Multitargeted Approach for the Optimization of Morphogenesis and Barrier Formation in Human Skin Equivalents
Abstract
:1. Introduction
2. Results
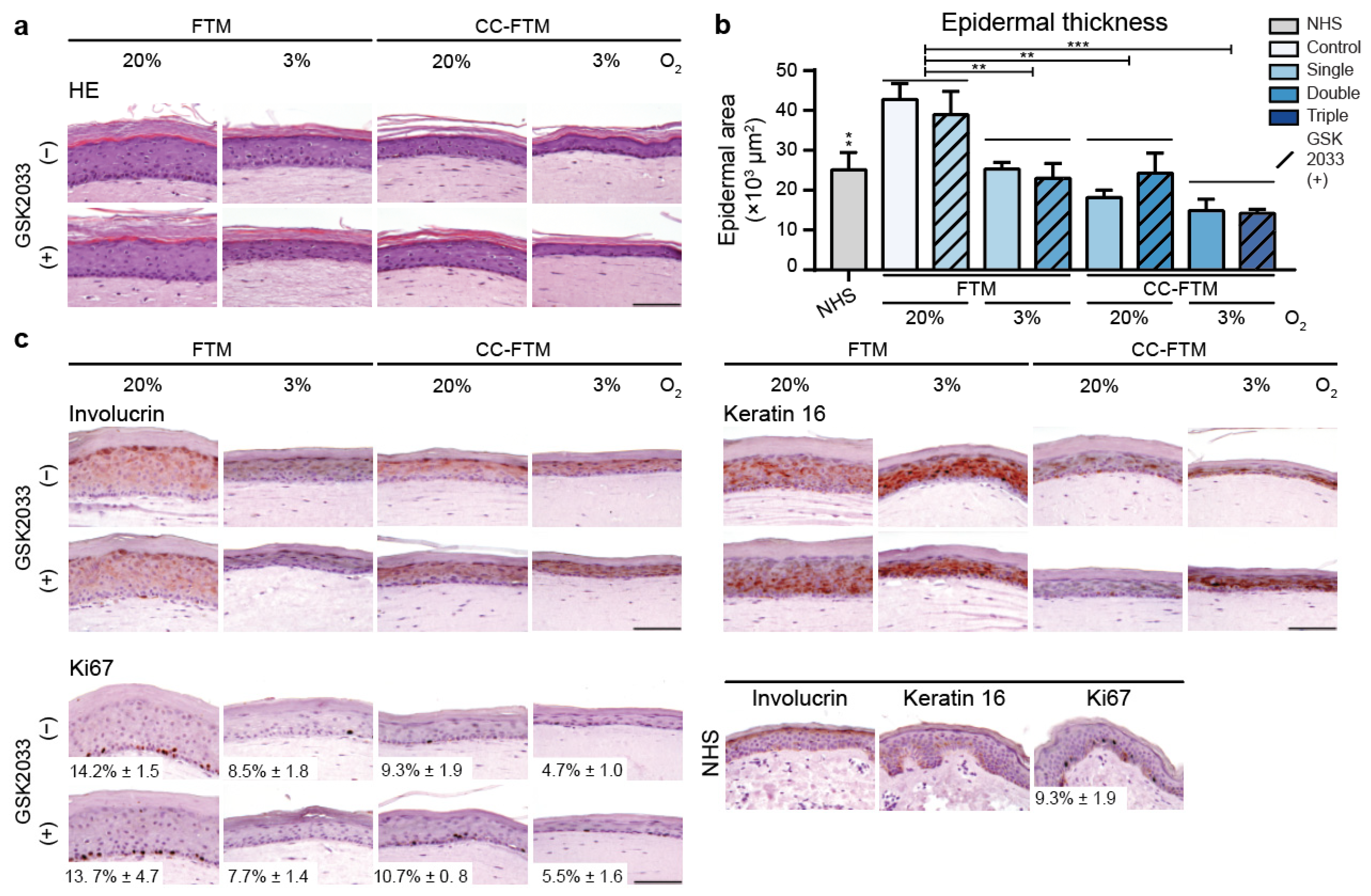
2.1. Multitargeted Optimization Approach Improved Epidermal Morphogenesis
2.2. Multitargeted Optimization Approach in FTMs Led to Enhanced Ceramide Composition
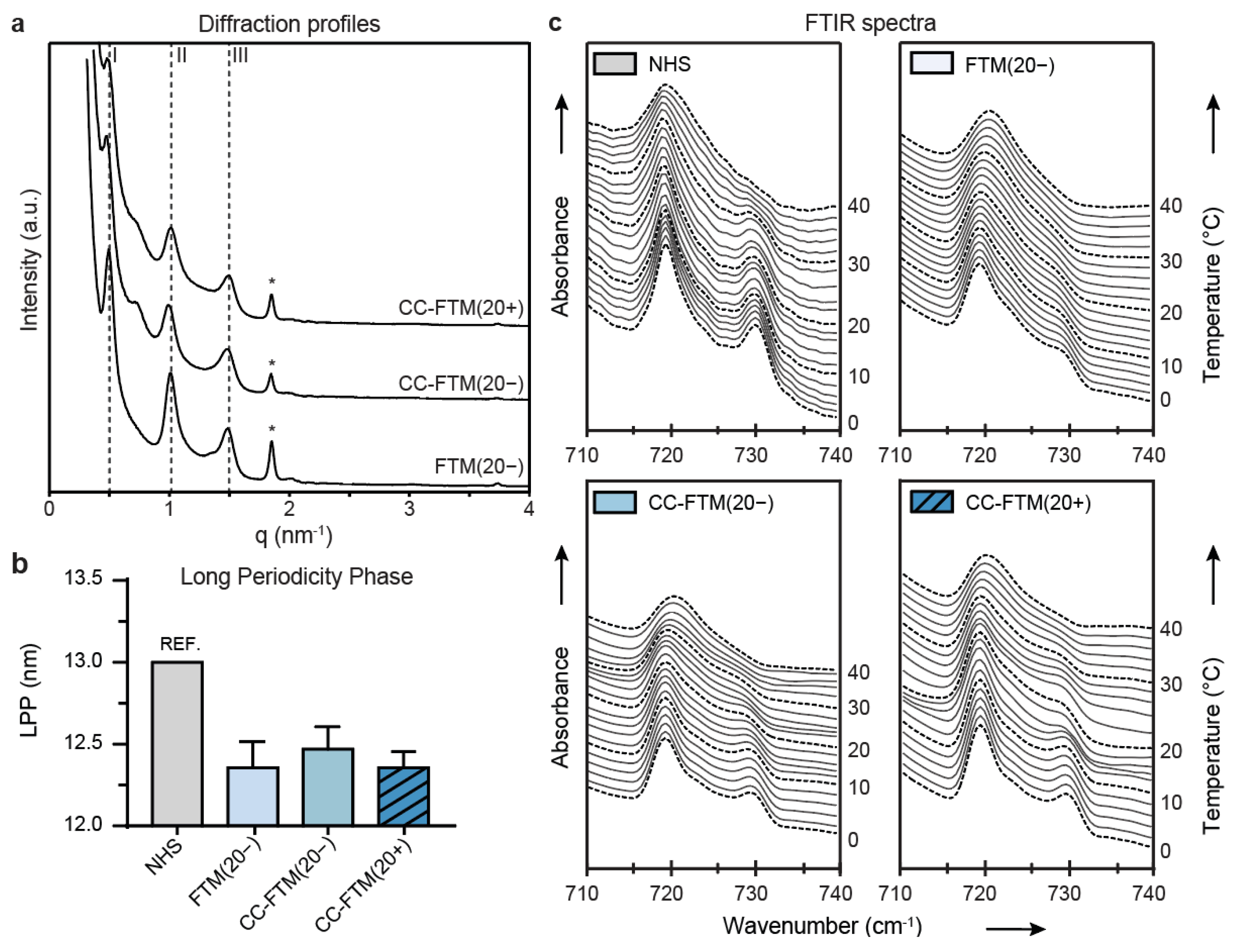
2.3. Leading Optimization Approaches Improved Organization of the Lipid Matrix
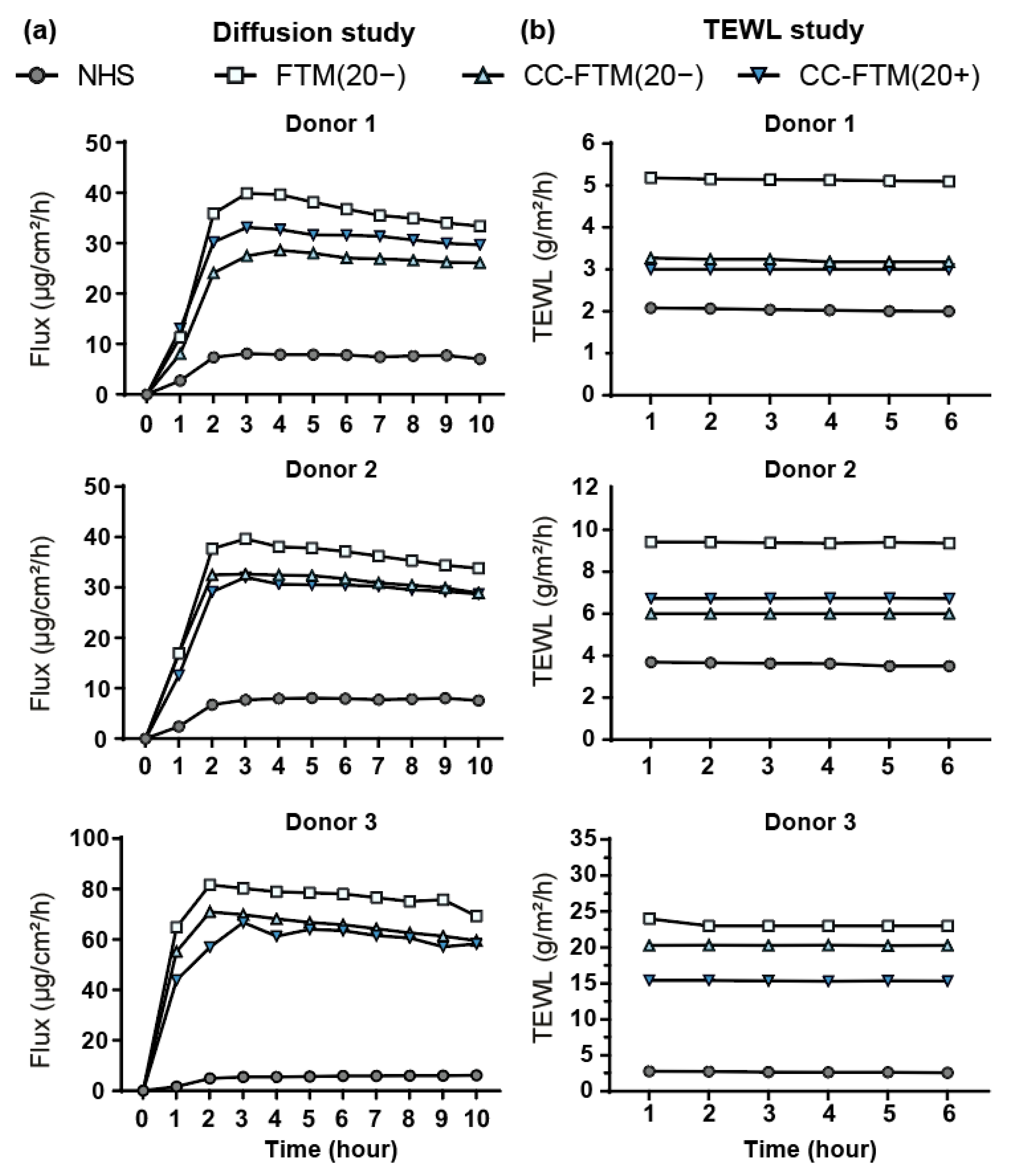
2.4. Optimized FTMs Displayed Enhanced Barrier Functionality
3. Discussion
4. Materials and Methods
4.1. Generation of Human Skin Equivalents
4.2. NHS Used in this Study
4.3. Immunohistochemical Analyses
4.4. Liquid Chromatography Coupled to Mass Spectrometry Analyses
4.5. SAXD
4.6. FTIR Spectroscopy
4.7. Barrier Functionality Assays
4.8. Statistics
4.9. Data Availability
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mathes, S.H.; Ruffner, H.; Graf-Hausner, U. The use of skin models in drug development. Adv. Drug Deliv. Rev. 2014, 69–70, 81–102. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S. Non-animal models of epithelial barriers (skin, intestine and lung) in research, industrial applications and regulatory toxicology. ALTEX 2015, 32, 327–378. [Google Scholar] [CrossRef]
- Niehues, H.; Bouwstra, J.A.; El Ghalbzouri, A.; Brandner, J.M.; Zeeuwen, P.L.J.M.; Van Den Bogaard, E.H. 3D skin models for 3R research: The potential of 3D reconstructed skin models to study skin barrier function. Exp. Dermatol. 2018, 27, 501–511. [Google Scholar] [CrossRef] [Green Version]
- El Ghalbzouri, A.; Siamari, R.; Willemze, R.; Ponec, M. Leiden reconstructed human epidermal model as a tool for the evaluation of the skin corrosion and irritation potential according to the ECVAM guidelines. Toxicol. Vitr. 2008, 22, 1311–1320. [Google Scholar] [CrossRef]
- Wufuer, M.; Lee, G.; Hur, W.; Jeon, B.; Kim, B.J.; Choi, T.H.; Lee, S. Skin-on-a-chip model simulating inflammation, edema and drug-based treatment. Sci. Rep. 2016, 6, 37471. [Google Scholar] [CrossRef] [Green Version]
