191–216. [Google Scholar] [CrossRef] [Green Version]
- Chames, P.; Van Regenmortel, M.; Weiss, E.; Baty, D. Therapeutic antibodies: Successes, limitations and hopes for the future. Br. J. Pharmacol. 2009, 157, 220–233. [Google Scholar] [CrossRef]
- Lu, R.-M.; Hwang, Y.-C.; Liu, I.J.; Lee, C.-C.; Tsai, H.-Z.; Li, H.-J.; Wu, H.-C. Development of therapeutic antibodies for the treatment of diseases. J. Biomed. Sci. 2020, 27, 1–30. [Google Scholar] [CrossRef]
- Di Minno, M.N.D.; Iervolino, S.; Peluso, R.; Scarpa, R.; Di Minno, G. Carotid Intima-Media Thickness in Psoriatic Arthritis. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 705–712. [Google Scholar] [CrossRef] [Green Version]
- Mease, P.J.; McInnes, I.B.; Kirkham, B.; Kavanaugh, A.; Rahman, P.; Van Der Heijde, D.; Landewé, R.; Nash, P.; Pricop, L.; Yuan, J.; et al. Secukinumab Inhibition of Interleukin-17A in Patients with Psoriatic Arthritis. N. Engl. J. Med. 2015, 373, 1329–1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langley, R.G.; Papp, K.; Gottlieb, A.B.; Krueger, G.G.; Gordon, K.B.; Williams, D.; Valdes, J.; Setze, C.; Strober, B. Safety results from a pooled analysis of randomized, controlled phase II and III clinical trials and interim data from an open-label extension trial of the interleukin-12/23 monoclonal antibody, briakinumab, in moderate to severe psoriasis. J. Eur. Acad. Derm. Venereol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Fredrikson, G.N.; Hedblad, B.; Berglund, G.; Alm, R.; Ares, M.; Cercek, B.; Chyu, K.Y.; Shah, P.K.; Nilsson, J. Identification of immune responses against aldehyde-modified peptide sequences in apoB associated with cardiovascular disease. Arter. Thromb. Vasc. Biol. 2003, 23, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, I.; Nitulescu, M.; Ares, M.P.S.; Fredrikson, G.N.; Jansson, B.; Li, Z.-C.; Nilsson, J. Identification of the target for therapeutic recombinant anti-apoB-100 peptide antibodies in human atherosclerotic lesions. Atherosclerosis 2009, 205, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Schiopu, A.; Bengtsson, J.; Soderberg, I.; Janciauskiene, S.; Lindgren, S.; Ares, M.P.; Shah, P.K.; Carlsson, R.; Nilsson, J.; Fredrikson, G.N. Recombinant human antibodies against aldehyde-modified apolipoprotein B-100 peptide sequences inhibit atherosclerosis. Circulation 2004, 110, 2047–2052. [Google Scholar] [CrossRef] [Green Version]
- Schiopu, A.; Frendéus, B.; Jansson, B.; Söderberg, I.; Ljungcrantz, I.; Araya, Z.; Shah, P.K.; Carlsson, R.; Nilsson, J.; Fredrikson, G.N. Recombinant Antibodies to an Oxidized Low-Density Lipoprotein Epitope Induce Rapid Regression of Atherosclerosis in Apobec-1−/−/Low-Density Lipoprotein Receptor−/−Mice. J. Am. Coll. Cardiol. 2007, 50, 2313–2318. [Google Scholar] [CrossRef] [PubMed]
- Fredrikson, G.N.; Andersson, L.; Soderberg, I.; Dimayuga, P.; Chyu, K.Y.; Shah, P.K.; Nilsson, J. Atheroprotective immunization with MDA-modified apo B-100 peptide sequences is associated with activation of Th2 specific antibody expression. Autoimmunity 2005, 38, 171–179. [Google Scholar] [CrossRef]
- Li, S.; Kievit, P.; Robertson, A.-K.; Kolumam, G.; Li, X.; Von Wachenfeldt, K.; Valfridsson, C.; Bullens, S.; Messaoudi, I.; Bader, L.; et al. Targeting oxidized LDL improves insulin sensitivity and immune cell function in obese Rhesus macaques. Mol. Metab. 2013, 2, 256–269. [Google Scholar] [CrossRef]
- Björkbacka, H.; Alm, R.; Persson, M.; Hedblad, B.; Nilsson, J.; Fredrikson, G.N. Low Levels of Apolipoprotein B-100 Autoantibodies Are Associated With Increased Risk of Coronary Events. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 765–771. [Google Scholar] [CrossRef] [Green Version]
- Asciutto, G.; Wigren, M.; Fredrikson, G.N.; Mattisson, I.Y.; Grönberg, C.; Alm, R.; Björkbacka, H.; Dias, N.V.; Edsfeldt, A.; Gonçalves, I.; et al. Apolipoprotein B-100 Antibody Interaction With Atherosclerotic Plaque Inflammation and Repair Processes. Stroke 2016, 47, 1140–1143. [Google Scholar] [CrossRef] [Green Version]
- Lehrer-Graiwer, J.; Singh, P.; Abdelbaky, A.; Vucic, E.; Korsgren, M.; Baruch, A.; Fredrickson, J.; Van Bruggen, N.; Tang, M.T.; Frendeus, B.; et al. FDG-PET Imaging for Oxidized LDL in Stable Atherosclerotic Disease: A Phase II Study of Safety, Tolerability, and Anti-Inflammatory Activity. JACC Cardiovasc. Imaging 2015, 8, 493–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunér, P.; Mattisson, I.Y.; Fogelstrand, P.; Glise, L.; Ruiz, S.; Farina, C.; Borén, J.; Nilsson, J.; Bengtsson, E. Antibodies against apoB100 peptide 210 inhibit atherosclerosis in apoE-/- mice. Sci. Rep. 2021, 11, 9022. [Google Scholar] [CrossRef]
