The Role of IL-17-Producing Cells in Cutaneous Fungal Infections
Abstract
:1. Introduction
1.1. Th17 Cells and Antimicrobial Action against Fungi
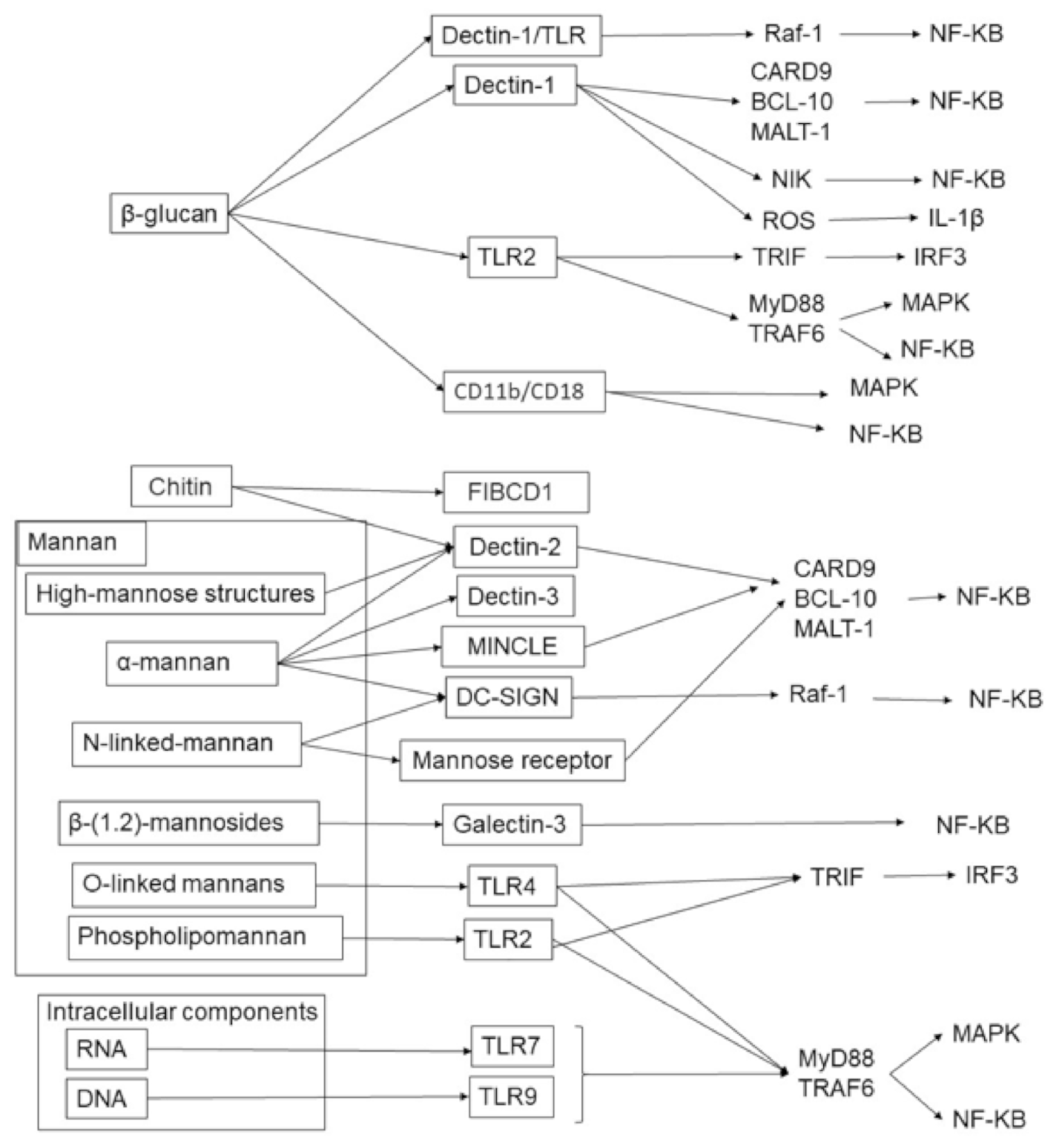
1.2. Pattern Recognition for IL-17 Induction against Fungi
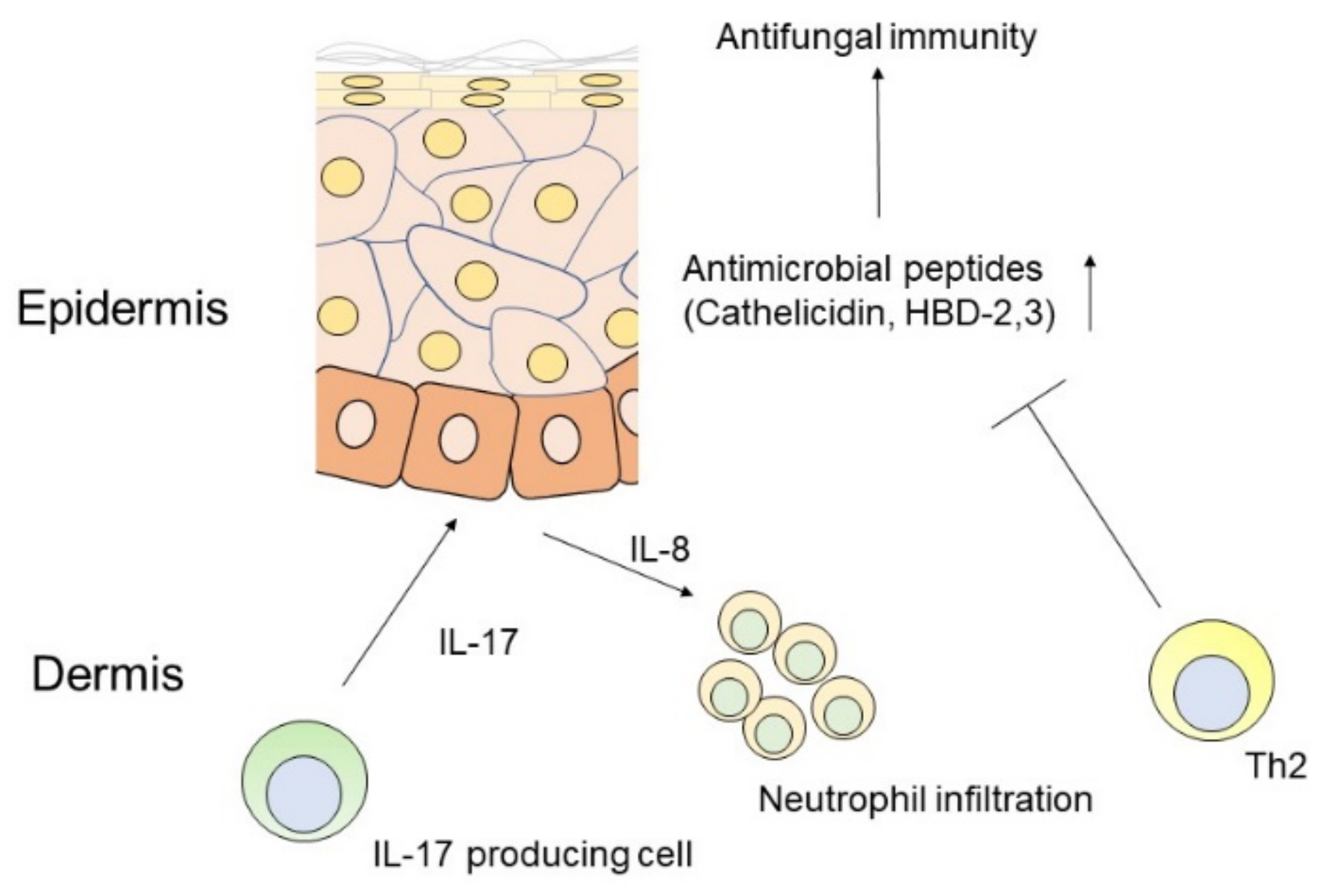
1.3. IL-17 and Antimicrobial Action in the Skin
1.4. Skin Fungal Infection and Th17 Cells
2. Candidiasis
3. Dermatophytosis
4. Malassezia
5. Sporotrichosis
6. Chromoblastomycosis
7. Mycetoma
8. Paracoccidioidomycosis
9. Coccidioidomycosis
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dainichi, T.; Kitoh, A.; Otsuka, A.; Nakajima, S.; Nomura, T.; Kaplan, D.H.; Kabashima, K. The epithelial immune microenvironment (EIME) in atopic dermatitis and psoriasis. Nat. Immunol. 2018, 19, 1286–1298. [Google Scholar] [CrossRef]
- Egawa, G.; Kabashima, K. Skin as a peripheral lymphoid organ: Revisiting the concept of skin-associated lymphoid tissues. J. Investig. Dermatol. 2011, 131, 2178–2185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernard, J.J.; Gallo, R.L.; Krutmann, J. Photoimmunology: How ultraviolet radiation affects the immune system. Nat. Rev. Immunol. 2019, 19, 688–701. [Google Scholar] [CrossRef] [PubMed]
- Sakuragi, Y.; Sawada, Y.; Nakamura, M. Leukoderma following allergic contact dermatitis caused by the silicone component silprene-30A/B in swimming goggles. Contact Dermat. 2017, 77, 418–419. [Google Scholar] [CrossRef] [PubMed]
- Honda, T.; Egawa, G.; Grabbe, S.; Kabashima, K. Update of immune events in the murine contact hypersensitivity model: Toward the understanding of allergic contact dermatitis. J. Investig. Dermatol. 2013, 133, 303–315. [Google Scholar] [CrossRef] [Green Version]
- Nakatsuji, T.; Chen, T.H.; Narala, S.; Chun, K.A.; Two, A.M.; Yun, T.; Shafiq, F.; Kotol, P.F.; Bouslimani, A.; Melnik, A.V.; et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [Green Version]
- Harpf, V.; Rambach, G.; Würzner, R.; Lass-Flörl, C.; Speth, C. Candida and complement: New aspects in an old battle. Front. Immunol. 2020, 11, 1471. [Google Scholar] [CrossRef]
- Yoo, Y.J.; Kim, A.R.; Perinpanayagam, H.; Han, S.H.; Kum, K.Y. Candida albicans virulence factors and pathogenicity for endodontic infections. Microorganisms 2020, 8, 1300. [Google Scholar] [CrossRef] [PubMed]
- Kabashima, K.; Honda, T.; Ginhoux, F.; Egawa, G. The immunological anatomy of the skin. Nat. Rev. Immunol. 2019, 19, 19–30. [Google Scholar] [CrossRef]
- Hube, B.; Hay, R.; Brasch, J.; Veraldi, S.; Schaller, M. Dermatomycoses and inflammation: The adaptive balance between growth, damage, and survival. J. Mycol. Med. 2015, 25, e44–e58. [Google Scholar] [CrossRef]
- Hay, R.J. Tinea capitis: Current status. Mycopathologia 2017, 182, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Kashem, S.W.; Kaplan, D.H. Skin immunity to Candida albicans. Trends Immunol. 2016, 37, 440–450. [Google Scholar] [CrossRef] [Green Version]
- Lanternier, F.; Pathan, S.; Vincent, Q.B.; Liu, L.; Cypowyj, S.; Prando, C.; Migaud, M.; Taibi, L.; Ammar-Khodja, A.; Stambouli, O.B.; et al. Deep dermatophytosis and inherited CARD9 deficiency. N. Engl. J. Med. 2013, 369, 1704–1714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, D.; Tjon, E.C.; Andersson, K.M.; Molica, G.M.; Pham, M.C.; Healy, B.; Murugaiyan, G.; Pochet, N.; Kuchroo, V.K.; Bokarewa, M.I.; et al. Aberrant expression of USF2 in refractory rheumatoid arthritis and its regulation of proinflammatory cytokines in Th17 cells. Proc. Natl. Acad. Sci. USA 2020, 117, 30639–30648. [Google Scholar] [CrossRef]
- Saito-Sasaki, N.; Sawada, Y.; Omoto, D.; Ohmori, S.; Haruyama, S.; Yoshioka, M.; Nishio, D.; Nakamura, M. A possible pathogenetic role of IL-23/IL-17 axis in rheumatoid nodules in patients with rheumatoid arthritis. Clin. Immunol. 2016, 170, 20–21. [Google Scholar] [CrossRef]
- Hawkes, J.E.; Chan, T.C.; Krueger, J.G. Psoriasis pathogenesis and the development of novel targeted immune therapies. J. Allergy Clin. Immunol. 2017, 140, 645–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almradi, A.; Hanzel, J.; Sedano, R.; Parker, C.E.; Feagan, B.G.; Ma, C.; Jairath, V. Clinical trials of IL-12/IL-23 inhibitors in inflammatory bowel disease. BioDrugs 2020, 34, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Huang, L.; Lv, P.; Li, X.; Liu, G.; Chen, Y.; Wang, Z.; Qian, X.; Shen, Y.; Li, Y.; et al. The role of Th17 cells in psoriasis. Immunol. Res. 2020, 68, 296–309. [Google Scholar] [CrossRef]
- Leung, S.; Liu, X.; Fang, L.; Chen, X.; Guo, T.; Zhang, J. The cytokine milieu in the interplay of pathogenic Th1/Th17 cells and regulatory T cells in autoimmune disease. Cell. Mol. Immunol. 2010, 7, 182–189. [Google Scholar] [CrossRef]