- Sriram, G.; Alberti, M.; Dancik, Y.; Wu, B.; Wu, R.; Feng, Z.; Ramasamy, S.; Bigliardi, P.L.; Bigliardi-Qi, M.; Wang, Z. Full-thickness human skin-on-chip with enhanced epidermal morphogenesis and barrier function. Mater. Today 2018, 21, 326–340. [Google Scholar] [CrossRef]
- Broek, L.J.V.D.; Bergers, L.I.J.C.; Reijnders, C.M.A.; Gibbs, S. Progress and Future Prospectives in Skin-on-Chip Development with Emphasis on the use of Different Cell Types and Technical Challenges. Stem Cell Rev. Rep. 2017, 13, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Thakoersing, V.S.; Gooris, G.S.; Mulder, A.; Rietveld, M.; El Ghalbzouri, A.; Bouwstra, J.A. Unraveling Barrier Properties of Three Different In-House Human Skin Equivalents. Tissue Eng. Part C Methods 2012, 18, 1–11. [Google Scholar] [CrossRef]
- Schäfer-Korting, M.; Bock, U.; Gamer, A.; Haberland, A.; Haltner-Ukomadu, E.; Kaca, M.; Kamp, H.; Kietzmann, M.; Korting, H.C.; Krächter, H.-U.; et al. Reconstructed human epidermis for skin absorption testing: Results of the German prevalidation study. Altern. Lab. Anim. 2006, 34, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Van Gele, M.; Geusens, B.; Brochez, L.; Speeckaert, R.; Lambert, J. Three-dimensional skin models as tools for transdermal drug delivery: Challenges and limitations. Expert Opin. Drug Deliv. 2011, 8, 705–720. [Google Scholar] [CrossRef] [PubMed]
- Flaten, G.E.; Palac, Z.; Engesland, A.; Filipović-Grčić, J.; Vanić, Ž.; Škalko-Basnet, N. In vitro skin models as a tool in optimization of drug formulation. Eur. J. Pharm. Sci. 2015, 75, 10–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Planz, V.; Lehr, C.-M.; Windbergs, M. In vitro models for evaluating safety and efficacy of novel technologies for skin drug delivery. J. Control. Release 2016, 242, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Proksch, E.; Brandner, J.M.; Jensen, J.-M. The skin: An indispensable barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R.; Elias, P.M. Role of lipids in the formation and maintenance of the cutaneous permeability barrier. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2014, 1841, 280–294. [Google Scholar] [CrossRef]