- Lin, X.-L.; Xiao, L.-L.; Tang, Z.-H.; Jiang, Z.-S.; Liu, M.-H. Role of PCSK9 in lipid metabolism and atherosclerosis. Biomed. Pharmacother. 2018, 104, 36–44. [Google Scholar] [CrossRef]
- Tang, Y.; Li, S.-L.; Hu, J.-H.; Sun, K.-J.; Liu, L.-L.; Xu, D.-Y. Research progress on alternative non-classical mechanisms of PCSK9 in atherosclerosis in patients with and without diabetes. Cardiovasc. Diabetol. 2020, 19, 33. [Google Scholar] [CrossRef] [Green Version]
- Morelli, M.B.; Wang, X.; Santulli, G. Functional role of gut microbiota and PCSK9 in the pathogenesis of diabetes mellitus and cardiovascular disease. Atherosclerosis 2019, 289, 176–178. [Google Scholar] [CrossRef]
- Horton, J.D.; Cohen, J.C.; Hobbs, H.H. PCSK9: A convertase that coordinates LDL catabolism. J. Lipid Res. 2009, 50, S172–S177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasichayanula, S.; Grover, A.; Emery, M.G.; Gibbs, M.A.; Somaratne, R.; Wasserman, S.M.; Gibbs, J.P. Clinical Pharmacokinetics and Pharmacodynamics of Evolocumab, a PCSK9 Inhibitor. Clin. Pharmacokinet. 2018, 57, 769–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catapano, A.L.; Pirillo, A.; Norata, G.D. New Pharmacological Approaches to Target PCSK9. Curr. Atheroscler. Rep. 2020, 22, 24. [Google Scholar] [CrossRef]
- Kosenko, T.; Golder, M.; Leblond, G.; Weng, W.; Lagace, T.A. Low density lipoprotein binds to proprotein convertase subtilisin/kexin type-9 (PCSK9) in human plasma and inhibits PCSK9-mediated low density lipoprotein receptor degradation. J. Biol. Chem. 2013, 288, 8279–8288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.J.; Lee, Y.-H.; Park, S.W.; Kim, K.J.; Park, S.; Youn, J.-C.; Lee, S.-H.; Kang, S.-M.; Jang, Y. Association of serum proprotein convertase subtilisin/kexin type 9 with carotid intima media thickness in hypertensive subjects. Metabolism 2013, 62, 845–850. [Google Scholar] [CrossRef]
- Chen, S.N.; Ballantyne, C.M.; Gotto, A.M., Jr.; Tan, Y.; Willerson, J.T.; Marian, A.J. A common PCSK9 haplotype, encompassing the E670G coding single nucleotide polymorphism, is a novel genetic marker for plasma low-density lipoprotein cholesterol levels and severity of coronary atherosclerosis. J. Am. Coll. Cardiol. 2005, 45, 1611–1619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norata, G.D.; Garlaschelli, K.; Grigore, L.; Raselli, S.; Tramontana, S.; Meneghetti, F.; Artali, R.; Noto, D.; Cefalù, A.B.; Buccianti, G.; et al. Effects of PCSK9 variants on common carotid artery intima media thickness and relation to ApoE alleles. Atherosclerosis 2010, 208, 177–182. [Google Scholar] [CrossRef]
- Shapiro, M.D.; Tavori, H.; Fazio, S. PCSK9. Circ. Res. 2018, 122, 1420–1438. [Google Scholar] [CrossRef] [PubMed]
- Brandts, J.; Dharmayat, K.I.; Vallejo-Vaz, A.J.; Azar Sharabiani, M.T.; Jones, R.; Kastelein, J.J.P.; Raal, F.J.; Ray, K.K. A meta-analysis of medications directed against PCSK9 in familial hypercholesterolemia. Atherosclerosis 2021, 325, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Duff, C.J.; Scott, M.J.; Kirby, I.T.; Hutchinson, S.E.; Martin, S.L.; Hooper, N.M. Antibody-mediated disruption of the interaction between PCSK9 and the low-density lipoprotein receptor. Biochem. J. 2009, 419, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.C.Y.; Piper, D.E.; Cao, Q.; Liu, D.; King, C.; Wang, W.; Tang, J.; Liu, Q.; Higbee, J.; Xia, Z.; et al. A proprotein convertase subtilisin/kexin type 9 neutralizing antibody reduces serum cholesterol in mice and nonhuman primates. Proc. Natl. Acad. Sci. USA 2009, 106, 9820–9825. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, K.N.; Breslow, J.L. Antibodies to PCSK9. Circ. Res. 2012, 111, 274–277. [Google Scholar] [CrossRef] [Green Version]
- Stein, E.A.; Mellis, S.; Yancopoulos, G.D.; Stahl, N.; Logan, D.; Smith, W.B.; Lisbon, E.; Gutierrez, M.; Webb, C.; Wu, R.; et al. Effect of a Monoclonal Antibody to PCSK9 on LDL Cholesterol. N. Engl. J. Med. 2012, 366, 1108–1118. [Google Scholar] [CrossRef] [Green Version]
