A Computational Model of Kidney Function in a Patient with Diabetes
Abstract
:1. Introduction
2. Results
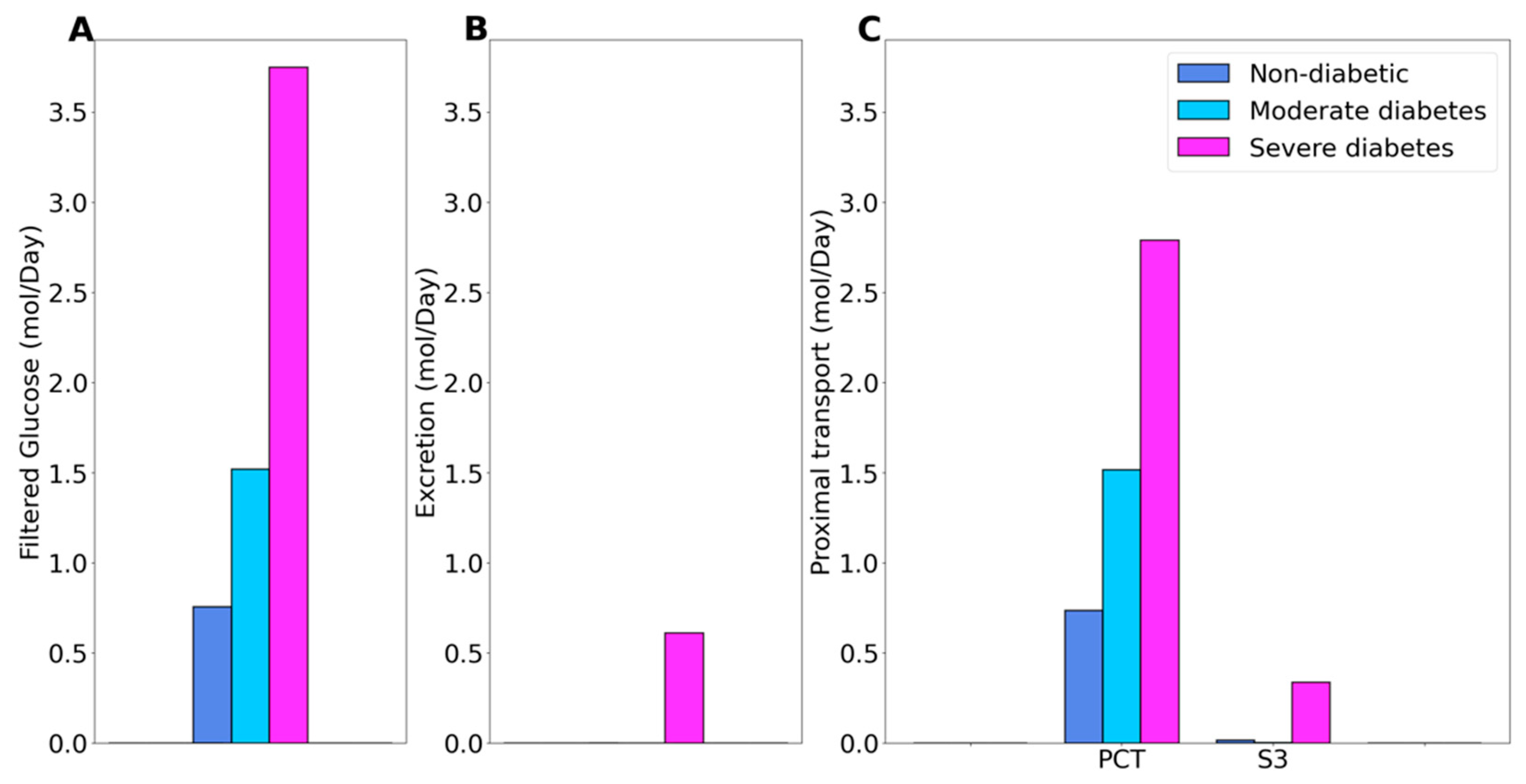
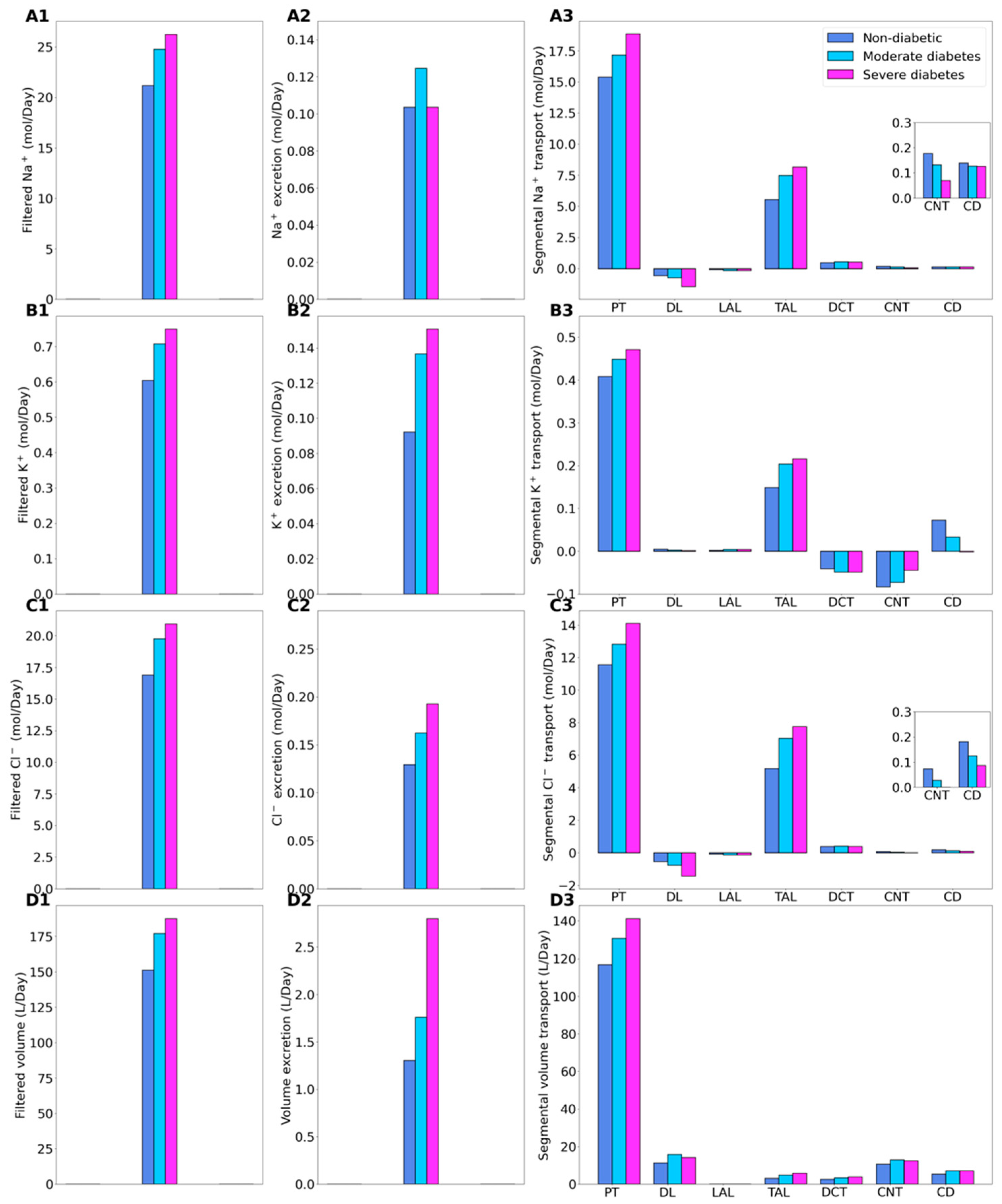
2.1. Kidney Function under Non-Diabetic and Diabetic Conditions
2.2. SGLT2 Inhibition in a Non-Diabetic Kidney
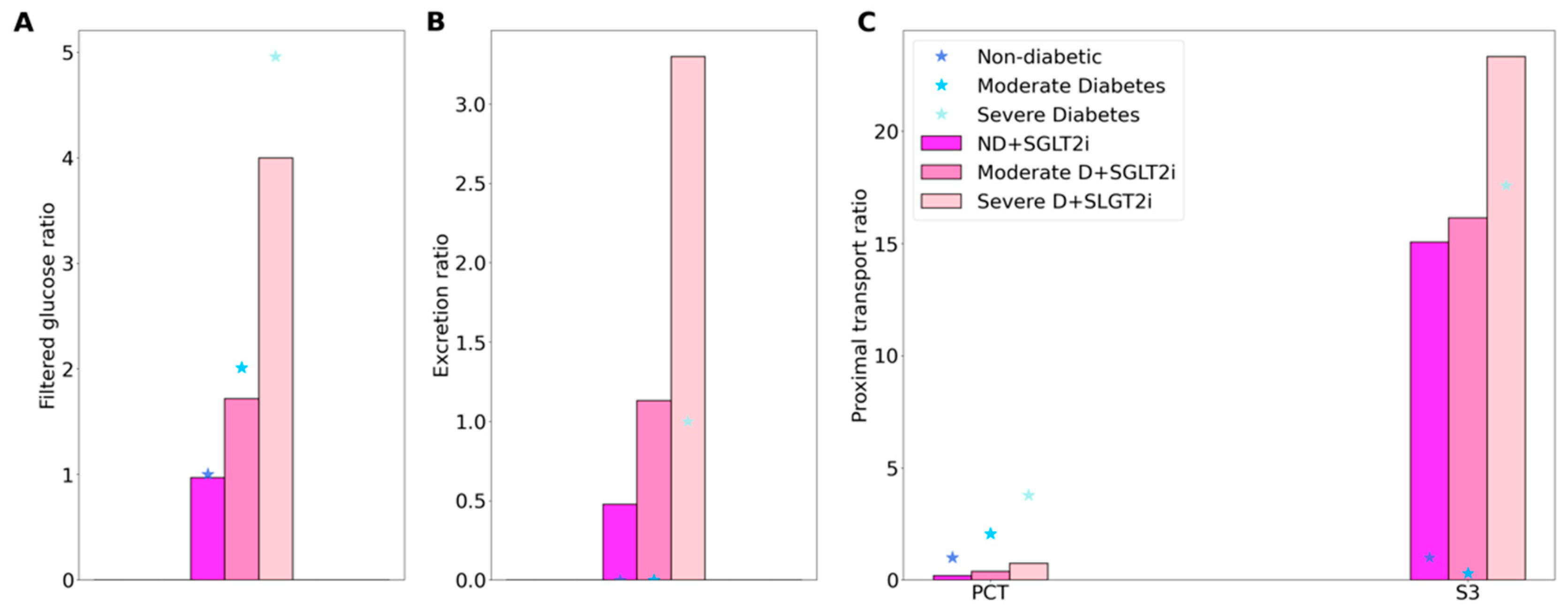
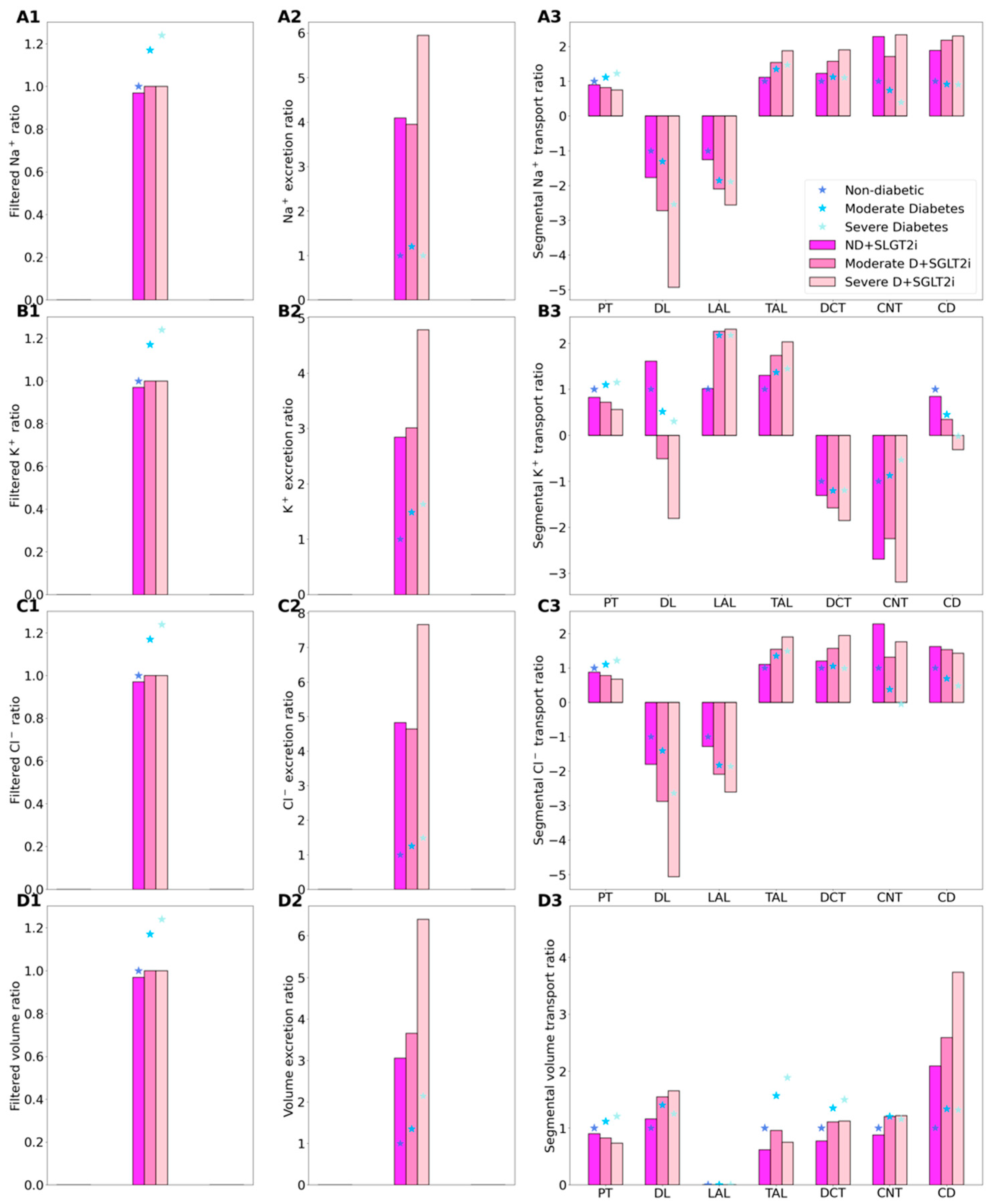
2.3. SGLT2 Inhibition in a Diabetic Kidney
3. Discussion
4. Materials and Methods
4.1. Glucose Transport in the Proximal Tubule
4.2. Simulating a Diabetic Kidney
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [Green Version]
- Foley, R.N.; Collins, A.J. End-stage renal disease in the United States: An update from the United States Renal Data System. J. Am. Soc. Nephrol. 2007, 18, 2644–2648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koye, D.N.; Magliano, D.J.; Nelson, R.G.; Pavkov, M.E. The global epidemiology of diabetes and kidney disease. Adv. Chronic Kidney Dis. 2018, 25, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, C. Glomerular filtration rate and renal plasma flow in short-term and long-term juvenile diabetes mellitus. Scand. J. Clin. Lab. Investig. 1971, 28, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Eaton, D.C.; Pooler, J.; Vander, A.J. Vander’s Renal Physiology, 7th ed.; McGraw-Hill Medical: New York, NY, USA, 2009. [Google Scholar]
- Hoenig, M.P.; Zeidel, M.L. Homeostasis, the milieu interieur, and the wisdom of the nephron. Clin. J. Am. Soc. Nephrol. 2014, 9, 1272–1281. [Google Scholar] [CrossRef] [Green Version]
- Dantzler, W.H.; Pannabecker, T.L.; Layton, A.T.; Layton, H.E. Urine concentrating mechanism in the inner medulla of the mammalian kidney: Role of three-dimensional architecture. Acta Physiol. 2011, 202, 361–378. [Google Scholar] [CrossRef] [Green Version]