- Van Smeden, J.; Bouwstra, J.A. Stratum Corneum Lipids: Their Role for the Skin Barrier Function in Healthy Subjects and Atopic Dermatitis Patients. Curr. Probl. Dermatol. 2016, 49, 8–26. [Google Scholar] [PubMed]
- Bouwstra, J.A.; Gooris, G.S. The lipid organisation in human stratum corneum and model systems. Open Dermatol. J. 2010, 4. [Google Scholar] [CrossRef]
- Boncheva, M. The physical chemistry of the stratum corneum lipids. Int. J. Cosmet. Sci. 2014, 36, 505–515. [Google Scholar] [CrossRef]
- Masukawa, Y.; Narita, H.; Sato, H.; Naoe, A.; Kondo, N.; Sugai, Y.; Oba, T.; Homma, R.; Ishikawa, J.; Takagi, Y.; et al. Comprehensive quantification of ceramide species in human stratum corneum. J. Lipid Res. 2009, 50, 1708–1719. [Google Scholar] [CrossRef] [Green Version]
- Boiten, W.; Absalah, S.; Vreeken, R.; Bouwstra, J.; Van Smeden, J. Quantitative analysis of ceramides using a novel lipidomics approach with three dimensional response modelling. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2016, 1861, 1652–1661. [Google Scholar] [CrossRef]
- T’Kindt, R.; Jorge, L.; Dumont, E.; Couturon, P.; David, F.; Sandra, P.; Sandra, K. Profiling and Characterizing Skin Ceramides Using Reversed-Phase Liquid Chromatography–Quadrupole Time-of-Flight Mass Spectrometry. Anal. Chem. 2011, 84, 403–411. [Google Scholar] [CrossRef]
- Mojumdar, E.H.; Helder, R.W.J.; Gooris, G.S.; Bouwstra, J.A. Monounsaturated Fatty Acids Reduce the Barrier of Stratum Corneum Lipid Membranes by Enhancing the Formation of a Hexagonal Lateral Packing. Langmuir 2014, 30, 6534–6543. [Google Scholar] [CrossRef]
- Janssens, M.; Van Smeden, J.; Gooris, G.S.; Bras, W.; Portale, G.; Caspers, P.J.; Vreeken, R.J.; Hankemeier, T.; Kezic, S.; Wolterbeek, R.; et al. Increase in short-chain ceramides correlates with an altered lipid organization and decreased barrier function in atopic eczema patients. J. Lipid Res. 2012, 53, 2755–2766. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.H.; Lim, B.; Kim, J.W.; An, E.J.; Lee, D. Effect of composition on water permeability of model stratum corneum lipid membranes. Soft Matter 2012, 8, 1539–1546. [Google Scholar] [CrossRef]