- Koga, C.; Kabashima, K.; Shiraishi, N.; Kobayashi, M.; Tokura, Y. Possible pathogenic role of Th17 cells for atopic dermatitis. J. Investig. Dermatol. 2008, 128, 2625–2630. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Danilenko, D.M.; Valdez, P.; Kasman, I.; Eastham-Anderson, J.; Wu, J.; Ouyang, W. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 2007, 445, 648–651. [Google Scholar] [CrossRef]
- Cheuk, S.; Wikén, M.; Blomqvist, L.; Nylén, S.; Talme, T.; Ståhle, M.; Eidsmo, L. Epidermal Th22 and Tc17 cells form a localized disease memory in clinically healed psoriasis. J. Immunol. 2014, 192, 3111–3120. [Google Scholar] [CrossRef] [Green Version]
- Ortega, C.; Fernández, A.S.; Carrillo, J.M.; Romero, P.; Molina, I.J.; Moreno, J.C.; Santamaría, M. IL-17-producing CD8+ T lymphocytes from psoriasis skin plaques are cytotoxic effector cells that secrete Th17-related cytokines. J. Leukoc. Biol. 2009, 86, 435–443. [Google Scholar] [CrossRef]
- Hijnen, D.; Knol, E.F.; Gent, Y.Y.; Giovannone, B.; Beijn, S.J.; Kupper, T.S.; Bruijnzeel-Koomen, C.A.; Clark, R.A. CD8(+) T cells in the lesional skin of atopic dermatitis and psoriasis patients are an important source of IFN-γ, IL-13, IL-17, and IL-22. J. Investig. Dermatol. 2013, 133, 973–979. [Google Scholar] [CrossRef] [Green Version]
- Yoshiki, R.; Kabashima, K.; Honda, T.; Nakamizo, S.; Sawada, Y.; Sugita, K.; Yoshioka, H.; Ohmori, S.; Malissen, B.; Tokura, Y.; et al. IL-23 from Langerhans cells is required for the development of imiquimod-induced psoriasis-like dermatitis by induction of IL-17A-producing γδ T cells. J. Investig. Dermatol. 2014, 134, 1912–1921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamizo, S.; Honda, T.; Adachi, A.; Nagatake, T.; Kunisawa, J.; Kitoh, A.; Otsuka, A.; Dainichi, T.; Nomura, T.; Ginhoux, F.; et al. High fat diet exacerbates murine psoriatic dermatitis by increasing the number of IL-17-producing γδ T cells. Sci. Rep. 2017, 7, 14076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito-Sasaki, N.; Sawada, Y.; Mashima, E.; Yamaguchi, T.; Ohmori, S.; Yoshioka, H.; Haruyama, S.; Okada, E.; Nakamura, M. Maresin-1 suppresses imiquimod-induced skin inflammation by regulating IL-23 receptor expression. Sci. Rep. 2018, 8, 5522. [Google Scholar] [CrossRef] [PubMed]
- Ueharaguchi, Y.; Honda, T.; Kusuba, N.; Hanakawa, S.; Adachi, A.; Sawada, Y.; Otsuka, A.; Kitoh, A.; Dainichi, T.; Egawa, G.; et al. Thromboxane A(2) facilitates IL-17A production from Vγ4(+) γδ T cells and promotes psoriatic dermatitis in mice. J. Allergy Clin. Immunol. 2018, 142, 680–683. [Google Scholar] [CrossRef] [Green Version]
- Sawada, Y.; Honda, T.; Nakamizo, S.; Otsuka, A.; Ogawa, N.; Kobayashi, Y.; Nakamura, M.; Kabashima, K. Resolvin E1 attenuates murine psoriatic dermatitis. Sci. Rep. 2018, 8, 11873. [Google Scholar] [CrossRef] [Green Version]
- Stockinger, B.; Omenetti, S. The dichotomous nature of T helper 17 cells. Nat. Rev. Immunol. 2017, 17, 535–544. [Google Scholar] [CrossRef]
- Shibue, Y.; Kimura, S.; Kajiwara, C.; Iwakura, Y.; Yamaguchi, K.; Tateda, K. Role of interleukin-17 in a murine community-associated methicillin-resistant Staphylococcus aureus pneumonia model. Microbes Infect. 2019, 21, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Thammahong, A.; Kiatsurayanon, C.; Edwards, S.W.; Rerknimitr, P.; Chiewchengchol, D. The clinical significance of fungi in atopic dermatitis. Int. J. Dermatol. 2020, 59, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Pontón, J.; Omaetxebarría, M.J.; Elguezabal, N.; Alvarez, M.; Moragues, M.D. Immunoreactivity of the fungal cell wall. Med. Mycol. 2001, 39 (Suppl. 1), 101–110. [Google Scholar] [CrossRef]
- Biondo, C.; Malara, A.; Costa, A.; Signorino, G.; Cardile, F.; Midiri, A.; Galbo, R.; Papasergi, S.; Domina, M.; Pugliese, M.; et al. Recognition of fungal RNA by TLR7 has a nonredundant role in host defense against experimental candidiasis. Eur. J. Immunol. 2012, 42, 2632–2643. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, C.S.; Dubey, L.K.; Colmorten, K.B.; Moeller, J.B.; Hammond, M.A.; Nielsen, O.; Schlosser, A.; Templeton, S.P.; Sorensen, G.L.; Holmskov, U. FIBCD1 binds Aspergillus fumigatus and regulates lung epithelial response to cell wall components. Front. Immunol. 2018, 9, 1967. [Google Scholar] [CrossRef] [Green Version]
- Moeller, J.B.; Leonardi, I.; Schlosser, A.; Flamar, A.L.; Bessman, N.J.; Putzel, G.G.; Thomsen, T.; Hammond, M.; Jepsen, C.S.; Skjødt, K.; et al. Modulation of the fungal mycobiome is regulated by the chitin-binding receptor FIBCD1. J. Exp. Med. 2019, 216, 2689–2700. [Google Scholar] [CrossRef]