- Palmer, L.G.; Schnermann, J. Integrated control of Na transport along the nephron. Clin. J. Am. Soc. Nephrol. 2015, 10, 676–687. [Google Scholar] [CrossRef] [Green Version]
- Layton, A.T. Feedback-mediated dynamics in a model of a compliant thick ascending limb. Math. Biosci. 2010, 228, 185–194. [Google Scholar] [CrossRef] [Green Version]
- Layton, A.T.; Layton, H.E. A Computational Model of Epithelial Solute and Water Transport along a Human Nephron. PLoS Comput. Biol. 2018, 15, e1006108. [Google Scholar] [CrossRef]
- Weinstein, A.M. A mathematical model of the rat kidney: K+-induced natriuresis. Am. J. Physiol. Renal Physiol. 2017, 312, F925–F950. [Google Scholar] [CrossRef] [Green Version]
- Layton, A.T.; Edwards, A.; Vallon, V. Renal potassium handling in rats with subtotal nephrectomy: Modeling and Analysis. Am. J. Physiol. Renal Physiol. 2017, 314, F643–F657. [Google Scholar] [CrossRef] [Green Version]
- Edwards, A.; Castrop, H.; Laghmani, K.; Vallon, V.; Layton, A.T. Effects of NKCC2 isoform regulation on NaCl transport in thick ascending limb and macula densa: A modeling study. Am. J. Physiol. Renal Physiol. 2014, 307, F137–F146. [Google Scholar] [CrossRef] [Green Version]
- Layton, A.T.; Laghmani, K.; Vallon, V.; Edwards, A. Solute transport and oxygen consumption along the nephrons: Effects of Na+ transport inhibitors. Am. J. Physiol. Renal Physiol. 2016, 311, F1217–F1229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Layton, A.T.; Vallon, V. SGLT2 Inhibition in a Kidney with Reduced Nephron Number: Modeling and Analysis of Solute Transport and Metabolism. Am. J. Physiol. Renal Physiol. 2018, 314, F969–F984. [Google Scholar] [CrossRef] [PubMed]