- Ponec, M.; Gibbs, S.; Pilgram, G.; Boelsma, E.; Koerten, H.; Bouwstra, J.; Mommaas, M. Barrier function in reconstructed epidermis and its resemblance to native human skin. Ski. Pharmacol. Appl. Ski. Physiol. 2001, 14, 63–71. [Google Scholar] [CrossRef]
- Thakoersing, V.S.; Van Smeden, J.; Mulder, A.A.; Vreeken, R.J.; El Ghalbzouri, A.; Bouwstra, J.A. Increased Presence of Monounsaturated Fatty Acids in the Stratum Corneum of Human Skin Equivalents. J. Investig. Dermatol. 2013, 133, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Netzlaff, F.; Lehr, C.-M.; Wertz, P.; Schaefer, U. The human epidermis models EpiSkin®, SkinEthic® and EpiDerm®: An evaluation of morphology and their suitability for testing phototoxicity, irritancy, corrosivity, and substance transport. Eur. J. Pharm. Biopharm. 2005, 60, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Mieremet, A.; Rietveld, M.; Absalah, S.; Van Smeden, J.; Bouwstra, J.A.; El Ghalbzouri, A. Improved epidermal barrier formation in human skin models by chitosan modulated dermal matrices. PLoS ONE 2017, 12, e0174478. [Google Scholar] [CrossRef] [Green Version]
- Helder, R.W.; Boiten, W.A.; van Dijk, R.; Gooris, G.S.; El Ghalbzouri, A.; Bouwstra, J.A. The effects of LXR agonist T0901317 and LXR antagonist GSK2033 on morphogenesis and lipid properties in full thickness skin models. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158546. [Google Scholar] [CrossRef] [PubMed]
- Mieremet, A.; García, A.V.; Boiten, W.; Van Dijk, R.; Gooris, G.; Bouwstra, J.A.; El Ghalbzouri, A. Human skin equivalents cultured under hypoxia display enhanced epidermal morphogenesis and lipid barrier formation. Sci. Rep. 2019, 9, 7811. [Google Scholar] [CrossRef] [PubMed]
- Bouwstra, J.; Gooris, G.S.; Van Der Spek, J.; Bras, W. Structural Investigations of Human Stratum Corneum by Small-Angle X-Ray Scattering. J. Investig. Dermatol. 1991, 97, 1005–1012. [Google Scholar] [CrossRef] [Green Version]
- Howling, G.; Dettmar, P.W.; Goddard, P.; Hampson, F.C.; Dornish, M.; Wood, E.J. The effect of chitin and chitosan on the proliferation of human skin fibroblasts and keratinocytes in vitro. Biomaterials 2001, 22, 2959–2966. [Google Scholar] [CrossRef]