- Haley, K.; Igyártó, B.Z.; Ortner, D.; Bobr, A.; Kashem, S.; Schenten, D.; Kaplan, D.H. Langerhans cells require MyD88-dependent signals for Candida albicans response but not for contact hypersensitivity or migration. J. Immunol. 2012, 188, 4334–4339. [Google Scholar] [CrossRef] [Green Version]
- Wcisło-Dziadecka, D.; Kaźmierczak, A.; Grabarek, B.; Zbiciak-Nylec, M.; Brzezińska-Wcisło, L. Are new variants of psoriasis therapy (IL-17 inhibitors) safe? Int. J. Dermatol. 2019, 58, 1360–1365. [Google Scholar] [CrossRef]
- Papini, M.; Natalini, Y. Candida infections in psoriatic patients on anti-IL17 therapy: A case series. J. Dermatol. Treat. 2018, 29 (Suppl. 2), 3–4. [Google Scholar] [CrossRef]
- Peric, M.; Koglin, S.; Dombrowski, Y.; Gross, K.; Bradac, E.; Büchau, A.; Steinmeyer, A.; Zügel, U.; Ruzicka, T.; Schauber, J. Vitamin D analogs differentially control antimicrobial peptide/“alarmin” expression in psoriasis. PLoS ONE 2009, 4, e6340. [Google Scholar] [CrossRef] [Green Version]
- Peric, M.; Koglin, S.; Kim, S.M.; Morizane, S.; Besch, R.; Prinz, J.C.; Ruzicka, T.; Gallo, R.L.; Schauber, J. IL-17A enhances vitamin D3-induced expression of cathelicidin antimicrobial peptide in human keratinocytes. J. Immunol. 2008, 181, 8504–8512. [Google Scholar] [CrossRef] [Green Version]
- Eyerich, K.; Pennino, D.; Scarponi, C.; Foerster, S.; Nasorri, F.; Behrendt, H.; Ring, J.; Traidl-Hoffmann, C.; Albanesi, C.; Cavani, A. IL-17 in atopic eczema: Linking allergen-specific adaptive and microbial-triggered innate immune response. J. Allergy Clin. Immunol. 2009, 123, 59–66. [Google Scholar] [CrossRef]
- Liang, S.C.; Tan, X.Y.; Luxenberg, D.P.; Karim, R.; Dunussi-Joannopoulos, K.; Collins, M.; Fouser, L.A. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 2006, 203, 2271–2279. [Google Scholar] [CrossRef]
- Zheng, N.X.; Wang, Y.; Hu, D.D.; Yan, L.; Jiang, Y.Y. The role of pattern recognition receptors in the innate recognition of Candida albicans. Virulence 2015, 6, 347–361. [Google Scholar] [CrossRef] [Green Version]
- Sirvent, S.; Soria, I.; Cirauqui, C.; Cases, B.; Manzano, A.I.; Diez-Rivero, C.M.; Reche, P.A.; López-Relaño, J.; Martínez-Naves, E.; Cañada, F.J.; et al. Novel vaccines targeting dendritic cells by coupling allergoids to nonoxidized mannan enhance allergen uptake and induce functional regulatory T cells through programmed death ligand 1. J. Allergy Clin. Immunol. 2016, 138, 558–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, K.; Naganuma, M.; Mizuno, S.; Suzuki, H.; Kitazume, M.T.; Shimamura, K.; Chiba, S.; Sugita, A.; Matsuoka, K.; Hisamatsu, T.; et al. β-(1,3)-Glucan derived from Candida albicans induces inflammatory cytokines from macrophages and lamina propria mononuclear cells derived from patients with Crohn’s disease. Intest. Res. 2018, 16, 384–392. [Google Scholar] [CrossRef] [Green Version]
- Netea, M.G.; Gow, N.A.; Munro, C.A.; Bates, S.; Collins, C.; Ferwerda, G.; Hobson, R.P.; Bertram, G.; Hughes, H.B.; Jansen, T.; et al. Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. J. Clin. Investig. 2006, 116, 1642–1650. [Google Scholar] [CrossRef]
- Means, T.K.; Mylonakis, E.; Tampakakis, E.; Colvin, R.A.; Seung, E.; Puckett, L.; Tai, M.F.; Stewart, C.R.; Pukkila-Worley, R.; Hickman, S.E.; et al. Evolutionarily conserved recognition and innate immunity to fungal pathogens by the scavenger receptors SCARF1 and CD36. J. Exp. Med. 2009, 206, 637–653. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.N.Y.; Padungros, P.; Wongsrisupphakul, P.; Sa-Ard-Iam, N.; Mahanonda, R.; Matangkasombut, O.; Choo, M.K.; Ritprajak, P. Cell wall mannan of Candida krusei mediates dendritic cell apoptosis and orchestrates Th17 polarization via TLR-2/MyD88-dependent pathway. Sci. Rep. 2018, 8, 17123. [Google Scholar] [CrossRef] [PubMed]
- Choteau, L.; Parny, M.; François, N.; Bertin, B.; Fumery, M.; Dubuquoy, L.; Takahashi, K.; Colombel, J.F.; Jouault, T.; Poulain, D.; et al. Role of mannose-binding lectin in intestinal homeostasis and fungal elimination. Mucosal Immunol. 2016, 9, 767–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linden, J.R.; Kunkel, D.; Laforce-Nesbitt, S.S.; Bliss, J.M. The role of galectin-3 in phagocytosis of Candida albicans and Candida parapsilosis by human neutrophils. Cell Microbiol. 2013, 15, 1127–1142. [Google Scholar] [CrossRef] [Green Version]