- Layton, A.T.; Vallon, V.; Edwards, A. Modeling oxygen consumption in the proximal tubule: Effects of NHE and SGLT2 inhibition. Am. J. Physiol. Renal Physiol. 2015, 308, F1343–F1357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Layton, A.T.; Vallon, V.; Edwards, A. Predicted consequences of diabetes and SGLT inhibition on transport and oxygen consumption along a rat nephron. Am. J. Physiol. Renal Physiol. 2016, 310, F1269–F1283. [Google Scholar] [CrossRef] [Green Version]
- Layton, A.T.; Vallon, V.; Edwards, A. Adaptive Changes in GFR, Tubular Morphology and Transport in Subtotal Nephrectomized Kidneys: Modeling and Analysis. Am. J. Physiol. Renal Physiol. 2017, 313, F199–F209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sgouralis, I.; Evans, R.G.; Gardiner, B.S.; Smith, J.A.; Fry, B.C.; Layton, A.T. Renal hemodynamics, function, and oxygenation during cardiac surgery performed on cardiopulmonary bypass: A modeling study. Physiol. Rep. 2015, 3, e12260. [Google Scholar] [CrossRef]
- Layton, A.T. Sweet success? SGLT2 inhibitors and diabetes. Am. J. Physiol. Renal Physiol. 2018, 314, F1034–F1035. [Google Scholar] [CrossRef]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Vallon, V.; Thomson, S.C. Targeting renal glucose reabsorption to treat hyperglycaemia: The pleiotropic effects of SGLT2 inhibition. Diabetologia 2017, 60, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Layton, A.T.; Vallon, V. Cardiovascular benefits of SGLT2 inhibition in diabetes and chronic kidney diseases. Acta Physiol. 2018, 222, e13050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, R.; McDonough, A.A.; Layton, T.A. Sex differences in solute and water handling in the human kidney: Modeling and functional implications. iScience. 2021. [Google Scholar]
- Nauck, M.A. Update on developments with SGLT2 inhibitors in the management of type 2 diabetes. Drug Des. Dev. Ther. 2014, 8, 1335. [Google Scholar] [CrossRef] [Green Version]