- Catapano, A.L.; Papadopoulos, N. The safety of therapeutic monoclonal antibodies: Implications for cardiovascular disease and targeting the PCSK9 pathway. Atherosclerosis 2013, 228, 18–28. [Google Scholar] [CrossRef] [Green Version]
- Solanki, A.; Bhatt, L.K.; Johnston, T.P. Evolving targets for the treatment of atherosclerosis. Pharmacol. Ther. 2018, 187, 1–12. [Google Scholar] [CrossRef]
- Gallego-Colon, E.; Daum, A.; Yosefy, C. Statins and PCSK9 inhibitors: A new lipid-lowering therapy. Eur. J. Pharmacol. 2020, 878, 173114. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Lorenz, P.; Ballantyne, C.M. Poststatin Lipid Therapeutics: A Review. Methodist Debakey Cardiovasc. J. 2019, 15, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef]
- Schwartz, G.G.; Steg, P.G.; Szarek, M.; Bhatt, D.L.; Bittner, V.A.; Diaz, R.; Edelberg, J.M.; Goodman, S.G.; Hanotin, C.; Harrington, R.A.; et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N. Engl. J. Med. 2018, 379, 2097–2107. [Google Scholar] [CrossRef]
- Whayne, T.F. PCSK9 inhibitors in the current management of atherosclerosis. Arch. Cardiol. México 2017, 87, 43–48. [Google Scholar] [CrossRef]
- Adamstein, N.H.; Macfadyen, J.G.; Rose, L.M.; Glynn, R.J.; Dey, A.K.; Libby, P.; Tabas, I.A.; Mehta, N.N.; Ridker, P.M. The neutrophil–lymphocyte ratio and incident atherosclerotic events: Analyses from five contemporary randomized trials. Eur. Heart J. 2021, 42, 896–903. [Google Scholar] [CrossRef]
- Ridker, P.M.; Revkin, J.; Amarenco, P.; Brunell, R.; Curto, M.; Civeira, F.; Flather, M.; Glynn, R.J.; Gregoire, J.; Jukema, J.W.; et al. Cardiovascular Efficacy and Safety of Bococizumab in High-Risk Patients. N. Engl. J. Med. 2017, 376, 1527–1539. [Google Scholar] [CrossRef] [Green Version]
- Wiciński, M.; Żak, J.; Malinowski, B.; Popek, G.; Grześk, G. PCSK9 signaling pathways and their potential importance in clinical practice. EPMA J. 2017, 8, 391–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, X.; Al Rifai, M.; Birnbaum, Y.; Smith, S.C.; Virani, S.S. The 2018 Cholesterol Management Guidelines: Topics in Secondary ASCVD Prevention Clinicians Need to Know. Curr. Atheroscler. Rep. 2019, 21, 20. [Google Scholar] [CrossRef] [PubMed]
- Wong, N.D.; Shapiro, M.D. Interpreting the Findings From the Recent PCSK9 Monoclonal Antibody Cardiovascular Outcomes Trials. Front. Cardiovasc. Med. 2019, 6, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruscica, M.; Tokgözoğlu, L.; Corsini, A.; Sirtori, C.R. PCSK9 inhibition and inflammation: A narrative review. Atherosclerosis 2019, 288, 146–155. [Google Scholar] [CrossRef] [Green Version]
- Khambhati, J.; Engels, M.; Allard-Ratick, M.; Sandesara, P.B.; Quyyumi, A.A.; Sperling, L. Immunotherapy for the prevention of atherosclerotic cardiovascular disease: Promise and possibilities. Atherosclerosis 2018, 276, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rayment, N.; Moss, E.; Faulkner, L.; Brickell, P.; Davies, M.; Woolf, N.; Katz, D. Synthesis of TNFα and TGFβ mRNA in the different micro-environments within atheromatous plaques. Cardiovasc. Res. 1996, 32, 1123–1130. [Google Scholar] [CrossRef] [Green Version]
- Ridker, P.M.; Rifai, N.; Pfeffer, M.; Sacks, F.; Lepage, S.; Braunwald, E. Elevation of Tumor Necrosis Factor-α and Increased Risk of Recurrent Coronary Events After Myocardial Infarction. Circulation 2000, 101, 2149–2153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohta, H.; Wada, H.; Niwa, T.; Kirii, H.; Iwamoto, N.; Fujii, H.; Saito, K.; Sekikawa, K.; Seishima, M. Disruption of tumor necrosis factor-α gene diminishes the development of atherosclerosis in ApoE-deficient mice. Atherosclerosis 2005, 180, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Schreyer, S.A.; Peschon, J.J.; Leboeuf, R.C. Accelerated Atherosclerosis in Mice Lacking Tumor Necrosis Factor Receptor p55. J. Biol. Chem. 1996, 271, 26174–26178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ait-Oufella, H.; Libby, P.; Tedgui, A. Anticytokine Immune Therapy and Atherothrombotic Cardiovascular Risk. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1510–1519. [Google Scholar] [CrossRef]
- Jacobsson, L.T.; Turesson, C.; Gulfe, A.; Kapetanovic, M.C.; Petersson, I.F.; Saxne, T.; Geborek, P. Treatment with tumor necrosis factor blockers is associated with a lower incidence of first cardiovascular events in patients with rheumatoid arthritis. J. Rheumatol. 2005, 32, 1213–1218. [Google Scholar]