- Oharaseki, T.; Yokouchi, Y.; Enomoto, Y.; Sato, W.; Ishibashi, K.; Miura, N.; Ohno, N.; Takahashi, K. Recognition of alpha-mannan by dectin 2 is essential for onset of Kawasaki disease-like murine vasculitis induced by Candida albicans cell-wall polysaccharide. Mod. Rheumatol. 2020, 30, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.L.; Zhao, X.Q.; Jiang, C.; You, Y.; Chen, X.P.; Jiang, Y.Y.; Jia, X.M.; Lin, X. C-Type lectin receptors Dectin-3 and Dectin-2 form a heterodimeric pattern-recognition receptor for host defense against fungal infection. Immunity 2013, 39, 324–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cambi, A.; Netea, M.G.; Mora-Montes, H.M.; Gow, N.A.; Hato, S.V.; Lowman, D.W.; Kullberg, B.J.; Torensma, R.; Williams, D.L.; Figdor, C.G. Dendritic cell interaction with Candida albicans critically depends on N-linked mannan. J. Biol. Chem. 2008, 283, 20590–20599. [Google Scholar] [CrossRef] [Green Version]
- Van de Veerdonk, F.L.; Marijnissen, R.J.; Kullberg, B.J.; Koenen, H.J.; Cheng, S.C.; Joosten, I.; van den Berg, W.B.; Williams, D.L.; van der Meer, J.W.; Joosten, L.A.; et al. The macrophage mannose receptor induces IL-17 in response to Candida albicans. Cell Host Microbe 2009, 5, 329–340. [Google Scholar] [CrossRef] [Green Version]
- Miyazato, A.; Nakamura, K.; Yamamoto, N.; Mora-Montes, H.M.; Tanaka, M.; Abe, Y.; Tanno, D.; Inden, K.; Gang, X.; Ishii, K.; et al. Toll-Like receptor 9-dependent activation of myeloid dendritic cells by deoxynucleic acids from Candida albicans. Infect. Immun. 2009, 77, 3056–3064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashem, S.W.; Riedl, M.S.; Yao, C.; Honda, C.N.; Vulchanova, L.; Kaplan, D.H. Nociceptive sensory fibers drive interleukin-23 production from CD301b+ dermal dendritic cells and drive protective cutaneous immunity. Immunity 2015, 43, 515–526. [Google Scholar] [CrossRef] [Green Version]
- Trautwein-Weidner, K.; Gladiator, A.; Kirchner, F.R.; Becattini, S.; Rülicke, T.; Sallusto, F.; LeibundGut-Landmann, S. Antigen-specific Th17 cells are primed by distinct and complementary dendritic cell subsets in oropharyngeal candidiasis. PLoS Pathog. 2015, 11, e1005164. [Google Scholar] [CrossRef] [Green Version]
- Campois, T.G.; Zucoloto, A.Z.; de Almeida Araujo, E.J.; Svidizinski, T.I.E.; Almeida, R.S.; da Silva Quirino, G.F.; Harano, R.M.; Conchon-Costa, I.; Felipe, I. Immunological and histopathological characterization of cutaneous candidiasis. J. Med. Microbiol. 2015, 64, 810–817. [Google Scholar] [CrossRef] [Green Version]
- Park, C.O.; Fu, X.; Jiang, X.; Pan, Y.; Teague, J.E.; Collins, N.; Tian, T.; O’Malley, J.T.; Emerson, R.O.; Kim, J.H.; et al. Staged development of long-lived T-cell receptor αβ T(H)17 resident memory T-cell population to Candida albicans after skin infection. J. Allergy Clin. Immunol. 2018, 142, 647–662. [Google Scholar] [CrossRef] [Green Version]
- Alves de Medeiros, A.K.; Lodewick, E.; Bogaert, D.J.; Haerynck, F.; Van Daele, S.; Lambrecht, B.; Bosma, S.; Vanderdonckt, L.; Lortholary, O.; Migaud, M.; et al. Chronic and invasive fungal infections in a family with CARD9 deficiency. J. Clin. Immunol. 2016, 36, 204–209. [Google Scholar] [CrossRef]
- Glocker, E.O.; Hennigs, A.; Nabavi, M.; Schäffer, A.A.; Woellner, C.; Salzer, U.; Pfeifer, D.; Veelken, H.; Warnatz, K.; Tahami, F.; et al. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N. Engl. J. Med. 2009, 361, 1727–1735. [Google Scholar] [CrossRef] [Green Version]
- Puel, A.; Cypowyj, S.; Bustamante, J.; Wright, J.F.; Liu, L.; Lim, H.K.; Migaud, M.; Israel, L.; Chrabieh, M.; Audry, M.; et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science 2011, 332, 65–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kärner, J.; Pihlap, M.; Ranki, A.; Krohn, K.; Trebusak Podkrajsek, K.; Bratanic, N.; Battelino, T.; Willcox, N.; Peterson, P.; Kisand, K. IL-6-Specific autoantibodies among APECED and thymoma patients. Immun. Inflamm. Dis. 2016, 4, 235–243. [Google Scholar] [CrossRef] [Green Version]
- Ling, Y.; Cypowyj, S.; Aytekin, C.; Galicchio, M.; Camcioglu, Y.; Nepesov, S.; Ikinciogullari, A.; Dogu, F.; Belkadi, A.; Levy, R.; et al. Inherited IL-17RC deficiency in patients with chronic mucocutaneous candidiasis. J. Exp. Med. 2015, 212, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.; Kofod-Olsen, E.; Spaun, E.; Larsen, C.S.; Christiansen, M.; Mogensen, T.H. A STAT1-gain-of-function mutation causing Th17 deficiency with chronic mucocutaneous candidiasis, psoriasiform hyperkeratosis and dermatophytosis. BMJ Case Rep. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Van de Veerdonk, F.L.; Plantinga, T.S.; Hoischen, A.; Smeekens, S.P.; Joosten, L.A.; Gilissen, C.; Arts, P.; Rosentul, D.C.; Carmichael, A.J.; Smits-van der Graaf, C.A.; et al. STAT1 mutations in autosomal dominant chronic mucocutaneous candidiasis. N. Engl. J. Med. 2011, 365, 54–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abusleme, L.; Diaz, P.I.; Freeman, A.F.; Greenwell-Wild, T.; Brenchley, L.; Desai, J.V.; Ng, W.I.; Holland, S.M.; Lionakis, M.S.; Segre, J.A.; et al. Human defects in STAT3 promote oral mucosal fungal and bacterial dysbiosis. JCI Insight 2018, 3, e122061. [Google Scholar] [CrossRef] [PubMed]