- Straseski, J.A.; Gibson, A.L.; Thomas-Virnig, C.L.; Allen-Hoffmann, B.L. Oxygen deprivation inhibits basal keratinocyte proliferation in a model of human skin and induces regio-specific changes in the distribution of epidermal adherens junction proteins, aquaporin-3, and glycogen. Wound Repair Regen. 2009, 17, 606–616. [Google Scholar] [CrossRef] [Green Version]
- Chatelet, C.; Damour, O.; Domard, A. Influence of the degree of acetylation on some biological properties of chitosan films. Biomaterials 2001, 22, 261–268. [Google Scholar] [CrossRef]
- Gooris, G.; Bouwstra, J. Infrared Spectroscopic Study of Stratum Corneum Model Membranes Prepared from Human Ceramides, Cholesterol, and Fatty Acids. Biophys. J. 2007, 92, 2785–2795. [Google Scholar] [CrossRef] [Green Version]
- Van Smeden, J.; Janssens, M.; Gooris, G.; Bouwstra, J. The important role of stratum corneum lipids for the cutaneous barrier function. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2014, 1841, 295–313. [Google Scholar] [CrossRef] [PubMed]
- Mieremet, A.; Helder, R.; Nadaban, A.; Gooris, G.; Boiten, W.; El Ghalbzouri, A.; Bouwstra, J.A. Contribution of Palmitic Acid to Epidermal Morphogenesis and Lipid Barrier Formation in Human Skin Equivalents. Int. J. Mol. Sci. 2019, 20, 6069. [Google Scholar] [CrossRef] [Green Version]
- Pullmannová, P.; Pavlíková, L.; Kováčik, A.; Sochorová, M.; Školová, B.; Slepička, P.; Maixner, J.; Zbytovská, J.; Vávrová, K. Permeability and microstructure of model stratum corneum lipid membranes containing ceramides with long (C16) and very long (C24) acyl chains. Biophys. Chem. 2017, 224, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Bouwstra, J.A.; Gooris, G.S.; Dubbelaar, F.E.; Ponec, M. Phase Behavior of Stratum Corneum Lipid Mixtures Based on Human Ceramides: The Role of Natural and Synthetic Ceramide 1. J. Investig. Dermatol. 2002, 118, 606–617. [Google Scholar] [CrossRef] [Green Version]
- Wertz, P.W.; Cho, E.S.; Downing, D.T. Effect of essential fatty acid deficiency on the epidermal sphingolipids of the rat. Biochim. Biophys. Acta Lipids Lipid Metab. 1983, 753, 350–355. [Google Scholar] [CrossRef]