- Seman, L.; Macha, S.; Nehmiz, G.; Simons, G.; Ren, B.; Pinnetti, S.; Woerle, H.J.; Dugi, K. Empagliflozin (BI 10773), a potent and selective SGLT2 inhibitor, induces dose-dependent glucosuria in healthy subjects. Clin. Pharmacol. Drug Dev. 2013, 2, 152–161. [Google Scholar] [CrossRef]
- Cherney, D.Z.I.; Perkins, B.A.; Soleymanlou, N.; Maione, M.; Lai, V.; Lee, A.; Fagan, N.M.; Woerle, H.J.; Johansen, O.E.; Broedl, U.C.; et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 2014, 129, 587–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- List, J.F.; Whaley, J.M. Glucose dynamics and mechanistic implications of SGLT2 inhibitors in animals and humans. Kidney Int. 2011, 79, S20–S27. [Google Scholar] [CrossRef] [Green Version]
- Mordi, N.A.; Mordi, I.R.; Singh, J.S.; McCrimmon, R.J.; Struthers, A.D.; Lang, C.C. Renal and cardiovascular effects of SGLT2 inhibition in combination with loop diuretics in patients with type 2 diabetes and chronic heart failure: The Recede-CHF trial. Circulation 2020, 142, 1713–1724. [Google Scholar] [CrossRef]
- Vallon, V. The proximal tubule in the pathophysiology of the diabetic kidney. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R1009–R1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baines, A.; Ho, P. Glucose stimulates O2 consumption, NOS, and Na/H exchange in diabetic rat proximal tubules. Am. J. Physiol. Renal Physiol. 2002, 283, F286–F293. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Edwards, A.; Layton, A.T. Effects of pH and medullary blood flow on oxygen transport and sodium reabsorption in the rat outer medulla. Am. J. Physiol. Renal Physiol. 2010, 298, F1369–F1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fry, B.C.; Edwards, A.; Layton, A.T. Impacts of nitric oxide and superoxide on renal medullary oxygen transport and urine concentration. Am. J. Physiol. Renal Physiol. 2015, 308, F967–F980. [Google Scholar] [CrossRef]