- Dahl, M.V. Dermatophytosis and the immune response. J. Am. Acad. Dermatol. 1994, 31, S34–S41. [Google Scholar] [CrossRef]
- Dahl, M.V.; Grando, S.A. Chronic dermatophytosis: What is special about Trichophyton rubrum? Adv. Dermatol. 1994, 9, 97–109. [Google Scholar]
- Sakuragi, Y.; Sawada, Y.; Hara, Y.; Ohmori, S.; Omoto, D.; Haruyama, S.; Yoshioka, M.; Nishio, D.; Nakamura, M. Increased circulating Th17 cell in a patient with tinea capitis caused by Microsporum canis. Allergol. Int. Off. J. Jpn. Soc. Allergol. 2016, 65, 215–216. [Google Scholar] [CrossRef] [Green Version]
- Goto, Y.; Suzuki, T.; Suzuki, Y.; Anzawa, K.; Mochizuki, T.; Tamura, T.; Makimura, K.; Aoshima, M.; Ito, T.; Tokura, Y. Trichophyton tonsurans-induced kerion celsi with decreased defensin expression and paradoxically increased interleukin-17A production. J. Dermatol. 2019, 46, 794–797. [Google Scholar] [CrossRef]
- Tokura, Y.; Sawada, Y.; Shimauchi, T. Skin manifestations of adult T-cell leukemia/lymphoma: Clinical, cytological and immunological features. J. Dermatol. 2014, 41, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Sawada, Y.; Nakamura, M.; Kabashima-Kubo, R.; Shimauchi, T.; Kobayashi, M.; Tokura, Y. Defective epidermal innate immunity and resultant superficial dermatophytosis in adult T-cell leukemia/lymphoma. Clin. Cancer Res. 2012, 18, 3772–3779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawada, Y.; Nakamura, M.; Kabashima-Kubo, R.; Shimauchi, T.; Kobayashi, M.; Tokura, Y. Defective epidermal induction of S100A7/psoriasin associated with low frequencies of skin-infiltrating Th17 cells in dermatophytosis-prone adult T cell leukemia/lymphoma. Clin. Immunol. 2013, 148, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Burstein, V.L.; Guasconi, L.; Beccacece, I.; Theumer, M.G.; Mena, C.; Prinz, I.; Cervi, L.; Herrero, M.; Masih, D.T.; Chiapello, L.S. IL-17-Mediated immunity controls skin infection and T helper 1 response during experimental Microsporum canis dermatophytosis. J. Investig. Dermatol. 2018, 138, 1744–1753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshikawa, F.S.; Yabe, R.; Iwakura, Y.; de Almeida, S.R.; Saijo, S. Dectin-1 and Dectin-2 promote control of the fungal pathogen Trichophyton rubrum independently of IL-17 and adaptive immunity in experimental deep dermatophytosis. Innate Immun. 2016, 22, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, F.S.; Ferreira, L.G.; de Almeida, S.R. IL-1 signaling inhibits Trichophyton rubrum conidia development and modulates the IL-17 response in vivo. Virulence 2015, 6, 449–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stalhberger, T.; Simenel, C.; Clavaud, C.; Eijsink, V.G.; Jourdain, R.; Delepierre, M.; Latgé, J.P.; Breton, L.; Fontaine, T. Chemical organization of the cell wall polysaccharide core of Malassezia restricta. J. Biol. Chem. 2014, 289, 12647–12656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baroni, A.; Orlando, M.; Donnarumma, G.; Farro, P.; Iovene, M.R.; Tufano, M.A.; Buommino, E. Toll-Like receptor 2 (TLR2) mediates intracellular signalling in human keratinocytes in response to Malassezia furfur. Arch. Dermatol. Res. 2006, 297, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Roesner, L.M.; Ernst, M.; Chen, W.; Begemann, G.; Kienlin, P.; Raulf, M.K.; Lepenies, B.; Werfel, T. Human thioredoxin, a damage-associated molecular pattern and Malassezia-crossreactive autoallergen, modulates immune responses via the C-type lectin receptors Dectin-1 and Dectin-2. Sci. Rep. 2019, 9, 11210. [Google Scholar] [CrossRef] [Green Version]
- Haider, M.; Dambuza, I.M.; Asamaphan, P.; Stappers, M.; Reid, D.; Yamasaki, S.; Brown, G.D.; Gow, N.A.R.; Erwig, L.P. The pattern recognition receptors dectin-2, mincle, and FcRγ impact the dynamics of phagocytosis of Candida, Saccharomyces, Malassezia, and Mucor species. PLoS ONE 2019, 14, e0220867. [Google Scholar] [CrossRef] [Green Version]
- Wollenberg, A.; Mommaas, M.; Oppel, T.; Schottdorf, E.M.; Günther, S.; Moderer, M. Expression and function of the mannose receptor CD206 on epidermal dendritic cells in inflammatory skin diseases. J. Investig. Dermatol. 2002, 118, 327–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasperkovitz, P.V.; Khan, N.S.; Tam, J.M.; Mansour, M.K.; Davids, P.J.; Vyas, J.M. Toll-Like receptor 9 modulates macrophage antifungal effector function during innate recognition of Candida albicans and Saccharomyces cerevisiae. Infect. Immun. 2011, 79, 4858–4867. [Google Scholar] [CrossRef] [Green Version]