- Ahlehoff, O.; Skov, L.; Gislason, G.; Gniadecki, R.; Iversen, L.; Bryld, L.E.; Lasthein, S.; Lindhardsen, J.; Kristensen, S.L.; Torp-Pedersen, C.; et al. Cardiovascular outcomes and systemic anti-inflammatory drugs in patients with severe psoriasis: 5-year follow-up of a Danish nationwide cohort. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1128–1134. [Google Scholar] [CrossRef]
- Lebwohl, M.; Blauvelt, A.; Paul, C.; Sofen, H.; Węgłowska, J.; Piguet, V.; Burge, D.; Rolleri, R.; Drew, J.; Peterson, L.; et al. Certolizumab pegol for the treatment of chronic plaque psoriasis: Results through 48 weeks of a phase 3, multicenter, randomized, double-blind, etanercept- and placebo-controlled study (CIMPACT). J. Am. Acad. Dermatol. 2018, 79, 266–276.e265. [Google Scholar] [CrossRef] [Green Version]
- Gkalpakiotis, S.; Arenbergerova, M.; Gkalpakioti, P.; Potockova, J.; Arenberger, P.; Kraml, P. Long-term impact of adalimumab therapy on biomarkers of systemic inflammation in psoriasis: Results of a 2 year study. Dermatol. Ther. 2020, 33. [Google Scholar] [CrossRef]
- Lee, A.; Scott, L.J. Certolizumab Pegol: A Review in Moderate to Severe Plaque Psoriasis. BioDrugs 2020, 34, 235–244. [Google Scholar] [CrossRef]
- Frampton, J.E. Golimumab: A Review in Inflammatory Arthritis. BioDrugs 2017, 31, 263–274. [Google Scholar] [CrossRef]
- Pelechas, E.; Voulgari, P.; Drosos, A. Golimumab for Rheumatoid Arthritis. J. Clin. Med. 2019, 8, 387. [Google Scholar] [CrossRef] [Green Version]
- Chung, E.S.; Packer, M.; Lo, K.H.; Fasanmade, A.A.; Willerson, J.T. Randomized, Double-Blind, Placebo-Controlled, Pilot Trial of Infliximab, a Chimeric Monoclonal Antibody to Tumor Necrosis Factor-α, in Patients With Moderate-to-Severe Heart Failure. Circulation 2003, 107, 3133–3140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ait-Oufella, H.; Libby, P.; Tedgui, A. Antibody-based immunotherapy targeting cytokines and atherothrombotic cardiovascular diseases. Arch. Cardiovasc. Dis. 2020, 113, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Lukens, J.R.; Gross, J.M.; Kanneganti, T.-D. IL-1 family cytokines trigger sterile inflammatory disease. Front. Immunol. 2012, 3, 315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moriya, J. Critical roles of inflammation in atherosclerosis. J. Cardiol. 2019, 73, 22–27. [Google Scholar] [CrossRef] [Green Version]
- Singer, I.I.; Scott, S.; Chin, J.; Bayne, E.K.; Limjuco, G.; Weidner, J.; Miller, D.K.; Chapman, K.; Kostura, M.J. The interleukin-1 beta-converting enzyme (ICE) is localized on the external cell surface membranes and in the cytoplasmic ground substance of human monocytes by immuno-electron microscopy. J. Exp. Med. 1995, 182, 1447–1459. [Google Scholar] [CrossRef] [Green Version]
- Libby, P. Interleukin-1 Beta as a Target for Atherosclerosis Therapy. J. Am. Coll. Cardiol. 2017, 70, 2278–2289. [Google Scholar] [CrossRef] [PubMed]
- Kirii, H.; Niwa, T.; Yamada, Y.; Wada, H.; Saito, K.; Iwakura, Y.; Asano, M.; Moriwaki, H.; Seishima, M. Lack of Interleukin-1β Decreases the Severity of Atherosclerosis in ApoE-Deficient Mice. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 656–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimokawa, H.; Ito, A.; Fukumoto, Y.; Kadokami, T.; Nakaike, R.; Sakata, M.; Takayanagi, T.; Egashira, K.; Takeshita, A. Chronic treatment with interleukin-1 beta induces coronary intimal lesions and vasospastic responses in pigs in vivo. The role of platelet-derived growth factor. J. Clin. Investig. 1996, 97, 769–776. [Google Scholar] [CrossRef] [Green Version]
- Dinarello, C.A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 2011, 117, 3720–3732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinarello, C.A.; Simon, A.; Van Der Meer, J.W.M. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat. Rev. Drug Discov. 2012, 11, 633–652. [Google Scholar] [CrossRef] [Green Version]
- Kedor, C.; Listing, J.; Zernicke, J.; Weiß, A.; Behrens, F.; Blank, N.; Henes, J.C.; Kekow, J.; Rubbert-Roth, A.; Schulze-Koops, H.; et al. Canakinumab for Treatment of Adult-Onset Still’s Disease to Achieve Reduction of Arthritic Manifestation (CONSIDER): Phase II, randomised, double-blind, placebo-controlled, multicentre, investigator-initiated trial. Ann. Rheum. Dis. 2020, 79, 1090–1097. [Google Scholar] [CrossRef]