- Hansen, H.S.; Jensen, B. Essential function of linoleic acid esterified in acylglucosylceramide and acylceramide in maintaining the epidermal water permeability barrier. Evidence from feeding studies with oleate, linoleate, arachidonate, columbinate and α-linolenate. Biochim. Biophys. Acta Lipids Lipid Metab. 1985, 834, 357–363. [Google Scholar] [CrossRef]
- Vičanová, J.; Weerheim, A.M.; Kempenaar, J.A.; Ponec, M. Incorporation of linoleic acid by cultured human keratinocytes. Arch. Dermatol. Res. 1999, 291, 405–412. [Google Scholar] [CrossRef]
- Simard, M.; Julien, P.; Fradette, J.; Pouliot, R. Modulation of the Lipid Profile of Reconstructed Skin Substitutes after Essential Fatty Acid Supplementation Affects Testosterone Permeability. Cells 2019, 8, 1142. [Google Scholar] [CrossRef] [Green Version]
- Boiten, W.; Helder, R.; Van Smeden, J.; Bouwstra, J. Selectivity in cornified envelop binding of ceramides in human skin and the role of LXR inactivation on ceramide binding. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 1206–1213. [Google Scholar] [CrossRef]
- Ohno, Y.; Kamiyama, N.; Nakamichi, S.; Kihara, A. PNPLA1 is a transacylase essential for the generation of the skin barrier lipid ω-O-acylceramide. Nat. Commun. 2017, 8, 14610. [Google Scholar] [CrossRef]
- Ohno, Y.; Nakamichi, S.; Ohkuni, A.; Kamiyama, N.; Naoe, A.; Tsujimura, H.; Yokose, U.; Sugiura, K.; Ishikawa, J.; Akiyama, M.; et al. Essential role of the cytochrome P450 CYP4F22 in the production of acylceramide, the key lipid for skin permeability barrier formation. Proc. Natl. Acad. Sci. USA 2015, 112, 7707–7712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elias, P.M.; Gruber, R.; Crumrine, D.; Menon, G.; Williams, M.L.; Wakefield, J.S.; Holleran, W.M.; Uchida, Y. Formation and functions of the corneocyte lipid envelope (CLE). Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2014, 1841, 314–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naik, A.; Kalia, Y.N.; Guy, R.H. Transdermal drug delivery: Overcoming the skin’s barrier function. Pharm. Sci. Technol. Today 2000, 3, 318–326. [Google Scholar] [CrossRef]