- Balaji, H.; Heratizadeh, A.; Wichmann, K.; Niebuhr, M.; Crameri, R.; Scheynius, A.; Werfel, T. Malassezia sympodialis thioredoxin-specific T cells are highly cross-reactive to human thioredoxin in atopic dermatitis. J. Allergy Clin. Immunol. 2011, 128, 92–99. [Google Scholar] [CrossRef]
- Sato, Y.; Fujimura, T.; Tanita, K.; Chunbing, L.; Matsushita, S.; Fujisawa, Y.; Otsuka, A.; Yamamoto, Y.; Hidaka, T.; Aiba, S. Malassezia-Derived aryl hydrocarbon receptor ligands enhance the CCL20/Th17/soluble CD163 pathogenic axis in extra-mammary Paget’s disease. Exp. Dermatol. 2019, 28, 933–939. [Google Scholar] [CrossRef]
- Sparber, F.; De Gregorio, C.; Steckholzer, S.; Ferreira, F.M.; Dolowschiak, T.; Ruchti, F.; Kirchner, F.R.; Mertens, S.; Prinz, I.; Joller, N.; et al. The skin commensal yeast Malassezia triggers a type 17 response that coordinates anti-fungal immunity and exacerbates skin inflammation. Cell Host Microbe 2019, 25, 389–403. [Google Scholar] [CrossRef] [Green Version]
- Sizar, O.; Talati, R. Sporotrichosis. In StatPearls; StatPearls Publishing Copyright; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2020. [Google Scholar]
- Chakrabarti, A.; Bonifaz, A.; Gutierrez-Galhardo, M.C.; Mochizuki, T.; Li, S. Global epidemiology of sporotrichosis. Med. Mycol. 2015, 53, 3–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coles, F.B.; Schuchat, A.; Hibbs, J.R.; Kondracki, S.F.; Salkin, I.F.; Dixon, D.M.; Chang, H.G.; Duncan, R.A.; Hurd, N.J.; Morse, D.L. A multistate outbreak of sporotrichosis associated with sphagnum moss. Am. J. Epidemiol. 1992, 136, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Jellmayer, J.A.; Ferreira, L.S.; Manente, F.A.; Gonçalves, A.C.; Polesi, M.C.; Batista-Duharte, A.; Carlos, I.Z. Dectin-1 expression by macrophages and related antifungal mechanisms in a murine model of Sporothrix schenckii sensu stricto systemic infection. Microb. Pathog. 2017, 110, 78–84. [Google Scholar] [CrossRef] [Green Version]
- Sassá, M.F.; Saturi, A.E.; Souza, L.F.; Ribeiro, L.C.; Sgarbi, D.B.; Carlos, I.Z. Response of macrophage Toll-like receptor 4 to a Sporothrix schenckii lipid extract during experimental sporotrichosis. Immunology 2009, 128, 301–309. [Google Scholar] [CrossRef]
- Li, M.; Chen, Q.; Sun, J.; Shen, Y.; Liu, W. Inflammatory response of human keratinocytes triggered by Sporothrix schenckii via Toll-like receptor 2 and 4. J. Dermatol. Sci. 2012, 66, 80–82. [Google Scholar] [CrossRef]
- García-Lozano, A.; Toriello, C.; Antonio-Herrera, L.; Bonifaz, L.C. Sporothrix schenckii Immunization, but not infection, induces protective Th17 responses mediated by circulating memory CD4(+) T cells. Front. Microbiol. 2018, 9, 1275. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Huang, H.; Xue, R.; Hu, X.; Li, M.; Zhong, Y.; Yuan, L. Taenia taeniaeformis in rat favors protracted skin lesions caused by Sporothrix schenckii infection: Dectin-1 and IL-17 are dispensable for clearance of this fungus. PLoS ONE 2012, 7, e52514. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, L.S.; Gonçalves, A.C.; Portuondo, D.L.; Maia, D.C.; Placeres, M.C.; Batista-Duharte, A.; Carlos, I.Z. Optimal clearance of Sporothrix schenckii requires an intact Th17 response in a mouse model of systemic infection. Immunobiology 2015, 220, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.C.; Ferreira, L.S.; Manente, F.A.; de Faria, C.; Polesi, M.C.; de Andrade, C.R.; Zamboni, D.S.; Carlos, I.Z. The NLRP3 inflammasome contributes to host protection during Sporothrix schenckii infection. Immunology 2017, 151, 154–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negrini, T.D.C.; Ferreira, L.S.; Arthur, R.A.; Alegranci, P.; Placeres, M.C.; Spolidorio, L.C.; Carlos, I.Z. Influence of TLR-2 in the immune response in the infection induced by fungus Sporothrix schenckii. Immunol. Investig. 2014, 43, 370–390. [Google Scholar] [CrossRef] [PubMed]
- Breda, L.C.D.; Breda, C.N.S.; de Almeida, J.R.F.; Paulo, L.N.M.; Jannuzzi, G.P.; Menezes, I.G.; Albuquerque, R.C.; Câmara, N.O.S.; Ferreira, K.S.; de Almeida, S.R. Fonsecaea pedrosoi conidia and hyphae activate neutrophils distinctly: Requirement of TLR-2 and TLR-4 in neutrophil effector functions. Front. Immunol. 2020, 11, 540064. [Google Scholar] [CrossRef] [PubMed]