- Ridker, P.M.; Howard, C.P.; Walter, V.; Everett, B.; Libby, P.; Hensen, J.; Thuren, T. Effects of Interleukin-1β Inhibition With Canakinumab on Hemoglobin A1c, Lipids, C-Reactive Protein, Interleukin-6, and Fibrinogen. Circulation 2012, 126, 2739–2748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ridker, P.M.; Thuren, T.; Zalewski, A.; Libby, P. Interleukin-1β inhibition and the prevention of recurrent cardiovascular events: Rationale and Design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS). Am. Heart J. 2011, 162, 597–605. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; Macfadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Everett, B.M.; Macfadyen, J.G.; Thuren, T.; Libby, P.; Glynn, R.J.; Ridker, P.M. Inhibition of Interleukin-1β and Reduction in Atherothrombotic Cardiovascular Events in the CANTOS Trial. J. Am. Coll. Cardiol. 2020, 76, 1660–1670. [Google Scholar] [CrossRef]
- Libby, P.; Rocha, V.Z. All roads lead to IL-6: A central hub of cardiometabolic signaling. Int. J. Cardiol. 2018, 259, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Saremi, A.; Anderson, R.J.; Luo, P.; Moritz, T.E.; Schwenke, D.C.; Allison, M.; Reaven, P.D. Association between IL-6 and the extent of coronary atherosclerosis in the veterans affairs diabetes trial (VADT). Atherosclerosis 2009, 203, 610–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huber, S.A.; Sakkinen, P.; Conze, D.; Hardin, N.; Tracy, R. Interleukin-6 Exacerbates Early Atherosclerosis in Mice. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 2364–2367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schieffer, B.; Selle, T.; Hilfiker, A.; Hilfiker-Kleiner, D.; Grote, K.; Tietge, U.J.F.; Trautwein, C.; Luchtefeld, M.; Schmittkamp, C.; Heeneman, S.; et al. Impact of Interleukin-6 on Plaque Development and Morphology in Experimental Atherosclerosis. Circulation 2004, 110, 3493–3500. [Google Scholar] [CrossRef]
- Elhage, R. Involvement of interleukin-6 in atherosclerosis but not in the prevention of fatty streak formation by 17β-estradiol in apolipoprotein E-deficient mice. Atherosclerosis 2001, 156, 315–320. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rifai, N.; Stampfer, M.J.; Hennekens, C.H. Plasma Concentration of Interleukin-6 and the Risk of Future Myocardial Infarction Among Apparently Healthy Men. Circulation 2000, 101, 1767–1772. [Google Scholar] [CrossRef] [Green Version]
- The interleukin-6 receptor as a target for prevention of coronary heart disease: A mendelian randomisation analysis. Lancet 2012, 379, 1214–1224. [CrossRef] [Green Version]
- Gabay, C.; Riek, M.; Hetland, M.L.; Hauge, E.-M.; Pavelka, K.; Tomšič, M.; Canhao, H.; Chatzidionysiou, K.; Lukina, G.; Nordström, D.C.; et al. Effectiveness of tocilizumab with and without synthetic disease-modifying antirheumatic drugs in rheumatoid arthritis: Results from a European collaborative study. Ann. Rheum. Dis. 2016, 75, 1336–1342. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.C.; Solomon, D.H.; Rogers, J.R.; Gale, S.; Klearman, M.; Sarsour, K.; Schneeweiss, S. Cardiovascular Safety of Tocilizumab Versus Tumor Necrosis Factor Inhibitors in Patients With Rheumatoid Arthritis: A Multi-Database Cohort Study. Arthritis Rheumatol. 2017, 69, 1154–1164. [Google Scholar] [CrossRef] [Green Version]
- Generali, E.; Carrara, G.; Selmi, C.; Verstappen, S.M.M.; Zambon, A.; Bortoluzzi, A.; Silvagni, E.; Scire, C.A. Comparison of the risks of hospitalisation for cardiovascular events in patients with rheumatoid arthritis treated with tocilizumab and etanercept. Clin. Exp. Rheumatol. 2018, 36, 310–313. [Google Scholar]
- Yamamoto, K.; Goto, H.; Hirao, K.; Nakajima, A.; Origasa, H.; Tanaka, K.; Tomobe, M.; Totsuka, K. Longterm Safety of Tocilizumab: Results from 3 Years of Followup Postmarketing Surveillance of 5573 Patients with Rheumatoid Arthritis in Japan. J. Rheumatol. 2015, 42, 1368–1375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anstensrud, A.K.; Woxholt, S.; Sharma, K.; Broch, K.; Bendz, B.; Aakhus, S.; Ueland, T.; Amundsen, B.H.; Damås, J.K.; Hopp, E.; et al. Rationale for the ASSAIL-MI-trial: A randomised controlled trial designed to assess the effect of tocilizumab on myocardial salvage in patients with acute ST-elevation myocardial infarction (STEMI). Open Heart 2019, 6, e001108. [Google Scholar] [CrossRef] [PubMed]