- Fine, L.G.; Norman, J.T. Chronic hypoxia as a mechanism of progression of chronic kidney diseases: From hypothesis to novel therapeutics. Kidney Int. 2008, 74, 867–872. [Google Scholar] [CrossRef] [Green Version]
- Vallon, V.; Richter, K.; Blantz, R.C.; Thomson, S.; Osswald, H. Glomerular hyperfiltration in experimental diabetes mellitus: Potential role of tubular reabsorption. J. Am. Soc. Nephrol. 1999, 10, 2569–2576. [Google Scholar] [CrossRef] [PubMed]
- Thomson, S.C.; Vallon, V. Effects of SGLT2 Inhibitor and Dietary NaCl on Glomerular Hemodynamics Assessed by Micropuncture in Diabetic Rats. Am. J. Physiol. Renal Physiol. 2021, 320, F761–F771. [Google Scholar] [CrossRef] [PubMed]
- Barnett, A.H.; Mithal, A.; Manassie, J.; Jones, R.; Rattunde, H.; Woerle, H.J.; Broedl, U.C. EMPA-REG RENAL Trial Investigators Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: A randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2014, 2, 369–384. [Google Scholar] [CrossRef]
- Layton, A.T.; Vallon, V.; Edwards, A. A computational model for simulating solute transport and oxygen consumption along the nephrons. Am. J. Physiol. Renal Physiol. 2016, 311, F1378–F1390. [Google Scholar] [CrossRef] [PubMed]
- Veiras, L.C.; Girardi, A.C.C.; Curry, J.; Pei, L.; Ralph, D.L.; Tran, A.; Castelo-Branco, R.C.; Pastor-Soler, N.; Arranz, C.T.; Yu, A.S.L.; et al. Sexual Dimorphic Pattern of Renal Transporters and Electrolyte Homeostasis. J. Am. Soc. Nephrol. 2017, 28, 3504–3517. [Google Scholar] [CrossRef] [PubMed]