- De Castro, R.J.A.; Siqueira, I.M.; Jerônimo, M.S.; Basso, A.M.M.; Veloso Junior, P.H.H.; Magalhães, K.G.; Leonhardt, L.C.; de Oliveira, S.A.M.; Bürgel, P.H.; Tavares, A.H.; et al. The major Chromoblastomycosis etiologic agent Fonsecaea pedrosoi activates the NLRP3 inflammasome. Front. Immunol. 2017, 8, 1572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wevers, B.A.; Kaptein, T.M.; Zijlstra-Willems, E.M.; Theelen, B.; Boekhout, T.; Geijtenbeek, T.B.; Gringhuis, S.I. Fungal engagement of the C-type lectin mincle suppresses dectin-1-induced antifungal immunity. Cell Host Microbe 2014, 15, 494–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siqueira, I.M.; Wüthrich, M.; Li, M.; Wang, H.; Las-Casas, L.O.; de Castro, R.J.A.; Klein, B.; Bocca, A.L. Early immune response against Fonsecaea pedrosoi requires Dectin-2-mediated Th17 activity, whereas Th1 response, aided by Treg cells, is crucial for fungal clearance in later stage of experimental chromoblastomycosis. PLoS Negl. Trop. Dis. 2020, 14, e0008386. [Google Scholar] [CrossRef]
- Dong, B.; Tong, Z.; Li, R.; Chen, S.C.; Liu, W.; Liu, W.; Chen, Y.; Zhang, X.; Duan, Y.; Li, D.; et al. Transformation of Fonsecaea pedrosoi into sclerotic cells links to the refractoriness of experimental chromoblastomycosis in BALB/c mice via a mechanism involving a chitin-induced impairment of IFN-γ production. PLoS Negl. Trop. Dis. 2018, 12, e0006237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, A.A.; Criado, P.R.; Nunes, R.S.; da Silva, W.L.; Kanashiro-Galo, L.; Duarte, M.I.; Sotto, M.N.; Pagliari, C. In situ immune response in human chromoblastomycosis—A possible role for regulatory and Th17 T cells. PLoS Negl. Trop. Dis. 2014, 8, e3162. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Li, D.; Li, R.; Chen, S.C.; Liu, W.; Liu, W.; Chen, L.; Chen, Y.; Zhang, X.; Tong, Z.; et al. A chitin-like component on sclerotic cells of Fonsecaea pedrosoi inhibits Dectin-1-mediated murine Th17 development by masking β-glucans. PLoS ONE 2014, 9, e114113. [Google Scholar] [CrossRef]
- Sousa Mda, G.; Reid, D.M.; Schweighoffer, E.; Tybulewicz, V.; Ruland, J.; Langhorne, J.; Yamasaki, S.; Taylor, P.R.; Almeida, S.R.; Brown, G.D. Restoration of pattern recognition receptor costimulation to treat chromoblastomycosis, a chronic fungal infection of the skin. Cell Host Microbe 2011, 9, 436–443. [Google Scholar] [CrossRef]
- Figueiredo, R.T.; Bittencourt, V.C.; Lopes, L.C.; Sassaki, G.; Barreto-Bergter, E. Toll-Like receptors (TLR2 and TLR4) recognize polysaccharides of Pseudallescheria boydii cell wall. Carbohydr. Res. 2012, 356, 260–264. [Google Scholar] [CrossRef]
- Figueiredo, R.T.; Fernandez, P.L.; Dutra, F.F.; González, Y.; Lopes, L.C.; Bittencourt, V.C.; Sassaki, G.L.; Barreto-Bergter, E.; Bozza, M.T. TLR4 recognizes Pseudallescheria boydii conidia and purified rhamnomannans. J. Biol. Chem. 2010, 285, 40714–40723. [Google Scholar] [CrossRef] [Green Version]
- Leeyaphan, C.; Hau, C.; Takeoka, S.; Tada, Y.; Bunyaratavej, S.; Pattanaprichakul, P.; Sitthinamsuwan, P.; Chaiprasert, A.; Sasajima, Y.; Makimura, K.; et al. Immune response in human chromoblastomycosis and eumycetoma-focusing on human interleukin-17A, interferon-gamma, tumour necrosis factor-alpha, interleukin-1 beta and human beta-defensin-2. Mycoses 2016, 59, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Vieira, I.R.; Fernandes, R.K.; Rodrigues, D.R.; Gorgulho, C.M.; Kaneno, R.; Soares, Â.M.V.C. TLR9 stimulation induces increase in fungicidal activity of human dendritic cells challenged with Paracoccidioides brasiliensis. Med. Mycol. 2018, 56, 911–915. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M.S.; Oliveira, A.F.; da Silva, T.A.; Fernandes, F.F.; Gonçales, R.A.; Almeida, F.; Roque-Barreira, M.C. Paracoccin induces M1 polarization of macrophages via interaction with TLR4. Front. Microbiol. 2016, 7, 1003. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Echeverri, C.; Puerta-Arias, J.D.; González, Á. Paracoccidioides brasiliensis activates mesenchymal stem cells through TLR2, TLR4, and Dectin-1. Med. Mycol. 2020, 59, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Peres da Silva, R.; Heiss, C.; Black, I.; Azadi, P.; Gerlach, J.Q.; Travassos, L.R.; Joshi, L.; Kilcoyne, M.; Puccia, R. Extracellular vesicles from Paracoccidioides pathogenic species transport polysaccharide and expose ligands for DC-SIGN receptors. Sci. Rep. 2015, 5, 14213. [Google Scholar] [CrossRef]
- Ruas, L.P.; Bernardes, E.S.; Fermino, M.L.; de Oliveira, L.L.; Hsu, D.K.; Liu, F.T.; Chammas, R.; Roque-Barreira, M.C. Lack of galectin-3 drives response to Paracoccidioides brasiliensis toward a Th2-biased immunity. PLoS ONE 2009, 4, e4519. [Google Scholar] [CrossRef]