- Kurschus, F.C.; Moos, S. IL-17 for therapy. J. Dermatol. Sci. 2017, 87, 221–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, P.; Rodriguez, F.H.; Kanaly, S.; Stocking, K.L.; Schurr, J.; Schwarzenberger, P.; Oliver, P.; Huang, W.; Zhang, P.; Zhang, J.; et al. Requirement of Interleukin 17 Receptor Signaling for Lung Cxc Chemokine and Granulocyte Colony-Stimulating Factor Expression, Neutrophil Recruitment, and Host Defense. J. Exp. Med. 2001, 194, 519–528. [Google Scholar] [CrossRef]
- O’Connor, W., Jr.; Kamanaka, M.; Booth, C.J.; Town, T.; Nakae, S.; Iwakura, Y.; Kolls, J.K.; Flavell, R.A. A protective function for interleukin 17A in T cell–mediated intestinal inflammation. Nat. Immunol. 2009, 10, 603–609. [Google Scholar] [CrossRef]
- Hawkes, J.E.; Yan, B.Y.; Chan, T.C.; Krueger, J.G. Discovery of the IL-23/IL-17 Signaling Pathway and the Treatment of Psoriasis. J. Immunol. 2018, 201, 1605–1613. [Google Scholar] [CrossRef]
- Erbel, C.; Chen, L.; Bea, F.; Wangler, S.; Celik, S.; Lasitschka, F.; Wang, Y.; Böckler, D.; Katus, H.A.; Dengler, T.J. Inhibition of IL-17A Attenuates Atherosclerotic Lesion Development in ApoE-Deficient Mice. J. Immunol. 2009, 183, 8167–8175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, E.; Prasad, K.-M.R.; Butcher, M.; Dobrian, A.; Kolls, J.K.; Ley, K.; Galkina, E. Blockade of Interleukin-17A Results in Reduced Atherosclerosis in Apolipoprotein E–Deficient Mice. Circulation 2010, 121, 1746–1755. [Google Scholar] [CrossRef]
- Butcher, M.J.; Gjurich, B.N.; Phillips, T.; Galkina, E.V. The IL-17A/IL-17RA Axis Plays a Proatherogenic Role via the Regulation of Aortic Myeloid Cell Recruitment. Circ. Res. 2012, 110, 675–687. [Google Scholar] [CrossRef] [Green Version]
- Madhur, M.S.; Funt, S.A.; Li, L.; Vinh, A.; Chen, W.; Lob, H.E.; Iwakura, Y.; Blinder, Y.; Rahman, A.; Quyyumi, A.A.; et al. Role of Interleukin 17 in Inflammation, Atherosclerosis, and Vascular Function in Apolipoprotein E–Deficient Mice. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1565–1572. [Google Scholar] [CrossRef] [Green Version]
- Danzaki, K.; Matsui, Y.; Ikesue, M.; Ohta, D.; Ito, K.; Kanayama, M.; Kurotaki, D.; Morimoto, J.; Iwakura, Y.; Yagita, H.; et al. Interleukin-17A Deficiency Accelerates Unstable Atherosclerotic Plaque Formation in Apolipoprotein E-Deficient Mice. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 273–280. [Google Scholar] [CrossRef] [Green Version]
- Ge, S.; Hertel, B.; Koltsova, E.K.; Sörensen-Zender, I.; Kielstein, J.T.; Ley, K.; Haller, H.; Von Vietinghoff, S. Increased Atherosclerotic Lesion Formation and Vascular Leukocyte Accumulation in Renal Impairment Are Mediated by Interleukin-17A. Circ. Res. 2013, 113, 965–974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taleb, S.; Romain, M.; Ramkhelawon, B.; Uyttenhove, C.; Pasterkamp, G.; Herbin, O.; Esposito, B.; Perez, N.; Yasukawa, H.; Van Snick, J.; et al. Loss of SOCS3 expression in T cells reveals a regulatory role for interleukin-17 in atherosclerosis. J. Exp. Med. 2009, 206, 2067–2077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gisterå, A.; Robertson, A.-K.L.; Andersson, J.; Ketelhuth, D.F.J.; Ovchinnikova, O.; Nilsson, S.K.; Lundberg, A.M.; Li, M.O.; Flavell, R.A.; Hansson, G.K. Transforming Growth Factor–β Signaling in T Cells Promotes Stabilization of Atherosclerotic Plaques Through an Interleukin-17–Dependent Pathway. Sci. Transl. Med. 2013, 5, 196ra100–196ra191. [Google Scholar] [CrossRef]
- Simon, T.; Taleb, S.; Danchin, N.; Laurans, L.; Rousseau, B.; Cattan, S.; Montely, J.-M.; Dubourg, O.; Tedgui, A.; Kotti, S.; et al. Circulating levels of interleukin-17 and cardiovascular outcomes in patients with acute myocardial infarction. Eur. Heart J. 2013, 34, 570–577. [Google Scholar] [CrossRef] [Green Version]
- Langley, R.G.; Elewski, B.E.; Lebwohl, M.; Reich, K.; Griffiths, C.E.M.; Papp, K.; Puig, L.; Nakagawa, H.; Spelman, L.; Sigurgeirsson, B.; et al. Secukinumab in Plaque Psoriasis—Results of Two Phase 3 Trials. N. Engl. J. Med. 2014, 371, 326–338. [Google Scholar] [CrossRef] [Green Version]