- Sabolic, I.; Vrhovac, I.; Eror, D.B.; Gerasimova, M.; Rose, M.; Breljak, D.; Ljubojevic, M.; Brzica, H.; Sebastiani, A.; Thal, S.C. Expression of Na+-d-glucose cotransporter SGLT2 in rodents is kidney-specific and exhibits sex and species differences. Am. J. Physiol. Cell Physiol. 2012, 302, C1174–C1188. [Google Scholar] [CrossRef] [Green Version]
- Stamler, J.; Stamler, R.; Riedlinger, W.F.; Algera, G.; Roberts, R.H. Hypertension screening of 1 million Americans: Community hypertension evaluation clinic (CHEC) program, 1973 through 1975. JAMA 1976, 235, 2299–2306. [Google Scholar] [CrossRef]
- Wenger, N.K.; Arnold, A.; Merz, C.N.B.; Cooper-DeHoff, R.M.; Ferdinand, K.C.; Fleg, J.L.; Gulati, M.; Isiadinso, I.; Itchhaporia, D.; Light-McGroary, K. Hypertension across a woman’s life cycle. J. Am. Coll. Cardiol. 2018, 71, 1797–1813. [Google Scholar] [CrossRef]
- Leete, J.; Layton, A.T. Sex-specific long-term blood pressure regulation: Modeling and analysis. Comput. Biol. Med. 2019, 104, 139–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leete, J.G.S.; Layton, A.T. Modeling Sex Differences in the Renin Angiotensin System and the Efficacy of Antihypertensive Therapies. Comput. Chem. Eng. 2018, 112, 253–264. [Google Scholar] [CrossRef]
- Ahmed, S.; Layton, A.T. Sex-specific computational models for blood pressure regulation in the rat. Am. J. Physiol. Renal Physiol. 2019, 318, F888–F900. [Google Scholar] [CrossRef]
- Hu, R.; McDonough, A.A.; Layton, A.T. Functional implications of the sex differences in transporter abundance along the rat nephron: Modeling and analysis. Am. J. Physiol. Renal Physiol. 2019, 317, F1462–F1474. [Google Scholar] [CrossRef]