- Bolzinger, M.-A.; Briançon, S.; Pelletier, J.; Chevalier, Y. Penetration of drugs through skin, a complex rate-controlling membrane. Curr. Opin. Colloid Interface Sci. 2012, 17, 156–165. [Google Scholar] [CrossRef]
- de Jager, M.; Groenink, W.; Bielsa i Guivernau, R.; Andersson, E.; Angelova, N.; Ponec, M.; Bouwstra, J.A. A novel in vitro percutaneous penetration model: Evaluation of barrier properties with p-aminobenzoic acid and two of its derivatives. Pharm. Res. 2006, 23, 951–960. [Google Scholar] [CrossRef]
- Akdeniz, M.; Gabriel, S.; Lichterfeld-Kottner, A.; Blume-Peytavi, U.; Kottner, J. Transepidermal water loss in healthy adults: A systematic review and meta-analysis update. Br. J. Dermatol. 2018, 179, 1049–1055. [Google Scholar] [CrossRef]
- Hadgraft, J.; Lane, M.E. Transepidermal water loss and skin site: A hypothesis. Int. J. Pharm. 2009, 373, 1–3. [Google Scholar] [CrossRef]
- Chilcott, R.P.; Dalton, C.H.; Emmanuel, A.J.; Allen, C.E.; Bradley, S.T. Transepidermal Water Loss Does Not Correlate with Skin Barrier Function In Vitro. J. Investig. Dermatol. 2002, 118, 871–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levin, J.; Maibach, H. The correlation between transepidermal water loss and percutaneous absorption: An overview. J. Control. Release 2005, 103, 291–299. [Google Scholar] [CrossRef]
- Lotte, C.; Rougier, A.; Wilson, D.R.; Maibach, H.I. In vivo relationship between transepidermal water loss and percutaneous penetration of some organic com-pounds in man: Effect of anatomic site. Arch. Dermatol. Res. 1987, 279, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Haisma, E.M.; Rietveld, M.H.; De Breij, A.; Van Dissel, J.T.; El Ghalbzouri, A.; Nibbering, P.H. Inflammatory and Antimicrobial Responses to Methicillin-Resistant Staphylococcus aureus in an In Vitro Wound Infection Model. PLoS ONE 2013, 8, e82800. [Google Scholar] [CrossRef] [Green Version]