- McInnes, I.B.; Sieper, J.; Braun, J.; Emery, P.; Van Der Heijde, D.; Isaacs, J.D.; Dahmen, G.; Wollenhaupt, J.; Schulze-Koops, H.; Kogan, J.; et al. Efficacy and safety of secukinumab, a fully human anti-interleukin-17A monoclonal antibody, in patients with moderate-to-severe psoriatic arthritis: A 24-week, randomised, double-blind, placebo-controlled, phase II proof-of-concept trial. Ann. Rheum. Dis. 2014, 73, 349–356. [Google Scholar] [CrossRef]
- Gisondi, P.; Dalle Vedove, C.; Girolomoni, G. Efficacy and Safety of Secukinumab in Chronic Plaque Psoriasis and Psoriatic Arthritis Therapy. Dermatol. Ther. 2014, 4, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baeten, D.; Baraliakos, X.; Braun, J.; Sieper, J.; Emery, P.; Van Der Heijde, D.; McInnes, I.; Van Laar, J.M.; Landewé, R.; Wordsworth, P.; et al. Anti-interleukin-17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis: A randomised, double-blind, placebo-controlled trial. Lancet 2013, 382, 1705–1713. [Google Scholar] [CrossRef]
- Burkett, P.R.; Kuchroo, V.K. IL-17 Blockade in Psoriasis. Cell 2016, 167, 1669. [Google Scholar] [CrossRef]
- Nash, P.; McInnes, I.B.; Mease, P.; Thom, H.; Cure, S.; Palaka, E.; Gandhi, K.; Mpofu, S.; Jugl, S. Secukinumab for the Treatment of Psoriatic Arthritis: Comparative Effectiveness Results Versus Adalimumab up to 48 Weeks Using a Matching-Adjusted Indirect Comparison. Ann. Rheum. Dis. 2016, 75, 353–354. [Google Scholar] [CrossRef]
- Gordon, K.B.; Blauvelt, A.; Papp, K.A.; Langley, R.G.; Luger, T.; Ohtsuki, M.; Reich, K.; Amato, D.; Ball, S.G.; Braun, D.K.; et al. Phase 3 Trials of Ixekizumab in Moderate-to-Severe Plaque Psoriasis. N. Engl. J. Med. 2016, 375, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Papp, K.A.; Leonardi, C.; Menter, A.; Ortonne, J.-P.; Krueger, J.G.; Kricorian, G.; Aras, G.; Li, J.; Russell, C.B.; Thompson, E.H.Z.; et al. Brodalumab, an Anti–Interleukin-17–Receptor Antibody for Psoriasis. N. Engl. J. Med. 2012, 366, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Kunwar, S.; Dahal, K.; Sharma, S. Anti-IL-17 therapy in treatment of rheumatoid arthritis: A systematic literature review and meta-analysis of randomized controlled trials. Rheumatol. Int. 2016, 36, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Kugyelka, R.; Kohl, Z.; Olasz, K.; Mikecz, K.; Rauch, T.A.; Glant, T.T.; Boldizsar, F. Enigma of IL-17 and Th17 Cells in Rheumatoid Arthritis and in Autoimmune Animal Models of Arthritis. Mediat. Inflamm. 2016, 2016, 6145810. [Google Scholar] [CrossRef] [Green Version]
- Moschen, A.R.; Tilg, H.; Raine, T. IL-12, IL-23 and IL-17 in IBD: Immunobiology and therapeutic targeting. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Wang, Y.; Wang, Z.; Liu, L.; Yang, Z.; Wang, M.; Xu, Y.; Ye, D.; Zhang, J.; Zhou, Q.; et al. The Expression of IL-12 Family Members in Patients with Hypertension and Its Association with the Occurrence of Carotid Atherosclerosis. Mediat. Inflamm. 2020, 2020, 2369279. [Google Scholar] [CrossRef] [Green Version]
- Jääskeläinen, A.E.; Seppälä, S.; Kakko, T.; Jaakkola, U.; Kallio, J. Systemic treatment with neuropeptide Y receptor Y1-antagonist enhances atherosclerosis and stimulates IL-12 expression in ApoE deficient mice. Neuropeptides 2013, 47, 67–73. [Google Scholar] [CrossRef]
- Lee, T.-S.; Yen, H.-C.; Pan, C.-C.; Chau, L.-Y. The Role of Interleukin 12 in the Development of Atherosclerosis in ApoE-Deficient Mice. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 734–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davenport, P.; Tipping, P.G. The Role of Interleukin-4 and Interleukin-12 in the Progression of Atherosclerosis in Apolipoprotein E-Deficient Mice. Am. J. Pathol. 2003, 163, 1117–1125. [Google Scholar] [CrossRef] [Green Version]
- Hauer, A.D.; Uyttenhove, C.; De Vos, P.; Stroobant, V.; Renauld, J.C.; Van Berkel, T.J.C.; Van Snick, J.; Kuiper, J. Blockade of Interleukin-12 Function by Protein Vaccination Attenuates Atherosclerosis. Circulation 2005, 112, 1054–1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Yu, H.; Liu, H.; Pu, D.; Gao, J.; Jin, X.; Liu, X.; Yan, A. Correlation of pre-operative circulating inflammatory cytokines with restenosis and rapid angiographic stenotic progression risk in coronary artery disease patients underwent percutaneous coronary intervention with drug-eluting stents. J. Clin. Lab. Anal. 2020, 34. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Wang, Y.; Wang, Z.; Liu, L.; Yang, Z.; Wang, M.; Xu, Y.; Ye, D.; Zhang, J.; Lin, Y.; et al. Roles and Mechanisms of Interleukin-12 Family Members in Cardiovascular Diseases: Opportunities and Challenges. Front. Pharmacol. 