- Loures, F.V.; Araújo, E.F.; Feriotti, C.; Bazan, S.B.; Calich, V.L. TLR-4 cooperates with Dectin-1 and mannose receptor to expand Th17 and Tc17 cells induced by Paracoccidioides brasiliensis stimulated dendritic cells. Front. Microbiol. 2015, 6, 261. [Google Scholar] [CrossRef]
- De Castro, L.F.; Longhi, L.N.A.; Paião, M.R.; Justo-Júnior, A.D.S.; de Jesus, M.B.; Blotta, M.; Mamoni, R.L. NLRP3 inflammasome is involved in the recognition of Paracoccidioides brasiliensis by human dendritic cells and in the induction of Th17 cells. J. Infect. 2018, 77, 137–144. [Google Scholar] [CrossRef]
- Ketelut-Carneiro, N.; Souza, C.O.S.; Benevides, L.; Gardinassi, L.G.; Silva, M.C.; Tavares, L.A.; Zamboni, D.S.; Silva, J.S. Caspase-11-Dependent IL-1α release boosts Th17 immunity against Paracoccidioides brasiliensis. PLoS Pathog. 2019, 15, e1007990. [Google Scholar] [CrossRef] [Green Version]
- De Araújo, E.F.; Feriotti, C.; Galdino, N.A.L.; Preite, N.W.; Calich, V.L.G.; Loures, F.V. The IDO-AhR axis controls Th17/Treg immunity in a pulmonary model of fungal infection. Front. Immunol. 2017, 8, 880. [Google Scholar] [CrossRef] [Green Version]
- Pagliari, C.; Fernandes, E.R.; Stegun, F.W.; da Silva, W.L.; Seixas Duarte, M.I.; Sotto, M.N. Paracoccidioidomycosis: Cells expressing IL17 and Foxp3 in cutaneous and mucosal lesions. Microb. Pathog. 2011, 50, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Loures, F.V.; Pina, A.; Felonato, M.; Feriotti, C.; de Araújo, E.F.; Calich, V.L. MyD88 signaling is required for efficient innate and adaptive immune responses to Paracoccidioides brasiliensis infection. Infect. Immun. 2011, 79, 2470–2480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loures, F.V.; Araújo, E.F.; Feriotti, C.; Bazan, S.B.; Costa, T.A.; Brown, G.D.; Calich, V.L. Dectin-1 induces M1 macrophages and prominent expansion of CD8+IL-17+ cells in pulmonary paracoccidioidomycosis. J. Infect. Dis. 2014, 210, 762–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feriotti, C.; de Araújo, E.F.; Loures, F.V.; da Costa, T.A.; Galdino, N.A.L.; Zamboni, D.S.; Calich, V.L.G. NOD-Like receptor P3 inflammasome controls protective Th1/Th17 immunity against pulmonary paracoccidioidomycosis. Front. Immunol. 2017, 8, 786. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, S. Susceptibility of TLR4-defective C3H/HeJ mice to Coccidioides posadasii infection. Med. Mycol. 2010, 48, 470–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; LeBert, V.; Hung, C.Y.; Galles, K.; Saijo, S.; Lin, X.; Cole, G.T.; Klein, B.S.; Wüthrich, M. C-Type lectin receptors differentially induce th17 cells and vaccine immunity to the endemic mycosis of North America. J. Immunol. 2014, 192, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.Y.; Castro-Lopez, N.; Cole, G.T. Card9- and MyD88-mediated gamma interferon and nitric oxide production is essential for resistance to subcutaneous Coccidioides posadasii infection. Infect. Immun. 2016, 84, 1166–1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wüthrich, M.; Gern, B.; Hung, C.Y.; Ersland, K.; Rocco, N.; Pick-Jacobs, J.; Galles, K.; Filutowicz, H.; Warner, T.; Evans, M.; et al. Vaccine-Induced protection against 3 systemic mycoses endemic to North America requires Th17 cells in mice. J. Clin. Investig. 2011, 121, 554–568. [Google Scholar] [CrossRef]
- Hung, C.Y.; Gonzalez, A.; Wüthrich, M.; Klein, B.S.; Cole, G.T. Vaccine immunity to coccidioidomycosis occurs by early activation of three signal pathways of T helper cell response (Th1, Th2, and Th17). Infect. Immun. 2011, 79, 4511–4522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawada, Y.; Setoyama, A.; Sakuragi, Y.; Saito-Sasaki, N.; Yoshioka, H.; Nakamura, M. The Role of IL-17-Producing Cells in Cutaneous Fungal Infections. Int. J. Mol. Sci. 2021, 22, 5794. https://doi.org/10.3390/ijms22115794
Sawada Y, Setoyama A, Sakuragi Y, Saito-Sasaki N, Yoshioka H, Nakamura M. The Role of IL-17-Producing Cells in Cutaneous Fungal Infections. International Journal of Molecular Sciences. 2021; 22(11):5794. https://doi.org/10.3390/ijms22115794
Chicago/Turabian StyleSawada, Yu, Ayako Setoyama, Yumiko Sakuragi, Natsuko Saito-Sasaki, Haruna Yoshioka, and Motonobu Nakamura. 2021. "The Role of IL-17-Producing Cells in Cutaneous Fungal Infections" International Journal of Molecular Sciences 22, no. 11: 5794. https://doi.org/10.3390/ijms22115794
APA StyleSawada, Y., Setoyama, A., Sakuragi, Y., Saito-Sasaki, N., Yoshioka, H., & Nakamura, M. (2021). The Role of IL-17-Producing Cells in Cutaneous Fungal Infections. International Journal of Molecular Sciences, 22(11), 5794. https://doi.org/10.3390/ijms22115794