- Hu, R.; McDonough, A.A.; Layton, A.T. Sex-Differences in Solute Transport along the Nephrons: Effects of Na+ Transport Inhibition. Am. J. Physiol. Renal Physiol. 2020, 319, F487–F505. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; McDonough, A.A.; Layton, H.E.; Layton, A.T. Functional implications of sexual dimorphism of transporter patterns along the rat proximal tubule: Modeling and analysis. Am. J. Physiol. Renal Physiol. 2018, 315, F692–F700. [Google Scholar] [CrossRef] [PubMed]
- Maric-Bilkan, C. Sex Differences in Diabetic Kidney Disease. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Oliver, J. Nephrons and Kidneys: A Qualitative Study of Development and Evolutionary Mammalian Renal Architecture; Harper and Row: New York, NY, USA, 1968. [Google Scholar]
- Abdul-Ghani, M.A.; DeFronzo, R.A.; Norton, L. Novel hypothesis to explain why SGLT2 inhibitors inhibit only 30–50% of filtered glucose load in humans. Diabetes 2013, 62, 3324–3328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumgartl, H.-J.; Sigl, G.; Banholzer, P.; Haslbeck, M.; Standl, E. On the prognosis of IDDM patients with large kidneys. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Renal Assoc. 1998, 13, 630–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, R.; Layton, A. A Computational Model of Kidney Function in a Patient with Diabetes. Int. J. Mol. Sci. 2021, 22, 5819. https://doi.org/10.3390/ijms22115819
Hu R, Layton A. A Computational Model of Kidney Function in a Patient with Diabetes. International Journal of Molecular Sciences. 2021; 22(11):5819. https://doi.org/10.3390/ijms22115819
Chicago/Turabian StyleHu, Rui, and Anita Layton. 2021. "A Computational Model of Kidney Function in a Patient with Diabetes" International Journal of Molecular Sciences 22, no. 11: 5819. https://doi.org/10.3390/ijms22115819
APA StyleHu, R., & Layton, A. (2021). A Computational Model of Kidney Function in a Patient with Diabetes. International Journal of Molecular Sciences, 22(11), 5819. https://doi.org/10.3390/ijms22115819