2020, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mannon, P.J.; Fuss, I.J.; Mayer, L.; Elson, C.O.; Sandborn, W.J.; Present, D.; Dolin, B.; Goodman, N.; Groden, C.; Hornung, R.L.; et al. Anti–Interleukin-12 Antibody for Active Crohn’s Disease. N. Engl. J. Med. 2004, 351, 2069–2079. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Gasink, C.; Gao, L.L.; Blank, M.A.; Johanns, J.; Guzzo, C.; Sands, B.E.; Hanauer, S.B.; Targan, S.; Rutgeerts, P.; et al. Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N. Engl. J. Med. 2012, 367, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Feagan, B.G.; Sandborn, W.J.; Gasink, C.; Jacobstein, D.; Lang, Y.; Friedman, J.R.; Blank, M.A.; Johanns, J.; Gao, L.-L.; Miao, Y.; et al. Ustekinumab as Induction and Maintenance Therapy for Crohn’s Disease. N. Engl. J. Med. 2016, 375, 1946–1960. [Google Scholar] [CrossRef]
- Marovt, M.; Marko, P.B.; Pirnat, M.; Ekart, R. Effect of biologics targeting interleukin-23/-17 axis on subclinical atherosclerosis: Results of a pilot study. Clin. Exp. Dermatol. 2020, 45, 560–564. [Google Scholar] [CrossRef]
- Morelli, M.B.; Chavez, C.; Santulli, G. Angiopoietin-like proteins as therapeutic targets for cardiovascular disease: Focus on lipid disorders. Expert Opin. Ther. Targets 2020, 24, 79–88. [Google Scholar] [CrossRef]
- Geladari, E.; Tsamadia, P.; Vallianou, N.G. ANGPTL3 Inhibitors- Their Role in Cardiovascular Disease Through Regulation of Lipid Metabolism. Circ. J. 2019, 83, 267–273. [Google Scholar] [CrossRef] [Green Version]
- Jarr, K.-U.; Nakamoto, R.; Doan, B.H.; Kojima, Y.; Weissman, I.L.; Advani, R.H.; Iagaru, A.; Leeper, N.J. Effect of CD47 Blockade on Vascular Inflammation. N. Engl. J. Med. 2021, 384, 382–383. [Google Scholar] [CrossRef]
- Caligiuri, G. CD31 as a Therapeutic Target in Atherosclerosis. Circ. Res. 2020, 126, 1178–1189. [Google Scholar] [CrossRef] [PubMed]
| Therapeutic/Study Name | Antibody Name | Target | Patients | Result |
|---|---|---|---|---|
| GLACIER | MLDL1278A | oxLDL (MDA-modified human ApoB-100) | CVD patients | Non significantly reduce carotid plaque |
| FOURIER | Evolocumab | PCSK9 | patients with clinically evident CVD(prior MI, stroke or PAD) | LDL-C level and primary outcomes (MI, stroke, cardiovascular death, coronary revascularization, unstable angina) reduction |
| ODYSSEY | Alirocumab | PCSK9 | patients diagnosed with ACS | LDL-C level and primary outcomes (non-fatal MI, ischemic stroke, unstable angina) reduction |
| SPIRE | Bococizumab | PCSK9 | CV or high risk patients | LDL-C level and primary ennpoint reduction in LDL-C >100 mg/dL group |
| ATTACH | Infliximab | TNF-α | Heart failure | Deteriorated heart failure |
| STROBE (follow up study) | Infliximab | TNF-α | Psoriasis | Significantly reduce the cardiovascular risk |
| Di Minno et al. [21] | Adalimumab, Infliximab | TNF-A | Psoriatic arthritis | Decreased atherosclerosis of carotid artery |
| CANTOS | Canakinumab | IL-1B | CAD after MI + hsCRP | Decreased hsCRP level and incidence of the primary endpoint (nonfatal myocardial infarction, stroke, cardiovascular death) |
| ASSIL-MI | Tocilizumab | IL-6 | ACS | Increased myocardial salvage |
| Mease et al. [22] | Secukinumab | IL-17 | Psoriatic arthritis | Non significant increased MACE |
| Uncover | Ixekizumab | IL-17 | Moderate to severe psoriasis | Reduced Psoriasis Area and Severity Index (PASI) score |
| Langley et al. [23] | Briakinumab | IL-12/23 | Psoriasis | Increased MACE |
| Uniti | Ustekinumab | IL-12/23 | Moderate to severe Crohn’s disease | Significantly higher rate of response |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, E.; Lee, S. Antibody-Based Therapeutics for Atherosclerosis and Cardiovascular Diseases. Int. J. Mol. Sci. 2021, 22, 5770. https://doi.org/10.3390/ijms22115770
Ji E, Lee S. Antibody-Based Therapeutics for Atherosclerosis and Cardiovascular Diseases. International Journal of Molecular Sciences. 2021; 22(11):5770. https://doi.org/10.3390/ijms22115770
Chicago/Turabian StyleJi, Eunhye, and Sahmin Lee. 2021. "Antibody-Based Therapeutics for Atherosclerosis and Cardiovascular Diseases" International Journal of Molecular Sciences 22, no. 11: 5770. https://doi.org/10.3390/ijms22115770