- Ponec, M.; Weerheim, A.; Kempenaar, J.; Mulder, A.; Gooris, G.S.; Bouwstra, J.; Mommaas, A.M. The Formation of Competent Barrier Lipids in Reconstructed Human Epidermis Requires the Presence of Vitamin C. J. Investig. Dermatol. 1997, 109, 348–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berkers, T.; Van Dijk, L.; Absalah, S.; Van Smeden, J.; Bouwstra, J.A. Topically applied fatty acids are elongated before incorporation in the stratum corneum lipid matrix in compromised skin. Exp. Dermatol. 2017, 26, 36–43. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. 1959, 37, 911–917. [Google Scholar]

| Number | HSE Type | O2 | GSK2033 | Treatment | Abbreviation |
|---|---|---|---|---|---|
| 1 | FTM | 20% | (−) | Control | FTM(20−) |
| 2 | FTM | 20% | (+) | Single | FTM(20+) |
| 3 | FTM | 3% | (−) | Single | FTM(3−) |
| 4 | FTM | 3% | (+) | Double | FTM(3+) |
| 5 | CC-FTM | 20% | (−) | Single | CC-FTM(20−) |
| 6 | CC-FTM | 20% | (+) | Double | CC-FTM(20+) |
| 7 | CC-FTM | 3% | (−) | Double | CC-FTM(3−) |
| 8 | CC-FTM | 3% | (+) | Triple | CC-FTM(3+) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mieremet, A.; Helder, R.W.J.; Nadaban, A.; Boiten, W.A.; Gooris, G.S.; El Ghalbzouri, A.; Bouwstra, J.A. Multitargeted Approach for the Optimization of Morphogenesis and Barrier Formation in Human Skin Equivalents. Int. J. Mol. Sci. 2021, 22, 5790. https://doi.org/10.3390/ijms22115790
Mieremet A, Helder RWJ, Nadaban A, Boiten WA, Gooris GS, El Ghalbzouri A, Bouwstra JA. Multitargeted Approach for the Optimization of Morphogenesis and Barrier Formation in Human Skin Equivalents. International Journal of Molecular Sciences. 2021; 22(11):5790. https://doi.org/10.3390/ijms22115790
Chicago/Turabian StyleMieremet, Arnout, Richard W. J. Helder, Andreea Nadaban, Walter A. Boiten, Gert S. Gooris, Abdoelwaheb El Ghalbzouri, and Joke A. Bouwstra. 2021. "Multitargeted Approach for the Optimization of Morphogenesis and Barrier Formation in Human Skin Equivalents" International Journal of Molecular Sciences 22, no. 11: 5790. https://doi.org/10.3390/ijms22115790
APA StyleMieremet, A., Helder, R. W. J., Nadaban, A., Boiten, W. A., Gooris, G. S., El Ghalbzouri, A., & Bouwstra, J. A. (2021). Multitargeted Approach for the Optimization of Morphogenesis and Barrier Formation in Human Skin Equivalents. International Journal of Molecular Sciences, 22(11), 5790. https://doi.org/10.3390/ijms22115790






